 Found 367 hits with Last Name = 'bornemeier' and Initial = 'da'
Found 367 hits with Last Name = 'bornemeier' and Initial = 'da' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Collagenase 3
(Homo sapiens (Human)) | BDBM50234334

(BENZYL 6-BENZYL-5,7-DIOXO-6,7-DIHYDRO-5H-[1,3]THIA...)Show SMILES O=C(OCc1ccccc1)c1cn2c(cc(=O)n(Cc3ccccc3)c2=O)s1 Show InChI InChI=1S/C21H16N2O4S/c24-18-11-19-23(21(26)22(18)12-15-7-3-1-4-8-15)13-17(28-19)20(25)27-14-16-9-5-2-6-10-16/h1-11,13H,12,14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length MMP13 by steady state kinetic assay |
J Biol Chem 282: 27781-91 (2007)
Article DOI: 10.1074/jbc.M703286200
BindingDB Entry DOI: 10.7270/Q2Z89C58 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Collagenase 3
(Homo sapiens (Human)) | BDBM50265079

((4-[1-methyl-2,4-dioxo-6-(3-phenyl-prop-1-ynyl)-1,...)Show SMILES Cn1c2ccc(cc2c(=O)n(Cc2ccc(cc2)C(O)=O)c1=O)C#CCc1ccccc1 Show InChI InChI=1S/C26H20N2O4/c1-27-23-15-12-19(9-5-8-18-6-3-2-4-7-18)16-22(23)24(29)28(26(27)32)17-20-10-13-21(14-11-20)25(30)31/h2-4,6-7,10-16H,8,17H2,1H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP13 catalytic domain |
J Biol Chem 282: 27781-91 (2007)
Article DOI: 10.1074/jbc.M703286200
BindingDB Entry DOI: 10.7270/Q2Z89C58 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP1 |
J Biol Chem 282: 27781-91 (2007)
Article DOI: 10.1074/jbc.M703286200
BindingDB Entry DOI: 10.7270/Q2Z89C58 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-17
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP17 catalytic domain |
J Biol Chem 282: 27781-91 (2007)
Article DOI: 10.1074/jbc.M703286200
BindingDB Entry DOI: 10.7270/Q2Z89C58 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP14 catalytic domain |
J Biol Chem 282: 27781-91 (2007)
Article DOI: 10.1074/jbc.M703286200
BindingDB Entry DOI: 10.7270/Q2Z89C58 |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP12 catalytic domain |
J Biol Chem 282: 27781-91 (2007)
Article DOI: 10.1074/jbc.M703286200
BindingDB Entry DOI: 10.7270/Q2Z89C58 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP13 catalytic domain |
J Biol Chem 282: 27781-91 (2007)
Article DOI: 10.1074/jbc.M703286200
BindingDB Entry DOI: 10.7270/Q2Z89C58 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50044072
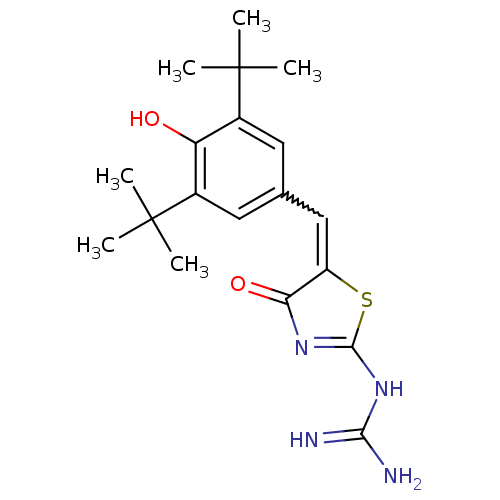
(CHEMBL16434 | N-[5-(3,5-Di-tert-butyl-4-hydroxy-be...)Show SMILES CC(C)(C)c1cc(C=C2SC(NC(N)=N)=NC2=O)cc(c1O)C(C)(C)C |w:7.6,c:14| Show InChI InChI=1S/C19H26N4O2S/c1-18(2,3)11-7-10(8-12(14(11)24)19(4,5)6)9-13-15(25)22-17(26-13)23-16(20)21/h7-9,24H,1-6H3,(H4,20,21,22,23,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
IC50 against ovine Prostaglandin G/H synthase 1 |
J Med Chem 42: 1151-60 (1999)
Article DOI: 10.1021/jm9805081
BindingDB Entry DOI: 10.7270/Q24X56Z3 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
IC50 value was determined against Prostaglandin G/H synthase 2 of J7744A.1 cell lines. |
J Med Chem 42: 1151-60 (1999)
Article DOI: 10.1021/jm9805081
BindingDB Entry DOI: 10.7270/Q24X56Z3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
IC50 value against Prostaglandin G/H synthase 2 of murine J774A.1 cell line |
J Med Chem 42: 1161-9 (1999)
Article DOI: 10.1021/jm980570y
BindingDB Entry DOI: 10.7270/Q2154G6N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
J Biol Chem 282: 27781-91 (2007)
Article DOI: 10.1074/jbc.M703286200
BindingDB Entry DOI: 10.7270/Q2Z89C58 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 catalytic domain |
J Biol Chem 282: 27781-91 (2007)
Article DOI: 10.1074/jbc.M703286200
BindingDB Entry DOI: 10.7270/Q2Z89C58 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
IC50 against Prostaglandin G/H synthase 1 from human platelet rich plasma |
J Med Chem 42: 1151-60 (1999)
Article DOI: 10.1021/jm9805081
BindingDB Entry DOI: 10.7270/Q24X56Z3 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
IC50 value against Prostaglandin G/H synthase 1 of human platelet rich plasma |
J Med Chem 42: 1161-9 (1999)
Article DOI: 10.1021/jm980570y
BindingDB Entry DOI: 10.7270/Q2154G6N |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50046902

(2,6-Di-tert-butyl-4-(5-methylsulfanyl-[1,3,4]thiad...)Show SMILES CSc1nnc(s1)-c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C17H24N2OS2/c1-16(2,3)11-8-10(14-18-19-15(21-7)22-14)9-12(13(11)20)17(4,5)6/h8-9,20H,1-7H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
IC50 value against Prostaglandin G/H synthase 2 of murine J774A.1 cell line |
J Med Chem 42: 1161-9 (1999)
Article DOI: 10.1021/jm980570y
BindingDB Entry DOI: 10.7270/Q2154G6N |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP8 catalytic domain |
J Biol Chem 282: 27781-91 (2007)
Article DOI: 10.1074/jbc.M703286200
BindingDB Entry DOI: 10.7270/Q2Z89C58 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
J Biol Chem 282: 27781-91 (2007)
Article DOI: 10.1074/jbc.M703286200
BindingDB Entry DOI: 10.7270/Q2Z89C58 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50075529
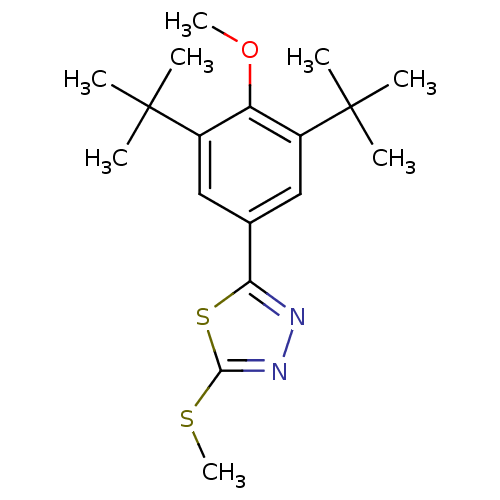
(2-(3,5-Di-tert-butyl-4-methoxy-phenyl)-5-methylsul...)Show InChI InChI=1S/C18H26N2OS2/c1-17(2,3)12-9-11(15-19-20-16(22-8)23-15)10-13(14(12)21-7)18(4,5)6/h9-10H,1-8H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
IC50 value against Prostaglandin G/H synthase 2 of murine J774A.1 cell line |
J Med Chem 42: 1161-9 (1999)
Article DOI: 10.1021/jm980570y
BindingDB Entry DOI: 10.7270/Q2154G6N |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
IC50 value against ovine Prostaglandin G/H synthase 1 |
J Med Chem 42: 1161-9 (1999)
Article DOI: 10.1021/jm980570y
BindingDB Entry DOI: 10.7270/Q2154G6N |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
IC50 against ovine Prostaglandin G/H synthase 1 |
J Med Chem 42: 1151-60 (1999)
Article DOI: 10.1021/jm9805081
BindingDB Entry DOI: 10.7270/Q24X56Z3 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50075548
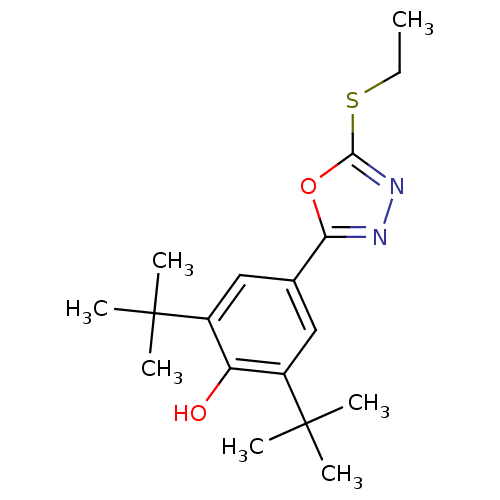
(2,6-Di-tert-butyl-4-(5-ethylsulfanyl-[1,3,4]oxadia...)Show SMILES CCSc1nnc(o1)-c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C18H26N2O2S/c1-8-23-16-20-19-15(22-16)11-9-12(17(2,3)4)14(21)13(10-11)18(5,6)7/h9-10,21H,8H2,1-7H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
IC50 value against Prostaglandin G/H synthase 2 of murine J774A.1 cell line |
J Med Chem 42: 1161-9 (1999)
Article DOI: 10.1021/jm980570y
BindingDB Entry DOI: 10.7270/Q2154G6N |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50075551
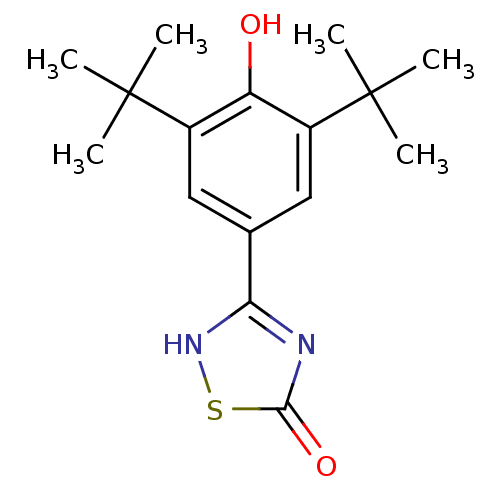
(3-(3,5-Di-tert-butyl-4-hydroxy-phenyl)-[1,2,4]thia...)Show SMILES CC(C)(C)c1cc(cc(c1O)C(C)(C)C)-c1nc(=O)s[nH]1 Show InChI InChI=1S/C16H22N2O2S/c1-15(2,3)10-7-9(13-17-14(20)21-18-13)8-11(12(10)19)16(4,5)6/h7-8,19H,1-6H3,(H,17,18,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
IC50 value against Prostaglandin G/H synthase 2 of murine J774A.1 cell line |
J Med Chem 42: 1161-9 (1999)
Article DOI: 10.1021/jm980570y
BindingDB Entry DOI: 10.7270/Q2154G6N |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50075541

(4,6-Di-tert-butyl-2-(5-ethylsulfanyl-[1,3,4]thiadi...)Show SMILES CCSc1nnc(s1)-c1nc(c(O)c(n1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C16H24N4OS2/c1-8-22-14-20-19-13(23-14)12-17-10(15(2,3)4)9(21)11(18-12)16(5,6)7/h21H,8H2,1-7H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
IC50 value against Prostaglandin G/H synthase 2 of murine J774A.1 cell line |
J Med Chem 42: 1161-9 (1999)
Article DOI: 10.1021/jm980570y
BindingDB Entry DOI: 10.7270/Q2154G6N |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50029593

(CHEMBL7162 | N-(2-(cyclohexyloxy)-4-nitrophenyl)me...)Show InChI InChI=1S/C13H18N2O5S/c1-21(18,19)14-12-8-7-10(15(16)17)9-13(12)20-11-5-3-2-4-6-11/h7-9,11,14H,2-6H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
IC50 value was determined against Prostaglandin G/H synthase 2 of J7744A.1 cell lines. |
J Med Chem 42: 1151-60 (1999)
Article DOI: 10.1021/jm9805081
BindingDB Entry DOI: 10.7270/Q24X56Z3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50029593

(CHEMBL7162 | N-(2-(cyclohexyloxy)-4-nitrophenyl)me...)Show InChI InChI=1S/C13H18N2O5S/c1-21(18,19)14-12-8-7-10(15(16)17)9-13(12)20-11-5-3-2-4-6-11/h7-9,11,14H,2-6H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
IC50 value against Prostaglandin G/H synthase 2 of murine J774A.1 cell line |
J Med Chem 42: 1161-9 (1999)
Article DOI: 10.1021/jm980570y
BindingDB Entry DOI: 10.7270/Q2154G6N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50075519
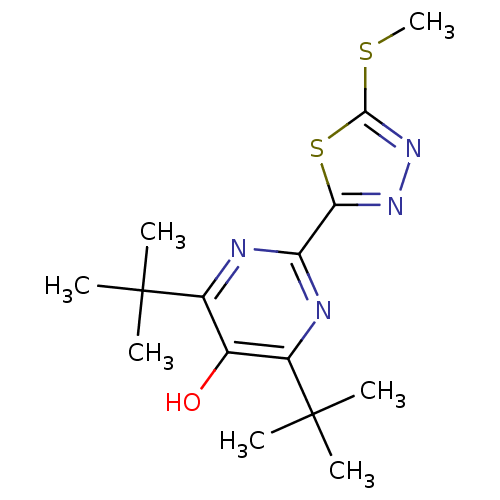
(4,6-Di-tert-butyl-2-(5-methylsulfanyl-[1,3,4]thiad...)Show SMILES CSc1nnc(s1)-c1nc(c(O)c(n1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C15H22N4OS2/c1-14(2,3)9-8(20)10(15(4,5)6)17-11(16-9)12-18-19-13(21-7)22-12/h20H,1-7H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
IC50 value against Prostaglandin G/H synthase 2 of murine J774A.1 cell line |
J Med Chem 42: 1161-9 (1999)
Article DOI: 10.1021/jm980570y
BindingDB Entry DOI: 10.7270/Q2154G6N |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50075539

(3-(3,5-Di-tert-butyl-4-hydroxy-phenyl)-[1,2,4]thia...)Show SMILES CC(C)(C)c1cc(cc(c1O)C(C)(C)C)-c1nsc(NC#N)n1 Show InChI InChI=1S/C17H22N4OS/c1-16(2,3)11-7-10(8-12(13(11)22)17(4,5)6)14-20-15(19-9-18)23-21-14/h7-8,22H,1-6H3,(H,19,20,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
IC50 value against Prostaglandin G/H synthase 2 of murine J774A.1 cell line |
J Med Chem 42: 1161-9 (1999)
Article DOI: 10.1021/jm980570y
BindingDB Entry DOI: 10.7270/Q2154G6N |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50044068

(CHEMBL15904 | N-[5-(3,5-Di-tert-butyl-4-hydroxy-be...)Show SMILES CN1C(NC(N)=N)=NC(=O)C1=Cc1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C |w:11.12,c:6| Show InChI InChI=1S/C20H29N5O2/c1-19(2,3)12-8-11(9-13(15(12)26)20(4,5)6)10-14-16(27)23-18(25(14)7)24-17(21)22/h8-10,26H,1-7H3,(H4,21,22,23,24,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
IC50 against ovine Prostaglandin G/H synthase 1 |
J Med Chem 42: 1151-60 (1999)
Article DOI: 10.1021/jm9805081
BindingDB Entry DOI: 10.7270/Q24X56Z3 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50234334

(BENZYL 6-BENZYL-5,7-DIOXO-6,7-DIHYDRO-5H-[1,3]THIA...)Show SMILES O=C(OCc1ccccc1)c1cn2c(cc(=O)n(Cc3ccccc3)c2=O)s1 Show InChI InChI=1S/C21H16N2O4S/c24-18-11-19-23(21(26)22(18)12-15-7-3-1-4-8-15)13-17(28-19)20(25)27-14-16-9-5-2-6-10-16/h1-11,13H,12,14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP13 catalytic domain |
J Biol Chem 282: 27781-91 (2007)
Article DOI: 10.1074/jbc.M703286200
BindingDB Entry DOI: 10.7270/Q2Z89C58 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Matrilysin
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP7 |
J Biol Chem 282: 27781-91 (2007)
Article DOI: 10.1074/jbc.M703286200
BindingDB Entry DOI: 10.7270/Q2Z89C58 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50075539

(3-(3,5-Di-tert-butyl-4-hydroxy-phenyl)-[1,2,4]thia...)Show SMILES CC(C)(C)c1cc(cc(c1O)C(C)(C)C)-c1nsc(NC#N)n1 Show InChI InChI=1S/C17H22N4OS/c1-16(2,3)11-7-10(8-12(13(11)22)17(4,5)6)14-20-15(19-9-18)23-21-14/h7-8,22H,1-6H3,(H,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human prostaglandin G/H synthase 2 (COX-2) |
J Med Chem 42: 1161-9 (1999)
Article DOI: 10.1021/jm980570y
BindingDB Entry DOI: 10.7270/Q2154G6N |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50234334

(BENZYL 6-BENZYL-5,7-DIOXO-6,7-DIHYDRO-5H-[1,3]THIA...)Show SMILES O=C(OCc1ccccc1)c1cn2c(cc(=O)n(Cc3ccccc3)c2=O)s1 Show InChI InChI=1S/C21H16N2O4S/c24-18-11-19-23(21(26)22(18)12-15-7-3-1-4-8-15)13-17(28-19)20(25)27-14-16-9-5-2-6-10-16/h1-11,13H,12,14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length MMP13-mediated type 2 collagen cleavage |
J Biol Chem 282: 27781-91 (2007)
Article DOI: 10.1074/jbc.M703286200
BindingDB Entry DOI: 10.7270/Q2Z89C58 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50044059
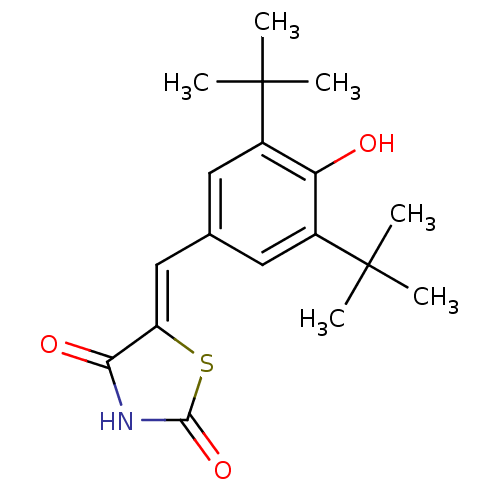
(5-(3,5-Di-tert-butyl-4-hydroxy-benzylidene)-thiazo...)Show SMILES CC(C)(C)c1cc(\C=C2/SC(=O)NC2=O)cc(c1O)C(C)(C)C Show InChI InChI=1S/C18H23NO3S/c1-17(2,3)11-7-10(8-12(14(11)20)18(4,5)6)9-13-15(21)19-16(22)23-13/h7-9,20H,1-6H3,(H,19,21,22)/b13-9- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
IC50 value was determined against Prostaglandin G/H synthase 2 of J7744A.1 cell lines. |
J Med Chem 42: 1151-60 (1999)
Article DOI: 10.1021/jm9805081
BindingDB Entry DOI: 10.7270/Q24X56Z3 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50075526
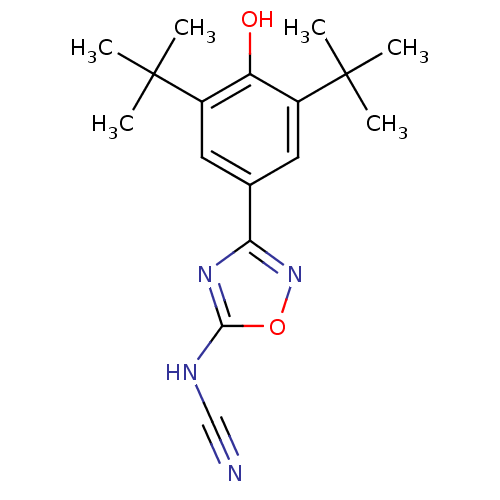
(3-(3,5-Di-tert-butyl-4-hydroxy-phenyl)-[1,2,4]oxad...)Show SMILES CC(C)(C)c1cc(cc(c1O)C(C)(C)C)-c1noc(NC#N)n1 Show InChI InChI=1S/C17H22N4O2/c1-16(2,3)11-7-10(8-12(13(11)22)17(4,5)6)14-20-15(19-9-18)23-21-14/h7-8,22H,1-6H3,(H,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human prostaglandin G/H synthase 2 (COX-2) |
J Med Chem 42: 1161-9 (1999)
Article DOI: 10.1021/jm980570y
BindingDB Entry DOI: 10.7270/Q2154G6N |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50044080
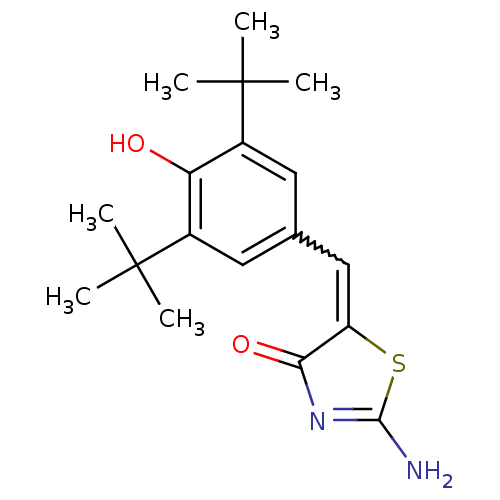
(2-Amino-5-[1-(3,5-di-tert-butyl-4-hydroxy-phenyl)-...)Show SMILES CC(C)(C)c1cc(C=C2SC(N)=NC2=O)cc(c1O)C(C)(C)C |w:7.6,c:11| Show InChI InChI=1S/C18H24N2O2S/c1-17(2,3)11-7-10(8-12(14(11)21)18(4,5)6)9-13-15(22)20-16(19)23-13/h7-9,21H,1-6H3,(H2,19,20,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
IC50 value was determined against Prostaglandin G/H synthase 2 of J7744A.1 cell lines. |
J Med Chem 42: 1151-60 (1999)
Article DOI: 10.1021/jm9805081
BindingDB Entry DOI: 10.7270/Q24X56Z3 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50075527

(2,6-Di-tert-butyl-4-(5-ethoxy-[1,3,4]thiadiazol-2-...)Show SMILES CCOc1nnc(s1)-c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C18H26N2O2S/c1-8-22-16-20-19-15(23-16)11-9-12(17(2,3)4)14(21)13(10-11)18(5,6)7/h9-10,21H,8H2,1-7H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
IC50 value against Prostaglandin G/H synthase 2 of murine J774A.1 cell line |
J Med Chem 42: 1161-9 (1999)
Article DOI: 10.1021/jm980570y
BindingDB Entry DOI: 10.7270/Q2154G6N |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50029616
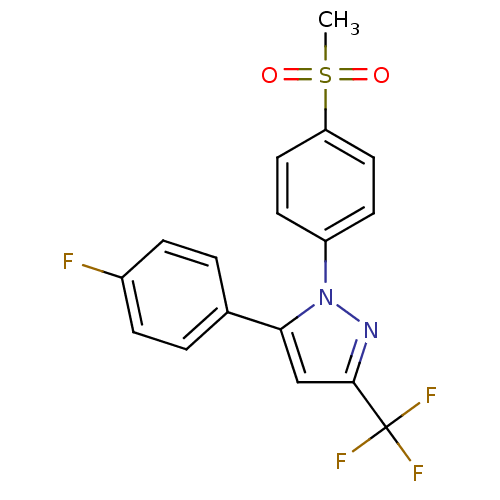
(5-(4-Fluoro-phenyl)-1-(4-methanesulfonyl-phenyl)-3...)Show SMILES CS(=O)(=O)c1ccc(cc1)-n1nc(cc1-c1ccc(F)cc1)C(F)(F)F Show InChI InChI=1S/C17H12F4N2O2S/c1-26(24,25)14-8-6-13(7-9-14)23-15(10-16(22-23)17(19,20)21)11-2-4-12(18)5-3-11/h2-10H,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
IC50 value against Prostaglandin G/H synthase 2 of murine J774A.1 cell line |
J Med Chem 42: 1161-9 (1999)
Article DOI: 10.1021/jm980570y
BindingDB Entry DOI: 10.7270/Q2154G6N |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50029616

(5-(4-Fluoro-phenyl)-1-(4-methanesulfonyl-phenyl)-3...)Show SMILES CS(=O)(=O)c1ccc(cc1)-n1nc(cc1-c1ccc(F)cc1)C(F)(F)F Show InChI InChI=1S/C17H12F4N2O2S/c1-26(24,25)14-8-6-13(7-9-14)23-15(10-16(22-23)17(19,20)21)11-2-4-12(18)5-3-11/h2-10H,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
IC50 value was determined against Prostaglandin G/H synthase 2 of J7744A.1 cell lines. |
J Med Chem 42: 1151-60 (1999)
Article DOI: 10.1021/jm9805081
BindingDB Entry DOI: 10.7270/Q24X56Z3 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50044081

(5-(3,5-Di-tert-butyl-4-hydroxy-benzylidene)-oxazol...)Show SMILES CC(C)(C)c1cc(\C=C2/OC(=O)NC2=O)cc(c1O)C(C)(C)C Show InChI InChI=1S/C18H23NO4/c1-17(2,3)11-7-10(8-12(14(11)20)18(4,5)6)9-13-15(21)19-16(22)23-13/h7-9,20H,1-6H3,(H,19,21,22)/b13-9- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
IC50 value was determined against Prostaglandin G/H synthase 2 of J7744A.1 cell lines. |
J Med Chem 42: 1151-60 (1999)
Article DOI: 10.1021/jm9805081
BindingDB Entry DOI: 10.7270/Q24X56Z3 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50075514

(5-[1-(3,5-Di-tert-butyl-4-hydroxy-phenyl)-meth-(Z)...)Show SMILES CC(C)(C)c1cc(\C=C2/NC(=S)NC2=O)cc(c1O)C(C)(C)C Show InChI InChI=1S/C18H24N2O2S/c1-17(2,3)11-7-10(8-12(14(11)21)18(4,5)6)9-13-15(22)20-16(23)19-13/h7-9,21H,1-6H3,(H2,19,20,22,23)/b13-9- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
IC50 value was determined against Prostaglandin G/H synthase 2 of J7744A.1 cell lines. |
J Med Chem 42: 1151-60 (1999)
Article DOI: 10.1021/jm9805081
BindingDB Entry DOI: 10.7270/Q24X56Z3 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50044081

(5-(3,5-Di-tert-butyl-4-hydroxy-benzylidene)-oxazol...)Show SMILES CC(C)(C)c1cc(\C=C2/OC(=O)NC2=O)cc(c1O)C(C)(C)C Show InChI InChI=1S/C18H23NO4/c1-17(2,3)11-7-10(8-12(14(11)20)18(4,5)6)9-13-15(21)19-16(22)23-13/h7-9,20H,1-6H3,(H,19,21,22)/b13-9- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
IC50 against ovine Prostaglandin G/H synthase 1 |
J Med Chem 42: 1151-60 (1999)
Article DOI: 10.1021/jm9805081
BindingDB Entry DOI: 10.7270/Q24X56Z3 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50075519

(4,6-Di-tert-butyl-2-(5-methylsulfanyl-[1,3,4]thiad...)Show SMILES CSc1nnc(s1)-c1nc(c(O)c(n1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C15H22N4OS2/c1-14(2,3)9-8(20)10(15(4,5)6)17-11(16-9)12-18-19-13(21-7)22-12/h20H,1-7H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
IC50 value against ovine Prostaglandin G/H synthase 1 |
J Med Chem 42: 1161-9 (1999)
Article DOI: 10.1021/jm980570y
BindingDB Entry DOI: 10.7270/Q2154G6N |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(RAT) | BDBM22971
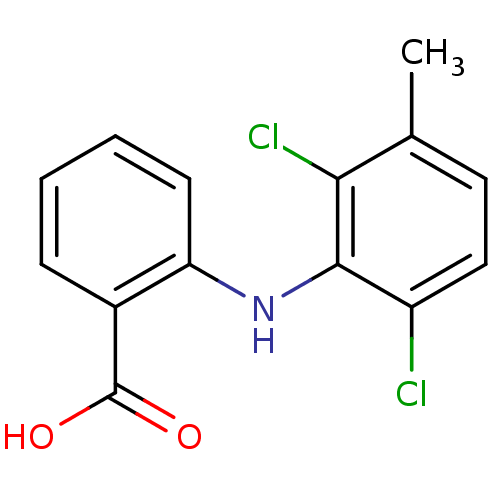
(2-[(2,6-dichloro-3-methylphenyl)amino]benzoic acid...)Show InChI InChI=1S/C14H11Cl2NO2/c1-8-6-7-10(15)13(12(8)16)17-11-5-3-2-4-9(11)14(18)19/h2-7,17H,1H3,(H,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of Prostaglandin G/H synthase mediated PGF2-alpha formation in rat basophilic leukemia (RBL-1) cells |
J Med Chem 36: 1802-10 (1993)
BindingDB Entry DOI: 10.7270/Q2542MPQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 1/2
(RAT) | BDBM22971

(2-[(2,6-dichloro-3-methylphenyl)amino]benzoic acid...)Show InChI InChI=1S/C14H11Cl2NO2/c1-8-6-7-10(15)13(12(8)16)17-11-5-3-2-4-9(11)14(18)19/h2-7,17H,1H3,(H,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated in an intact RBL-1 cell line for inhibition of 5-Cyclooxygenase |
Bioorg Med Chem Lett 2: 69-72 (1992)
Article DOI: 10.1016/S0960-894X(00)80657-5
BindingDB Entry DOI: 10.7270/Q29K4B4Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50046910
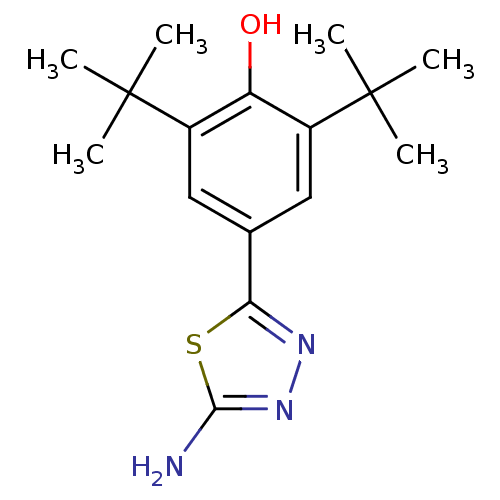
(4-(5-Amino-[1,3,4]thiadiazol-2-yl)-2,6-di-tert-but...)Show InChI InChI=1S/C16H23N3OS/c1-15(2,3)10-7-9(13-18-19-14(17)21-13)8-11(12(10)20)16(4,5)6/h7-8,20H,1-6H3,(H2,17,19) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
IC50 value against Prostaglandin G/H synthase 2 of murine J774A.1 cell line |
J Med Chem 42: 1161-9 (1999)
Article DOI: 10.1021/jm980570y
BindingDB Entry DOI: 10.7270/Q2154G6N |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50075532

(2,6-Di-tert-butyl-4-(5-methylsulfanyl-[1,3,4]oxadi...)Show SMILES CSc1nnc(o1)-c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C17H24N2O2S/c1-16(2,3)11-8-10(14-18-19-15(21-14)22-7)9-12(13(11)20)17(4,5)6/h8-9,20H,1-7H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
IC50 value against Prostaglandin G/H synthase 2 of murine J774A.1 cell line |
J Med Chem 42: 1161-9 (1999)
Article DOI: 10.1021/jm980570y
BindingDB Entry DOI: 10.7270/Q2154G6N |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50075535
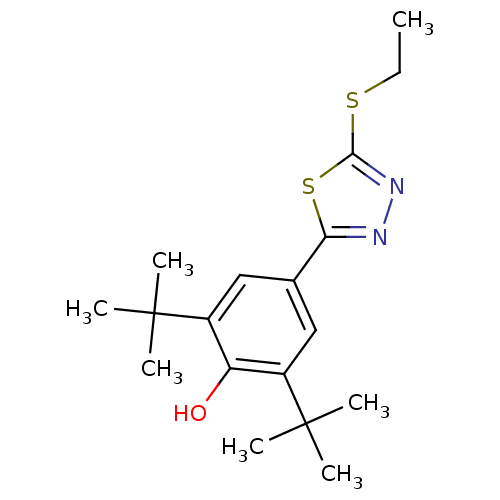
(2,6-Di-tert-butyl-4-(5-ethylsulfanyl-[1,3,4]thiadi...)Show SMILES CCSc1nnc(s1)-c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C18H26N2OS2/c1-8-22-16-20-19-15(23-16)11-9-12(17(2,3)4)14(21)13(10-11)18(5,6)7/h9-10,21H,8H2,1-7H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human prostaglandin G/H synthase 2 (COX-2) |
J Med Chem 42: 1161-9 (1999)
Article DOI: 10.1021/jm980570y
BindingDB Entry DOI: 10.7270/Q2154G6N |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50046902

(2,6-Di-tert-butyl-4-(5-methylsulfanyl-[1,3,4]thiad...)Show SMILES CSc1nnc(s1)-c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C17H24N2OS2/c1-16(2,3)11-8-10(14-18-19-15(21-7)22-14)9-12(13(11)20)17(4,5)6/h8-9,20H,1-7H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human prostaglandin G/H synthase 2 (COX-2) |
J Med Chem 42: 1161-9 (1999)
Article DOI: 10.1021/jm980570y
BindingDB Entry DOI: 10.7270/Q2154G6N |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50044075

(5-[1-(3,5-Di-tert-butyl-4-hydroxy-phenyl)-meth-(Z)...)Show SMILES CC(C)(C)c1cc(C=C2SC(NO)=NC2=O)cc(c1O)C(C)(C)C |w:7.6,c:12| Show InChI InChI=1S/C18H24N2O3S/c1-17(2,3)11-7-10(8-12(14(11)21)18(4,5)6)9-13-15(22)19-16(20-23)24-13/h7-9,21,23H,1-6H3,(H,19,20,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
IC50 value was determined against Prostaglandin G/H synthase 2 of J7744A.1 cell lines. |
J Med Chem 42: 1151-60 (1999)
Article DOI: 10.1021/jm9805081
BindingDB Entry DOI: 10.7270/Q24X56Z3 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50075510
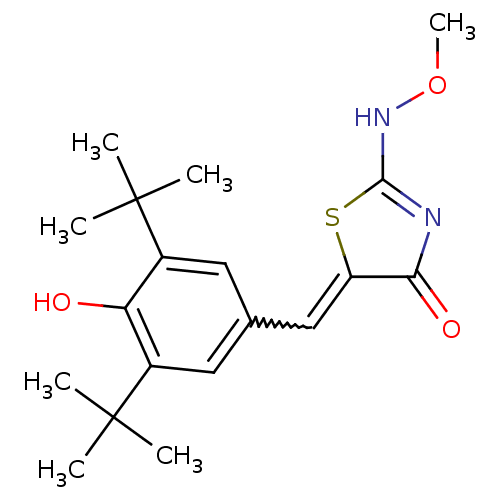
(5-[1-(3,5-Di-tert-butyl-4-hydroxy-phenyl)-meth-(Z)...)Show SMILES CONC1=NC(=O)C(S1)=Cc1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C |w:9.10,t:3| Show InChI InChI=1S/C19H26N2O3S/c1-18(2,3)12-8-11(9-13(15(12)22)19(4,5)6)10-14-16(23)20-17(25-14)21-24-7/h8-10,22H,1-7H3,(H,20,21,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
IC50 value was determined against Prostaglandin G/H synthase 2 of J7744A.1 cell lines. |
J Med Chem 42: 1151-60 (1999)
Article DOI: 10.1021/jm9805081
BindingDB Entry DOI: 10.7270/Q24X56Z3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data