

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50581548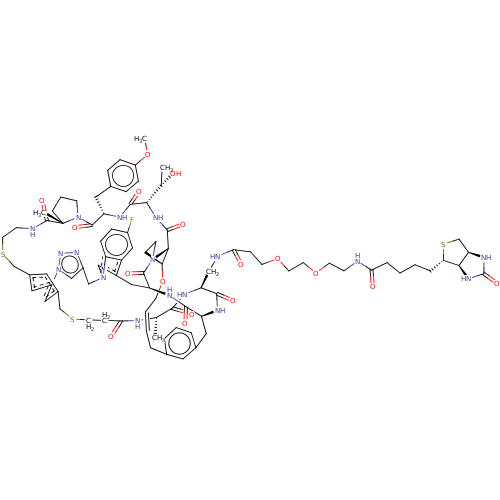 (CHEMBL5085124) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.000600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET ultra assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01599 BindingDB Entry DOI: 10.7270/Q2TM7G0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50581547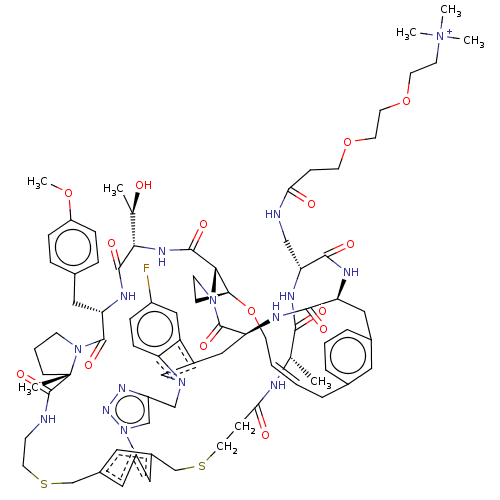 (CHEMBL5081349) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.000930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET ultra assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01599 BindingDB Entry DOI: 10.7270/Q2TM7G0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50581546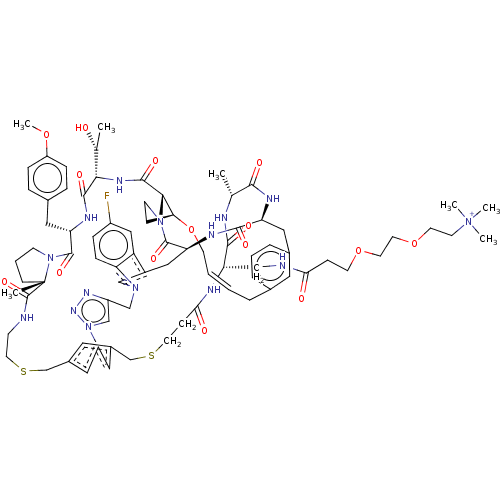 (CHEMBL5084416) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.00239 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET ultra assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01599 BindingDB Entry DOI: 10.7270/Q2TM7G0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50581547 (CHEMBL5081349) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00736 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01599 BindingDB Entry DOI: 10.7270/Q2TM7G0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50581545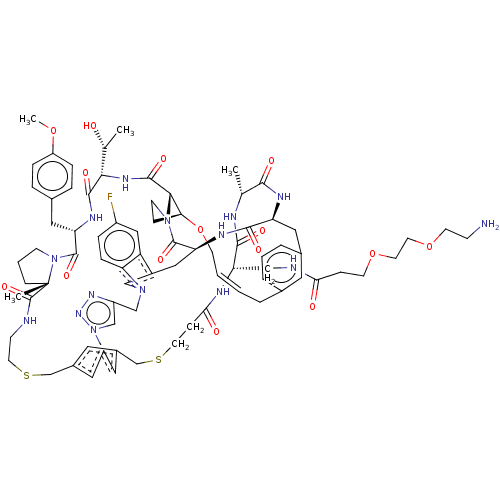 (CHEMBL5084902) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00813 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET ultra assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01599 BindingDB Entry DOI: 10.7270/Q2TM7G0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50581544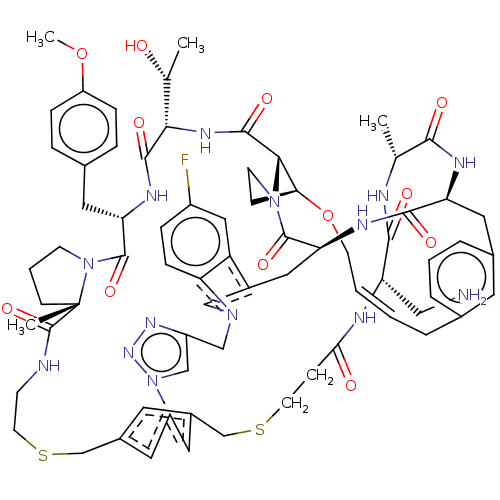 (CHEMBL5086475) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00826 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01599 BindingDB Entry DOI: 10.7270/Q2TM7G0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50581544 (CHEMBL5086475) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET ultra assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01599 BindingDB Entry DOI: 10.7270/Q2TM7G0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50581548 (CHEMBL5085124) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01599 BindingDB Entry DOI: 10.7270/Q2TM7G0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50581546 (CHEMBL5084416) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.0114 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01599 BindingDB Entry DOI: 10.7270/Q2TM7G0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50581549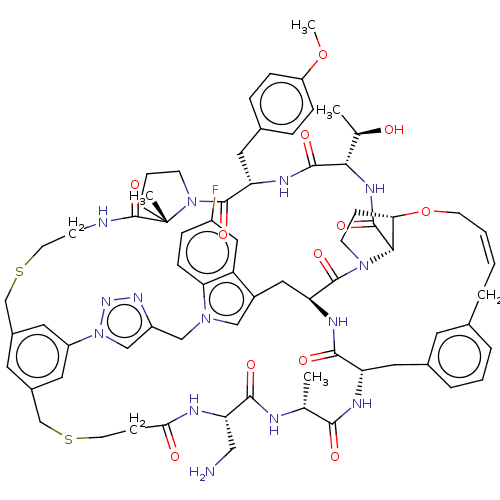 (CHEMBL5082483) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01599 BindingDB Entry DOI: 10.7270/Q2TM7G0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50581548 (CHEMBL5085124) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >0.129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01599 BindingDB Entry DOI: 10.7270/Q2TM7G0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50581547 (CHEMBL5081349) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >0.129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01599 BindingDB Entry DOI: 10.7270/Q2TM7G0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50581545 (CHEMBL5084902) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >0.129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01599 BindingDB Entry DOI: 10.7270/Q2TM7G0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50581544 (CHEMBL5086475) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >0.129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01599 BindingDB Entry DOI: 10.7270/Q2TM7G0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50581546 (CHEMBL5084416) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | >0.129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01599 BindingDB Entry DOI: 10.7270/Q2TM7G0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50581542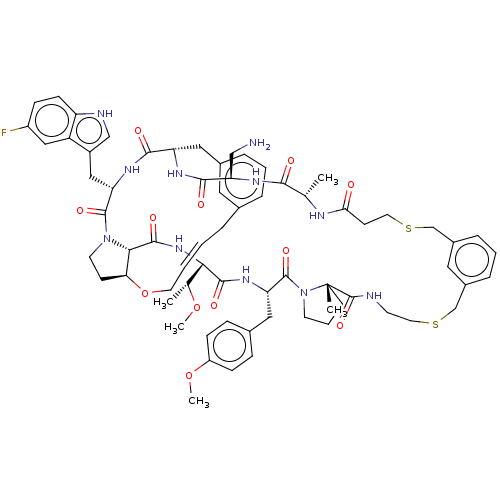 (CHEMBL5081587) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01599 BindingDB Entry DOI: 10.7270/Q2TM7G0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50581542 (CHEMBL5081587) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01599 BindingDB Entry DOI: 10.7270/Q2TM7G0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50413761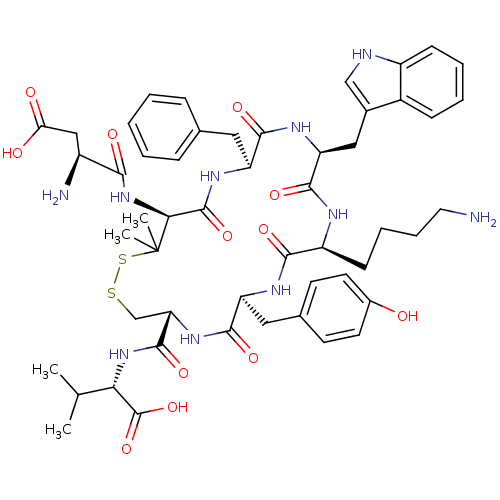 (CHEMBL390094) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Federico II Curated by ChEMBL | Assay Description Displacement of [125I]urotensin-2 from human UT2 receptor expressed in CHO-K1 cells by liquid scintillation counting | J Med Chem 52: 3927-40 (2009) Article DOI: 10.1021/jm900148c BindingDB Entry DOI: 10.7270/Q27W6DFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50581540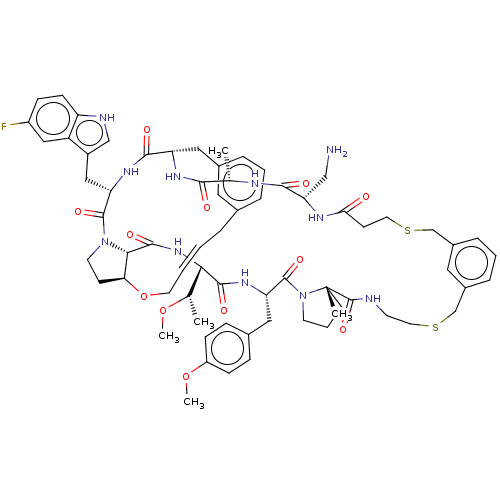 (CHEMBL5087487) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01599 BindingDB Entry DOI: 10.7270/Q2TM7G0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50581537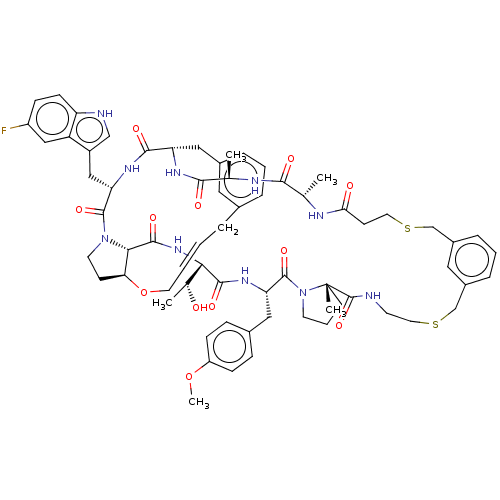 (CHEMBL5086286) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01599 BindingDB Entry DOI: 10.7270/Q2TM7G0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50413760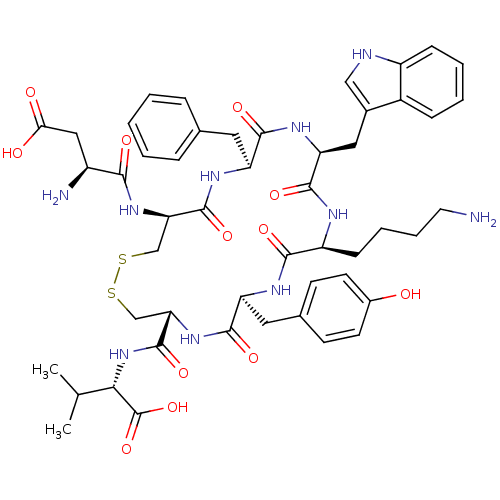 (CHEMBL426020) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Federico II Curated by ChEMBL | Assay Description Displacement of [125I]urotensin-2 from human UT2 receptor expressed in CHO-K1 cells by liquid scintillation counting | J Med Chem 52: 3927-40 (2009) Article DOI: 10.1021/jm900148c BindingDB Entry DOI: 10.7270/Q27W6DFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50413764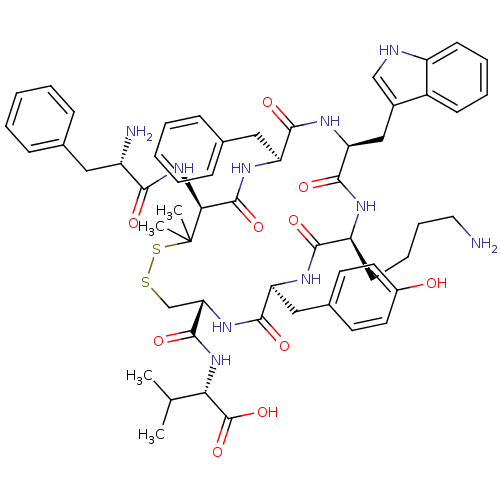 (CHEMBL504097) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.282 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Federico II Curated by ChEMBL | Assay Description Displacement of [125I]urotensin-2 from human UT2 receptor expressed in CHO-K1 cells by liquid scintillation counting | J Med Chem 52: 3927-40 (2009) Article DOI: 10.1021/jm900148c BindingDB Entry DOI: 10.7270/Q27W6DFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50581535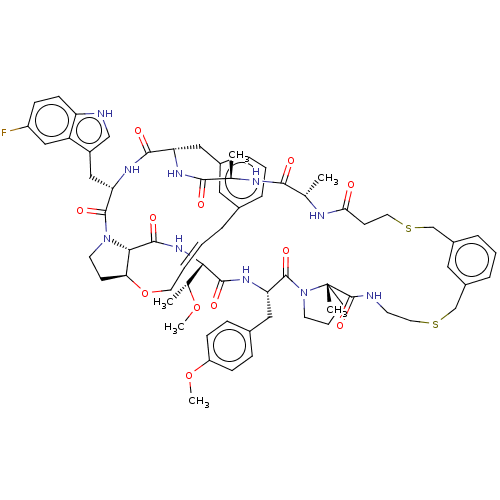 (CHEMBL5091040) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01599 BindingDB Entry DOI: 10.7270/Q2TM7G0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50581537 (CHEMBL5086286) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01599 BindingDB Entry DOI: 10.7270/Q2TM7G0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50581540 (CHEMBL5087487) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01599 BindingDB Entry DOI: 10.7270/Q2TM7G0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50581535 (CHEMBL5091040) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01599 BindingDB Entry DOI: 10.7270/Q2TM7G0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50413762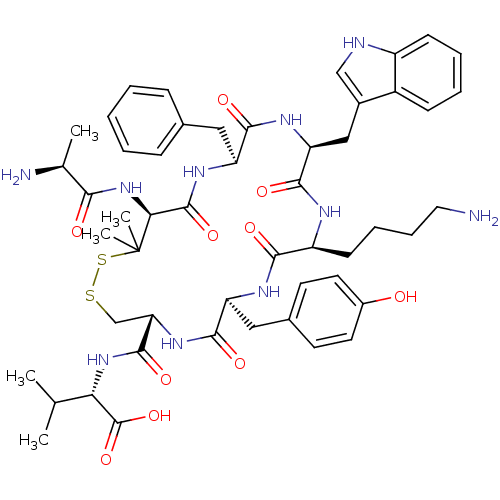 (CHEMBL509604) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Federico II Curated by ChEMBL | Assay Description Displacement of [125I]urotensin-2 from human UT2 receptor expressed in CHO-K1 cells by liquid scintillation counting | J Med Chem 52: 3927-40 (2009) Article DOI: 10.1021/jm900148c BindingDB Entry DOI: 10.7270/Q27W6DFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50378580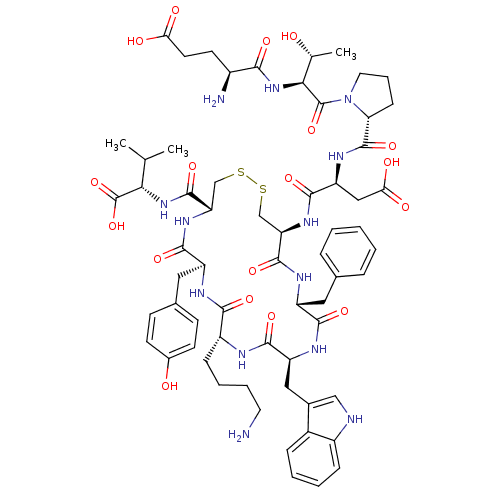 (CHEMBL437430) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Federico II Curated by ChEMBL | Assay Description Displacement of [125I]urotensin-2 from human UT2 receptor expressed in CHO-K1 cells by liquid scintillation counting | J Med Chem 52: 3927-40 (2009) Article DOI: 10.1021/jm900148c BindingDB Entry DOI: 10.7270/Q27W6DFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50413766 (CHEMBL510618) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.891 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Federico II Curated by ChEMBL | Assay Description Displacement of [125I]urotensin-2 from human UT2 receptor expressed in CHO-K1 cells by liquid scintillation counting | J Med Chem 52: 3927-40 (2009) Article DOI: 10.1021/jm900148c BindingDB Entry DOI: 10.7270/Q27W6DFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50581543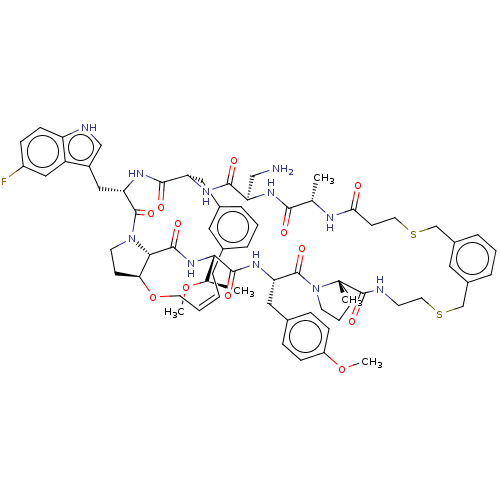 (CHEMBL5094648) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01599 BindingDB Entry DOI: 10.7270/Q2TM7G0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50554754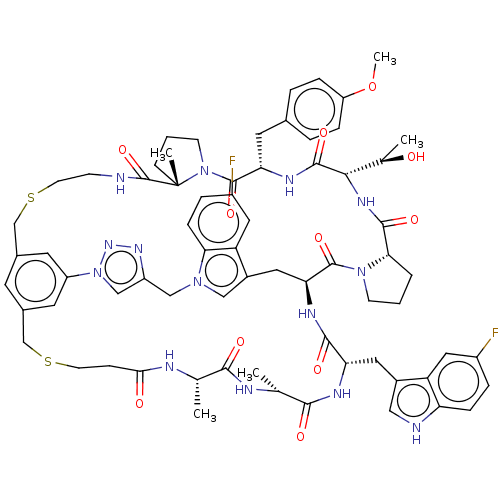 (CHEMBL4790764) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01599 BindingDB Entry DOI: 10.7270/Q2TM7G0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50554754 (CHEMBL4790764) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AlexaFluor647-tagged cyclic peptide binding to avi-tagged-biotinylated human PCSK9 measured after 2 hrs by Lance Streptavidin Europium ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01084 BindingDB Entry DOI: 10.7270/Q23N272N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50554755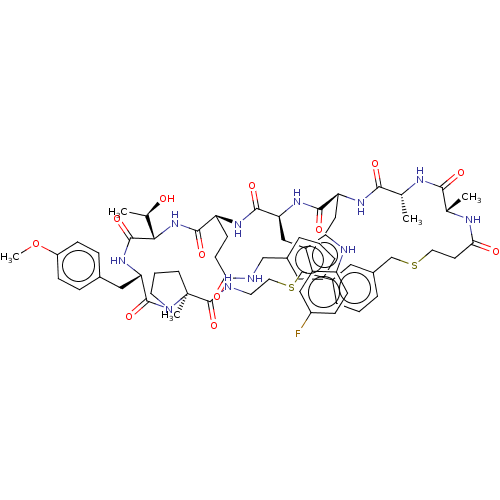 (CHEMBL4782157) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AlexaFluor647-tagged cyclic peptide binding to avi-tagged-biotinylated human PCSK9 measured after 2 hrs by Lance Streptavidin Europium ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01084 BindingDB Entry DOI: 10.7270/Q23N272N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50554734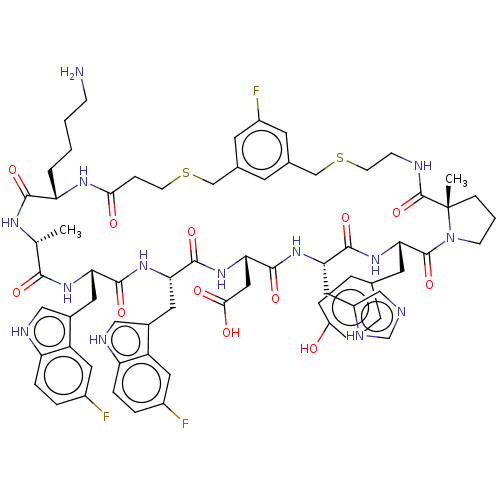 (CHEMBL4761415 | US11530244, Compound 289) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AlexaFluor647-tagged cyclic peptide binding to avi-tagged-biotinylated human PCSK9 measured after 2 hrs by Lance Streptavidin Europium ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01084 BindingDB Entry DOI: 10.7270/Q23N272N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50413763 (CHEMBL448403) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Federico II Curated by ChEMBL | Assay Description Displacement of [125I]urotensin-2 from human UT2 receptor expressed in CHO-K1 cells by liquid scintillation counting | J Med Chem 52: 3927-40 (2009) Article DOI: 10.1021/jm900148c BindingDB Entry DOI: 10.7270/Q27W6DFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50517320 (CHEMBL4568153) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II" Curated by ChEMBL | Assay Description Displacement of [125I]-URP or human [125I[-urotensin-2 from human urotensin 2 receptor expressed in HEK293 or CHOK1 cells after 2 hrs by gamma-counti... | J Med Chem 62: 1455-1467 (2019) Article DOI: 10.1021/acs.jmedchem.8b01601 BindingDB Entry DOI: 10.7270/Q2PV6PRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50517327 (CHEMBL4472928) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II" Curated by ChEMBL | Assay Description Displacement of [125I]-URP or human [125I[-urotensin-2 from human urotensin 2 receptor expressed in HEK293 or CHOK1 cells after 2 hrs by gamma-counti... | J Med Chem 62: 1455-1467 (2019) Article DOI: 10.1021/acs.jmedchem.8b01601 BindingDB Entry DOI: 10.7270/Q2PV6PRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50554743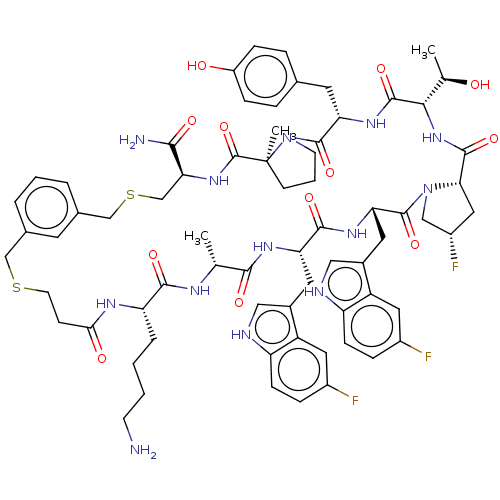 (CHEMBL4759174) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AlexaFluor647-tagged cyclic peptide binding to avi-tagged-biotinylated human PCSK9 measured after 2 hrs by Lance Streptavidin Europium ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01084 BindingDB Entry DOI: 10.7270/Q23N272N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50581539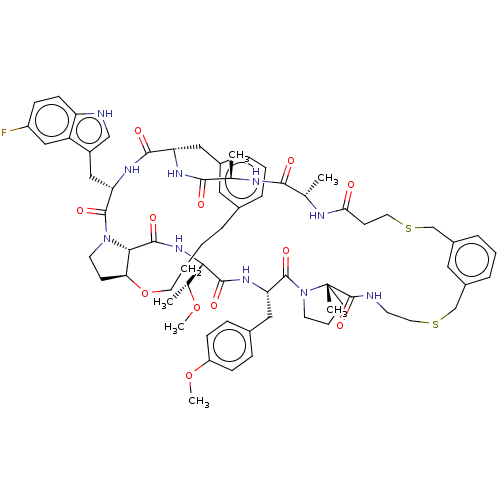 (CHEMBL5084586) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01599 BindingDB Entry DOI: 10.7270/Q2TM7G0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50517312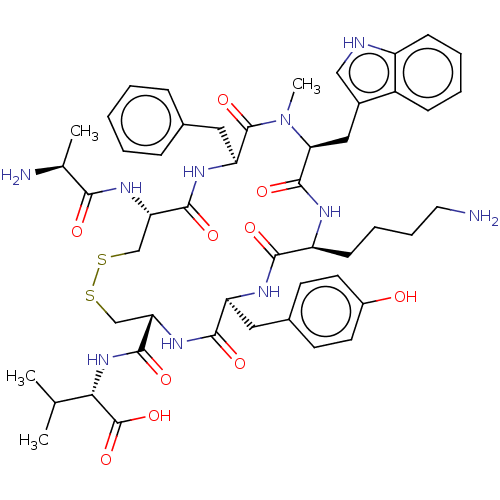 (CHEMBL4526244) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II" Curated by ChEMBL | Assay Description Displacement of [125I]-URP or human [125I[-urotensin-2 from human urotensin 2 receptor expressed in HEK293 or CHOK1 cells after 2 hrs by gamma-counti... | J Med Chem 62: 1455-1467 (2019) Article DOI: 10.1021/acs.jmedchem.8b01601 BindingDB Entry DOI: 10.7270/Q2PV6PRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50581543 (CHEMBL5094648) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01599 BindingDB Entry DOI: 10.7270/Q2TM7G0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50413777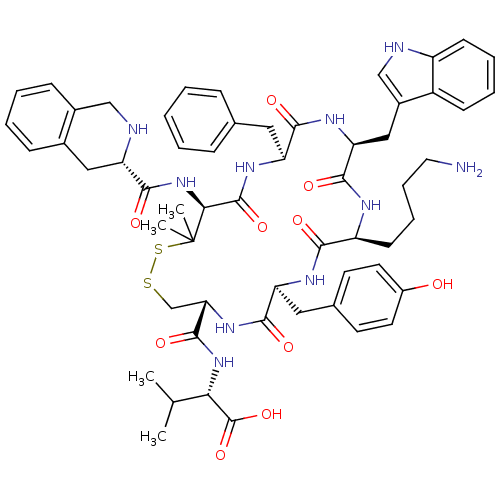 (CHEMBL524855) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Federico II Curated by ChEMBL | Assay Description Displacement of [125I]urotensin-2 from human UT2 receptor expressed in CHO-K1 cells by liquid scintillation counting | J Med Chem 52: 3927-40 (2009) Article DOI: 10.1021/jm900148c BindingDB Entry DOI: 10.7270/Q27W6DFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50554736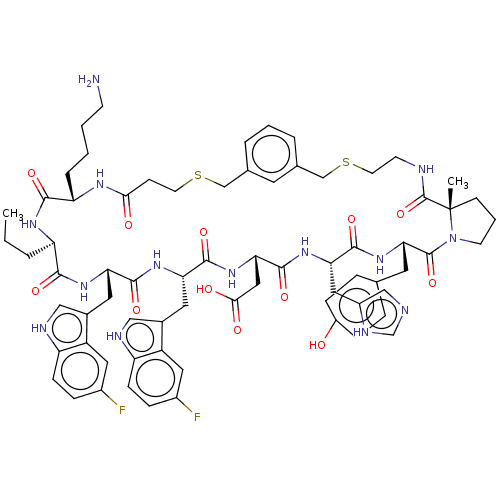 (CHEMBL4789838) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AlexaFluor647-tagged cyclic peptide binding to avi-tagged-biotinylated human PCSK9 measured after 2 hrs by Lance Streptavidin Europium ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01084 BindingDB Entry DOI: 10.7270/Q23N272N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50554760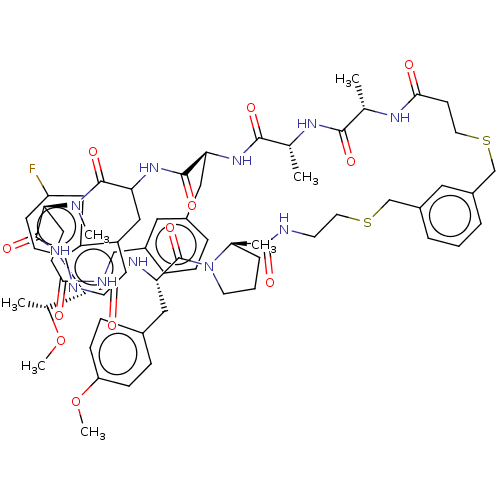 (CHEMBL4798026) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AlexaFluor647-tagged cyclic peptide binding to avi-tagged-biotinylated human PCSK9 measured after 2 hrs by Lance Streptavidin Europium ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01084 BindingDB Entry DOI: 10.7270/Q23N272N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50517325 (CHEMBL4590360) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II" Curated by ChEMBL | Assay Description Displacement of [125I]-URP or human [125I[-urotensin-2 from human urotensin 2 receptor expressed in HEK293 or CHOK1 cells after 2 hrs by gamma-counti... | J Med Chem 62: 1455-1467 (2019) Article DOI: 10.1021/acs.jmedchem.8b01601 BindingDB Entry DOI: 10.7270/Q2PV6PRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50517315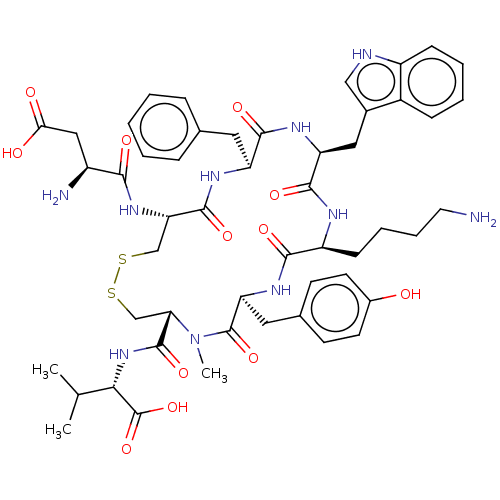 (CHEMBL4454498) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II" Curated by ChEMBL | Assay Description Displacement of [125I]-URP or human [125I[-urotensin-2 from human urotensin 2 receptor expressed in HEK293 or CHOK1 cells after 2 hrs by gamma-counti... | J Med Chem 62: 1455-1467 (2019) Article DOI: 10.1021/acs.jmedchem.8b01601 BindingDB Entry DOI: 10.7270/Q2PV6PRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50517316 (CHEMBL4462940) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II" Curated by ChEMBL | Assay Description Displacement of [125I]-URP or human [125I[-urotensin-2 from human urotensin 2 receptor expressed in HEK293 or CHOK1 cells after 2 hrs by gamma-counti... | J Med Chem 62: 1455-1467 (2019) Article DOI: 10.1021/acs.jmedchem.8b01601 BindingDB Entry DOI: 10.7270/Q2PV6PRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50413769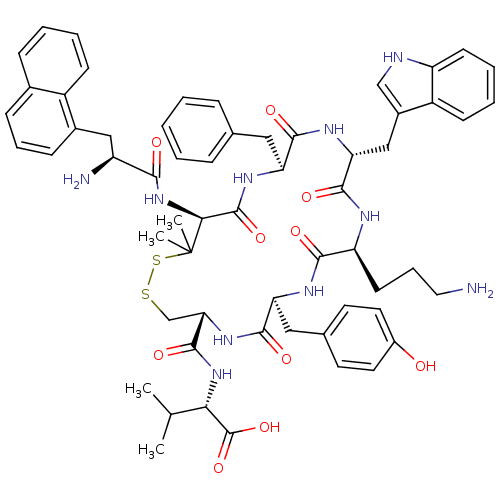 (CHEMBL507406) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Federico II Curated by ChEMBL | Assay Description Displacement of [125I]urotensin-2 from human UT2 receptor expressed in CHO-K1 cells by liquid scintillation counting | J Med Chem 52: 3927-40 (2009) Article DOI: 10.1021/jm900148c BindingDB Entry DOI: 10.7270/Q27W6DFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50554735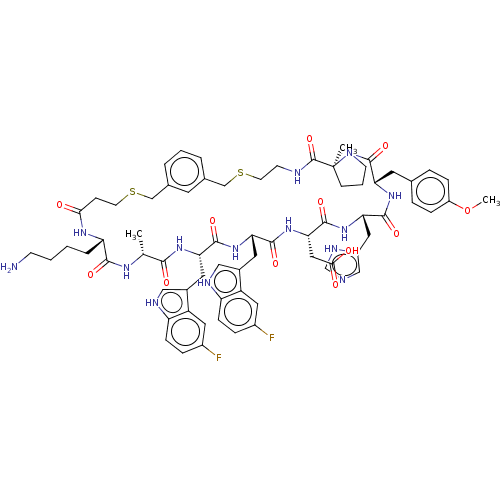 (CHEMBL4742833) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AlexaFluor647-tagged cyclic peptide binding to avi-tagged-biotinylated human PCSK9 measured after 2 hrs by Lance Streptavidin Europium ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01084 BindingDB Entry DOI: 10.7270/Q23N272N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50554759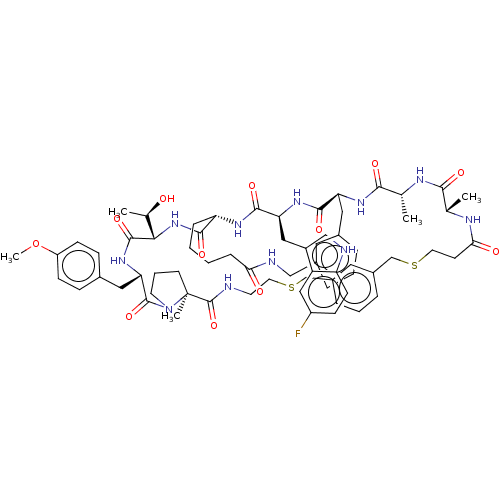 (CHEMBL4745053) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AlexaFluor647-tagged cyclic peptide binding to avi-tagged-biotinylated human PCSK9 measured after 2 hrs by Lance Streptavidin Europium ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01084 BindingDB Entry DOI: 10.7270/Q23N272N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1643 total ) | Next | Last >> |