 Found 1014 hits with Last Name = 'buckley' and Initial = 'd'
Found 1014 hits with Last Name = 'buckley' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine protease 1
(Homo sapiens (Human)) | BDBM50072292

((3S,3aS,6aS)-3-Phenylsulfanyl-hexahydro-cyclopenta...)Show InChI InChI=1S/C13H14O2S/c14-13-12(10-7-4-8-11(10)15-13)16-9-5-2-1-3-6-9/h1-3,5-6,10-12H,4,7-8H2/t10-,11-,12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of trypsin |
Bioorg Med Chem Lett 8: 2955-60 (1999)
Article DOI: 10.1016/S0960-894X(98)00531-9
BindingDB Entry DOI: 10.7270/Q2JQ105F |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50072285
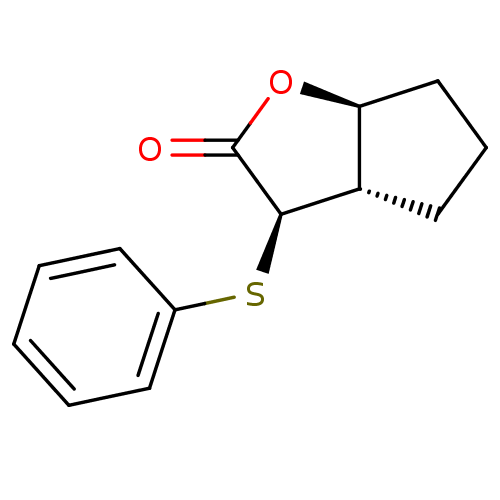
((3R,3aS,6aS)-3-Phenylsulfanyl-hexahydro-cyclopenta...)Show InChI InChI=1S/C13H14O2S/c14-13-12(10-7-4-8-11(10)15-13)16-9-5-2-1-3-6-9/h1-3,5-6,10-12H,4,7-8H2/t10-,11-,12+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of trypsin |
Bioorg Med Chem Lett 8: 2955-60 (1999)
Article DOI: 10.1016/S0960-894X(98)00531-9
BindingDB Entry DOI: 10.7270/Q2JQ105F |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50072289

((3S,3aR,6aS)-3-(4-Methyl-2-oxo-pent-3-enyl)-hexahy...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6](=O)-[#6]-[#6@H]-1-[#6@H]-2-[#6]-[#6]-[#6]-[#6@@H]-2-[#8]-[#6]-1=O Show InChI InChI=1S/C13H18O3/c1-8(2)6-9(14)7-11-10-4-3-5-12(10)16-13(11)15/h6,10-12H,3-5,7H2,1-2H3/t10-,11+,12+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of trypsin |
Bioorg Med Chem Lett 8: 2955-60 (1999)
Article DOI: 10.1016/S0960-894X(98)00531-9
BindingDB Entry DOI: 10.7270/Q2JQ105F |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50072283
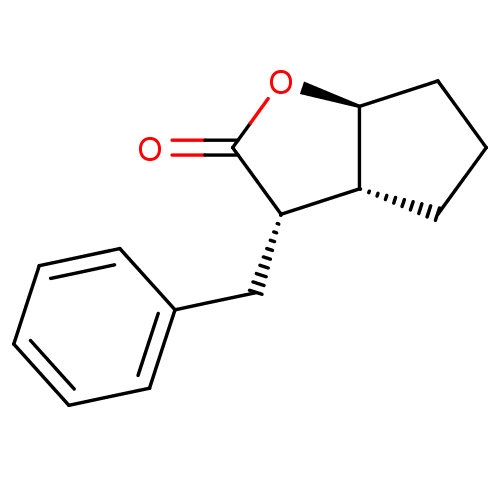
((3S,3aR,6aS)-3-Benzyl-hexahydro-cyclopenta[b]furan...)Show InChI InChI=1S/C14H16O2/c15-14-12(9-10-5-2-1-3-6-10)11-7-4-8-13(11)16-14/h1-3,5-6,11-13H,4,7-9H2/t11-,12+,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of trypsin |
Bioorg Med Chem Lett 8: 2955-60 (1999)
Article DOI: 10.1016/S0960-894X(98)00531-9
BindingDB Entry DOI: 10.7270/Q2JQ105F |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50458963
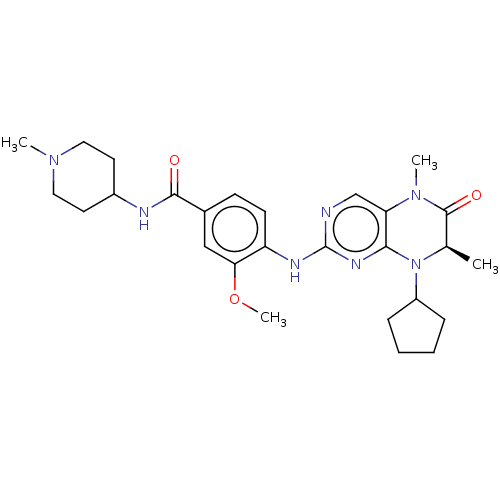
(CHEMBL4209902)Show SMILES COc1cc(ccc1Nc1ncc2N(C)C(=O)[C@@H](C)N(C3CCCC3)c2n1)C(=O)NC1CCN(C)CC1 |r| Show InChI InChI=1S/C27H37N7O3/c1-17-26(36)33(3)22-16-28-27(31-24(22)34(17)20-7-5-6-8-20)30-21-10-9-18(15-23(21)37-4)25(35)29-19-11-13-32(2)14-12-19/h9-10,15-17,19-20H,5-8,11-14H2,1-4H3,(H,29,35)(H,28,30,31)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts-Boston
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PLK1 by Z-Lyte assay |
J Med Chem 61: 7785-7795 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00765
BindingDB Entry DOI: 10.7270/Q2MP55WQ |
More data for this
Ligand-Target Pair | |
DNA damage-binding protein 1/Protein cereblon
(Homo sapiens (Human)) | BDBM511610
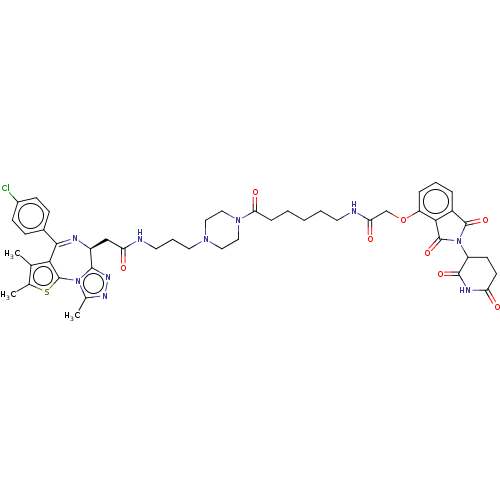
(US11059801, Compound dBET18)Show SMILES Cc1sc-2c(c1C)C(=N[C@@H](CC(=O)NCCCN1CCN(CC1)C(=O)CCCCCNC(=O)COc1cccc3C(=O)N(C4CCC(=O)NC4=O)C(=O)c13)c1nnc(C)n-21)c1ccc(Cl)cc1 |r,c:8| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.57 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A thalidomide competition AlphaScreen assay was employed to measure the binding affinity (IC50) of thalidomide conjugates and novel IMiDs to CRBN-DDB... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2G44TFM |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50072286
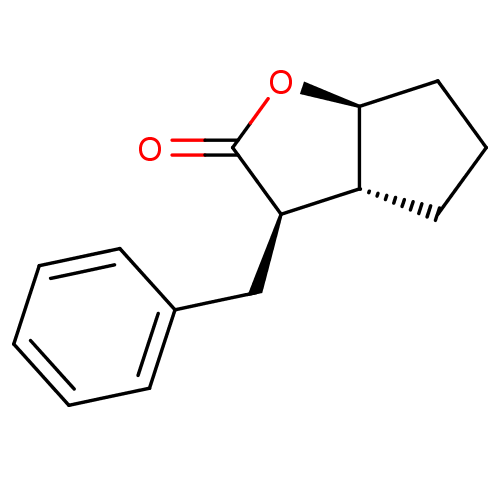
((3R,3aR,6aS)-3-Benzyl-hexahydro-cyclopenta[b]furan...)Show InChI InChI=1S/C14H16O2/c15-14-12(9-10-5-2-1-3-6-10)11-7-4-8-13(11)16-14/h1-3,5-6,11-13H,4,7-9H2/t11-,12-,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of trypsin |
Bioorg Med Chem Lett 8: 2955-60 (1999)
Article DOI: 10.1016/S0960-894X(98)00531-9
BindingDB Entry DOI: 10.7270/Q2JQ105F |
More data for this
Ligand-Target Pair | |
DNA damage-binding protein 1/Protein cereblon
(Homo sapiens (Human)) | BDBM50544938
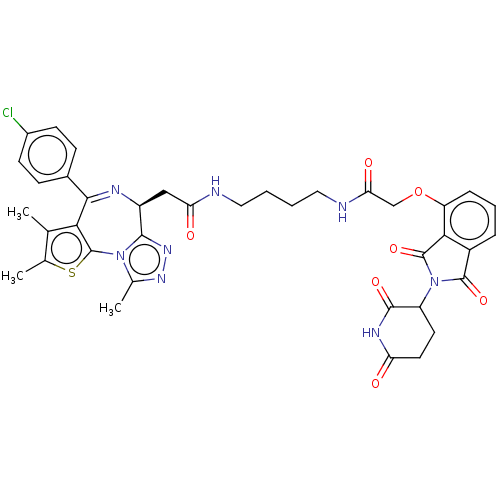
(CHEMBL4094346 | US11059801, Compound dBET1)Show SMILES Cc1sc-2c(c1C)C(=N[C@@H](CC(=O)NCCCCNC(=O)COc1cccc3C(=O)N(C4CCC(=O)NC4=O)C(=O)c13)c1nnc(C)n-21)c1ccc(Cl)cc1 |r,c:8| Show InChI InChI=1S/C38H37ClN8O7S/c1-19-20(2)55-38-31(19)33(22-9-11-23(39)12-10-22)42-25(34-45-44-21(3)46(34)38)17-29(49)40-15-4-5-16-41-30(50)18-54-27-8-6-7-24-32(27)37(53)47(36(24)52)26-13-14-28(48)43-35(26)51/h6-12,25-26H,4-5,13-18H2,1-3H3,(H,40,49)(H,41,50)(H,43,48,51)/t25-,26?/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.09 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A thalidomide competition AlphaScreen assay was employed to measure the binding affinity (IC50) of thalidomide conjugates and novel IMiDs to CRBN-DDB... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2G44TFM |
More data for this
Ligand-Target Pair | |
DNA damage-binding protein 1/Protein cereblon
(Homo sapiens (Human)) | BDBM511611

(US11059801, Compound dBET19)Show SMILES Cc1nnc2[C@H](CC(=O)NCCCCNC(=O)COc3cccc4C(=O)N(C5CCC(=O)NC5=O)C(=O)c34)N=C(c3c(C)c(CC#N)sc3-n12)c1ccc(Cl)cc1 |r,c:41| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.62 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A thalidomide competition AlphaScreen assay was employed to measure the binding affinity (IC50) of thalidomide conjugates and novel IMiDs to CRBN-DDB... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2G44TFM |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50458969
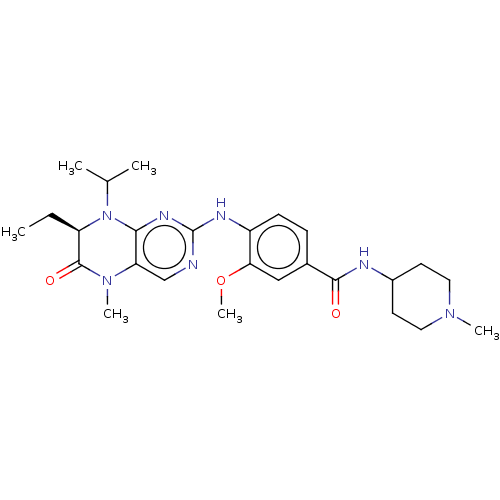
(CHEMBL4207165)Show SMILES CC[C@H]1N(C(C)C)c2nc(Nc3ccc(cc3OC)C(=O)NC3CCN(C)CC3)ncc2N(C)C1=O |r| Show InChI InChI=1S/C26H37N7O3/c1-7-20-25(35)32(5)21-15-27-26(30-23(21)33(20)16(2)3)29-19-9-8-17(14-22(19)36-6)24(34)28-18-10-12-31(4)13-11-18/h8-9,14-16,18,20H,7,10-13H2,1-6H3,(H,28,34)(H,27,29,30)/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts-Boston
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PLK1 by Z-Lyte assay |
J Med Chem 61: 7785-7795 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00765
BindingDB Entry DOI: 10.7270/Q2MP55WQ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072284

((1S,2R,4aR,6aS,6bS,7R,9aS,10aS)-2-Acetoxy-1,4a,6a,...)Show SMILES [H][C@@]12[#6]-[#6][C@]3([#6])[C@@]4([H])[#6]-[#6][C@@]5([#6])[C@]6([H])[#6@@H](-[#6]-[#6](=O)\[#6]=[#6](\[#6])-[#6])-[#6](=O)-[#8][C@@]6([H])[#6][C@]5([#6])[#6]4=[#6]-[#6][C@@]3([H])[C@]1([#6])[#6](=O)-[#8]2 |r,c:33| Show InChI InChI=1S/C30H40O5/c1-16(2)13-17(31)14-18-24-21(34-25(18)32)15-29(5)20-7-8-22-27(3,19(20)9-12-28(24,29)4)11-10-23-30(22,6)26(33)35-23/h7,13,18-19,21-24H,8-12,14-15H2,1-6H3/t18-,19+,21+,22-,23-,24-,27-,28+,29-,30+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of trypsin |
Bioorg Med Chem Lett 8: 2955-60 (1999)
Article DOI: 10.1016/S0960-894X(98)00531-9
BindingDB Entry DOI: 10.7270/Q2JQ105F |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM25121

(4-{[(7R)-8-cyclopentyl-7-ethyl-5-methyl-6-oxo-5,6,...)Show SMILES CC[C@H]1N(C2CCCC2)c2nc(Nc3ccc(cc3OC)C(=O)NC3CCN(C)CC3)ncc2N(C)C1=O |r| Show InChI InChI=1S/C28H39N7O3/c1-5-22-27(37)34(3)23-17-29-28(32-25(23)35(22)20-8-6-7-9-20)31-21-11-10-18(16-24(21)38-4)26(36)30-19-12-14-33(2)15-13-19/h10-11,16-17,19-20,22H,5-9,12-15H2,1-4H3,(H,30,36)(H,29,31,32)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts-Boston
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PLK1 by Z-Lyte assay |
J Med Chem 61: 7785-7795 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00765
BindingDB Entry DOI: 10.7270/Q2MP55WQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50458971
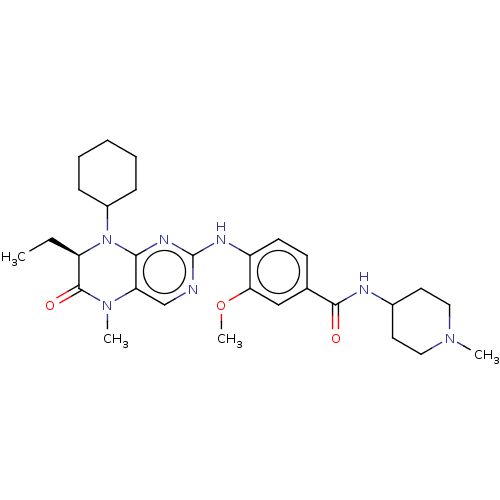
(CHEMBL4215729)Show SMILES CC[C@H]1N(C2CCCCC2)c2nc(Nc3ccc(cc3OC)C(=O)NC3CCN(C)CC3)ncc2N(C)C1=O |r| Show InChI InChI=1S/C29H41N7O3/c1-5-23-28(38)35(3)24-18-30-29(33-26(24)36(23)21-9-7-6-8-10-21)32-22-12-11-19(17-25(22)39-4)27(37)31-20-13-15-34(2)16-14-20/h11-12,17-18,20-21,23H,5-10,13-16H2,1-4H3,(H,31,37)(H,30,32,33)/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts-Boston
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PLK1 by Z-Lyte assay |
J Med Chem 61: 7785-7795 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00765
BindingDB Entry DOI: 10.7270/Q2MP55WQ |
More data for this
Ligand-Target Pair | |
DNA damage-binding protein 1/Protein cereblon
(Homo sapiens (Human)) | BDBM511608
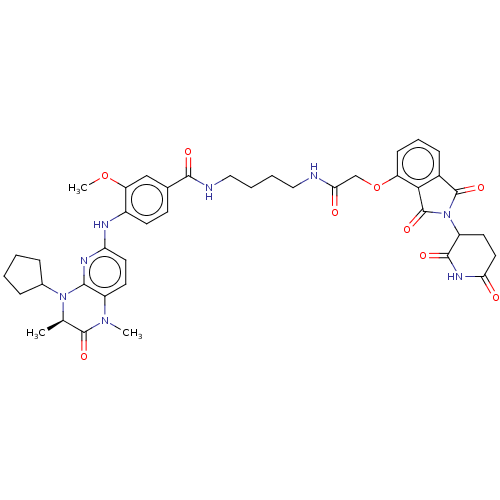
(US11059801, Compound dBET16)Show SMILES COc1cc(ccc1Nc1ccc2N(C)C(=O)[C@@H](C)N(C3CCCC3)c2n1)C(=O)NCCCCNC(=O)COc1cccc2C(=O)N(C3CCC(=O)NC3=O)C(=O)c12 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.84 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A thalidomide competition AlphaScreen assay was employed to measure the binding affinity (IC50) of thalidomide conjugates and novel IMiDs to CRBN-DDB... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2G44TFM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50530873

(CHEMBL4456215)Show SMILES CN(C1CCCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cnc(N)nc2)C1)S(C)(=O)=O Show InChI InChI=1S/C22H30N8O3S2/c1-28(35(2,31)32)16-4-3-5-29(13-16)14-17-10-18-19(34-17)21(30-6-8-33-9-7-30)27-20(26-18)15-11-24-22(23)25-12-15/h10-12,16H,3-9,13-14H2,1-2H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotin-PIP3 as substrate preincubated for 15 mins followed by substrate addition and measured after 60... |
J Med Chem 62: 10402-10422 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01499
BindingDB Entry DOI: 10.7270/Q27H1P12 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50530873

(CHEMBL4456215)Show SMILES CN(C1CCCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cnc(N)nc2)C1)S(C)(=O)=O Show InChI InChI=1S/C22H30N8O3S2/c1-28(35(2,31)32)16-4-3-5-29(13-16)14-17-10-18-19(34-17)21(30-6-8-33-9-7-30)27-20(26-18)15-11-24-22(23)25-12-15/h10-12,16H,3-9,13-14H2,1-2H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotin-PIP3 as substrate preincubated for 15 mins followed by substrate addition and measured after 60... |
J Med Chem 62: 10402-10422 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01499
BindingDB Entry DOI: 10.7270/Q27H1P12 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072285

((3R,3aS,6aS)-3-Phenylsulfanyl-hexahydro-cyclopenta...)Show InChI InChI=1S/C13H14O2S/c14-13-12(10-7-4-8-11(10)15-13)16-9-5-2-1-3-6-9/h1-3,5-6,10-12H,4,7-8H2/t10-,11-,12+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 8: 2955-60 (1999)
Article DOI: 10.1016/S0960-894X(98)00531-9
BindingDB Entry DOI: 10.7270/Q2JQ105F |
More data for this
Ligand-Target Pair | |
DNA damage-binding protein 1/Protein cereblon
(Homo sapiens (Human)) | BDBM511621
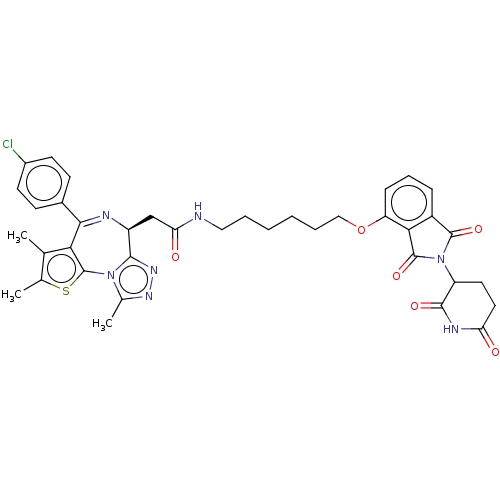
(US11059801, Compound dBET29)Show SMILES Cc1sc-2c(c1C)C(=N[C@@H](CC(=O)NCCCCCCOc1cccc3C(=O)N(C4CCC(=O)NC4=O)C(=O)c13)c1nnc(C)n-21)c1ccc(Cl)cc1 |r,c:8| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6.05 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A thalidomide competition AlphaScreen assay was employed to measure the binding affinity (IC50) of thalidomide conjugates and novel IMiDs to CRBN-DDB... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2G44TFM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50530887
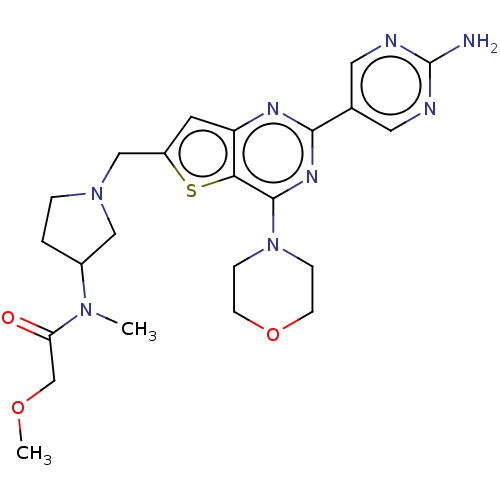
(CHEMBL4538734)Show SMILES COCC(=O)N(C)C1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cnc(N)nc2)C1 Show InChI InChI=1S/C23H30N8O3S/c1-29(19(32)14-33-2)16-3-4-30(12-16)13-17-9-18-20(35-17)22(31-5-7-34-8-6-31)28-21(27-18)15-10-25-23(24)26-11-15/h9-11,16H,3-8,12-14H2,1-2H3,(H2,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotin-PIP3 as substrate preincubated for 15 mins followed by substrate addition and measured after 60... |
J Med Chem 62: 10402-10422 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01499
BindingDB Entry DOI: 10.7270/Q27H1P12 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50530895
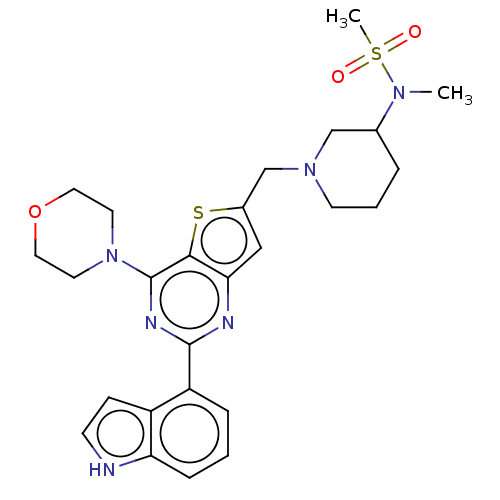
(CHEMBL4590897)Show SMILES CN(C1CCCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ccc23)C1)S(C)(=O)=O Show InChI InChI=1S/C26H32N6O3S2/c1-30(37(2,33)34)18-5-4-10-31(16-18)17-19-15-23-24(36-19)26(32-11-13-35-14-12-32)29-25(28-23)21-6-3-7-22-20(21)8-9-27-22/h3,6-9,15,18,27H,4-5,10-14,16-17H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotin-PIP3 as substrate preincubated for 15 mins followed by substrate addition and measured after 60... |
J Med Chem 62: 10402-10422 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01499
BindingDB Entry DOI: 10.7270/Q27H1P12 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50530895

(CHEMBL4590897)Show SMILES CN(C1CCCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ccc23)C1)S(C)(=O)=O Show InChI InChI=1S/C26H32N6O3S2/c1-30(37(2,33)34)18-5-4-10-31(16-18)17-19-15-23-24(36-19)26(32-11-13-35-14-12-32)29-25(28-23)21-6-3-7-22-20(21)8-9-27-22/h3,6-9,15,18,27H,4-5,10-14,16-17H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotin-PIP3 as substrate preincubated for 15 mins followed by substrate addition and measured after 60... |
J Med Chem 62: 10402-10422 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01499
BindingDB Entry DOI: 10.7270/Q27H1P12 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50530877
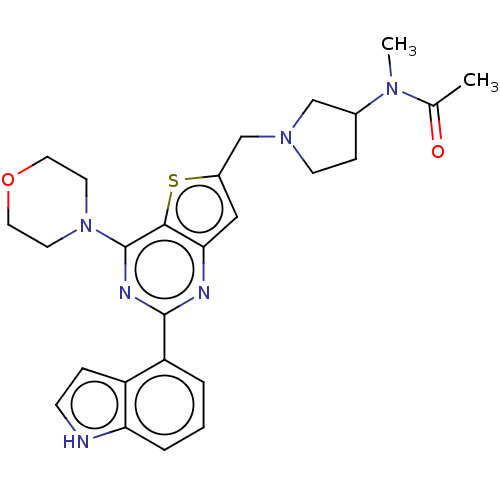
(CHEMBL4580111)Show SMILES CN(C1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ccc23)C1)C(C)=O Show InChI InChI=1S/C26H30N6O2S/c1-17(33)30(2)18-7-9-31(15-18)16-19-14-23-24(35-19)26(32-10-12-34-13-11-32)29-25(28-23)21-4-3-5-22-20(21)6-8-27-22/h3-6,8,14,18,27H,7,9-13,15-16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotin-PIP3 as substrate preincubated for 15 mins followed by substrate addition and measured after 60... |
J Med Chem 62: 10402-10422 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01499
BindingDB Entry DOI: 10.7270/Q27H1P12 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50530887

(CHEMBL4538734)Show SMILES COCC(=O)N(C)C1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cnc(N)nc2)C1 Show InChI InChI=1S/C23H30N8O3S/c1-29(19(32)14-33-2)16-3-4-30(12-16)13-17-9-18-20(35-17)22(31-5-7-34-8-6-31)28-21(27-18)15-10-25-23(24)26-11-15/h9-11,16H,3-8,12-14H2,1-2H3,(H2,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotin-PIP3 as substrate preincubated for 15 mins followed by substrate addition and measured after 60... |
J Med Chem 62: 10402-10422 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01499
BindingDB Entry DOI: 10.7270/Q27H1P12 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50530894
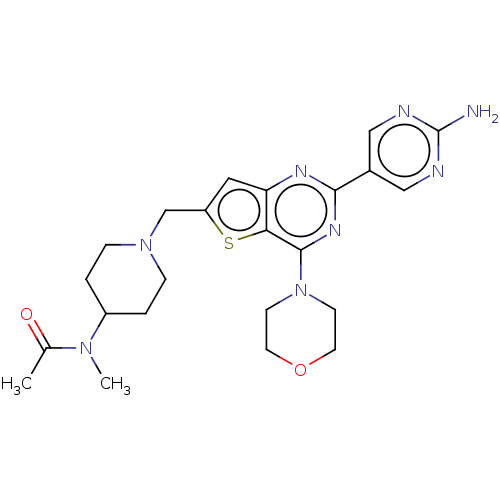
(CHEMBL4437468)Show SMILES CN(C1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cnc(N)nc2)CC1)C(C)=O Show InChI InChI=1S/C23H30N8O2S/c1-15(32)29(2)17-3-5-30(6-4-17)14-18-11-19-20(34-18)22(31-7-9-33-10-8-31)28-21(27-19)16-12-25-23(24)26-13-16/h11-13,17H,3-10,14H2,1-2H3,(H2,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotin-PIP3 as substrate preincubated for 15 mins followed by substrate addition and measured after 60... |
J Med Chem 62: 10402-10422 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01499
BindingDB Entry DOI: 10.7270/Q27H1P12 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50530928

(CHEMBL4534127)Show SMILES CN(C1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cnc(N)nc2)C1)S(C)(=O)=O Show InChI InChI=1S/C21H28N8O3S2/c1-27(34(2,30)31)15-3-4-28(12-15)13-16-9-17-18(33-16)20(29-5-7-32-8-6-29)26-19(25-17)14-10-23-21(22)24-11-14/h9-11,15H,3-8,12-13H2,1-2H3,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotin-PIP3 as substrate preincubated for 15 mins followed by substrate addition and measured after 60... |
J Med Chem 62: 10402-10422 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01499
BindingDB Entry DOI: 10.7270/Q27H1P12 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50530894

(CHEMBL4437468)Show SMILES CN(C1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cnc(N)nc2)CC1)C(C)=O Show InChI InChI=1S/C23H30N8O2S/c1-15(32)29(2)17-3-5-30(6-4-17)14-18-11-19-20(34-18)22(31-7-9-33-10-8-31)28-21(27-19)16-12-25-23(24)26-13-16/h11-13,17H,3-10,14H2,1-2H3,(H2,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotin-PIP3 as substrate preincubated for 15 mins followed by substrate addition and measured after 60... |
J Med Chem 62: 10402-10422 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01499
BindingDB Entry DOI: 10.7270/Q27H1P12 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50530928

(CHEMBL4534127)Show SMILES CN(C1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cnc(N)nc2)C1)S(C)(=O)=O Show InChI InChI=1S/C21H28N8O3S2/c1-27(34(2,30)31)15-3-4-28(12-15)13-16-9-17-18(33-16)20(29-5-7-32-8-6-29)26-19(25-17)14-10-23-21(22)24-11-14/h9-11,15H,3-8,12-13H2,1-2H3,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotin-PIP3 as substrate preincubated for 15 mins followed by substrate addition and measured after 60... |
J Med Chem 62: 10402-10422 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01499
BindingDB Entry DOI: 10.7270/Q27H1P12 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50530877

(CHEMBL4580111)Show SMILES CN(C1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ccc23)C1)C(C)=O Show InChI InChI=1S/C26H30N6O2S/c1-17(33)30(2)18-7-9-31(15-18)16-19-14-23-24(35-19)26(32-10-12-34-13-11-32)29-25(28-23)21-4-3-5-22-20(21)6-8-27-22/h3-6,8,14,18,27H,7,9-13,15-16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotin-PIP3 as substrate preincubated for 15 mins followed by substrate addition and measured after 60... |
J Med Chem 62: 10402-10422 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01499
BindingDB Entry DOI: 10.7270/Q27H1P12 |
More data for this
Ligand-Target Pair | |
DNA damage-binding protein 1/Protein cereblon
(Homo sapiens (Human)) | BDBM511609
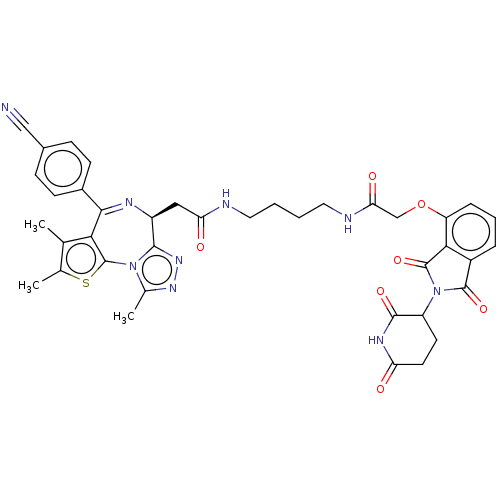
(US11059801, Compound dBET17)Show SMILES Cc1sc-2c(c1C)C(=N[C@@H](CC(=O)NCCCCNC(=O)COc1cccc3C(=O)N(C4CCC(=O)NC4=O)C(=O)c13)c1nnc(C)n-21)c1ccc(cc1)C#N |r,c:8| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6.41 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A thalidomide competition AlphaScreen assay was employed to measure the binding affinity (IC50) of thalidomide conjugates and novel IMiDs to CRBN-DDB... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2G44TFM |
More data for this
Ligand-Target Pair | |
Chymotrypsin-C
(Homo sapiens (Human)) | BDBM50072290

((3S,3aR,6aS)-3-Allyl-hexahydro-cyclopenta[b]furan-...)Show InChI InChI=1S/C10H14O2/c1-2-4-8-7-5-3-6-9(7)12-10(8)11/h2,7-9H,1,3-6H2/t7-,8+,9+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Chymotrypsinogen |
Bioorg Med Chem Lett 8: 2955-60 (1999)
Article DOI: 10.1016/S0960-894X(98)00531-9
BindingDB Entry DOI: 10.7270/Q2JQ105F |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4 [49-170]/DNA damage-binding protein 1/Protein cereblon
(Homo sapiens (Human)) | BDBM511627
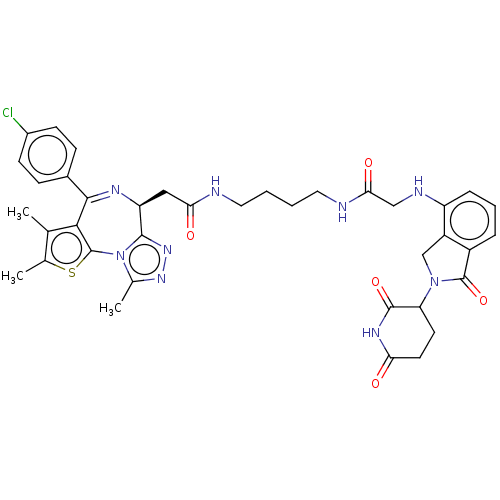
(US11059801, Compound dBET35)Show SMILES Cc1sc-2c(c1C)C(=N[C@@H](CC(=O)NCCCCNC(=O)CNc1cccc3C(=O)N(Cc13)C1CCC(=O)NC1=O)c1nnc(C)n-21)c1ccc(Cl)cc1 |r,c:8| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 7.21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
AlphaScreen technology was used to detect CRBN-DDB1/BRD4 dimerization induced by dBET molecules. In brief, GST-BRD4[49-170] (Sigma Aldrich) and CRBN-... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2G44TFM |
More data for this
Ligand-Target Pair | |
DNA damage-binding protein 1/Protein cereblon
(Homo sapiens (Human)) | BDBM511594
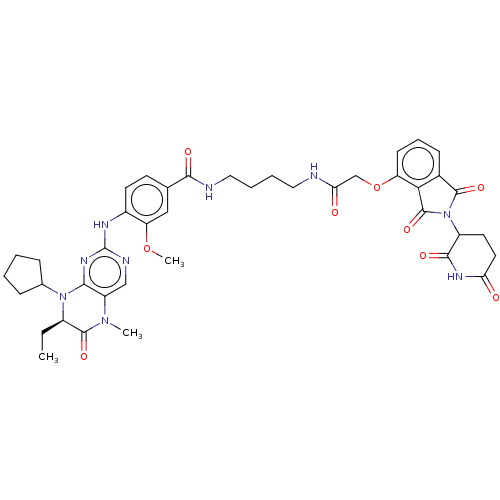
(US11059801, Compound dBET2)Show SMILES CC[C@H]1N(C2CCCC2)c2nc(Nc3ccc(cc3OC)C(=O)NCCCCNC(=O)COc3cccc4C(=O)N(C5CCC(=O)NC5=O)C(=O)c34)ncc2N(C)C1=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A thalidomide competition AlphaScreen assay was employed to measure the binding affinity (IC50) of thalidomide conjugates and novel IMiDs to CRBN-DDB... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2G44TFM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50530887

(CHEMBL4538734)Show SMILES COCC(=O)N(C)C1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cnc(N)nc2)C1 Show InChI InChI=1S/C23H30N8O3S/c1-29(19(32)14-33-2)16-3-4-30(12-16)13-17-9-18-20(35-17)22(31-5-7-34-8-6-31)28-21(27-18)15-10-25-23(24)26-11-15/h9-11,16H,3-8,12-14H2,1-2H3,(H2,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using biotin-PIP3 as substrate preincubated for 15 mins followed by substrate addition and measured after 60... |
J Med Chem 62: 10402-10422 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01499
BindingDB Entry DOI: 10.7270/Q27H1P12 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50530887

(CHEMBL4538734)Show SMILES COCC(=O)N(C)C1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cnc(N)nc2)C1 Show InChI InChI=1S/C23H30N8O3S/c1-29(19(32)14-33-2)16-3-4-30(12-16)13-17-9-18-20(35-17)22(31-5-7-34-8-6-31)28-21(27-18)15-10-25-23(24)26-11-15/h9-11,16H,3-8,12-14H2,1-2H3,(H2,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using biotin-PIP3 as substrate preincubated for 15 mins followed by substrate addition and measured after 60... |
J Med Chem 62: 10402-10422 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01499
BindingDB Entry DOI: 10.7270/Q27H1P12 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50530928

(CHEMBL4534127)Show SMILES CN(C1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cnc(N)nc2)C1)S(C)(=O)=O Show InChI InChI=1S/C21H28N8O3S2/c1-27(34(2,30)31)15-3-4-28(12-15)13-16-9-17-18(33-16)20(29-5-7-32-8-6-29)26-19(25-17)14-10-23-21(22)24-11-14/h9-11,15H,3-8,12-13H2,1-2H3,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using biotin-PIP3 as substrate preincubated for 15 mins followed by substrate addition and measured after 60... |
J Med Chem 62: 10402-10422 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01499
BindingDB Entry DOI: 10.7270/Q27H1P12 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50530905

(CHEMBL4453879)Show SMILES CN(C1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cnc(N)nc2)CC1)S(C)(=O)=O Show InChI InChI=1S/C22H30N8O3S2/c1-28(35(2,31)32)16-3-5-29(6-4-16)14-17-11-18-19(34-17)21(30-7-9-33-10-8-30)27-20(26-18)15-12-24-22(23)25-13-15/h11-13,16H,3-10,14H2,1-2H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotin-PIP3 as substrate preincubated for 15 mins followed by substrate addition and measured after 60... |
J Med Chem 62: 10402-10422 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01499
BindingDB Entry DOI: 10.7270/Q27H1P12 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50530905

(CHEMBL4453879)Show SMILES CN(C1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cnc(N)nc2)CC1)S(C)(=O)=O Show InChI InChI=1S/C22H30N8O3S2/c1-28(35(2,31)32)16-3-5-29(6-4-16)14-17-11-18-19(34-17)21(30-7-9-33-10-8-30)27-20(26-18)15-12-24-22(23)25-13-15/h11-13,16H,3-10,14H2,1-2H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotin-PIP3 as substrate preincubated for 15 mins followed by substrate addition and measured after 60... |
J Med Chem 62: 10402-10422 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01499
BindingDB Entry DOI: 10.7270/Q27H1P12 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50530928

(CHEMBL4534127)Show SMILES CN(C1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cnc(N)nc2)C1)S(C)(=O)=O Show InChI InChI=1S/C21H28N8O3S2/c1-27(34(2,30)31)15-3-4-28(12-15)13-16-9-17-18(33-16)20(29-5-7-32-8-6-29)26-19(25-17)14-10-23-21(22)24-11-14/h9-11,15H,3-8,12-13H2,1-2H3,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using biotin-PIP3 as substrate preincubated for 15 mins followed by substrate addition and measured after 60... |
J Med Chem 62: 10402-10422 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01499
BindingDB Entry DOI: 10.7270/Q27H1P12 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50530872
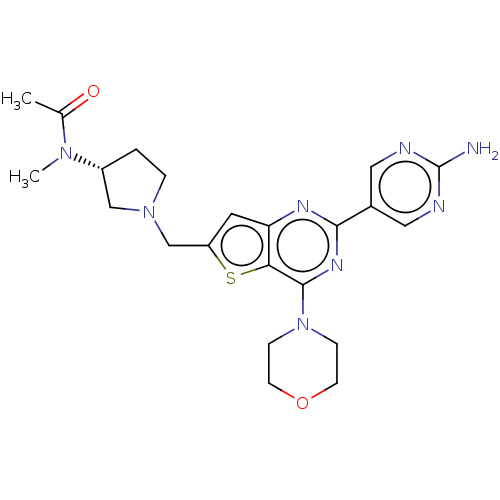
(CHEMBL4462986)Show SMILES CN([C@@H]1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cnc(N)nc2)C1)C(C)=O |r| Show InChI InChI=1S/C22H28N8O2S/c1-14(31)28(2)16-3-4-29(12-16)13-17-9-18-19(33-17)21(30-5-7-32-8-6-30)27-20(26-18)15-10-24-22(23)25-11-15/h9-11,16H,3-8,12-13H2,1-2H3,(H2,23,24,25)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotin-PIP3 as substrate preincubated for 15 mins followed by substrate addition and measured after 60... |
J Med Chem 62: 10402-10422 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01499
BindingDB Entry DOI: 10.7270/Q27H1P12 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50530872

(CHEMBL4462986)Show SMILES CN([C@@H]1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cnc(N)nc2)C1)C(C)=O |r| Show InChI InChI=1S/C22H28N8O2S/c1-14(31)28(2)16-3-4-29(12-16)13-17-9-18-19(33-17)21(30-5-7-32-8-6-30)27-20(26-18)15-10-24-22(23)25-11-15/h9-11,16H,3-8,12-13H2,1-2H3,(H2,23,24,25)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotin-PIP3 as substrate preincubated for 15 mins followed by substrate addition and measured after 60... |
J Med Chem 62: 10402-10422 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01499
BindingDB Entry DOI: 10.7270/Q27H1P12 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50458968
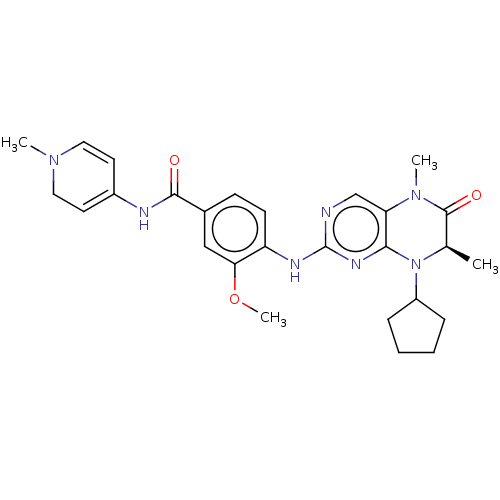
(CHEMBL4216442)Show SMILES COc1cc(ccc1Nc1ncc2N(C)C(=O)[C@@H](C)N(C3CCCC3)c2n1)C(=O)NC1=CCN(C)C=C1 |r,c:39,t:34| Show InChI InChI=1S/C27H33N7O3/c1-17-26(36)33(3)22-16-28-27(31-24(22)34(17)20-7-5-6-8-20)30-21-10-9-18(15-23(21)37-4)25(35)29-19-11-13-32(2)14-12-19/h9-13,15-17,20H,5-8,14H2,1-4H3,(H,29,35)(H,28,30,31)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts-Boston
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PLK1 by Z-Lyte assay |
J Med Chem 61: 7785-7795 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00765
BindingDB Entry DOI: 10.7270/Q2MP55WQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50458967

(CHEMBL4202820)Show SMILES COc1cc(ccc1Nc1ncc2N(C)C(=O)[C@@H](C)N(C3CCCCC3)c2n1)C(=O)NC1CCN(C)CC1 |r| Show InChI InChI=1S/C28H39N7O3/c1-18-27(37)34(3)23-17-29-28(32-25(23)35(18)21-8-6-5-7-9-21)31-22-11-10-19(16-24(22)38-4)26(36)30-20-12-14-33(2)15-13-20/h10-11,16-18,20-21H,5-9,12-15H2,1-4H3,(H,30,36)(H,29,31,32)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts-Boston
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PLK1 by Z-Lyte assay |
J Med Chem 61: 7785-7795 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00765
BindingDB Entry DOI: 10.7270/Q2MP55WQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50458960
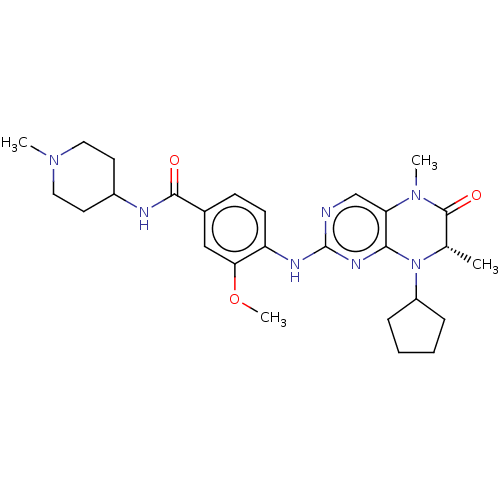
(CHEMBL4216875)Show SMILES COc1cc(ccc1Nc1ncc2N(C)C(=O)[C@H](C)N(C3CCCC3)c2n1)C(=O)NC1CCN(C)CC1 |r| Show InChI InChI=1S/C27H37N7O3/c1-17-26(36)33(3)22-16-28-27(31-24(22)34(17)20-7-5-6-8-20)30-21-10-9-18(15-23(21)37-4)25(35)29-19-11-13-32(2)14-12-19/h9-10,15-17,19-20H,5-8,11-14H2,1-4H3,(H,29,35)(H,28,30,31)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts-Boston
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PLK1 by Z-Lyte assay |
J Med Chem 61: 7785-7795 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00765
BindingDB Entry DOI: 10.7270/Q2MP55WQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50458962
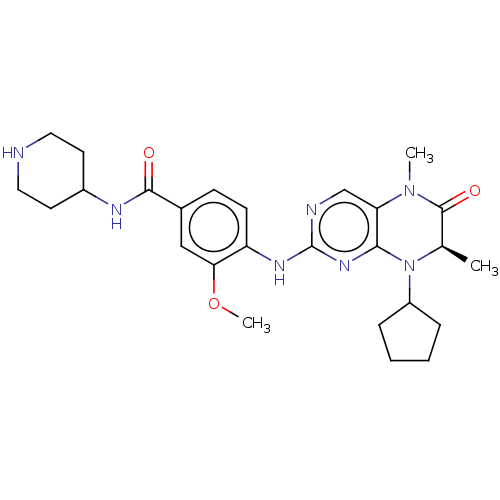
(CHEMBL4213276)Show SMILES COc1cc(ccc1Nc1ncc2N(C)C(=O)[C@@H](C)N(C3CCCC3)c2n1)C(=O)NC1CCNCC1 |r| Show InChI InChI=1S/C26H35N7O3/c1-16-25(35)32(2)21-15-28-26(31-23(21)33(16)19-6-4-5-7-19)30-20-9-8-17(14-22(20)36-3)24(34)29-18-10-12-27-13-11-18/h8-9,14-16,18-19,27H,4-7,10-13H2,1-3H3,(H,29,34)(H,28,30,31)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts-Boston
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PLK1 by Z-Lyte assay |
J Med Chem 61: 7785-7795 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00765
BindingDB Entry DOI: 10.7270/Q2MP55WQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50458951
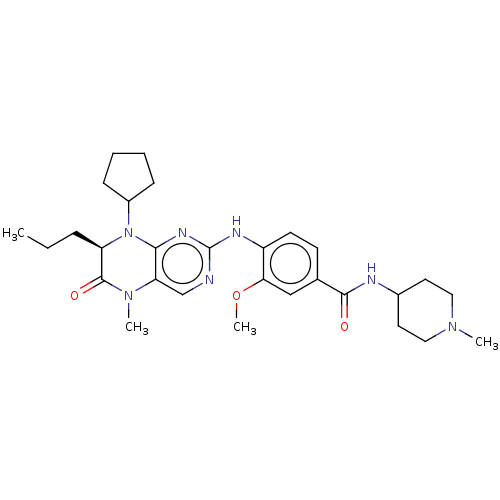
(CHEMBL4206171)Show SMILES CCC[C@H]1N(C2CCCC2)c2nc(Nc3ccc(cc3OC)C(=O)NC3CCN(C)CC3)ncc2N(C)C1=O |r| Show InChI InChI=1S/C29H41N7O3/c1-5-8-23-28(38)35(3)24-18-30-29(33-26(24)36(23)21-9-6-7-10-21)32-22-12-11-19(17-25(22)39-4)27(37)31-20-13-15-34(2)16-14-20/h11-12,17-18,20-21,23H,5-10,13-16H2,1-4H3,(H,31,37)(H,30,32,33)/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts-Boston
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PLK1 by Z-Lyte assay |
J Med Chem 61: 7785-7795 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00765
BindingDB Entry DOI: 10.7270/Q2MP55WQ |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM50503068

(CHEMBL4483587)Show SMILES COc1cc(ccc1Nc1ccc2N(C)C(=O)[C@@H](C)N(C3CCOCC3)c2n1)C(=O)NC1CCN(C)CC1 |r| Show InChI InChI=1S/C28H38N6O4/c1-18-28(36)33(3)23-7-8-25(31-26(23)34(18)21-11-15-38-16-12-21)30-22-6-5-19(17-24(22)37-4)27(35)29-20-9-13-32(2)14-10-20/h5-8,17-18,20-21H,9-16H2,1-4H3,(H,29,35)(H,30,31)/t18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Displacement of biotinylated probe from human TAF1 bromodomain 2 (1522 to 1656 residues) expressed in Escherichia coli BL21 (DE3) by alphascreen assa... |
ACS Med Chem Lett 10: 1443-1449 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00243
BindingDB Entry DOI: 10.7270/Q28P63T8 |
More data for this
Ligand-Target Pair | |
Transcription intermediary factor 1-alpha
(Homo sapiens (Human)) | BDBM50150808
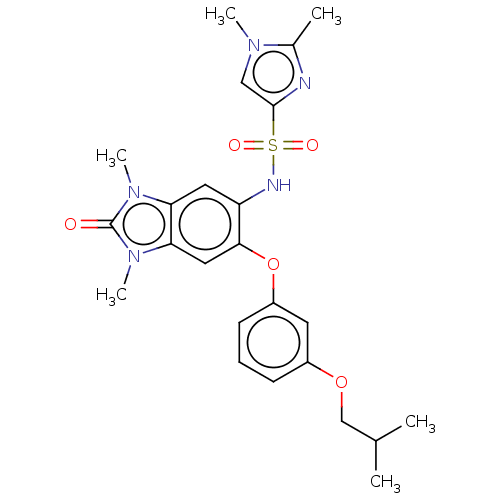
(CHEMBL3774507 | US10702504, Compound C)Show SMILES CC(C)COc1cccc(Oc2cc3n(C)c(=O)n(C)c3cc2NS(=O)(=O)c2cn(C)c(C)n2)c1 Show InChI InChI=1S/C24H29N5O5S/c1-15(2)14-33-17-8-7-9-18(10-17)34-22-12-21-20(28(5)24(30)29(21)6)11-19(22)26-35(31,32)23-13-27(4)16(3)25-23/h7-13,15,26H,14H2,1-6H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
DANA-FARBER CANCER INSTITUTE, INC.
US Patent
| Assay Description
The alpha assay was used to assess the binding of the compounds of the application to TRIM24 at various concentrations. All compounds were added at c... |
US Patent US10702504 (2020)
BindingDB Entry DOI: 10.7270/Q22B921C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Transcription intermediary factor 1-alpha
(Homo sapiens (Human)) | BDBM449307
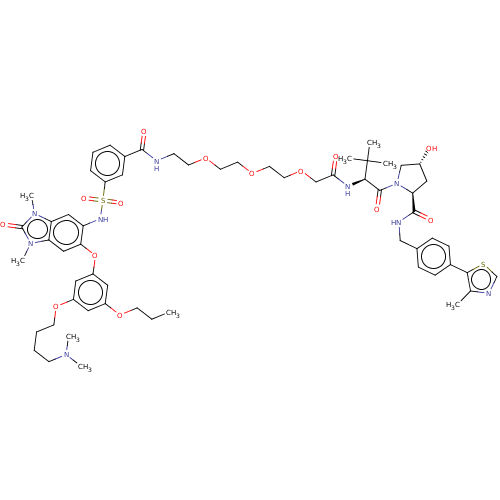
(US10702504, Compound I-B4)Show SMILES CCCOc1cc(OCCCCN(C)C)cc(Oc2cc3n(C)c(=O)n(C)c3cc2NS(=O)(=O)c2cccc(c2)C(=O)NCCOCCOCCOCC(=O)N[C@H](C(=O)N2C[C@H](O)C[C@H]2C(=O)NCc2ccc(cc2)-c2scnc2C)C(C)(C)C)c1 |r| Show InChI InChI=1S/C61H81N9O14S2/c1-10-22-82-45-31-46(83-23-12-11-21-67(6)7)33-47(32-45)84-53-35-51-50(68(8)60(76)69(51)9)34-49(53)66-86(77,78)48-15-13-14-43(29-48)57(73)62-20-24-79-25-26-80-27-28-81-38-54(72)65-56(61(3,4)5)59(75)70-37-44(71)30-52(70)58(74)63-36-41-16-18-42(19-17-41)55-40(2)64-39-85-55/h13-19,29,31-35,39,44,52,56,66,71H,10-12,20-28,30,36-38H2,1-9H3,(H,62,73)(H,63,74)(H,65,72)/t44-,52+,56-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
DANA-FARBER CANCER INSTITUTE, INC.
US Patent
| Assay Description
The alpha assay was used to assess the binding of the compounds of the application to TRIM24 at various concentrations. All compounds were added at c... |
US Patent US10702504 (2020)
BindingDB Entry DOI: 10.7270/Q22B921C |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50530893

(CHEMBL4532070)Show SMILES CN(C1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ccc23)C1)S(C)(=O)=O Show InChI InChI=1S/C25H30N6O3S2/c1-29(36(2,32)33)17-7-9-30(15-17)16-18-14-22-23(35-18)25(31-10-12-34-13-11-31)28-24(27-22)20-4-3-5-21-19(20)6-8-26-21/h3-6,8,14,17,26H,7,9-13,15-16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotin-PIP3 as substrate preincubated for 15 mins followed by substrate addition and measured after 60... |
J Med Chem 62: 10402-10422 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01499
BindingDB Entry DOI: 10.7270/Q27H1P12 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50530878
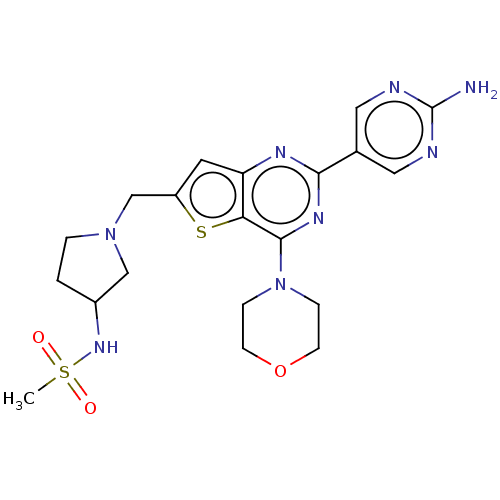
(CHEMBL4467891)Show SMILES CS(=O)(=O)NC1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cnc(N)nc2)C1 Show InChI InChI=1S/C20H26N8O3S2/c1-33(29,30)26-14-2-3-27(11-14)12-15-8-16-17(32-15)19(28-4-6-31-7-5-28)25-18(24-16)13-9-22-20(21)23-10-13/h8-10,14,26H,2-7,11-12H2,1H3,(H2,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using biotin-PIP3 as substrate preincubated for 15 mins followed by substrate addition and measured after 60... |
J Med Chem 62: 10402-10422 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01499
BindingDB Entry DOI: 10.7270/Q27H1P12 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data