 Found 452 hits with Last Name = 'bunker' and Initial = 'a'
Found 452 hits with Last Name = 'bunker' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Collagenase 3
(Homo sapiens (Human)) | BDBM50372652
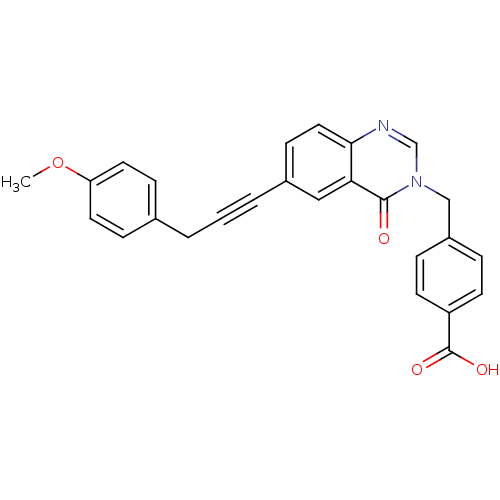
(CHEMBL410029)Show SMILES COc1ccc(CC#Cc2ccc3ncn(Cc4ccc(cc4)C(O)=O)c(=O)c3c2)cc1 Show InChI InChI=1S/C26H20N2O4/c1-32-22-12-7-18(8-13-22)3-2-4-19-9-14-24-23(15-19)25(29)28(17-27-24)16-20-5-10-21(11-6-20)26(30)31/h5-15,17H,3,16H2,1H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP13 catalytic domain |
J Med Chem 51: 835-41 (2008)
Article DOI: 10.1021/jm701274v
BindingDB Entry DOI: 10.7270/Q2J38TDZ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50230906
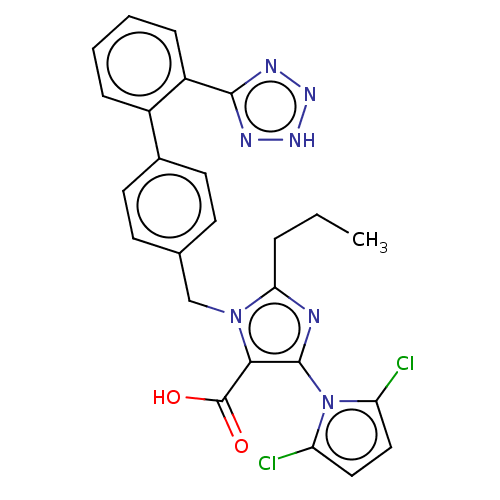
(CHEMBL308261)Show SMILES CCCc1nc(c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1)-n1c(Cl)ccc1Cl |(11.28,-11.6,;12.74,-12.07,;13.89,-11.03,;15.36,-11.5,;15.83,-12.96,;17.36,-12.96,;17.84,-11.47,;19.3,-11.03,;20.44,-12.04,;19.61,-9.53,;16.59,-10.6,;16.59,-9.05,;15.25,-8.29,;15.23,-6.73,;13.89,-5.99,;12.58,-6.76,;12.58,-8.29,;13.91,-9.05,;11.24,-6,;9.91,-6.76,;8.57,-6,;8.57,-4.44,;9.91,-3.67,;11.24,-4.44,;12.55,-3.66,;12.71,-2.13,;14.21,-1.79,;14.99,-3.13,;13.95,-4.28,;18.28,-14.2,;19.81,-14.18,;21.14,-13.41,;20.29,-15.64,;19.05,-16.57,;17.81,-15.66,;16.33,-16.06,)| Show InChI InChI=1S/C25H21Cl2N7O2/c1-2-5-21-28-24(34-19(26)12-13-20(34)27)22(25(35)36)33(21)14-15-8-10-16(11-9-15)17-6-3-4-7-18(17)23-29-31-32-30-23/h3-4,6-13H,2,5,14H2,1H3,(H,35,36)(H,29,30,31,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against binding of [125I]angiotensin II to rat liver expressing Angiotensin II receptor |
J Med Chem 36: 2253-65 (1993)
Article DOI: 10.1021/jm00068a002
BindingDB Entry DOI: 10.7270/Q25H7JGW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50230919
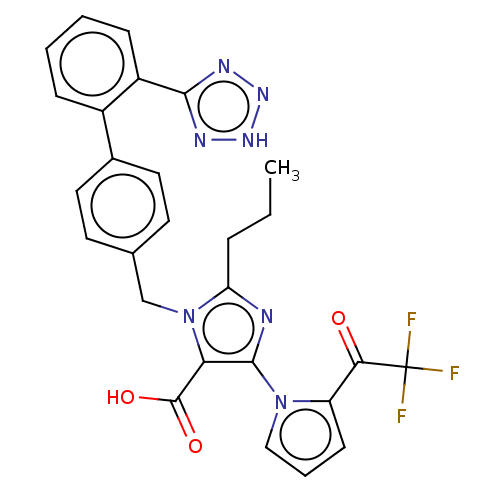
(CHEMBL307844 | CI-996)Show SMILES CCCc1nc(c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1)-n1cccc1C(=O)C(F)(F)F Show InChI InChI=1S/C27H22F3N7O3/c1-2-6-21-31-25(36-14-5-9-20(36)23(38)27(28,29)30)22(26(39)40)37(21)15-16-10-12-17(13-11-16)18-7-3-4-8-19(18)24-32-34-35-33-24/h3-5,7-14H,2,6,15H2,1H3,(H,39,40)(H,32,33,34,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against binding of [125I]angiotensin II to rat liver expressing Angiotensin II receptor |
J Med Chem 36: 2253-65 (1993)
Article DOI: 10.1021/jm00068a002
BindingDB Entry DOI: 10.7270/Q25H7JGW |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50372652

(CHEMBL410029)Show SMILES COc1ccc(CC#Cc2ccc3ncn(Cc4ccc(cc4)C(O)=O)c(=O)c3c2)cc1 Show InChI InChI=1S/C26H20N2O4/c1-32-22-12-7-18(8-13-22)3-2-4-19-9-14-24-23(15-19)25(29)28(17-27-24)16-20-5-10-21(11-6-20)26(30)31/h5-15,17H,3,16H2,1H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP13 |
J Med Chem 51: 835-41 (2008)
Article DOI: 10.1021/jm701274v
BindingDB Entry DOI: 10.7270/Q2J38TDZ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50230891

(CHEMBL76166)Show SMILES CCCc1nc(c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1)-n1cccc1C=O Show InChI InChI=1S/C26H23N7O3/c1-2-6-22-27-25(32-14-5-7-19(32)16-34)23(26(35)36)33(22)15-17-10-12-18(13-11-17)20-8-3-4-9-21(20)24-28-30-31-29-24/h3-5,7-14,16H,2,6,15H2,1H3,(H,35,36)(H,28,29,30,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against binding of [125I]angiotensin II to rat liver expressing Angiotensin II receptor |
J Med Chem 36: 2253-65 (1993)
Article DOI: 10.1021/jm00068a002
BindingDB Entry DOI: 10.7270/Q25H7JGW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50230883
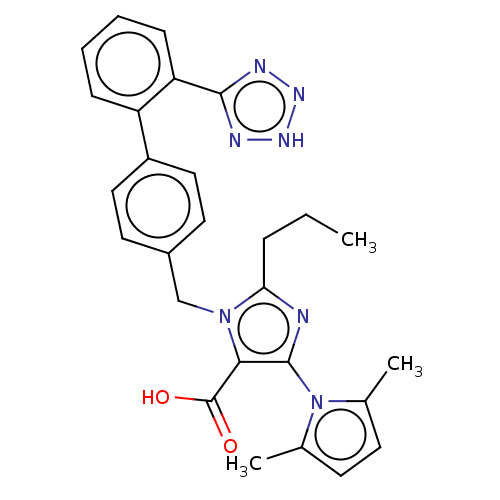
(CHEMBL309089)Show SMILES CCCc1nc(c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1)-n1c(C)ccc1C |(11.28,-11.6,;12.74,-12.07,;13.89,-11.03,;15.36,-11.5,;15.83,-12.96,;17.36,-12.96,;17.84,-11.47,;19.3,-11.03,;20.44,-12.04,;19.61,-9.53,;16.59,-10.6,;16.59,-9.05,;15.25,-8.29,;15.23,-6.73,;13.89,-5.99,;12.58,-6.76,;12.58,-8.29,;13.91,-9.05,;11.24,-6,;9.91,-6.76,;8.57,-6,;8.57,-4.44,;9.91,-3.67,;11.24,-4.44,;12.55,-3.66,;12.71,-2.13,;14.21,-1.79,;14.99,-3.13,;13.95,-4.28,;18.28,-14.2,;17.81,-15.66,;16.36,-16.14,;19.05,-16.57,;20.29,-15.64,;19.81,-14.18,;20.89,-13.6,)| Show InChI InChI=1S/C27H27N7O2/c1-4-7-23-28-26(34-17(2)10-11-18(34)3)24(27(35)36)33(23)16-19-12-14-20(15-13-19)21-8-5-6-9-22(21)25-29-31-32-30-25/h5-6,8-15H,4,7,16H2,1-3H3,(H,35,36)(H,29,30,31,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against binding of [125I]angiotensin II to rat liver expressing Angiotensin II receptor |
J Med Chem 36: 2253-65 (1993)
Article DOI: 10.1021/jm00068a002
BindingDB Entry DOI: 10.7270/Q25H7JGW |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50372636
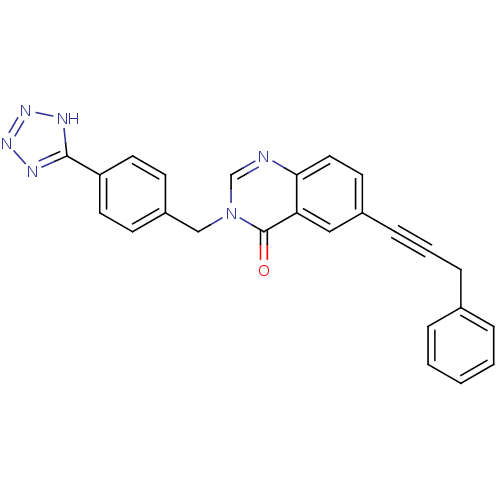
(CHEMBL429584)Show SMILES O=c1n(Cc2ccc(cc2)-c2nnn[nH]2)cnc2ccc(cc12)C#CCc1ccccc1 Show InChI InChI=1S/C25H18N6O/c32-25-22-15-19(8-4-7-18-5-2-1-3-6-18)11-14-23(22)26-17-31(25)16-20-9-12-21(13-10-20)24-27-29-30-28-24/h1-3,5-6,9-15,17H,7,16H2,(H,27,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP13 catalytic domain |
J Med Chem 51: 835-41 (2008)
Article DOI: 10.1021/jm701274v
BindingDB Entry DOI: 10.7270/Q2J38TDZ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50230915
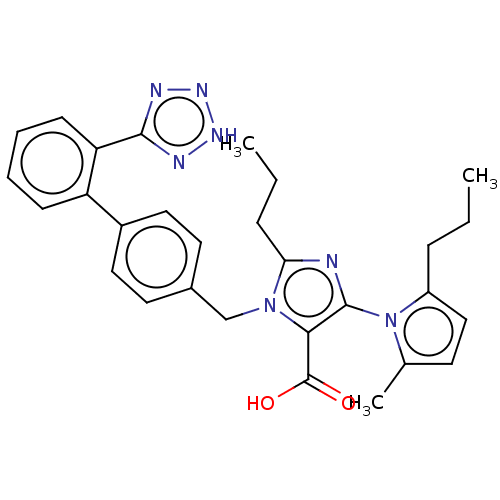
(CHEMBL76169)Show SMILES CCCc1ccc(C)n1-c1nc(CCC)n(Cc2ccc(cc2)-c2ccccc2-c2nn[nH]n2)c1C(O)=O |(13.76,-15.58,;15.23,-15.1,;16.36,-16.14,;17.81,-15.66,;19.05,-16.57,;20.29,-15.64,;19.81,-14.18,;20.89,-13.6,;18.28,-14.2,;17.36,-12.96,;15.83,-12.96,;15.36,-11.5,;13.89,-11.03,;12.74,-12.07,;11.28,-11.6,;16.59,-10.6,;16.59,-9.05,;15.25,-8.29,;15.23,-6.73,;13.89,-5.99,;12.58,-6.76,;12.58,-8.29,;13.91,-9.05,;11.24,-6,;9.91,-6.76,;8.57,-6,;8.57,-4.44,;9.91,-3.67,;11.24,-4.44,;12.55,-3.66,;12.71,-2.13,;14.21,-1.79,;14.99,-3.13,;13.95,-4.28,;17.84,-11.47,;19.3,-11.03,;20.44,-12.04,;19.61,-9.53,)| Show InChI InChI=1S/C29H31N7O2/c1-4-8-22-17-12-19(3)36(22)28-26(29(37)38)35(25(30-28)9-5-2)18-20-13-15-21(16-14-20)23-10-6-7-11-24(23)27-31-33-34-32-27/h6-7,10-17H,4-5,8-9,18H2,1-3H3,(H,37,38)(H,31,32,33,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against binding of [125I]angiotensin II to rat liver expressing Angiotensin II receptor |
J Med Chem 36: 2253-65 (1993)
Article DOI: 10.1021/jm00068a002
BindingDB Entry DOI: 10.7270/Q25H7JGW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50230887
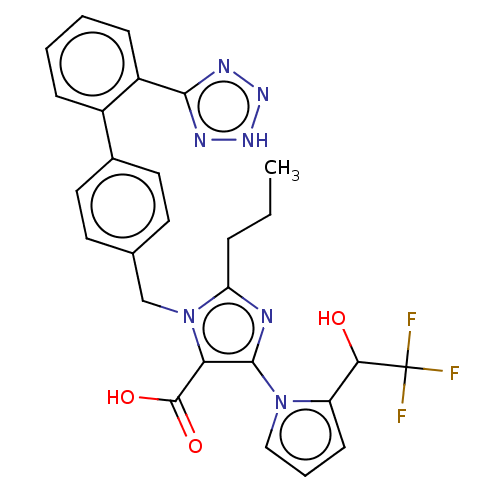
(CHEMBL306259)Show SMILES CCCc1nc(c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1)-n1cccc1C(O)C(F)(F)F Show InChI InChI=1S/C27H24F3N7O3/c1-2-6-21-31-25(36-14-5-9-20(36)23(38)27(28,29)30)22(26(39)40)37(21)15-16-10-12-17(13-11-16)18-7-3-4-8-19(18)24-32-34-35-33-24/h3-5,7-14,23,38H,2,6,15H2,1H3,(H,39,40)(H,32,33,34,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against binding of [125I]angiotensin II to rat liver expressing Angiotensin II receptor |
J Med Chem 36: 2253-65 (1993)
Article DOI: 10.1021/jm00068a002
BindingDB Entry DOI: 10.7270/Q25H7JGW |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(Homo sapiens (Human)) | BDBM50215294

(2-(N-(6-chloro-7-methyl-2,3-dioxo-1,2,3,4-tetrahyd...)Show SMILES Cc1cc2[nH]c(=O)c(=O)[nH]c2c(N(CC(O)=O)S(C)(=O)=O)c1Cl Show InChI InChI=1S/C12H12ClN3O6S/c1-5-3-6-9(15-12(20)11(19)14-6)10(8(5)13)16(4-7(17)18)23(2,21)22/h3H,4H2,1-2H3,(H,14,19)(H,15,20)(H,17,18) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human NMDA NR1 receptor |
Bioorg Med Chem Lett 17: 4599-603 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.083
BindingDB Entry DOI: 10.7270/Q2QR4WTR |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50230912
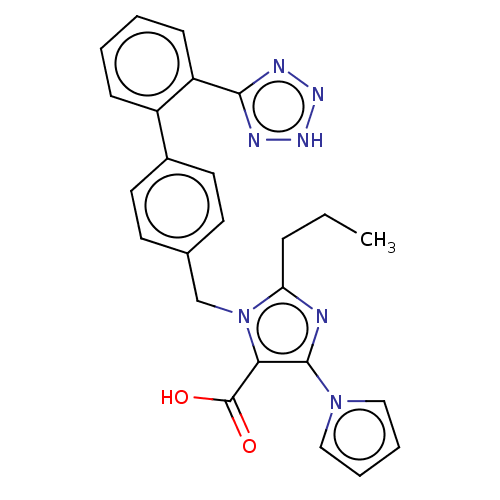
(CHEMBL72922)Show SMILES CCCc1nc(c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1)-n1cccc1 Show InChI InChI=1S/C25H23N7O2/c1-2-7-21-26-24(31-14-5-6-15-31)22(25(33)34)32(21)16-17-10-12-18(13-11-17)19-8-3-4-9-20(19)23-27-29-30-28-23/h3-6,8-15H,2,7,16H2,1H3,(H,33,34)(H,27,28,29,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against binding of [125I]angiotensin II to rat liver expressing Angiotensin II receptor |
J Med Chem 36: 2253-65 (1993)
Article DOI: 10.1021/jm00068a002
BindingDB Entry DOI: 10.7270/Q25H7JGW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50230886
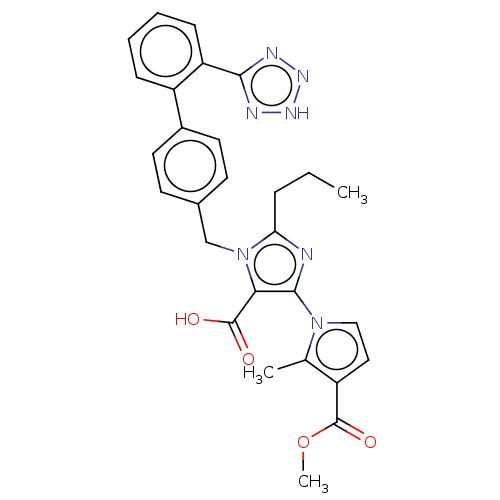
(CHEMBL307455)Show SMILES CCCc1nc(c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1)-n1ccc(C(=O)OC)c1C Show InChI InChI=1S/C28H27N7O4/c1-4-7-23-29-26(34-15-14-20(17(34)2)28(38)39-3)24(27(36)37)35(23)16-18-10-12-19(13-11-18)21-8-5-6-9-22(21)25-30-32-33-31-25/h5-6,8-15H,4,7,16H2,1-3H3,(H,36,37)(H,30,31,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against binding of [125I]angiotensin II to rat liver expressing Angiotensin II receptor |
J Med Chem 36: 2253-65 (1993)
Article DOI: 10.1021/jm00068a002
BindingDB Entry DOI: 10.7270/Q25H7JGW |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50372657
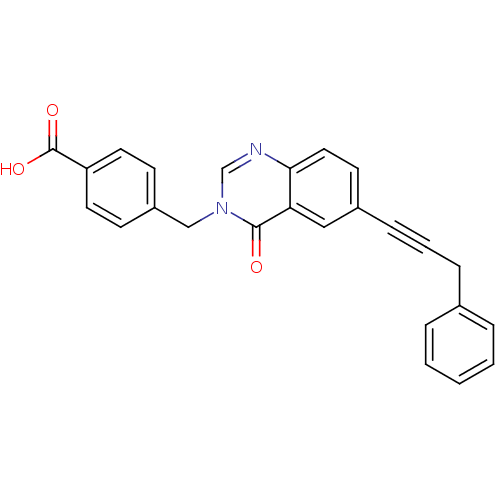
(CHEMBL273054)Show SMILES OC(=O)c1ccc(Cn2cnc3ccc(cc3c2=O)C#CCc2ccccc2)cc1 Show InChI InChI=1S/C25H18N2O3/c28-24-22-15-19(8-4-7-18-5-2-1-3-6-18)11-14-23(22)26-17-27(24)16-20-9-12-21(13-10-20)25(29)30/h1-3,5-6,9-15,17H,7,16H2,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.54 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP13 catalytic domain |
J Med Chem 51: 835-41 (2008)
Article DOI: 10.1021/jm701274v
BindingDB Entry DOI: 10.7270/Q2J38TDZ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50230882
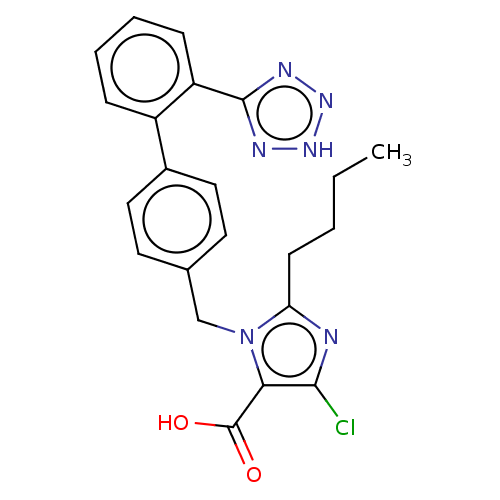
(CARBOXYLIC ACID METABOLITE | CHEBI:74125 | E-3174)Show SMILES CCCCc1nc(Cl)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1 Show InChI InChI=1S/C22H21ClN6O2/c1-2-3-8-18-24-20(23)19(22(30)31)29(18)13-14-9-11-15(12-10-14)16-6-4-5-7-17(16)21-25-27-28-26-21/h4-7,9-12H,2-3,8,13H2,1H3,(H,30,31)(H,25,26,27,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against binding of [125I]angiotensin II to rat liver expressing Angiotensin II receptor |
J Med Chem 36: 2253-65 (1993)
Article DOI: 10.1021/jm00068a002
BindingDB Entry DOI: 10.7270/Q25H7JGW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50230882

(CARBOXYLIC ACID METABOLITE | CHEBI:74125 | E-3174)Show SMILES CCCCc1nc(Cl)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1 Show InChI InChI=1S/C22H21ClN6O2/c1-2-3-8-18-24-20(23)19(22(30)31)29(18)13-14-9-11-15(12-10-14)16-6-4-5-7-17(16)21-25-27-28-26-21/h4-7,9-12H,2-3,8,13H2,1H3,(H,30,31)(H,25,26,27,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Tested for inhibitory concentration against AT1 receptor binding affinity in rat liver |
J Med Chem 36: 2253-65 (1993)
Article DOI: 10.1021/jm00068a002
BindingDB Entry DOI: 10.7270/Q25H7JGW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50230910

(CHEMBL306066)Show SMILES CCCCc1nc(c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1)-n1cccc1 Show InChI InChI=1S/C26H25N7O2/c1-2-3-10-22-27-25(32-15-6-7-16-32)23(26(34)35)33(22)17-18-11-13-19(14-12-18)20-8-4-5-9-21(20)24-28-30-31-29-24/h4-9,11-16H,2-3,10,17H2,1H3,(H,34,35)(H,28,29,30,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against binding of [125I]angiotensin II to rat liver expressing Angiotensin II receptor |
J Med Chem 36: 2253-65 (1993)
Article DOI: 10.1021/jm00068a002
BindingDB Entry DOI: 10.7270/Q25H7JGW |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50372633
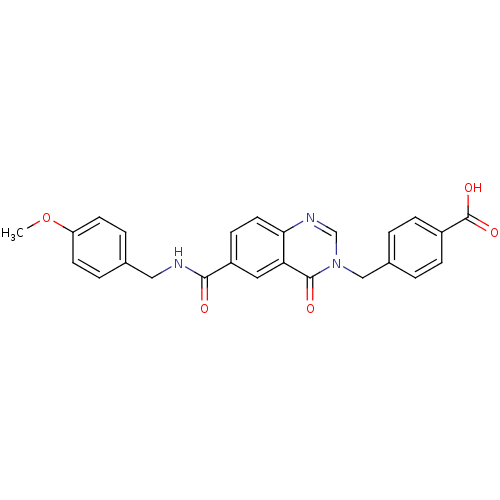
(CHEMBL270502)Show SMILES COc1ccc(CNC(=O)c2ccc3ncn(Cc4ccc(cc4)C(O)=O)c(=O)c3c2)cc1 Show InChI InChI=1S/C25H21N3O5/c1-33-20-9-4-16(5-10-20)13-26-23(29)19-8-11-22-21(12-19)24(30)28(15-27-22)14-17-2-6-18(7-3-17)25(31)32/h2-12,15H,13-14H2,1H3,(H,26,29)(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.61 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP13 catalytic domain |
J Med Chem 51: 835-41 (2008)
Article DOI: 10.1021/jm701274v
BindingDB Entry DOI: 10.7270/Q2J38TDZ |
More data for this
Ligand-Target Pair | |
Type-1/Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50230882

(CARBOXYLIC ACID METABOLITE | CHEBI:74125 | E-3174)Show SMILES CCCCc1nc(Cl)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1 Show InChI InChI=1S/C22H21ClN6O2/c1-2-3-8-18-24-20(23)19(22(30)31)29(18)13-14-9-11-15(12-10-14)16-6-4-5-7-17(16)21-25-27-28-26-21/h4-7,9-12H,2-3,8,13H2,1H3,(H,30,31)(H,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against [125I]angiotensin II(AII) induced contraction in rabbit aorta by 50% |
J Med Chem 36: 2253-65 (1993)
Article DOI: 10.1021/jm00068a002
BindingDB Entry DOI: 10.7270/Q25H7JGW |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(Homo sapiens (Human)) | BDBM50215284

(2-(N-(7-chloro-6-methyl-2,3-dioxo-1,2,3,4-tetrahyd...)Show SMILES Cc1c(Cl)cc2[nH]c(=O)c(=O)[nH]c2c1N(CC(O)=O)S(C)(=O)=O Show InChI InChI=1S/C12H12ClN3O6S/c1-5-6(13)3-7-9(15-12(20)11(19)14-7)10(5)16(4-8(17)18)23(2,21)22/h3H,4H2,1-2H3,(H,14,19)(H,15,20)(H,17,18) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human NMDA NR1 receptor |
Bioorg Med Chem Lett 17: 4599-603 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.083
BindingDB Entry DOI: 10.7270/Q2QR4WTR |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50287200
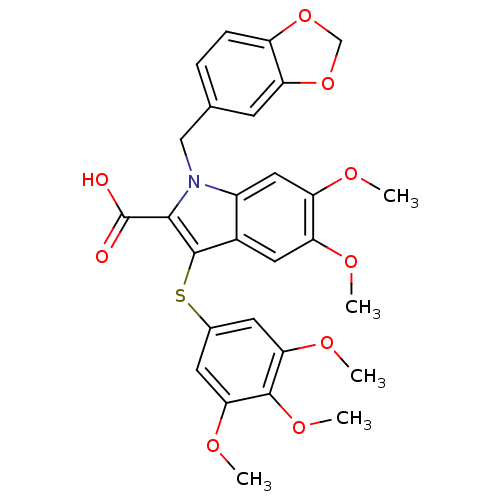
(1-Benzo[1,3]dioxol-5-ylmethyl-5,6-dimethoxy-3-(3,4...)Show SMILES COc1cc(Sc2c(C(O)=O)n(Cc3ccc4OCOc4c3)c3cc(OC)c(OC)cc23)cc(OC)c1OC Show InChI InChI=1S/C28H27NO9S/c1-32-20-11-17-18(12-21(20)33-2)29(13-15-6-7-19-22(8-15)38-14-37-19)25(28(30)31)27(17)39-16-9-23(34-3)26(36-5)24(10-16)35-4/h6-12H,13-14H2,1-5H3,(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Affinity for human endothelin A receptor expressed in Ltk cells. |
Bioorg Med Chem Lett 6: 1367-1370 (1996)
Article DOI: 10.1016/0960-894X(96)00232-6
BindingDB Entry DOI: 10.7270/Q2K35TMT |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(Homo sapiens (Human)) | BDBM50215283
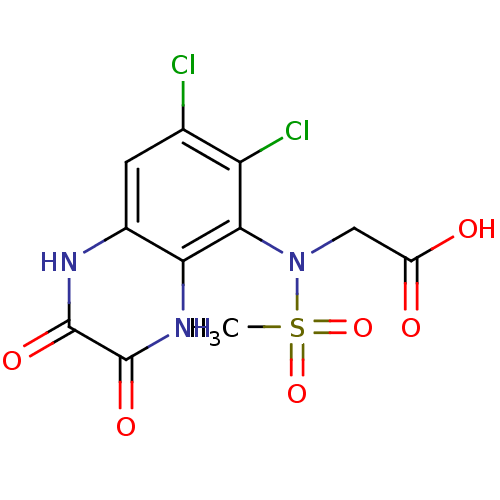
(2-(N-(6,7-dichloro-2,3-dioxo-1,2,3,4-tetrahydroqui...)Show SMILES CS(=O)(=O)N(CC(O)=O)c1c(Cl)c(Cl)cc2[nH]c(=O)c(=O)[nH]c12 Show InChI InChI=1S/C11H9Cl2N3O6S/c1-23(21,22)16(3-6(17)18)9-7(13)4(12)2-5-8(9)15-11(20)10(19)14-5/h2H,3H2,1H3,(H,14,19)(H,15,20)(H,17,18) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human NMDA NR1 receptor |
Bioorg Med Chem Lett 17: 4599-603 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.083
BindingDB Entry DOI: 10.7270/Q2QR4WTR |
More data for this
Ligand-Target Pair | |
Type-1/Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50230883

(CHEMBL309089)Show SMILES CCCc1nc(c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1)-n1c(C)ccc1C |(11.28,-11.6,;12.74,-12.07,;13.89,-11.03,;15.36,-11.5,;15.83,-12.96,;17.36,-12.96,;17.84,-11.47,;19.3,-11.03,;20.44,-12.04,;19.61,-9.53,;16.59,-10.6,;16.59,-9.05,;15.25,-8.29,;15.23,-6.73,;13.89,-5.99,;12.58,-6.76,;12.58,-8.29,;13.91,-9.05,;11.24,-6,;9.91,-6.76,;8.57,-6,;8.57,-4.44,;9.91,-3.67,;11.24,-4.44,;12.55,-3.66,;12.71,-2.13,;14.21,-1.79,;14.99,-3.13,;13.95,-4.28,;18.28,-14.2,;17.81,-15.66,;16.36,-16.14,;19.05,-16.57,;20.29,-15.64,;19.81,-14.18,;20.89,-13.6,)| Show InChI InChI=1S/C27H27N7O2/c1-4-7-23-28-26(34-17(2)10-11-18(34)3)24(27(35)36)33(23)16-19-12-14-20(15-13-19)21-8-5-6-9-22(21)25-29-31-32-30-25/h5-6,8-15H,4,7,16H2,1-3H3,(H,35,36)(H,29,30,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against [125I]angiotensin II(AII) induced contraction in rabbit aorta by 50% |
J Med Chem 36: 2253-65 (1993)
Article DOI: 10.1021/jm00068a002
BindingDB Entry DOI: 10.7270/Q25H7JGW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50230877
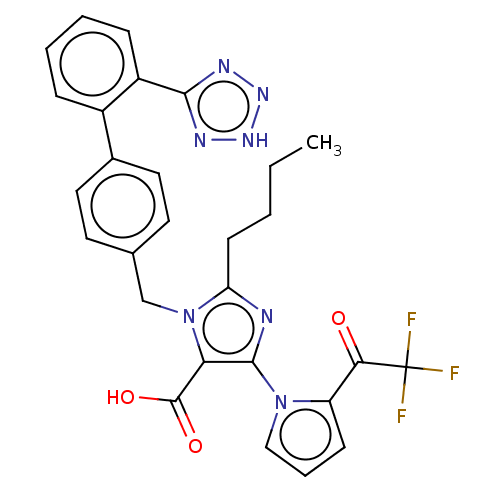
(CHEMBL70371)Show SMILES CCCCc1nc(c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1)-n1cccc1C(=O)C(F)(F)F Show InChI InChI=1S/C28H24F3N7O3/c1-2-3-10-22-32-26(37-15-6-9-21(37)24(39)28(29,30)31)23(27(40)41)38(22)16-17-11-13-18(14-12-17)19-7-4-5-8-20(19)25-33-35-36-34-25/h4-9,11-15H,2-3,10,16H2,1H3,(H,40,41)(H,33,34,35,36) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against binding of [125I]angiotensin II to rat liver expressing Angiotensin II receptor |
J Med Chem 36: 2253-65 (1993)
Article DOI: 10.1021/jm00068a002
BindingDB Entry DOI: 10.7270/Q25H7JGW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50230900
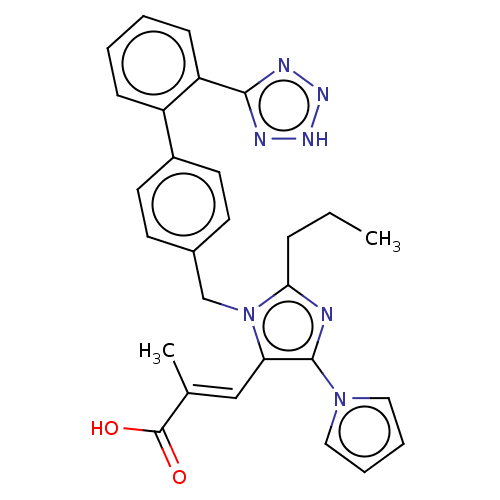
(CHEMBL73433)Show SMILES CCCc1nc(c(\C=C(/C)C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1)-n1cccc1 Show InChI InChI=1S/C28H27N7O2/c1-3-8-25-29-27(34-15-6-7-16-34)24(17-19(2)28(36)37)35(25)18-20-11-13-21(14-12-20)22-9-4-5-10-23(22)26-30-32-33-31-26/h4-7,9-17H,3,8,18H2,1-2H3,(H,36,37)(H,30,31,32,33)/b19-17+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against binding of [125I]angiotensin II to rat liver expressing Angiotensin II receptor |
J Med Chem 36: 2253-65 (1993)
Article DOI: 10.1021/jm00068a002
BindingDB Entry DOI: 10.7270/Q25H7JGW |
More data for this
Ligand-Target Pair | |
Type-1/Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50230891

(CHEMBL76166)Show SMILES CCCc1nc(c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1)-n1cccc1C=O Show InChI InChI=1S/C26H23N7O3/c1-2-6-22-27-25(32-14-5-7-19(32)16-34)23(26(35)36)33(22)15-17-10-12-18(13-11-17)20-8-3-4-9-21(20)24-28-30-31-29-24/h3-5,7-14,16H,2,6,15H2,1H3,(H,35,36)(H,28,29,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against [125I]angiotensin II(AII) induced contraction in rabbit aorta by 50% |
J Med Chem 36: 2253-65 (1993)
Article DOI: 10.1021/jm00068a002
BindingDB Entry DOI: 10.7270/Q25H7JGW |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50372647
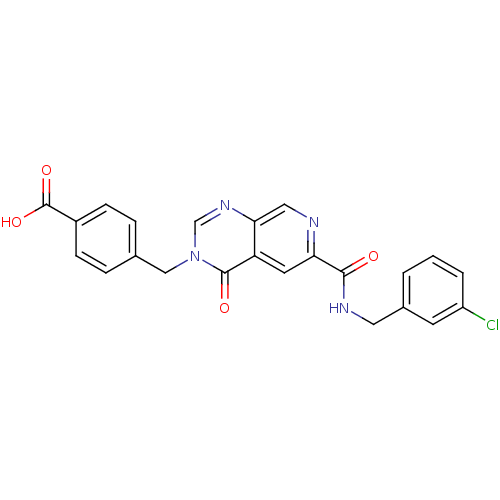
(CHEMBL272007)Show SMILES OC(=O)c1ccc(Cn2cnc3cnc(cc3c2=O)C(=O)NCc2cccc(Cl)c2)cc1 Show InChI InChI=1S/C23H17ClN4O4/c24-17-3-1-2-15(8-17)10-26-21(29)19-9-18-20(11-25-19)27-13-28(22(18)30)12-14-4-6-16(7-5-14)23(31)32/h1-9,11,13H,10,12H2,(H,26,29)(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.69 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP13 catalytic domain |
J Med Chem 51: 835-41 (2008)
Article DOI: 10.1021/jm701274v
BindingDB Entry DOI: 10.7270/Q2J38TDZ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50230902
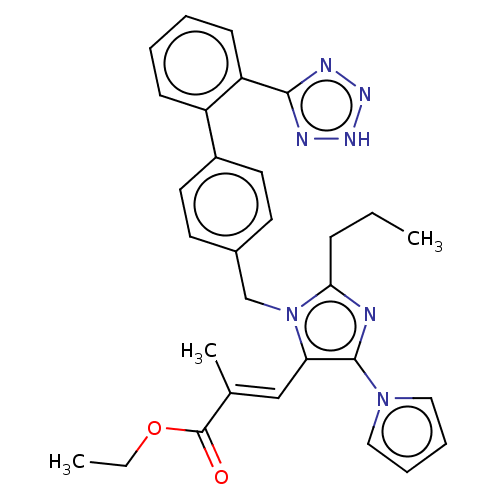
(CHEMBL75518)Show SMILES CCCc1nc(c(\C=C(/C)C(=O)OCC)n1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1)-n1cccc1 Show InChI InChI=1S/C30H31N7O2/c1-4-10-27-31-29(36-17-8-9-18-36)26(19-21(3)30(38)39-5-2)37(27)20-22-13-15-23(16-14-22)24-11-6-7-12-25(24)28-32-34-35-33-28/h6-9,11-19H,4-5,10,20H2,1-3H3,(H,32,33,34,35)/b21-19+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against binding of [125I]angiotensin II to rat liver expressing Angiotensin II receptor |
J Med Chem 36: 2253-65 (1993)
Article DOI: 10.1021/jm00068a002
BindingDB Entry DOI: 10.7270/Q25H7JGW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50230893
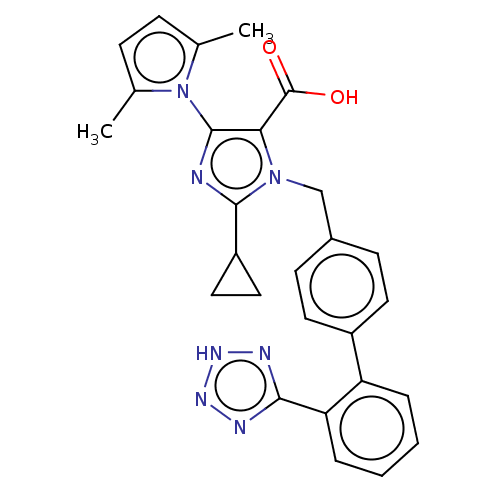
(CHEMBL308031)Show SMILES Cc1ccc(C)n1-c1nc(C2CC2)n(Cc2ccc(cc2)-c2ccccc2-c2nn[nH]n2)c1C(O)=O |(14.26,-14.55,;15.71,-14.07,;16.95,-14.97,;18.19,-14.05,;17.71,-12.59,;18.48,-11.85,;16.18,-12.61,;15.26,-11.37,;13.73,-11.37,;13.25,-9.91,;11.79,-9.43,;10.76,-8.29,;10.28,-9.75,;14.49,-9.01,;14.49,-7.46,;13.15,-6.7,;13.13,-5.14,;11.79,-4.39,;10.47,-5.17,;10.47,-6.7,;11.81,-7.46,;9.14,-4.4,;7.81,-5.17,;6.47,-4.4,;6.47,-2.84,;7.81,-2.08,;9.14,-2.84,;10.45,-2.07,;10.61,-.54,;12.11,-.2,;12.89,-1.54,;11.85,-2.68,;15.74,-9.9,;17.2,-9.43,;18.34,-10.45,;17.51,-7.94,)| Show InChI InChI=1S/C27H25N7O2/c1-16-7-8-17(2)34(16)26-23(27(35)36)33(25(28-26)20-13-14-20)15-18-9-11-19(12-10-18)21-5-3-4-6-22(21)24-29-31-32-30-24/h3-12,20H,13-15H2,1-2H3,(H,35,36)(H,29,30,31,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against binding of [125I]angiotensin II to rat liver expressing Angiotensin II receptor |
J Med Chem 36: 2253-65 (1993)
Article DOI: 10.1021/jm00068a002
BindingDB Entry DOI: 10.7270/Q25H7JGW |
More data for this
Ligand-Target Pair | |
Type-1/Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50230887

(CHEMBL306259)Show SMILES CCCc1nc(c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1)-n1cccc1C(O)C(F)(F)F Show InChI InChI=1S/C27H24F3N7O3/c1-2-6-21-31-25(36-14-5-9-20(36)23(38)27(28,29)30)22(26(39)40)37(21)15-16-10-12-17(13-11-16)18-7-3-4-8-19(18)24-32-34-35-33-24/h3-5,7-14,23,38H,2,6,15H2,1H3,(H,39,40)(H,32,33,34,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against [125I]angiotensin II(AII) induced contraction in rabbit aorta by 50% |
J Med Chem 36: 2253-65 (1993)
Article DOI: 10.1021/jm00068a002
BindingDB Entry DOI: 10.7270/Q25H7JGW |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM25960
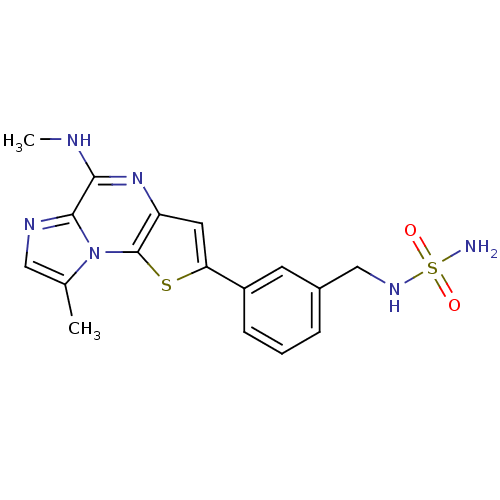
(amino-N-({3-[12-methyl-8-(methylamino)-3-thia-1,7,...)Show SMILES CNc1nc2cc(sc2n2c(C)cnc12)-c1cccc(CNS(N)(=O)=O)c1 Show InChI InChI=1S/C17H18N6O2S2/c1-10-8-20-16-15(19-2)22-13-7-14(26-17(13)23(10)16)12-5-3-4-11(6-12)9-21-27(18,24)25/h3-8,21H,9H2,1-2H3,(H,19,22)(H2,18,24,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
| Assay Description
Assays measuring the enzyme-catalyzed phosphorylation of GST-I kappa B alpha were performed. The phosphorylated substrate was detected using a Phosph... |
Bioorg Med Chem Lett 17: 4284-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.031
BindingDB Entry DOI: 10.7270/Q2QF8R50 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50093310

(3-{4-[5-(2,6-Dimethyl-piperidin-1-yl)-pentyl]-3-ox...)Show SMILES CC1CCCC(C)N1CCCCCN1C(=O)C(Oc2ccccc12)c1cccc(c1)C(N)=N Show InChI InChI=1S/C27H36N4O2/c1-19-10-8-11-20(2)30(19)16-6-3-7-17-31-23-14-4-5-15-24(23)33-25(27(31)32)21-12-9-13-22(18-21)26(28)29/h4-5,9,12-15,18-20,25H,3,6-8,10-11,16-17H2,1-2H3,(H3,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of coagulation factor Xa. |
J Med Chem 43: 4063-70 (2000)
BindingDB Entry DOI: 10.7270/Q2W66K1H |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50230881
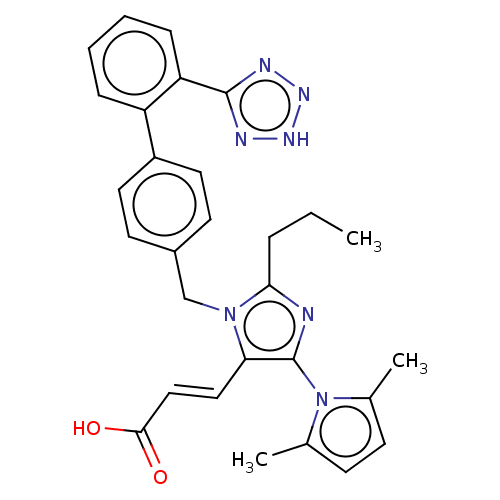
(CHEMBL308779)Show SMILES CCCc1nc(c(\C=C\C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1)-n1c(C)ccc1C |(11.3,-11.62,;12.77,-12.09,;13.92,-11.05,;15.38,-11.53,;15.86,-12.98,;17.39,-12.98,;17.87,-11.49,;19.34,-11.05,;19.65,-9.55,;21.09,-9.07,;22.24,-10.09,;21.41,-7.57,;16.62,-10.62,;16.62,-9.07,;15.28,-8.3,;15.26,-6.74,;13.92,-6,;12.6,-6.77,;12.6,-8.3,;13.94,-9.07,;11.26,-6.01,;9.93,-6.77,;8.59,-6.01,;8.59,-4.44,;9.93,-3.68,;11.26,-4.44,;12.58,-3.67,;12.74,-2.14,;14.24,-1.8,;15.02,-3.14,;13.98,-4.28,;18.32,-14.23,;19.85,-14.2,;20.74,-12.96,;20.33,-15.67,;19.08,-16.6,;17.84,-15.69,;16.39,-16.17,)| Show InChI InChI=1S/C29H29N7O2/c1-4-7-26-30-29(36-19(2)10-11-20(36)3)25(16-17-27(37)38)35(26)18-21-12-14-22(15-13-21)23-8-5-6-9-24(23)28-31-33-34-32-28/h5-6,8-17H,4,7,18H2,1-3H3,(H,37,38)(H,31,32,33,34)/b17-16+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against binding of [125I]angiotensin II to rat liver expressing Angiotensin II receptor |
J Med Chem 36: 2253-65 (1993)
Article DOI: 10.1021/jm00068a002
BindingDB Entry DOI: 10.7270/Q25H7JGW |
More data for this
Ligand-Target Pair | |
Type-1/Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50230906

(CHEMBL308261)Show SMILES CCCc1nc(c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1)-n1c(Cl)ccc1Cl |(11.28,-11.6,;12.74,-12.07,;13.89,-11.03,;15.36,-11.5,;15.83,-12.96,;17.36,-12.96,;17.84,-11.47,;19.3,-11.03,;20.44,-12.04,;19.61,-9.53,;16.59,-10.6,;16.59,-9.05,;15.25,-8.29,;15.23,-6.73,;13.89,-5.99,;12.58,-6.76,;12.58,-8.29,;13.91,-9.05,;11.24,-6,;9.91,-6.76,;8.57,-6,;8.57,-4.44,;9.91,-3.67,;11.24,-4.44,;12.55,-3.66,;12.71,-2.13,;14.21,-1.79,;14.99,-3.13,;13.95,-4.28,;18.28,-14.2,;19.81,-14.18,;21.14,-13.41,;20.29,-15.64,;19.05,-16.57,;17.81,-15.66,;16.33,-16.06,)| Show InChI InChI=1S/C25H21Cl2N7O2/c1-2-5-21-28-24(34-19(26)12-13-20(34)27)22(25(35)36)33(21)14-15-8-10-16(11-9-15)17-6-3-4-7-18(17)23-29-31-32-30-23/h3-4,6-13H,2,5,14H2,1H3,(H,35,36)(H,29,30,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against [125I]angiotensin II(AII) induced contraction in rabbit aorta by 50% |
J Med Chem 36: 2253-65 (1993)
Article DOI: 10.1021/jm00068a002
BindingDB Entry DOI: 10.7270/Q25H7JGW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50230908
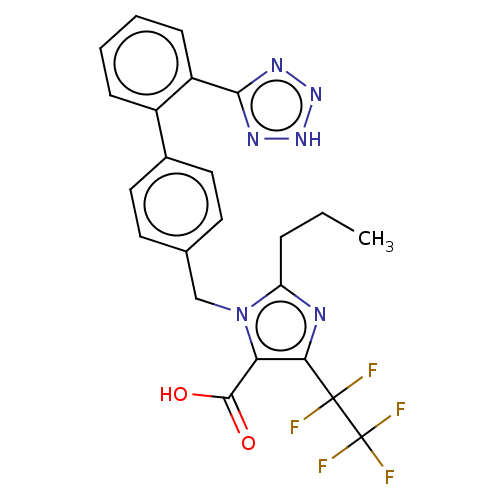
(CHEMBL443269 | DuP-532)Show SMILES CCCc1nc(c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1)C(F)(F)C(F)(F)F Show InChI InChI=1S/C23H19F5N6O2/c1-2-5-17-29-19(22(24,25)23(26,27)28)18(21(35)36)34(17)12-13-8-10-14(11-9-13)15-6-3-4-7-16(15)20-30-32-33-31-20/h3-4,6-11H,2,5,12H2,1H3,(H,35,36)(H,30,31,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Tested for inhibitory concentration against AT1 receptor binding affinity in rat liver |
J Med Chem 36: 2253-65 (1993)
Article DOI: 10.1021/jm00068a002
BindingDB Entry DOI: 10.7270/Q25H7JGW |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50372632
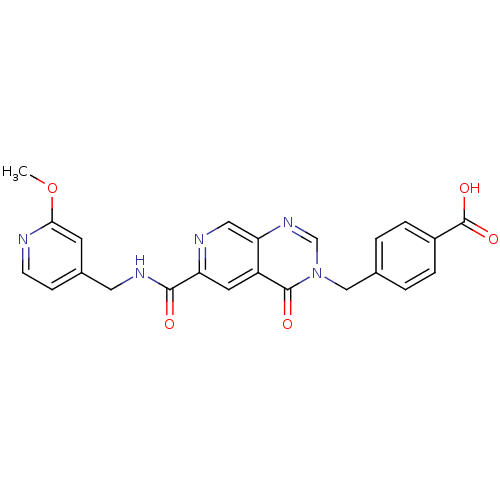
(CHEMBL273113)Show SMILES COc1cc(CNC(=O)c2cc3c(cn2)ncn(Cc2ccc(cc2)C(O)=O)c3=O)ccn1 Show InChI InChI=1S/C23H19N5O5/c1-33-20-8-15(6-7-24-20)10-26-21(29)18-9-17-19(11-25-18)27-13-28(22(17)30)12-14-2-4-16(5-3-14)23(31)32/h2-9,11,13H,10,12H2,1H3,(H,26,29)(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.46 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP13 catalytic domain |
J Med Chem 51: 835-41 (2008)
Article DOI: 10.1021/jm701274v
BindingDB Entry DOI: 10.7270/Q2J38TDZ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50230909
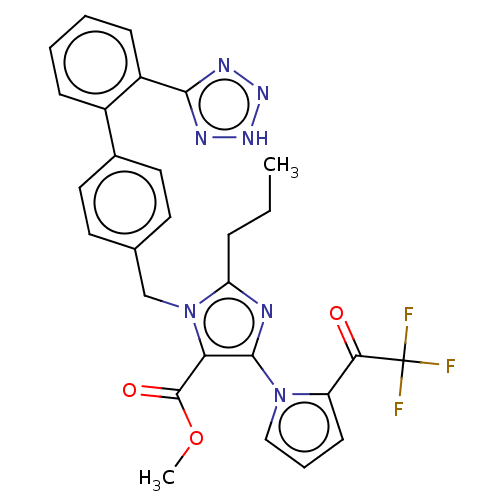
(CHEMBL306852)Show SMILES CCCc1nc(c(C(=O)OC)n1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1)-n1cccc1C(=O)C(F)(F)F Show InChI InChI=1S/C28H24F3N7O3/c1-3-7-22-32-26(37-15-6-10-21(37)24(39)28(29,30)31)23(27(40)41-2)38(22)16-17-11-13-18(14-12-17)19-8-4-5-9-20(19)25-33-35-36-34-25/h4-6,8-15H,3,7,16H2,1-2H3,(H,33,34,35,36) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against binding of [125I]angiotensin II to rat liver expressing Angiotensin II receptor |
J Med Chem 36: 2253-65 (1993)
Article DOI: 10.1021/jm00068a002
BindingDB Entry DOI: 10.7270/Q25H7JGW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50230897
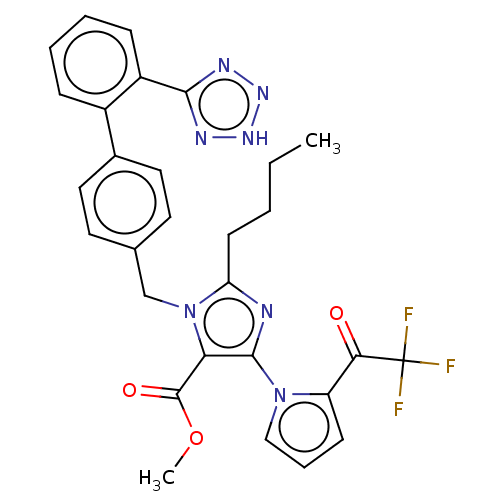
(CHEMBL275652)Show SMILES CCCCc1nc(c(C(=O)OC)n1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1)-n1cccc1C(=O)C(F)(F)F Show InChI InChI=1S/C29H26F3N7O3/c1-3-4-11-23-33-27(38-16-7-10-22(38)25(40)29(30,31)32)24(28(41)42-2)39(23)17-18-12-14-19(15-13-18)20-8-5-6-9-21(20)26-34-36-37-35-26/h5-10,12-16H,3-4,11,17H2,1-2H3,(H,34,35,36,37) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against binding of [125I]angiotensin II to rat liver expressing Angiotensin II receptor |
J Med Chem 36: 2253-65 (1993)
Article DOI: 10.1021/jm00068a002
BindingDB Entry DOI: 10.7270/Q25H7JGW |
More data for this
Ligand-Target Pair | |
Type-1/Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50230919

(CHEMBL307844 | CI-996)Show SMILES CCCc1nc(c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1)-n1cccc1C(=O)C(F)(F)F Show InChI InChI=1S/C27H22F3N7O3/c1-2-6-21-31-25(36-14-5-9-20(36)23(38)27(28,29)30)22(26(39)40)37(21)15-16-10-12-17(13-11-16)18-7-3-4-8-19(18)24-32-34-35-33-24/h3-5,7-14H,2,6,15H2,1H3,(H,39,40)(H,32,33,34,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against [125I]angiotensin II(AII) induced contraction in rabbit aorta by 50% |
J Med Chem 36: 2253-65 (1993)
Article DOI: 10.1021/jm00068a002
BindingDB Entry DOI: 10.7270/Q25H7JGW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50230914
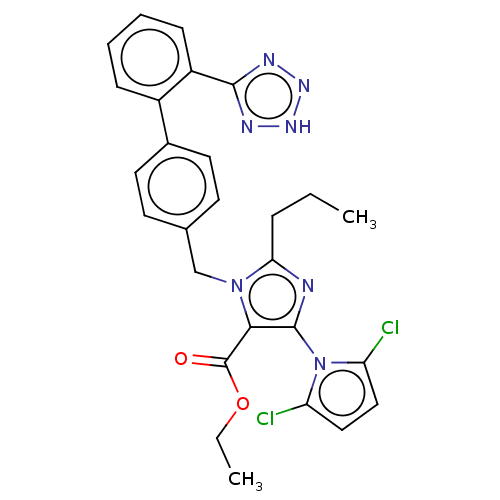
(CHEMBL431390)Show SMILES CCCc1nc(c(C(=O)OCC)n1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1)-n1c(Cl)ccc1Cl |(11.28,-11.6,;12.74,-12.07,;13.89,-11.03,;15.36,-11.5,;15.83,-12.96,;17.36,-12.96,;17.84,-11.47,;19.3,-11.03,;19.61,-9.53,;20.44,-12.04,;21.88,-11.57,;23.03,-12.59,;16.59,-10.6,;16.59,-9.05,;15.25,-8.29,;15.23,-6.73,;13.89,-5.99,;12.58,-6.76,;12.58,-8.29,;13.91,-9.05,;11.24,-6,;9.91,-6.76,;8.57,-6,;8.57,-4.44,;9.91,-3.67,;11.24,-4.44,;12.55,-3.66,;12.71,-2.13,;14.21,-1.79,;14.99,-3.13,;13.95,-4.28,;18.28,-14.2,;19.81,-14.18,;21.14,-13.41,;20.29,-15.64,;19.05,-16.57,;17.81,-15.66,;16.33,-16.06,)| Show InChI InChI=1S/C27H25Cl2N7O2/c1-3-7-23-30-26(36-21(28)14-15-22(36)29)24(27(37)38-4-2)35(23)16-17-10-12-18(13-11-17)19-8-5-6-9-20(19)25-31-33-34-32-25/h5-6,8-15H,3-4,7,16H2,1-2H3,(H,31,32,33,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against binding of [125I]angiotensin II to rat liver expressing Angiotensin II receptor |
J Med Chem 36: 2253-65 (1993)
Article DOI: 10.1021/jm00068a002
BindingDB Entry DOI: 10.7270/Q25H7JGW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50230888
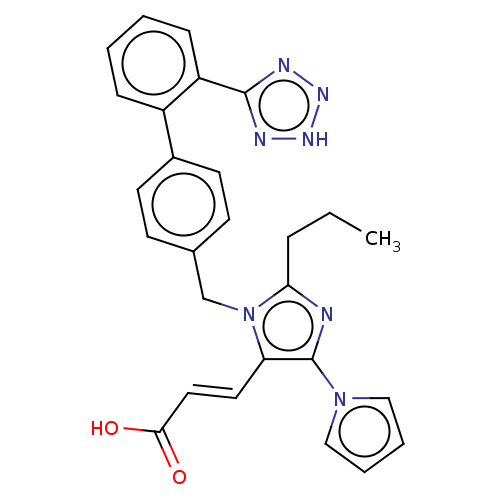
(CHEMBL306612)Show SMILES CCCc1nc(c(\C=C\C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1)-n1cccc1 Show InChI InChI=1S/C27H25N7O2/c1-2-7-24-28-27(33-16-5-6-17-33)23(14-15-25(35)36)34(24)18-19-10-12-20(13-11-19)21-8-3-4-9-22(21)26-29-31-32-30-26/h3-6,8-17H,2,7,18H2,1H3,(H,35,36)(H,29,30,31,32)/b15-14+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against binding of [125I]angiotensin II to rat liver expressing Angiotensin II receptor |
J Med Chem 36: 2253-65 (1993)
Article DOI: 10.1021/jm00068a002
BindingDB Entry DOI: 10.7270/Q25H7JGW |
More data for this
Ligand-Target Pair | |
Type-1/Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50230877

(CHEMBL70371)Show SMILES CCCCc1nc(c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1)-n1cccc1C(=O)C(F)(F)F Show InChI InChI=1S/C28H24F3N7O3/c1-2-3-10-22-32-26(37-15-6-9-21(37)24(39)28(29,30)31)23(27(40)41)38(22)16-17-11-13-18(14-12-17)19-7-4-5-8-20(19)25-33-35-36-34-25/h4-9,11-15H,2-3,10,16H2,1H3,(H,40,41)(H,33,34,35,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against [125I]angiotensin II(AII) induced contraction in rabbit aorta by 50% |
J Med Chem 36: 2253-65 (1993)
Article DOI: 10.1021/jm00068a002
BindingDB Entry DOI: 10.7270/Q25H7JGW |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50093310

(3-{4-[5-(2,6-Dimethyl-piperidin-1-yl)-pentyl]-3-ox...)Show SMILES CC1CCCC(C)N1CCCCCN1C(=O)C(Oc2ccccc12)c1cccc(c1)C(N)=N Show InChI InChI=1S/C27H36N4O2/c1-19-10-8-11-20(2)30(19)16-6-3-7-17-31-23-14-4-5-15-24(23)33-25(27(31)32)21-12-9-13-22(18-21)26(28)29/h4-5,9,12-15,18-20,25H,3,6-8,10-11,16-17H2,1-2H3,(H3,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Compound was tested for in vitro inhibitory activity against Prothrombinase |
J Med Chem 43: 4063-70 (2000)
BindingDB Entry DOI: 10.7270/Q2W66K1H |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(Homo sapiens (Human)) | BDBM50215282
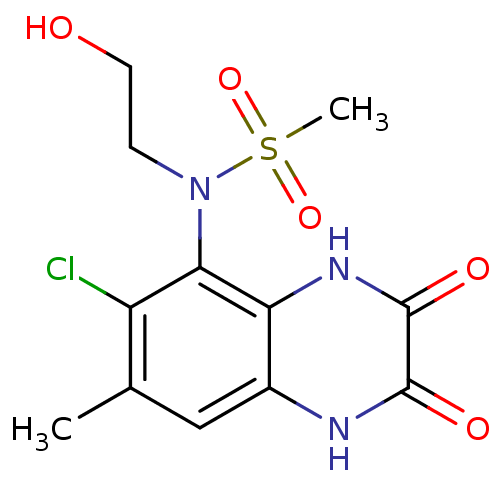
(CHEMBL429296 | N-(6-chloro-7-methyl-2,3-dioxo-1,2,...)Show SMILES Cc1cc2[nH]c(=O)c(=O)[nH]c2c(N(CCO)S(C)(=O)=O)c1Cl Show InChI InChI=1S/C12H14ClN3O5S/c1-6-5-7-9(15-12(19)11(18)14-7)10(8(6)13)16(3-4-17)22(2,20)21/h5,17H,3-4H2,1-2H3,(H,14,18)(H,15,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human NMDA NR1 receptor |
Bioorg Med Chem Lett 17: 4599-603 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.083
BindingDB Entry DOI: 10.7270/Q2QR4WTR |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50372639
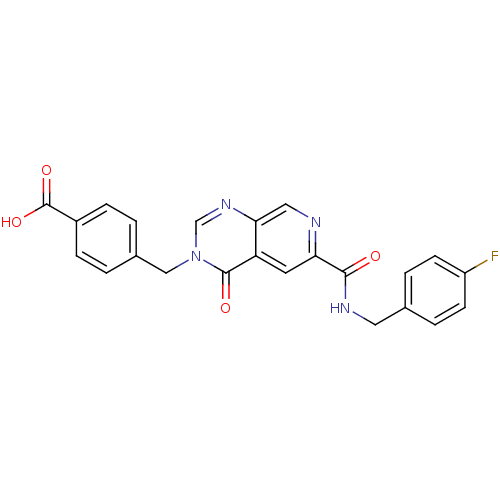
(CHEMBL271997)Show SMILES OC(=O)c1ccc(Cn2cnc3cnc(cc3c2=O)C(=O)NCc2ccc(F)cc2)cc1 Show InChI InChI=1S/C23H17FN4O4/c24-17-7-3-14(4-8-17)10-26-21(29)19-9-18-20(11-25-19)27-13-28(22(18)30)12-15-1-5-16(6-2-15)23(31)32/h1-9,11,13H,10,12H2,(H,26,29)(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.24 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP13 catalytic domain |
J Med Chem 51: 835-41 (2008)
Article DOI: 10.1021/jm701274v
BindingDB Entry DOI: 10.7270/Q2J38TDZ |
More data for this
Ligand-Target Pair | |
Type-1/Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50230915

(CHEMBL76169)Show SMILES CCCc1ccc(C)n1-c1nc(CCC)n(Cc2ccc(cc2)-c2ccccc2-c2nn[nH]n2)c1C(O)=O |(13.76,-15.58,;15.23,-15.1,;16.36,-16.14,;17.81,-15.66,;19.05,-16.57,;20.29,-15.64,;19.81,-14.18,;20.89,-13.6,;18.28,-14.2,;17.36,-12.96,;15.83,-12.96,;15.36,-11.5,;13.89,-11.03,;12.74,-12.07,;11.28,-11.6,;16.59,-10.6,;16.59,-9.05,;15.25,-8.29,;15.23,-6.73,;13.89,-5.99,;12.58,-6.76,;12.58,-8.29,;13.91,-9.05,;11.24,-6,;9.91,-6.76,;8.57,-6,;8.57,-4.44,;9.91,-3.67,;11.24,-4.44,;12.55,-3.66,;12.71,-2.13,;14.21,-1.79,;14.99,-3.13,;13.95,-4.28,;17.84,-11.47,;19.3,-11.03,;20.44,-12.04,;19.61,-9.53,)| Show InChI InChI=1S/C29H31N7O2/c1-4-8-22-17-12-19(3)36(22)28-26(29(37)38)35(25(30-28)9-5-2)18-20-13-15-21(16-14-20)23-10-6-7-11-24(23)27-31-33-34-32-27/h6-7,10-17H,4-5,8-9,18H2,1-3H3,(H,37,38)(H,31,32,33,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against [125I]angiotensin II(AII) induced contraction in rabbit aorta by 50% |
J Med Chem 36: 2253-65 (1993)
Article DOI: 10.1021/jm00068a002
BindingDB Entry DOI: 10.7270/Q25H7JGW |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50287200

(1-Benzo[1,3]dioxol-5-ylmethyl-5,6-dimethoxy-3-(3,4...)Show SMILES COc1cc(Sc2c(C(O)=O)n(Cc3ccc4OCOc4c3)c3cc(OC)c(OC)cc23)cc(OC)c1OC Show InChI InChI=1S/C28H27NO9S/c1-32-20-11-17-18(12-21(20)33-2)29(13-15-6-7-19-22(8-15)38-14-37-19)25(28(30)31)27(17)39-16-9-23(34-3)26(36-5)24(10-16)35-4/h6-12H,13-14H2,1-5H3,(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Affinity for human endothelin A receptor expressed in Ltk cells. |
Bioorg Med Chem Lett 6: 1367-1370 (1996)
Article DOI: 10.1016/0960-894X(96)00232-6
BindingDB Entry DOI: 10.7270/Q2K35TMT |
More data for this
Ligand-Target Pair | |
Type-1/Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50230893

(CHEMBL308031)Show SMILES Cc1ccc(C)n1-c1nc(C2CC2)n(Cc2ccc(cc2)-c2ccccc2-c2nn[nH]n2)c1C(O)=O |(14.26,-14.55,;15.71,-14.07,;16.95,-14.97,;18.19,-14.05,;17.71,-12.59,;18.48,-11.85,;16.18,-12.61,;15.26,-11.37,;13.73,-11.37,;13.25,-9.91,;11.79,-9.43,;10.76,-8.29,;10.28,-9.75,;14.49,-9.01,;14.49,-7.46,;13.15,-6.7,;13.13,-5.14,;11.79,-4.39,;10.47,-5.17,;10.47,-6.7,;11.81,-7.46,;9.14,-4.4,;7.81,-5.17,;6.47,-4.4,;6.47,-2.84,;7.81,-2.08,;9.14,-2.84,;10.45,-2.07,;10.61,-.54,;12.11,-.2,;12.89,-1.54,;11.85,-2.68,;15.74,-9.9,;17.2,-9.43,;18.34,-10.45,;17.51,-7.94,)| Show InChI InChI=1S/C27H25N7O2/c1-16-7-8-17(2)34(16)26-23(27(35)36)33(25(28-26)20-13-14-20)15-18-9-11-19(12-10-18)21-5-3-4-6-22(21)24-29-31-32-30-24/h3-12,20H,13-15H2,1-2H3,(H,35,36)(H,29,30,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against [125I]angiotensin II(AII) induced contraction in rabbit aorta by 50% |
J Med Chem 36: 2253-65 (1993)
Article DOI: 10.1021/jm00068a002
BindingDB Entry DOI: 10.7270/Q25H7JGW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM82258
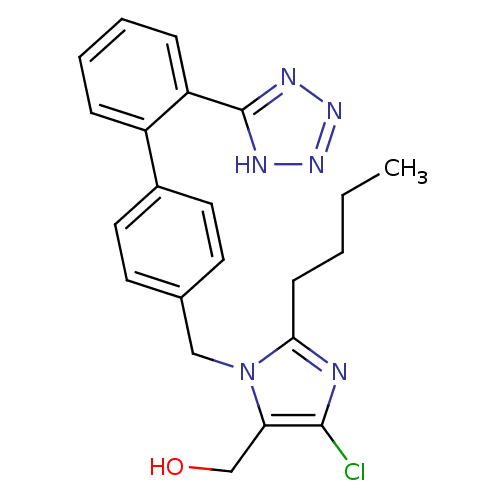
(CAS_114798-26-4 | Losartan | NSC_3961)Show SMILES CCCCc1nc(Cl)c(CO)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C22H23ClN6O/c1-2-3-8-20-24-21(23)19(14-30)29(20)13-15-9-11-16(12-10-15)17-6-4-5-7-18(17)22-25-27-28-26-22/h4-7,9-12,30H,2-3,8,13-14H2,1H3,(H,25,26,27,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Tested for inhibitory concentration against AT1 receptor binding affinity in rat liver |
J Med Chem 36: 2253-65 (1993)
Article DOI: 10.1021/jm00068a002
BindingDB Entry DOI: 10.7270/Q25H7JGW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM82258

(CAS_114798-26-4 | Losartan | NSC_3961)Show SMILES CCCCc1nc(Cl)c(CO)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C22H23ClN6O/c1-2-3-8-20-24-21(23)19(14-30)29(20)13-15-9-11-16(12-10-15)17-6-4-5-7-18(17)22-25-27-28-26-22/h4-7,9-12,30H,2-3,8,13-14H2,1H3,(H,25,26,27,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against binding of [125I]angiotensin II to rat liver expressing Angiotensin II receptor |
J Med Chem 36: 2253-65 (1993)
Article DOI: 10.1021/jm00068a002
BindingDB Entry DOI: 10.7270/Q25H7JGW |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50287183
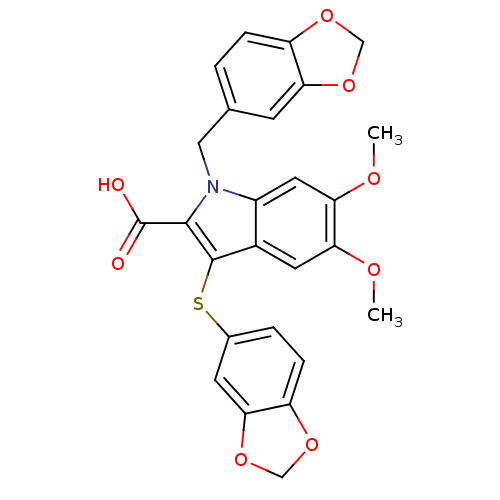
(1-Benzo[1,3]dioxol-5-ylmethyl-3-(benzo[1,3]dioxol-...)Show SMILES COc1cc2c(Sc3ccc4OCOc4c3)c(C(O)=O)n(Cc3ccc4OCOc4c3)c2cc1OC Show InChI InChI=1S/C26H21NO8S/c1-30-20-9-16-17(10-21(20)31-2)27(11-14-3-5-18-22(7-14)34-12-32-18)24(26(28)29)25(16)36-15-4-6-19-23(8-15)35-13-33-19/h3-10H,11-13H2,1-2H3,(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Affinity for human endothelin A receptor expressed in Ltk cells. |
Bioorg Med Chem Lett 6: 1367-1370 (1996)
Article DOI: 10.1016/0960-894X(96)00232-6
BindingDB Entry DOI: 10.7270/Q2K35TMT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data