 Found 82 hits with Last Name = 'burdette' and Initial = 'je'
Found 82 hits with Last Name = 'burdette' and Initial = 'je' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Progesterone receptor
(Homo sapiens (Human)) | BDBM8903
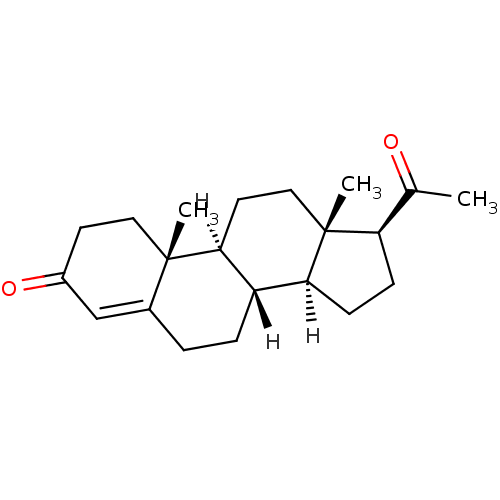
((1S,2R,10S,11S,14S,15S)-14-acetyl-2,15-dimethyltet...)Show SMILES [H][C@@]12CC[C@H](C(C)=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2=CC(=O)CC[C@]12C |r,t:20| Show InChI InChI=1S/C21H30O2/c1-13(22)17-6-7-18-16-5-4-14-12-15(23)8-10-20(14,2)19(16)9-11-21(17,18)3/h12,16-19H,4-11H2,1-3H3/t16-,17+,18-,19-,20-,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | 22 |
Northwestern University
| Assay Description
Competitive binding assay were performed using increasing dose of the contrast agents and fluorescently labeled progesterone as the competitor. |
Chem Biol 14: 824-34 (2007)
Article DOI: 10.1016/j.chembiol.2007.06.006
BindingDB Entry DOI: 10.7270/Q2HM56WH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM10755

(14C-5-hydroxy tryptamine creatinine disulfate | 2-...)Show InChI InChI=1S/C10H12N2O/c11-4-3-7-6-12-10-2-1-8(13)5-9(7)10/h1-2,5-6,12-13H,3-4,11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.55 | n/a | n/a | n/a | n/a | n/a | n/a |
Tom's of Maine
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT7 receptor expressed in CHO cells |
J Nat Prod 66: 535-7 (2003)
Article DOI: 10.1021/np0205102
BindingDB Entry DOI: 10.7270/Q2CZ36WZ |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM19459

(5,7-dihydroxy-3-(4-hydroxyphenyl)-4H-chromen-4-one...)Show InChI InChI=1S/C15H10O5/c16-9-3-1-8(2-4-9)11-7-20-13-6-10(17)5-12(18)14(13)15(11)19/h1-7,16-18H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lausanne
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from human recombinant ERbeta |
J Nat Prod 65: 1749-53 (2002)
Article DOI: 10.1021/np0201164
BindingDB Entry DOI: 10.7270/Q2S46RQX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Progesterone receptor
(Homo sapiens (Human)) | BDBM68290
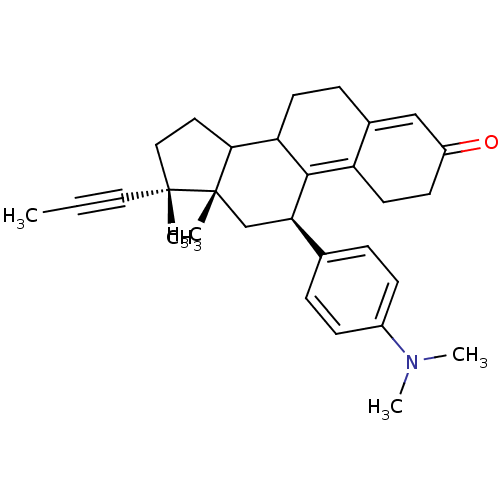
(RU486)Show SMILES CC#C[C@]1(C)CCC2C3CCC4=CC(=O)CCC4=C3[C@H](C[C@]12C)c1ccc(cc1)N(C)C |r,c:18,t:11| Show InChI InChI=1S/C30H37NO/c1-6-16-29(2)17-15-27-25-13-9-21-18-23(32)12-14-24(21)28(25)26(19-30(27,29)3)20-7-10-22(11-8-20)31(4)5/h7-8,10-11,18,25-27H,9,12-15,17,19H2,1-5H3/t25?,26-,27?,29-,30+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | 22 |
Northwestern University
| Assay Description
Competitive binding assay were performed using increasing dose of the contrast agents and fluorescently labeled progesterone as the competitor. |
Chem Biol 14: 824-34 (2007)
Article DOI: 10.1016/j.chembiol.2007.06.006
BindingDB Entry DOI: 10.7270/Q2HM56WH |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 2 group C member 2/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1
(Homo sapiens (Human)) | BDBM50129131
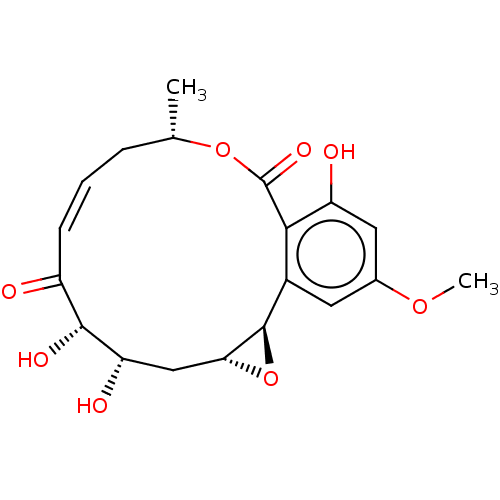
(CHEBI:83275 | Hypothemycin | US10434085, Compound ...)Show SMILES [H][C@@]12C[C@H](O)[C@H](O)C(=O)\C=C/C[C@H](C)OC(=O)c3c(O)cc(OC)cc3[C@@]1([H])O2 |r,c:9| Show InChI InChI=1S/C19H22O8/c1-9-4-3-5-12(20)17(23)14(22)8-15-18(27-15)11-6-10(25-2)7-13(21)16(11)19(24)26-9/h3,5-7,9,14-15,17-18,21-23H,4,8H2,1-2H3/b5-3-/t9-,14-,15+,17+,18+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.2c00434
BindingDB Entry DOI: 10.7270/Q21N854C |
More data for this
Ligand-Target Pair | |
Transcription factor p65
(Homo sapiens (Human)) | BDBM50148911

((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...)Show SMILES C[C@@H]1CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2[C@H]1C)C(O)=O |r,c:9| Show InChI InChI=1S/C30H48O3/c1-18-10-15-30(25(32)33)17-16-28(6)20(24(30)19(18)2)8-9-22-27(5)13-12-23(31)26(3,4)21(27)11-14-29(22,28)7/h8,18-19,21-24,31H,9-17H2,1-7H3,(H,32,33)/t18-,19+,21+,22-,23+,24+,27+,28-,29-,30+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University
Curated by ChEMBL
| Assay Description
Inhibition of biotinylated consensus sequence binding to NF-kB p65 in human HeLa nuclear extracts after 3 hrs by ELISA |
Bioorg Med Chem 26: 4452-4460 (2018)
Article DOI: 10.1016/j.bmc.2018.07.025
BindingDB Entry DOI: 10.7270/Q2N300MM |
More data for this
Ligand-Target Pair | |
Transcription factor p65
(Homo sapiens (Human)) | BDBM50196926

(CHEMBL438139 | Rocaglamide | US10085988, Compound ...)Show SMILES COc1ccc(cc1)[C@@]12Oc3cc(OC)cc(OC)c3[C@]1(O)[C@H](O)[C@@H]([C@H]2c1ccccc1)C(=O)N(C)C |r| Show InChI InChI=1S/C29H31NO7/c1-30(2)27(32)23-24(17-9-7-6-8-10-17)29(18-11-13-19(34-3)14-12-18)28(33,26(23)31)25-21(36-5)15-20(35-4)16-22(25)37-29/h6-16,23-24,26,31,33H,1-5H3/t23-,24-,26-,28+,29+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University
Curated by ChEMBL
| Assay Description
Inhibition of biotinylated consensus sequence binding to NF-kB p65 in human HeLa nuclear extracts after 3 hrs by ELISA |
Bioorg Med Chem 26: 4452-4460 (2018)
Article DOI: 10.1016/j.bmc.2018.07.025
BindingDB Entry DOI: 10.7270/Q2N300MM |
More data for this
Ligand-Target Pair | |
A5LHX3/O14818/P20618/P25786/P25787/P25788/P25789/P28062/P28065/P28066/P28070/P28072/P28074/P40306/P49720/P49721/P60900/Q8TAA3/Q99436
(Homo sapiens (Human)) | BDBM50593519
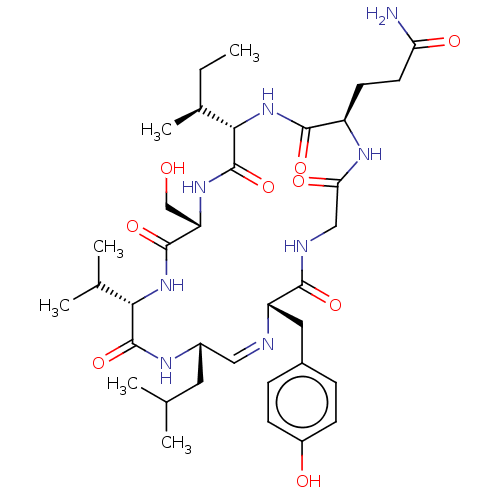
(CHEMBL5204252)Show SMILES [H][C@]1(NC(=O)[C@@H](CCC(N)=O)NC(=O)CNC(=O)[C@H](Cc2ccc(O)cc2)\N=C/[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](CO)NC1=O)C(C)C)[C@@H](C)CC |r,c:28| | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.2c00036
BindingDB Entry DOI: 10.7270/Q2J10752 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM68291

(Steroid-Gd (III) conjugate, 1)Show SMILES C1[N+]23CC[N+]45CC[N+]6(CC([C@@H]7[C@]8(C)C(CC7)C7C([C@@]9(C(CC7)=CC(CC9)=O)C)CC8)=O)[Gd-4]242([N+](CC6)(CC(O2)=O)C1)(OC(C5)=O)OC(=O)C3 |r,c:23| Show InChI InChI=1S/C35H54N4O8.Gd/c1-34-9-7-25(40)19-24(34)3-4-26-27-5-6-29(35(27,2)10-8-28(26)34)30(41)20-36-11-13-37(21-31(42)43)15-17-39(23-33(46)47)18-16-38(14-12-36)22-32(44)45;/h19,26-29H,3-18,20-23H2,1-2H3,(H,42,43)(H,44,45)(H,46,47);/q;+3/p-3/t26?,27?,28?,29-,34+,35+;/m1./s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | 22 |
Northwestern University
| Assay Description
Competitive binding assay were performed using increasing dose of the contrast agents and fluorescently labeled progesterone as the competitor. |
Chem Biol 14: 824-34 (2007)
Article DOI: 10.1016/j.chembiol.2007.06.006
BindingDB Entry DOI: 10.7270/Q2HM56WH |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM19459

(5,7-dihydroxy-3-(4-hydroxyphenyl)-4H-chromen-4-one...)Show InChI InChI=1S/C15H10O5/c16-9-3-1-8(2-4-9)11-7-20-13-6-10(17)5-12(18)14(13)15(11)19/h1-7,16-18H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lausanne
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from human recombinant ERalpha |
J Nat Prod 65: 1749-53 (2002)
Article DOI: 10.1021/np0201164
BindingDB Entry DOI: 10.7270/Q2S46RQX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nuclear receptor subfamily 2 group C member 2/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1
(Homo sapiens (Human)) | BDBM50594358
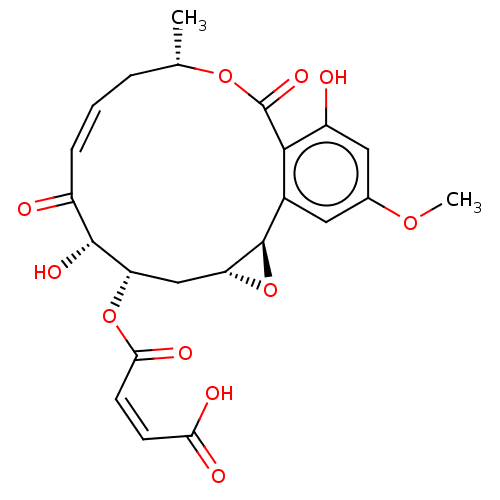
(CHEMBL5204235)Show SMILES [H][C@@]12C[C@H](OC(=O)\C=C/C(O)=O)[C@H](O)C(=O)\C=C/C[C@H](C)OC(=O)c3c(O)cc(OC)cc3[C@@]1([H])O2 |r,c:16| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.2c00434
BindingDB Entry DOI: 10.7270/Q21N854C |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 2 group C member 2/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1
(Homo sapiens (Human)) | BDBM50594353
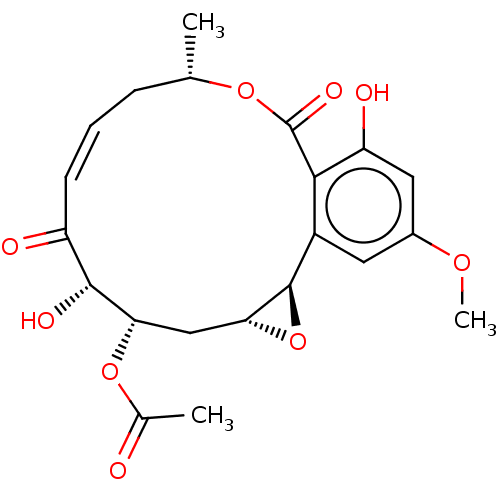
(CHEMBL5174288)Show SMILES [H][C@@]12C[C@H](OC(C)=O)[C@H](O)C(=O)\C=C/C[C@H](C)OC(=O)c3c(O)cc(OC)cc3[C@@]1([H])O2 |r,c:12| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.2c00434
BindingDB Entry DOI: 10.7270/Q21N854C |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 2 group C member 2/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1
(Homo sapiens (Human)) | BDBM50594354

(CHEMBL5186931)Show SMILES [H][C@@]12C[C@H](O)[C@H](OC(C)=O)C(=O)\C=C/C[C@H](C)OC(=O)c3c(O)cc(OC)cc3[C@@]1([H])O2 |r,c:12| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.2c00434
BindingDB Entry DOI: 10.7270/Q21N854C |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM68288
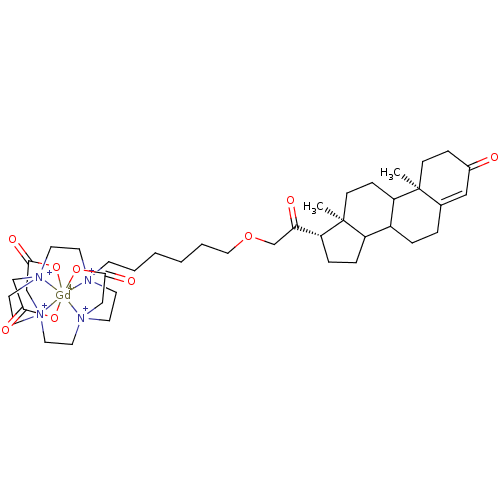
(Steroid-Gd (III) conjugate, 2)Show SMILES C1C[N+]23CC(O[Gd-4]22456OC(C[N+]12CC[N+]4(CCCCCCOCC([C@@H]1[C@]2(CCC4[C@@]7(C)CCC(=O)C=C7CCC4C2CC1)C)=O)CC[N+]5(CC3)CC(O6)=O)=O)=O |r,c:38| Show InChI InChI=1S/C41H66N4O9.Gd/c1-40-13-11-31(46)25-30(40)7-8-32-33-9-10-35(41(33,2)14-12-34(32)40)36(47)29-54-24-6-4-3-5-15-42-16-18-43(26-37(48)49)20-22-45(28-39(52)53)23-21-44(19-17-42)27-38(50)51;/h25,32-35H,3-24,26-29H2,1-2H3,(H,48,49)(H,50,51)(H,52,53);/q;+3/p-3/t32?,33?,34?,35-,40+,41+;/m1./s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | 22 |
Northwestern University
| Assay Description
Competitive binding assay were performed using increasing dose of the contrast agents and fluorescently labeled progesterone as the competitor. |
Chem Biol 14: 824-34 (2007)
Article DOI: 10.1016/j.chembiol.2007.06.006
BindingDB Entry DOI: 10.7270/Q2HM56WH |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 2 group C member 2/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1
(Homo sapiens (Human)) | BDBM50594367
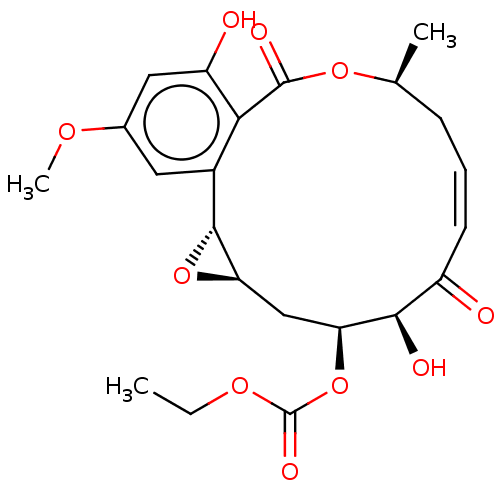
(CHEMBL5207996)Show SMILES [H][C@@]12C[C@H](OC(=O)OCC)[C@H](O)C(=O)\C=C/C[C@H](C)OC(=O)c3c(O)cc(OC)cc3[C@@]1([H])O2 |r,c:14| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.2c00434
BindingDB Entry DOI: 10.7270/Q21N854C |
More data for this
Ligand-Target Pair | |
Papain
(Carica papaya) | BDBM50513777

(CHEMBL4534212)Show SMILES CC(=O)Oc1ccc(cc1)C1Oc2cc(OC(C)=O)cc(O)c2C(=O)C1c1c(OC(C)=O)cc(O)c2c1oc(cc2=O)-c1ccc(OC(C)=O)c(OC(C)=O)c1 Show InChI InChI=1S/C40H30O16/c1-17(41)50-24-9-6-22(7-10-24)39-37(38(49)35-26(46)13-25(51-18(2)42)14-32(35)56-39)36-33(54-21(5)45)16-28(48)34-27(47)15-30(55-40(34)36)23-8-11-29(52-19(3)43)31(12-23)53-20(4)44/h6-16,37,39,46,48H,1-5H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University
Curated by ChEMBL
| Assay Description
Inhibition of Papaya papain using Z-FR-MCA as substrate by spectrofluorimetric method |
J Nat Prod 82: 657-679 (2019)
Article DOI: 10.1021/acs.jnatprod.9b00018
BindingDB Entry DOI: 10.7270/Q29W0JTR |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 2 group C member 2/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1
(Homo sapiens (Human)) | BDBM50594360

(CHEMBL5173905)Show SMILES [H][C@@]12C[C@H](OC(=O)OC)[C@H](O)C(=O)\C=C/C[C@H](C)OC(=O)c3c(O)cc(OC)cc3[C@@]1([H])O2 |r,c:13| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.2c00434
BindingDB Entry DOI: 10.7270/Q21N854C |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 2 group C member 2/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1
(Homo sapiens (Human)) | BDBM50594364
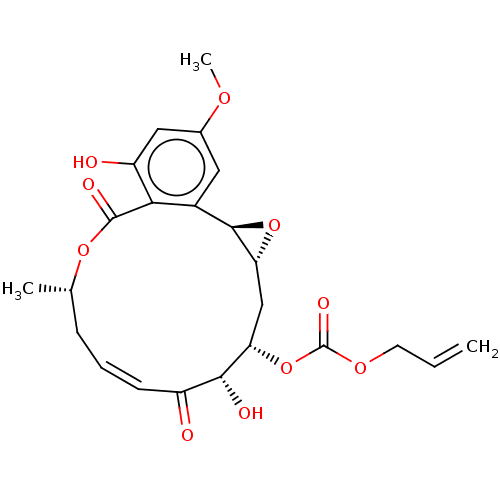
(CHEMBL5185893)Show SMILES [H][C@@]12C[C@H](OC(=O)OCC=C)[C@H](O)C(=O)\C=C/C[C@H](C)OC(=O)c3c(O)cc(OC)cc3[C@@]1([H])O2 |r,c:15| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.2c00434
BindingDB Entry DOI: 10.7270/Q21N854C |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 2 group C member 2/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1
(Homo sapiens (Human)) | BDBM50594356
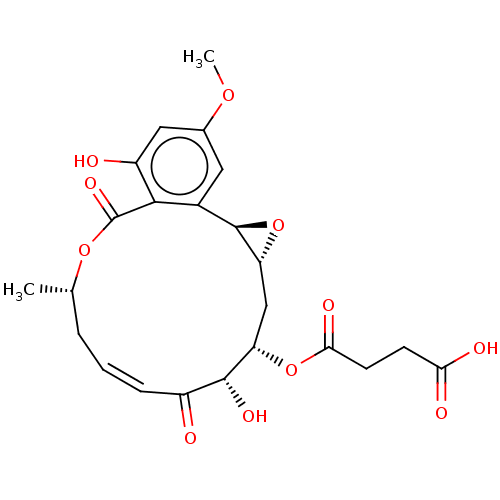
(CHEMBL5179846)Show SMILES [H][C@@]12C[C@H](OC(=O)CCC(O)=O)[C@H](O)C(=O)\C=C/C[C@H](C)OC(=O)c3c(O)cc(OC)cc3[C@@]1([H])O2 |r,c:16| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.2c00434
BindingDB Entry DOI: 10.7270/Q21N854C |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase 2A
(Homo sapiens (Human)) | BDBM50530867

(CHEMBL4472654)Show SMILES [H][C@]12Nc3ccccc3[C@]1([C@H](O)[C@]13SS[C@](C)(N(C)C1=O)C(=O)N23)[C@]12[C@H](O)[C@]34SS[C@](C)(N(C)C3=O)C(=O)N4[C@@]1([H])Nc1ccccc21 |r| Show InChI InChI=1S/C30H28N6O6S4/c1-25-21(39)35-19-27(13-9-5-7-11-15(13)31-19,17(37)29(35,45-43-25)23(41)33(25)3)28-14-10-6-8-12-16(14)32-20(28)36-22(40)26(2)34(4)24(42)30(36,18(28)38)46-44-26/h5-12,17-20,31-32,37-38H,1-4H3/t17-,18-,19+,20+,25-,26-,27+,28+,29-,30-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Greensboro
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MLL1 using [3H]S-adenosyl-methionine as substrate |
J Nat Prod 82: 3104-3110 (2019)
Article DOI: 10.1021/acs.jnatprod.9b00711
BindingDB Entry DOI: 10.7270/Q2C53QB2 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase 2A
(Homo sapiens (Human)) | BDBM50530867

(CHEMBL4472654)Show SMILES [H][C@]12Nc3ccccc3[C@]1([C@H](O)[C@]13SS[C@](C)(N(C)C1=O)C(=O)N23)[C@]12[C@H](O)[C@]34SS[C@](C)(N(C)C3=O)C(=O)N4[C@@]1([H])Nc1ccccc21 |r| Show InChI InChI=1S/C30H28N6O6S4/c1-25-21(39)35-19-27(13-9-5-7-11-15(13)31-19,17(37)29(35,45-43-25)23(41)33(25)3)28-14-10-6-8-12-16(14)32-20(28)36-22(40)26(2)34(4)24(42)30(36,18(28)38)46-44-26/h5-12,17-20,31-32,37-38H,1-4H3/t17-,18-,19+,20+,25-,26-,27+,28+,29-,30-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Greensboro
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MLL1 using [3H]S-adenosyl-methionine as substrate |
J Nat Prod 82: 3104-3110 (2019)
Article DOI: 10.1021/acs.jnatprod.9b00711
BindingDB Entry DOI: 10.7270/Q2C53QB2 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 2 group C member 2/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1
(Homo sapiens (Human)) | BDBM50594361

(CHEMBL5174742)Show SMILES [H][C@@]12C[C@H](O)[C@H](OC(=O)OC)C(=O)\C=C/C[C@H](C)OC(=O)c3c(O)cc(OC)cc3[C@@]1([H])O2 |r,c:13| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.2c00434
BindingDB Entry DOI: 10.7270/Q21N854C |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 2 group C member 2/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1
(Homo sapiens (Human)) | BDBM50594365

(CHEMBL5186989)Show SMILES [H][C@@]12C[C@H](O)[C@H](OC(=O)OCC=C)C(=O)\C=C/C[C@H](C)OC(=O)c3c(O)cc(OC)cc3[C@@]1([H])O2 |r,c:15| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.2c00434
BindingDB Entry DOI: 10.7270/Q21N854C |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM68289

(Steroid-Gd (III) conjugate, 3)Show SMILES [Gd-5]1234567[N+]8(CC(Cc9ccc(cc9)NC(=S)NCCCCCCOCC([C@@H]9[C@]%10(CCC%11C(CCC%12=CC(CC[C@]%11%12C)=O)C%10CC9)C)=O)[N+]1(CC(O2)=O)CC[N+]3(CC(O4)=O)CC[N+]5(CC8)CC(O6)=O)CC(O7)=O |r,t:33| Show InChI InChI=1S/C51H76N6O11S.Gd/c1-50-17-15-39(58)28-36(50)9-12-40-41-13-14-43(51(41,2)18-16-42(40)50)44(59)34-68-26-6-4-3-5-19-52-49(69)53-37-10-7-35(8-11-37)27-38-29-56(32-47(64)65)23-22-54(30-45(60)61)20-21-55(31-46(62)63)24-25-57(38)33-48(66)67;/h7-8,10-11,28,38,40-43H,3-6,9,12-27,29-34H2,1-2H3,(H,60,61)(H,62,63)(H,64,65)(H,66,67)(H2,52,53,69);/q;+3/p-4/t38?,40?,41?,42?,43-,50+,51+;/m1./s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | 22 |
Northwestern University
| Assay Description
Competitive binding assay were performed using increasing dose of the contrast agents and fluorescently labeled progesterone as the competitor. |
Chem Biol 14: 824-34 (2007)
Article DOI: 10.1016/j.chembiol.2007.06.006
BindingDB Entry DOI: 10.7270/Q2HM56WH |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50250906
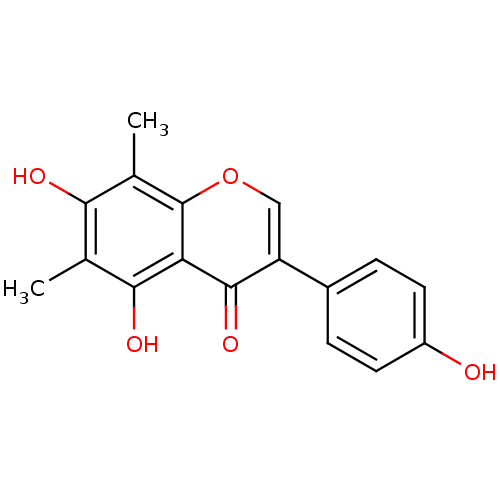
(4',5,7-trihydroxy-6,8-dimethylisoflavone | CHEMBL5...)Show InChI InChI=1S/C17H14O5/c1-8-14(19)9(2)17-13(15(8)20)16(21)12(7-22-17)10-3-5-11(18)6-4-10/h3-7,18-20H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lausanne
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from human recombinant ERbeta |
J Nat Prod 65: 1749-53 (2002)
Article DOI: 10.1021/np0201164
BindingDB Entry DOI: 10.7270/Q2S46RQX |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 2 group C member 2/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1
(Homo sapiens (Human)) | BDBM50594355
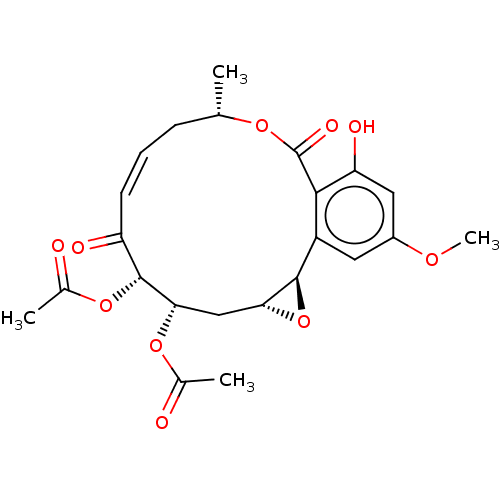
(CHEMBL5196981)Show SMILES [H][C@@]12C[C@H](OC(C)=O)[C@H](OC(C)=O)C(=O)\C=C/C[C@H](C)OC(=O)c3c(O)cc(OC)cc3[C@@]1([H])O2 |r,c:15| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.2c00434
BindingDB Entry DOI: 10.7270/Q21N854C |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 2 group C member 2/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1
(Homo sapiens (Human)) | BDBM50594357
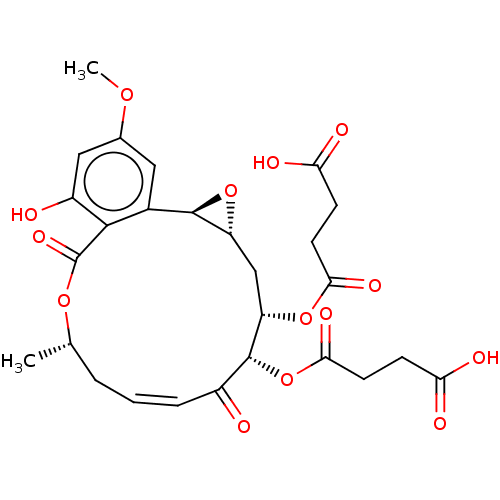
(CHEMBL5203951)Show SMILES [H][C@@]12C[C@H](OC(=O)CCC(O)=O)[C@H](OC(=O)CCC(O)=O)C(=O)\C=C/C[C@H](C)OC(=O)c3c(O)cc(OC)cc3[C@@]1([H])O2 |r,c:23| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.2c00434
BindingDB Entry DOI: 10.7270/Q21N854C |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 2 group C member 2/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1
(Homo sapiens (Human)) | BDBM50594362
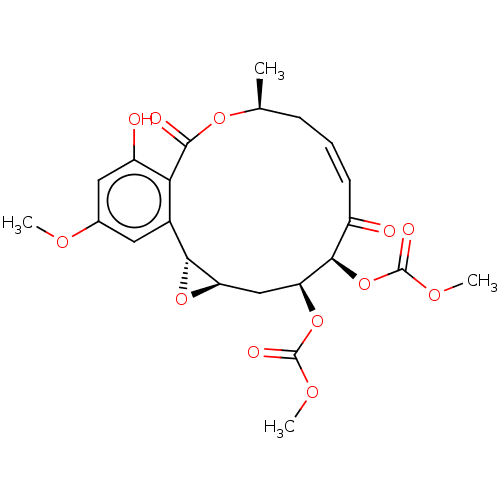
(CHEMBL5187912)Show SMILES [H][C@@]12C[C@H](OC(=O)OC)[C@H](OC(=O)OC)C(=O)\C=C/C[C@H](C)OC(=O)c3c(O)cc(OC)cc3[C@@]1([H])O2 |r,c:17| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.2c00434
BindingDB Entry DOI: 10.7270/Q21N854C |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 2 group C member 2/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1
(Homo sapiens (Human)) | BDBM50594363

(CHEMBL5196408)Show SMILES [H][C@@]12C[C@H](OC(=O)OC)[C@H](OC(=O)OC)C(=O)\C=C/C[C@H](C)OC(=O)c3c(OC(=O)OC)cc(OC)cc3[C@@]1([H])O2 |r,c:17| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.2c00434
BindingDB Entry DOI: 10.7270/Q21N854C |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 2 group C member 2/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1
(Homo sapiens (Human)) | BDBM50594366
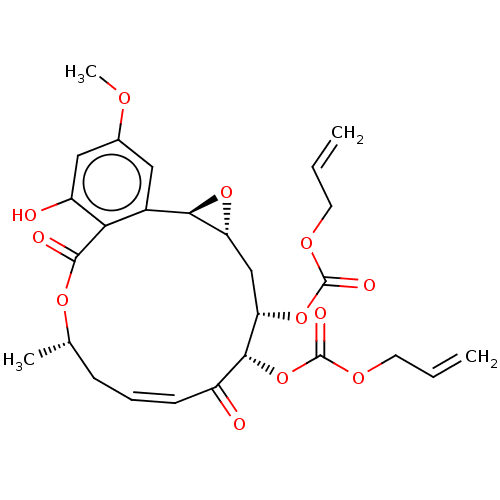
(CHEMBL5188775)Show SMILES [H][C@@]12C[C@H](OC(=O)OCC=C)[C@H](OC(=O)OCC=C)C(=O)\C=C/C[C@H](C)OC(=O)c3c(O)cc(OC)cc3[C@@]1([H])O2 |r,c:21| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.2c00434
BindingDB Entry DOI: 10.7270/Q21N854C |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 2 group C member 2/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1
(Homo sapiens (Human)) | BDBM50594369
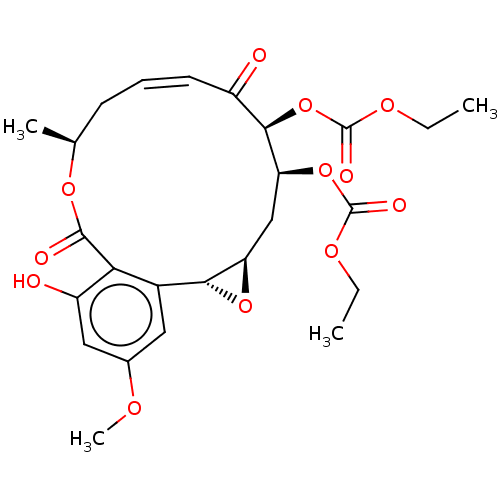
(CHEMBL5173639)Show SMILES [H][C@@]12C[C@H](OC(=O)OCC)[C@H](OC(=O)OCC)C(=O)\C=C/C[C@H](C)OC(=O)c3c(O)cc(OC)cc3[C@@]1([H])O2 |r,c:19| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.2c00434
BindingDB Entry DOI: 10.7270/Q21N854C |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 2 group C member 2/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1
(Homo sapiens (Human)) | BDBM50594370
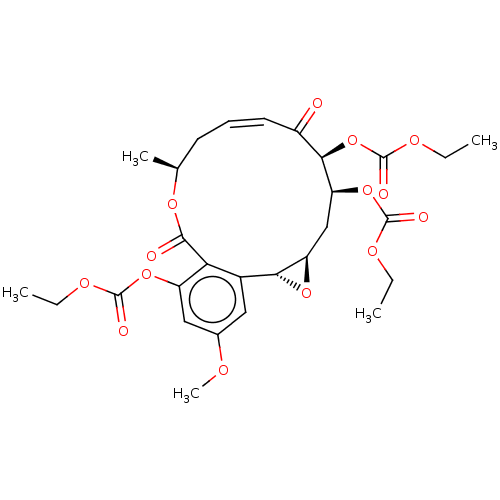
(CHEMBL5208768)Show SMILES [H][C@@]12C[C@H](OC(=O)OCC)[C@H](OC(=O)OCC)C(=O)\C=C/C[C@H](C)OC(=O)c3c(OC(=O)OCC)cc(OC)cc3[C@@]1([H])O2 |r,c:19| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.2c00434
BindingDB Entry DOI: 10.7270/Q21N854C |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 2 group C member 2/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1
(Homo sapiens (Human)) | BDBM50594371
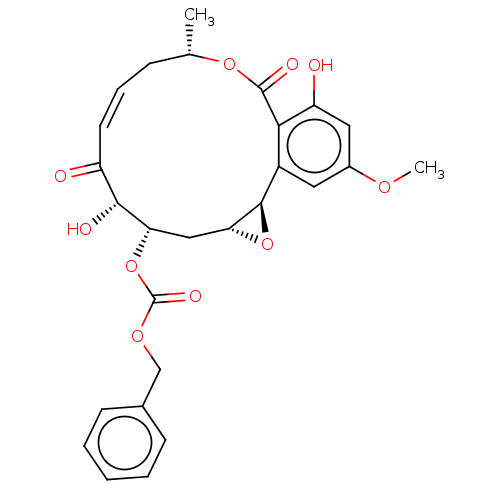
(CHEMBL5190572)Show SMILES [H][C@@]12C[C@H](OC(=O)OCc3ccccc3)[C@H](O)C(=O)\C=C/C[C@H](C)OC(=O)c3c(O)cc(OC)cc3[C@@]1([H])O2 |r,c:20| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.2c00434
BindingDB Entry DOI: 10.7270/Q21N854C |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 2 group C member 2/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1
(Homo sapiens (Human)) | BDBM50594372
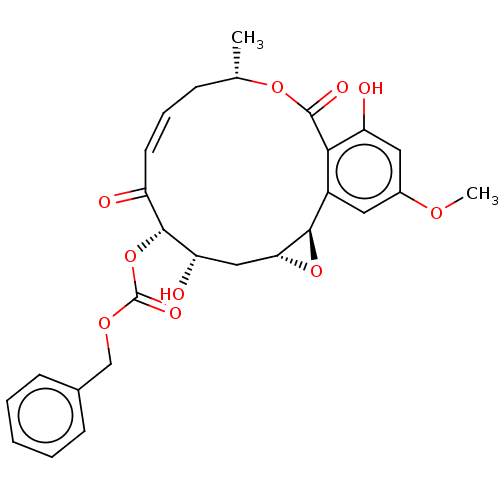
(CHEMBL5188253)Show SMILES [H][C@@]12C[C@H](O)[C@H](OC(=O)OCc3ccccc3)C(=O)\C=C/C[C@H](C)OC(=O)c3c(O)cc(OC)cc3[C@@]1([H])O2 |r,c:20| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.2c00434
BindingDB Entry DOI: 10.7270/Q21N854C |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 2 group C member 2/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1
(Homo sapiens (Human)) | BDBM50594373

(CHEMBL5198327)Show SMILES [H][C@@]12C[C@H](OC(=O)OCc3ccccc3)[C@H](OC(=O)OCc3ccccc3)C(=O)\C=C/C[C@H](C)OC(=O)c3c(O)cc(OC)cc3[C@@]1([H])O2 |r,c:31| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.2c00434
BindingDB Entry DOI: 10.7270/Q21N854C |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 2 group C member 2/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1
(Homo sapiens (Human)) | BDBM50594374
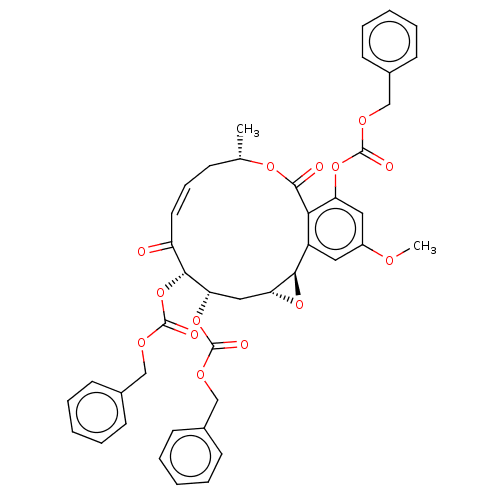
(CHEMBL5203686)Show SMILES [H][C@@]12C[C@H](OC(=O)OCc3ccccc3)[C@H](OC(=O)OCc3ccccc3)C(=O)\C=C/C[C@H](C)OC(=O)c3c(OC(=O)OCc4ccccc4)cc(OC)cc3[C@@]1([H])O2 |r,c:31| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.2c00434
BindingDB Entry DOI: 10.7270/Q21N854C |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 2 group C member 2/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1
(Homo sapiens (Human)) | BDBM50594375
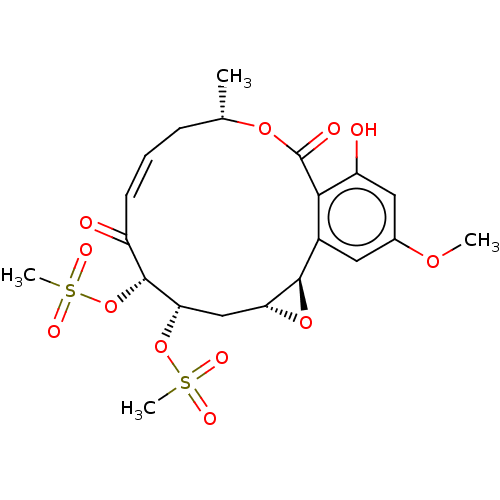
(CHEMBL5181612)Show SMILES [H][C@@]12C[C@H](OS(C)(=O)=O)[C@H](OS(C)(=O)=O)C(=O)\C=C/C[C@H](C)OC(=O)c3c(O)cc(OC)cc3[C@@]1([H])O2 |r,c:17| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.2c00434
BindingDB Entry DOI: 10.7270/Q21N854C |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 2 group C member 2/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1
(Homo sapiens (Human)) | BDBM50594376
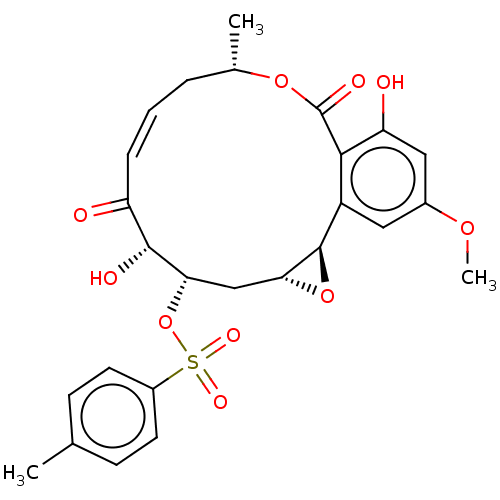
(CHEMBL5207341)Show SMILES [H][C@@]12C[C@H](OS(=O)(=O)c3ccc(C)cc3)[C@H](O)C(=O)\C=C/C[C@H](C)OC(=O)c3c(O)cc(OC)cc3[C@@]1([H])O2 |r,c:20| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.2c00434
BindingDB Entry DOI: 10.7270/Q21N854C |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 2 group C member 2/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1
(Homo sapiens (Human)) | BDBM50594377
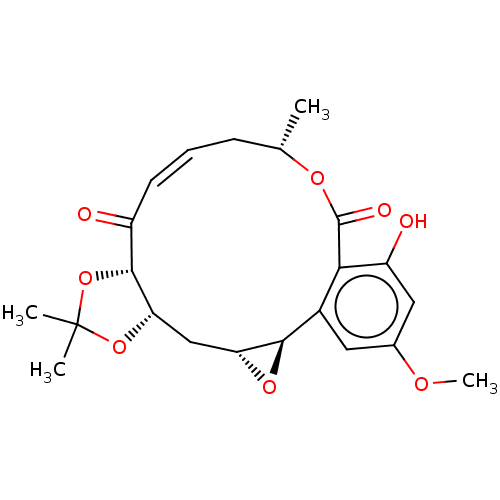
(CHEMBL5183713)Show SMILES [H][C@@]12C[C@]3([H])OC(C)(C)O[C@]3([H])C(=O)\C=C/C[C@H](C)OC(=O)c3c(O)cc(OC)cc3[C@@]1([H])O2 |r,c:15| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.2c00434
BindingDB Entry DOI: 10.7270/Q21N854C |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 2 group C member 2/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1
(Homo sapiens (Human)) | BDBM50594378
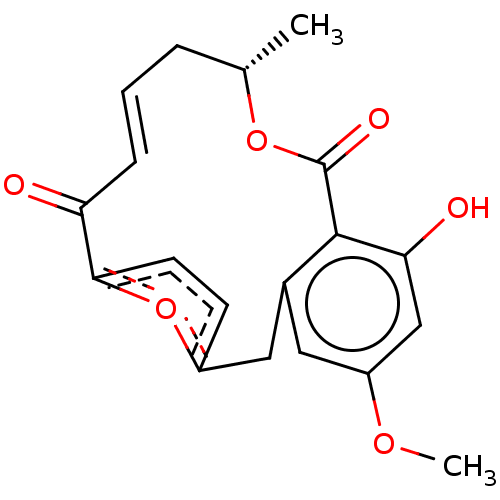
(CHEMBL5174017)Show SMILES COc1cc(O)c2c(Cc3ccc(o3)C(=O)\C=C/C[C@H](C)OC2=O)c1 |r,c:17| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.2c00434
BindingDB Entry DOI: 10.7270/Q21N854C |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 2 group C member 2/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1
(Homo sapiens (Human)) | BDBM50594359
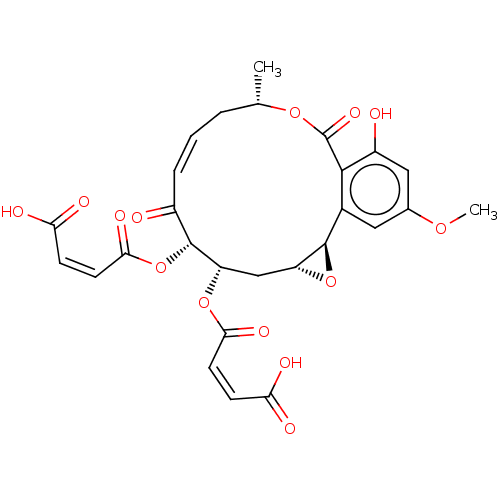
(CHEMBL5185951)Show SMILES [H][C@@]12C[C@H](OC(=O)\C=C/C(O)=O)[C@H](OC(=O)\C=C/C(O)=O)C(=O)\C=C/C[C@H](C)OC(=O)c3c(O)cc(OC)cc3[C@@]1([H])O2 |r,c:23| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.2c00434
BindingDB Entry DOI: 10.7270/Q21N854C |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM23420
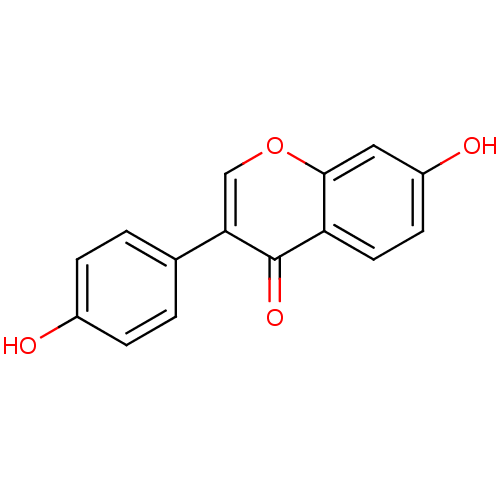
(7,4′-Dihydroxy-isoflavone (3a) | 7-hydroxy-3...)Show InChI InChI=1S/C15H10O4/c16-10-3-1-9(2-4-10)13-8-19-14-7-11(17)5-6-12(14)15(13)18/h1-8,16-17H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lausanne
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from human recombinant ERbeta |
J Nat Prod 65: 1749-53 (2002)
Article DOI: 10.1021/np0201164
BindingDB Entry DOI: 10.7270/Q2S46RQX |
More data for this
Ligand-Target Pair | |
Transcription factor p65
(Homo sapiens (Human)) | BDBM50463331
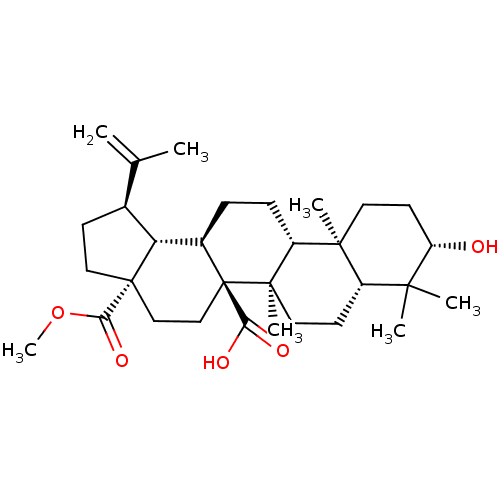
(CHEMBL4204109)Show SMILES [H][C@]12[C@@H](CC[C@@]1(CC[C@]1(C(O)=O)[C@]2([H])CC[C@]2([H])[C@@]3(C)CC[C@H](O)C(C)(C)[C@]3([H])CC[C@@]12C)C(=O)OC)C(C)=C |r| Show InChI InChI=1S/C31H48O5/c1-18(2)19-10-15-30(26(35)36-7)16-17-31(25(33)34)20(24(19)30)8-9-22-28(5)13-12-23(32)27(3,4)21(28)11-14-29(22,31)6/h19-24,32H,1,8-17H2,2-7H3,(H,33,34)/t19-,20+,21-,22+,23-,24+,28-,29+,30-,31+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University
Curated by ChEMBL
| Assay Description
Inhibition of biotinylated consensus sequence binding to NF-kB p65 in human HeLa nuclear extracts after 3 hrs by ELISA |
Bioorg Med Chem 26: 4452-4460 (2018)
Article DOI: 10.1016/j.bmc.2018.07.025
BindingDB Entry DOI: 10.7270/Q2N300MM |
More data for this
Ligand-Target Pair | |
Transcription factor p65
(Homo sapiens (Human)) | BDBM50463328

(CHEBI:1368 | CHEMBL451476)Show SMILES COc1cc2c3c(oc(=O)c4cc(O)c(OC)c(oc2=O)c34)c1OC Show InChI InChI=1S/C17H12O8/c1-21-9-5-7-11-10-6(16(19)25-15(11)13(9)23-3)4-8(18)12(22-2)14(10)24-17(7)20/h4-5,18H,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University
Curated by ChEMBL
| Assay Description
Inhibition of biotinylated consensus sequence binding to NF-kB p65 in human HeLa nuclear extracts after 3 hrs by ELISA |
Bioorg Med Chem 26: 4452-4460 (2018)
Article DOI: 10.1016/j.bmc.2018.07.025
BindingDB Entry DOI: 10.7270/Q2N300MM |
More data for this
Ligand-Target Pair | |
Trypsin
(Homo sapiens (Human)) | BDBM50513779
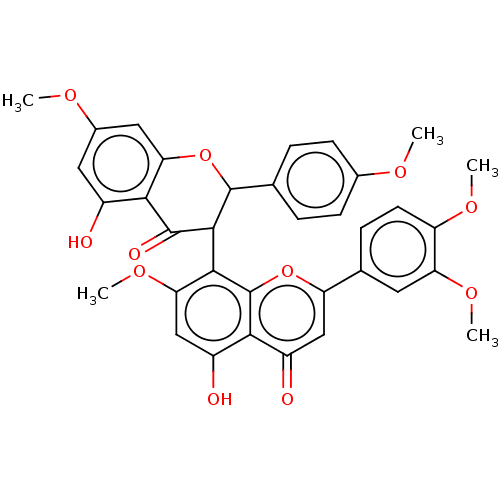
(CHEMBL4441556)Show SMILES COc1ccc(cc1)C1Oc2cc(OC)cc(O)c2C(=O)C1c1c(OC)cc(O)c2c1oc(cc2=O)-c1ccc(OC)c(OC)c1 Show InChI InChI=1S/C35H30O11/c1-40-19-9-6-17(7-10-19)34-32(33(39)30-21(36)13-20(41-2)14-28(30)46-34)31-27(44-5)16-23(38)29-22(37)15-25(45-35(29)31)18-8-11-24(42-3)26(12-18)43-4/h6-16,32,34,36,38H,1-5H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University
Curated by ChEMBL
| Assay Description
Inhibition of trypsin (unknown origin) using Z-FR-MCA as substrate by spectrofluorimetric method |
J Nat Prod 82: 657-679 (2019)
Article DOI: 10.1021/acs.jnatprod.9b00018
BindingDB Entry DOI: 10.7270/Q29W0JTR |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 2 group C member 2/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1
(Homo sapiens (Human)) | BDBM50594368

(CHEMBL5186228)Show SMILES [H][C@@]12C[C@H](O)[C@H](OC(=O)OCC)C(=O)\C=C/C[C@H](C)OC(=O)c3c(O)cc(OC)cc3[C@@]1([H])O2 |r,c:14| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.2c00434
BindingDB Entry DOI: 10.7270/Q21N854C |
More data for this
Ligand-Target Pair | |
Transcription factor p65
(Homo sapiens (Human)) | BDBM50346601

(NSC-114945 | OLEANOLIC_ACID | Oleanolic acid | Ole...)Show SMILES CC1(C)CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2C1)C(O)=O |r,c:10| Show InChI InChI=1S/C30H48O3/c1-25(2)14-16-30(24(32)33)17-15-28(6)19(20(30)18-25)8-9-22-27(5)12-11-23(31)26(3,4)21(27)10-13-29(22,28)7/h8,20-23,31H,9-18H2,1-7H3,(H,32,33)/t20-,21-,22+,23-,27-,28+,29+,30-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University
Curated by ChEMBL
| Assay Description
Inhibition of biotinylated consensus sequence binding to NF-kB p65 in human HeLa nuclear extracts after 3 hrs by ELISA |
Bioorg Med Chem 26: 4452-4460 (2018)
Article DOI: 10.1016/j.bmc.2018.07.025
BindingDB Entry DOI: 10.7270/Q2N300MM |
More data for this
Ligand-Target Pair | |
Trypsin
(Homo sapiens (Human)) | BDBM241952
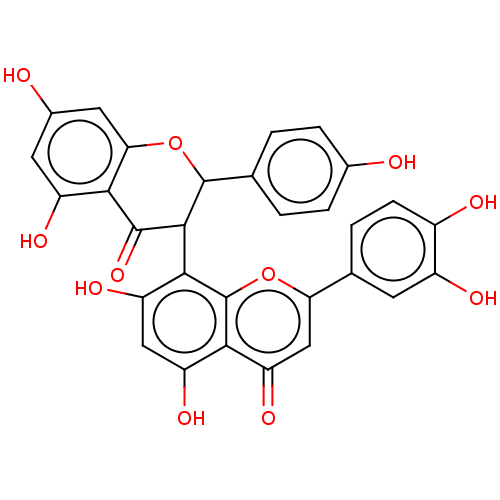
(Fukugetin)Show SMILES Oc1ccc(cc1)C1Oc2cc(O)cc(O)c2C(=O)C1c1c(O)cc(O)c2c1oc(cc2=O)-c1ccc(O)c(O)c1 Show InChI InChI=1S/C30H20O11/c31-14-4-1-12(2-5-14)29-27(28(39)25-18(35)8-15(32)9-23(25)41-29)26-20(37)10-19(36)24-21(38)11-22(40-30(24)26)13-3-6-16(33)17(34)7-13/h1-11,27,29,31-37H | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University
Curated by ChEMBL
| Assay Description
Inhibition of trypsin (unknown origin) using Z-FR-MCA as substrate by spectrofluorimetric method |
J Nat Prod 82: 657-679 (2019)
Article DOI: 10.1021/acs.jnatprod.9b00018
BindingDB Entry DOI: 10.7270/Q29W0JTR |
More data for this
Ligand-Target Pair | |
Transcription factor p65
(Homo sapiens (Human)) | BDBM50463333
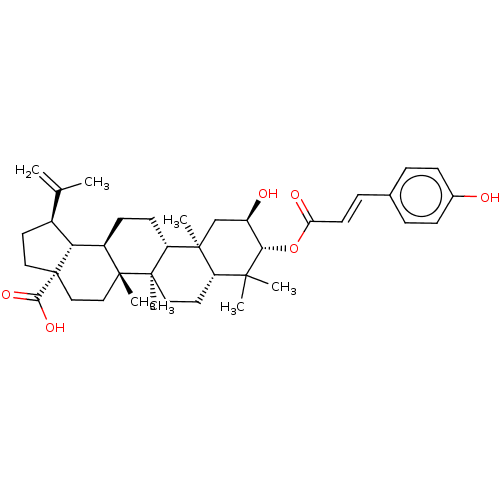
(CHEMBL4250573)Show SMILES [H][C@]12[C@@H](CC[C@@]1(CC[C@]1(C)[C@]2([H])CC[C@]2([H])[C@@]3(C)C[C@@H](O)[C@H](OC(=O)\C=C\c4ccc(O)cc4)C(C)(C)[C@]3([H])CC[C@@]12C)C(O)=O)C(C)=C |r| Show InChI InChI=1S/C39H54O6/c1-23(2)26-16-19-39(34(43)44)21-20-37(6)27(32(26)39)13-14-30-36(5)22-28(41)33(35(3,4)29(36)17-18-38(30,37)7)45-31(42)15-10-24-8-11-25(40)12-9-24/h8-12,15,26-30,32-33,40-41H,1,13-14,16-22H2,2-7H3,(H,43,44)/b15-10+/t26-,27+,28+,29-,30+,32+,33-,36-,37+,38+,39-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University
Curated by ChEMBL
| Assay Description
Inhibition of biotinylated consensus sequence binding to NF-kB p65 in human HeLa nuclear extracts after 3 hrs by ELISA |
Bioorg Med Chem 26: 4452-4460 (2018)
Article DOI: 10.1016/j.bmc.2018.07.025
BindingDB Entry DOI: 10.7270/Q2N300MM |
More data for this
Ligand-Target Pair | |
Transcription factor p65
(Homo sapiens (Human)) | BDBM50463337
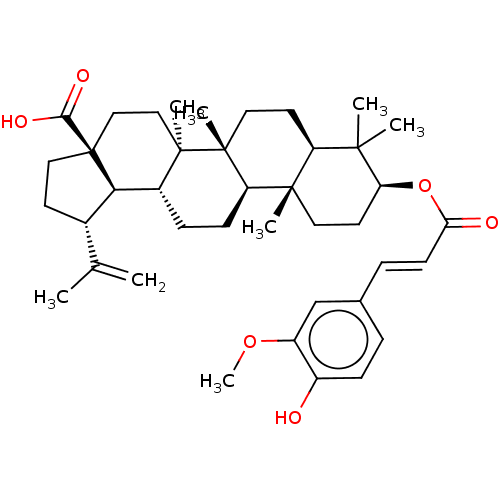
(3Beta-E-Feruloylbetulinic Acid | CHEMBL451046)Show SMILES [H][C@]12[C@@H](CC[C@@]1(CC[C@]1(C)[C@]2([H])CC[C@]2([H])[C@@]3(C)CC[C@H](OC(=O)\C=C\c4ccc(O)c(OC)c4)C(C)(C)[C@]3([H])CC[C@@]12C)C(O)=O)C(C)=C |r| Show InChI InChI=1S/C40H56O6/c1-24(2)26-15-20-40(35(43)44)22-21-38(6)27(34(26)40)11-13-31-37(5)18-17-32(36(3,4)30(37)16-19-39(31,38)7)46-33(42)14-10-25-9-12-28(41)29(23-25)45-8/h9-10,12,14,23,26-27,30-32,34,41H,1,11,13,15-22H2,2-8H3,(H,43,44)/b14-10+/t26-,27+,30-,31+,32-,34+,37-,38+,39+,40-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University
Curated by ChEMBL
| Assay Description
Inhibition of biotinylated consensus sequence binding to NF-kB p65 in human HeLa nuclear extracts after 3 hrs by ELISA |
Bioorg Med Chem 26: 4452-4460 (2018)
Article DOI: 10.1016/j.bmc.2018.07.025
BindingDB Entry DOI: 10.7270/Q2N300MM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data