

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50067678 ((6alpha)-17-(Acetyloxy)-6-methylpreg-4-ene-3,20-di...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as decrease in oxidation of 1-acenaphthenol substrate by spectrophotometric analysis | Eur J Med Chem 62: 89-97 (2013) Article DOI: 10.1016/j.ejmech.2012.12.045 BindingDB Entry DOI: 10.7270/Q2BR8TH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50067678 ((6alpha)-17-(Acetyloxy)-6-methylpreg-4-ene-3,20-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C1 expressed in Escherichia coli assessed as decrease in oxidation of 1-acenaphthenol substrate by spectrophotome... | Eur J Med Chem 62: 89-97 (2013) Article DOI: 10.1016/j.ejmech.2012.12.045 BindingDB Entry DOI: 10.7270/Q2BR8TH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50067678 ((6alpha)-17-(Acetyloxy)-6-methylpreg-4-ene-3,20-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C2 (unknown origin) assessed as decrease in oxidation of 1-acenaphthenol substrate by spectrophotometric analysis | Eur J Med Chem 62: 89-97 (2013) Article DOI: 10.1016/j.ejmech.2012.12.045 BindingDB Entry DOI: 10.7270/Q2BR8TH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50429341 (CHEMBL2334912) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as decrease in oxidation of 1-acenaphthenol substrate by spectrophotometric analysis | Eur J Med Chem 62: 89-97 (2013) Article DOI: 10.1016/j.ejmech.2012.12.045 BindingDB Entry DOI: 10.7270/Q2BR8TH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50429339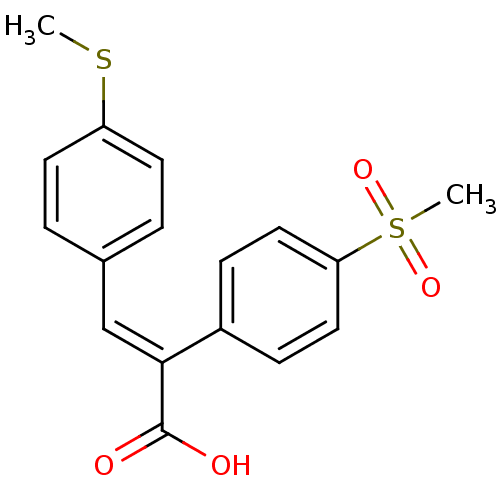 (CHEMBL2334914) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as decrease in oxidation of 1-acenaphthenol substrate by spectrophotometric analysis | Eur J Med Chem 62: 89-97 (2013) Article DOI: 10.1016/j.ejmech.2012.12.045 BindingDB Entry DOI: 10.7270/Q2BR8TH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50429332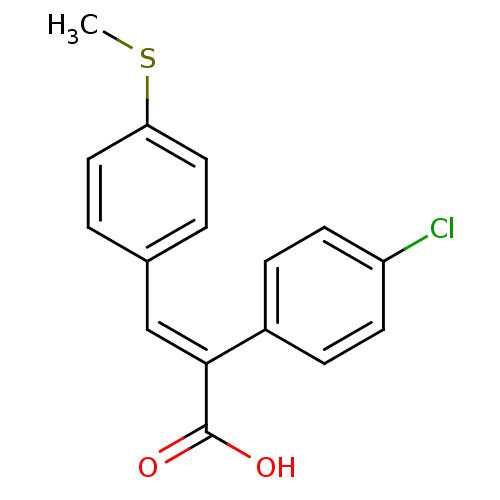 (CHEMBL2334932) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C1 expressed in Escherichia coli assessed as decrease in oxidation of 1-acenaphthenol substrate by spectrophotome... | Eur J Med Chem 62: 89-97 (2013) Article DOI: 10.1016/j.ejmech.2012.12.045 BindingDB Entry DOI: 10.7270/Q2BR8TH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50429337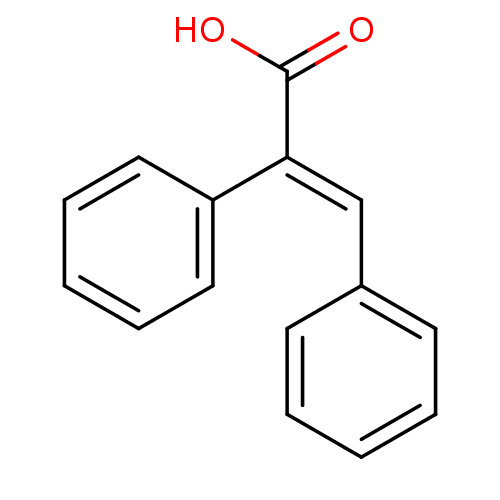 ((E)-2,3-Diphenylacrylic Acid | CHEMBL1980291) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C1 expressed in Escherichia coli assessed as decrease in oxidation of 1-acenaphthenol substrate by spectrophotome... | Eur J Med Chem 62: 89-97 (2013) Article DOI: 10.1016/j.ejmech.2012.12.045 BindingDB Entry DOI: 10.7270/Q2BR8TH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50429332 (CHEMBL2334932) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as decrease in oxidation of 1-acenaphthenol substrate by spectrophotometric analysis | Eur J Med Chem 62: 89-97 (2013) Article DOI: 10.1016/j.ejmech.2012.12.045 BindingDB Entry DOI: 10.7270/Q2BR8TH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50429333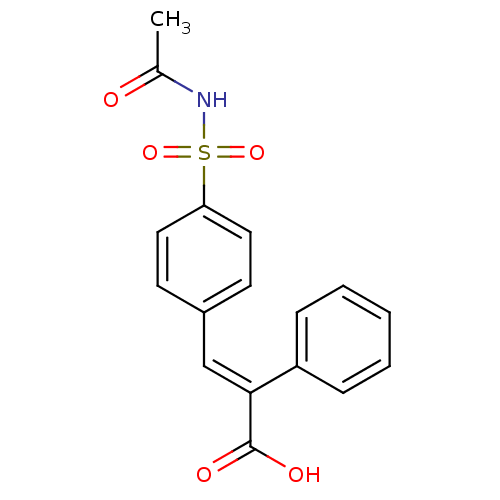 (CHEMBL2334931) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C1 expressed in Escherichia coli assessed as decrease in oxidation of 1-acenaphthenol substrate by spectrophotome... | Eur J Med Chem 62: 89-97 (2013) Article DOI: 10.1016/j.ejmech.2012.12.045 BindingDB Entry DOI: 10.7270/Q2BR8TH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50429349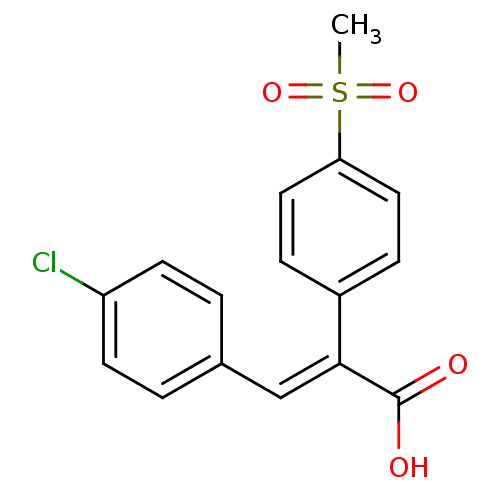 (CHEMBL2334934) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as decrease in oxidation of 1-acenaphthenol substrate by spectrophotometric analysis | Eur J Med Chem 62: 89-97 (2013) Article DOI: 10.1016/j.ejmech.2012.12.045 BindingDB Entry DOI: 10.7270/Q2BR8TH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50429334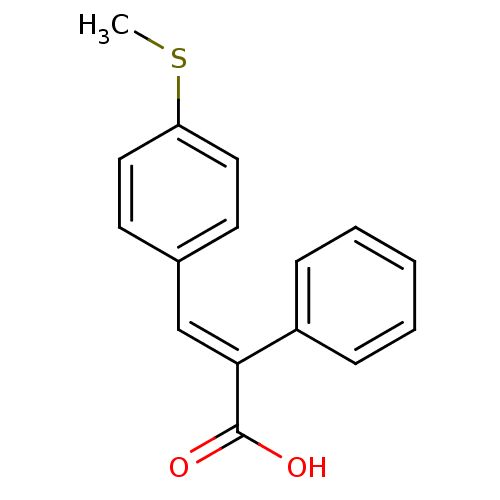 (CHEMBL2334929) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C1 expressed in Escherichia coli assessed as decrease in oxidation of 1-acenaphthenol substrate by spectrophotome... | Eur J Med Chem 62: 89-97 (2013) Article DOI: 10.1016/j.ejmech.2012.12.045 BindingDB Entry DOI: 10.7270/Q2BR8TH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50429346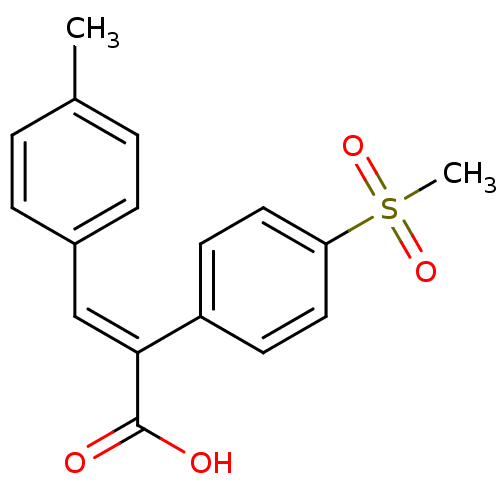 (CHEMBL2334903) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as decrease in oxidation of 1-acenaphthenol substrate by spectrophotometric analysis | Eur J Med Chem 62: 89-97 (2013) Article DOI: 10.1016/j.ejmech.2012.12.045 BindingDB Entry DOI: 10.7270/Q2BR8TH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50429345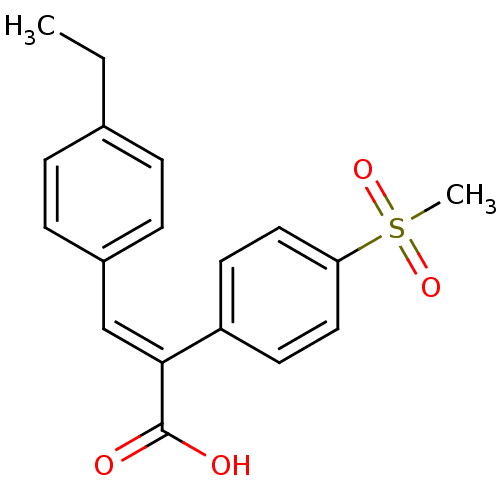 (CHEMBL2334904) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as decrease in oxidation of 1-acenaphthenol substrate by spectrophotometric analysis | Eur J Med Chem 62: 89-97 (2013) Article DOI: 10.1016/j.ejmech.2012.12.045 BindingDB Entry DOI: 10.7270/Q2BR8TH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50429328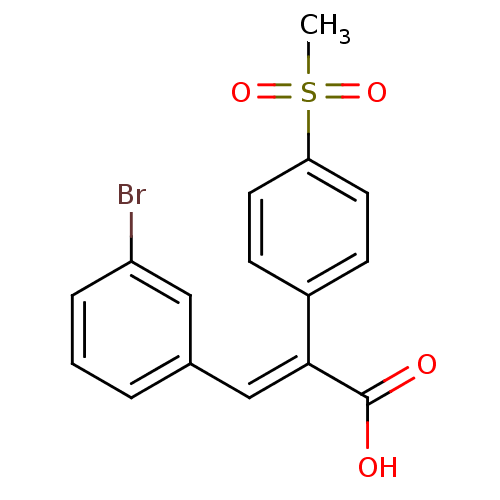 (CHEMBL2334911) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as decrease in oxidation of 1-acenaphthenol substrate by spectrophotometric analysis | Eur J Med Chem 62: 89-97 (2013) Article DOI: 10.1016/j.ejmech.2012.12.045 BindingDB Entry DOI: 10.7270/Q2BR8TH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50429331 (CHEMBL2334933) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C1 expressed in Escherichia coli assessed as decrease in oxidation of 1-acenaphthenol substrate by spectrophotome... | Eur J Med Chem 62: 89-97 (2013) Article DOI: 10.1016/j.ejmech.2012.12.045 BindingDB Entry DOI: 10.7270/Q2BR8TH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50429348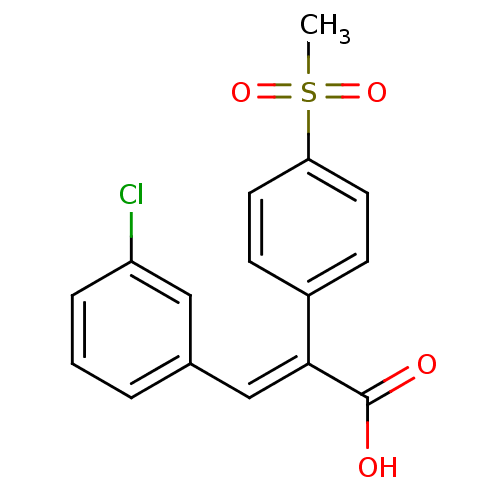 (CHEMBL2334937) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as decrease in oxidation of 1-acenaphthenol substrate by spectrophotometric analysis | Eur J Med Chem 62: 89-97 (2013) Article DOI: 10.1016/j.ejmech.2012.12.045 BindingDB Entry DOI: 10.7270/Q2BR8TH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50429330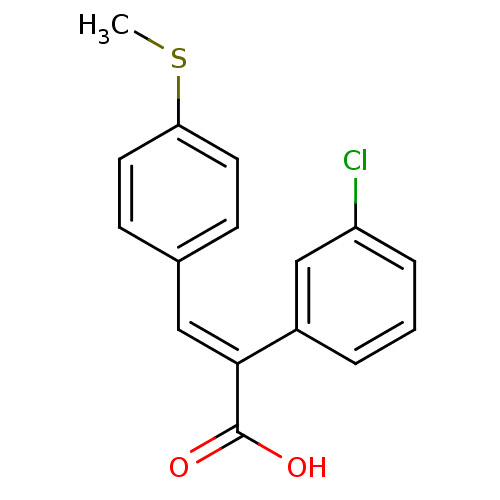 (CHEMBL2334935) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as decrease in oxidation of 1-acenaphthenol substrate by spectrophotometric analysis | Eur J Med Chem 62: 89-97 (2013) Article DOI: 10.1016/j.ejmech.2012.12.045 BindingDB Entry DOI: 10.7270/Q2BR8TH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50429330 (CHEMBL2334935) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.91E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C1 expressed in Escherichia coli assessed as decrease in oxidation of 1-acenaphthenol substrate by spectrophotome... | Eur J Med Chem 62: 89-97 (2013) Article DOI: 10.1016/j.ejmech.2012.12.045 BindingDB Entry DOI: 10.7270/Q2BR8TH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50429329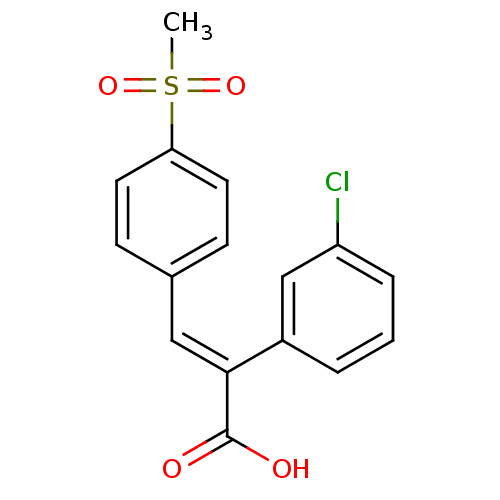 (CHEMBL2334936) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C1 expressed in Escherichia coli assessed as decrease in oxidation of 1-acenaphthenol substrate by spectrophotome... | Eur J Med Chem 62: 89-97 (2013) Article DOI: 10.1016/j.ejmech.2012.12.045 BindingDB Entry DOI: 10.7270/Q2BR8TH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50429330 (CHEMBL2334935) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C2 (unknown origin) assessed as decrease in oxidation of 1-acenaphthenol substrate by spectrophotometric analysis | Eur J Med Chem 62: 89-97 (2013) Article DOI: 10.1016/j.ejmech.2012.12.045 BindingDB Entry DOI: 10.7270/Q2BR8TH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50429329 (CHEMBL2334936) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C2 (unknown origin) assessed as decrease in oxidation of 1-acenaphthenol substrate by spectrophotometric analysis | Eur J Med Chem 62: 89-97 (2013) Article DOI: 10.1016/j.ejmech.2012.12.045 BindingDB Entry DOI: 10.7270/Q2BR8TH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50429340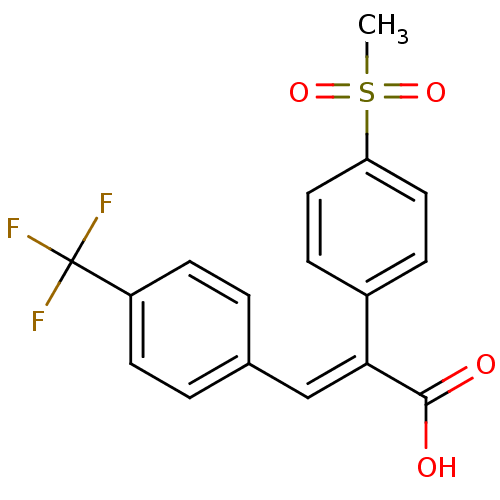 (CHEMBL2334913) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as decrease in oxidation of 1-acenaphthenol substrate by spectrophotometric analysis | Eur J Med Chem 62: 89-97 (2013) Article DOI: 10.1016/j.ejmech.2012.12.045 BindingDB Entry DOI: 10.7270/Q2BR8TH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50374770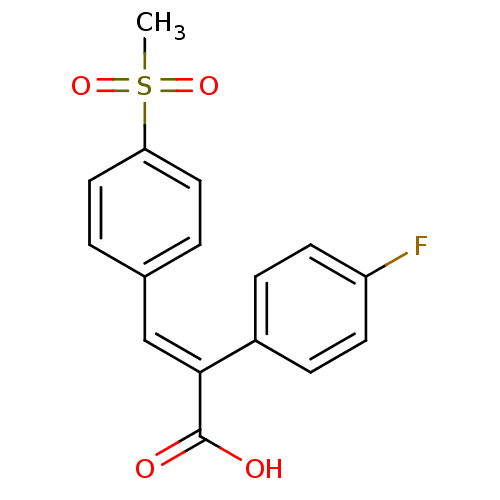 (CHEMBL407035) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C1 expressed in Escherichia coli assessed as decrease in oxidation of 1-acenaphthenol substrate by spectrophotome... | Eur J Med Chem 62: 89-97 (2013) Article DOI: 10.1016/j.ejmech.2012.12.045 BindingDB Entry DOI: 10.7270/Q2BR8TH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50429335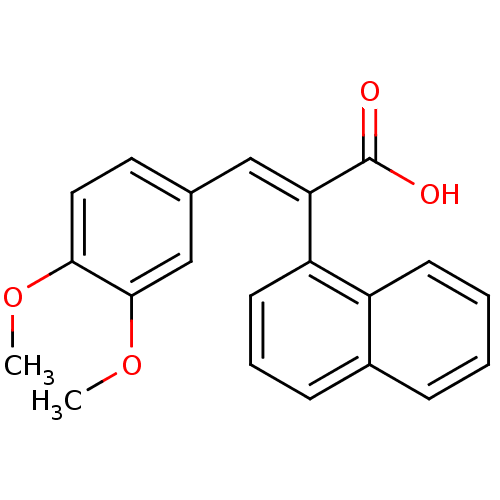 (CHEMBL2334927) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C2 (unknown origin) assessed as decrease in oxidation of 1-acenaphthenol substrate by spectrophotometric analysis | Eur J Med Chem 62: 89-97 (2013) Article DOI: 10.1016/j.ejmech.2012.12.045 BindingDB Entry DOI: 10.7270/Q2BR8TH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50429333 (CHEMBL2334931) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C2 (unknown origin) assessed as decrease in oxidation of 1-acenaphthenol substrate by spectrophotometric analysis | Eur J Med Chem 62: 89-97 (2013) Article DOI: 10.1016/j.ejmech.2012.12.045 BindingDB Entry DOI: 10.7270/Q2BR8TH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50429331 (CHEMBL2334933) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C2 (unknown origin) assessed as decrease in oxidation of 1-acenaphthenol substrate by spectrophotometric analysis | Eur J Med Chem 62: 89-97 (2013) Article DOI: 10.1016/j.ejmech.2012.12.045 BindingDB Entry DOI: 10.7270/Q2BR8TH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50429332 (CHEMBL2334932) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C2 (unknown origin) assessed as decrease in oxidation of 1-acenaphthenol substrate by spectrophotometric analysis | Eur J Med Chem 62: 89-97 (2013) Article DOI: 10.1016/j.ejmech.2012.12.045 BindingDB Entry DOI: 10.7270/Q2BR8TH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50429334 (CHEMBL2334929) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as decrease in oxidation of 1-acenaphthenol substrate by spectrophotometric analysis | Eur J Med Chem 62: 89-97 (2013) Article DOI: 10.1016/j.ejmech.2012.12.045 BindingDB Entry DOI: 10.7270/Q2BR8TH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50374772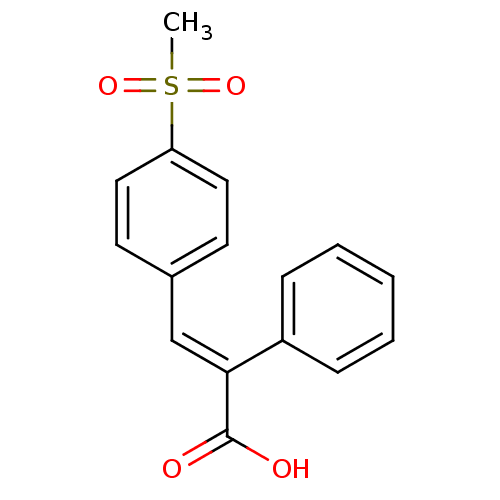 (CHEMBL405127) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C1 expressed in Escherichia coli assessed as decrease in oxidation of 1-acenaphthenol substrate by spectrophotome... | Eur J Med Chem 62: 89-97 (2013) Article DOI: 10.1016/j.ejmech.2012.12.045 BindingDB Entry DOI: 10.7270/Q2BR8TH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50429343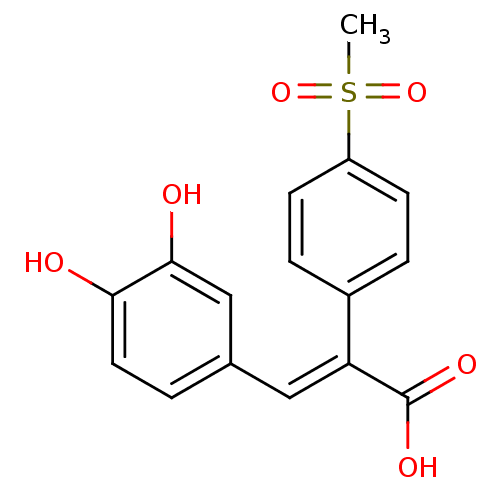 (CHEMBL2334908) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as decrease in oxidation of 1-acenaphthenol substrate by spectrophotometric analysis | Eur J Med Chem 62: 89-97 (2013) Article DOI: 10.1016/j.ejmech.2012.12.045 BindingDB Entry DOI: 10.7270/Q2BR8TH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50374772 (CHEMBL405127) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C2 (unknown origin) assessed as decrease in oxidation of 1-acenaphthenol substrate by spectrophotometric analysis | Eur J Med Chem 62: 89-97 (2013) Article DOI: 10.1016/j.ejmech.2012.12.045 BindingDB Entry DOI: 10.7270/Q2BR8TH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50429344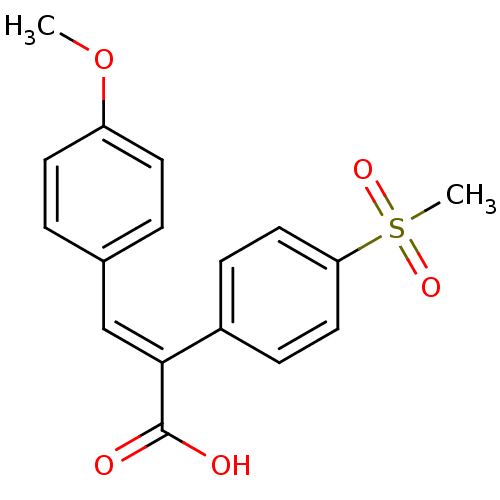 (CHEMBL2334905) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as decrease in oxidation of 1-acenaphthenol substrate by spectrophotometric analysis | Eur J Med Chem 62: 89-97 (2013) Article DOI: 10.1016/j.ejmech.2012.12.045 BindingDB Entry DOI: 10.7270/Q2BR8TH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50429351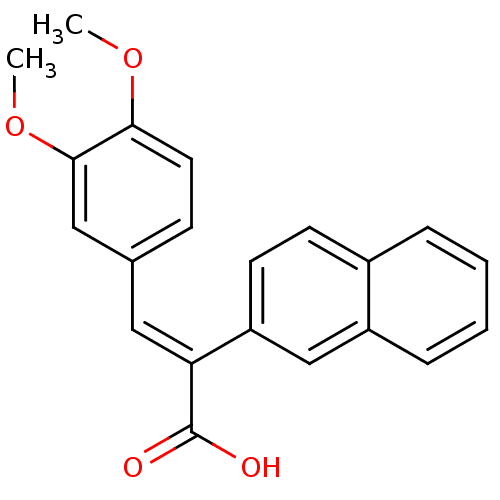 (CHEMBL2334928) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.87E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as decrease in oxidation of 1-acenaphthenol substrate by spectrophotometric analysis | Eur J Med Chem 62: 89-97 (2013) Article DOI: 10.1016/j.ejmech.2012.12.045 BindingDB Entry DOI: 10.7270/Q2BR8TH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50429342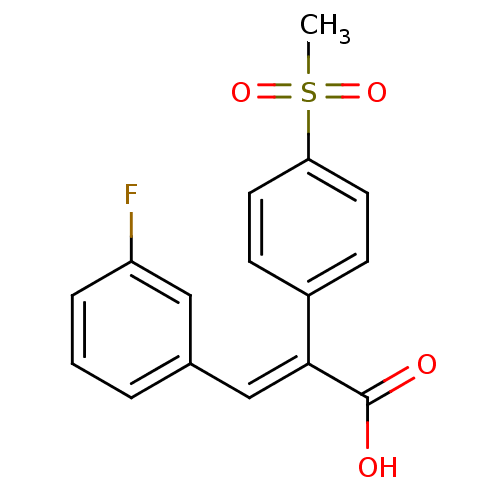 (CHEMBL2334910) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as decrease in oxidation of 1-acenaphthenol substrate by spectrophotometric analysis | Eur J Med Chem 62: 89-97 (2013) Article DOI: 10.1016/j.ejmech.2012.12.045 BindingDB Entry DOI: 10.7270/Q2BR8TH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50429334 (CHEMBL2334929) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C2 (unknown origin) assessed as decrease in oxidation of 1-acenaphthenol substrate by spectrophotometric analysis | Eur J Med Chem 62: 89-97 (2013) Article DOI: 10.1016/j.ejmech.2012.12.045 BindingDB Entry DOI: 10.7270/Q2BR8TH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50429347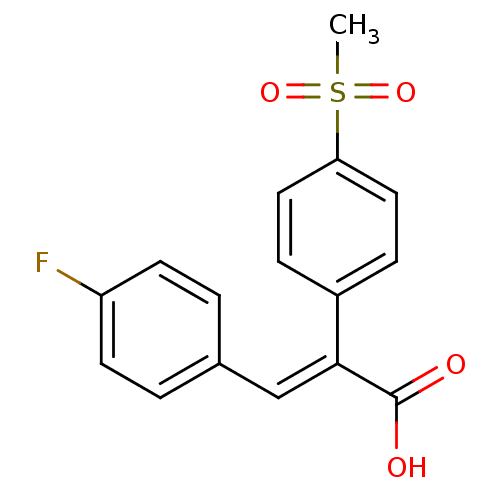 (CHEMBL2334902) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as decrease in oxidation of 1-acenaphthenol substrate by spectrophotometric analysis | Eur J Med Chem 62: 89-97 (2013) Article DOI: 10.1016/j.ejmech.2012.12.045 BindingDB Entry DOI: 10.7270/Q2BR8TH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50374770 (CHEMBL407035) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C2 (unknown origin) assessed as decrease in oxidation of 1-acenaphthenol substrate by spectrophotometric analysis | Eur J Med Chem 62: 89-97 (2013) Article DOI: 10.1016/j.ejmech.2012.12.045 BindingDB Entry DOI: 10.7270/Q2BR8TH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50429338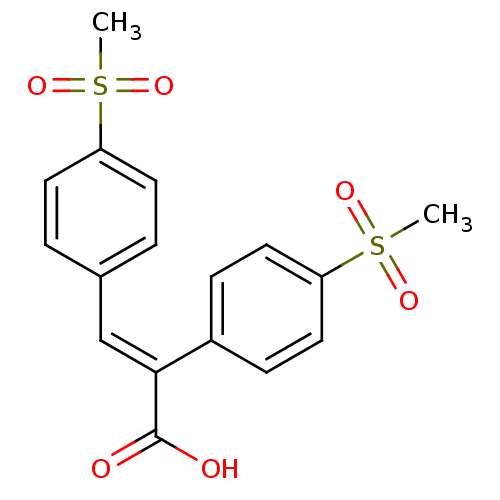 (CHEMBL2334915) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as decrease in oxidation of 1-acenaphthenol substrate by spectrophotometric analysis | Eur J Med Chem 62: 89-97 (2013) Article DOI: 10.1016/j.ejmech.2012.12.045 BindingDB Entry DOI: 10.7270/Q2BR8TH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50429350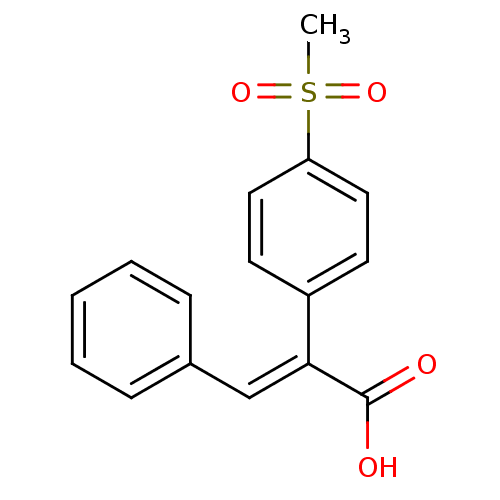 (CHEMBL1682539) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as decrease in oxidation of 1-acenaphthenol substrate by spectrophotometric analysis | Eur J Med Chem 62: 89-97 (2013) Article DOI: 10.1016/j.ejmech.2012.12.045 BindingDB Entry DOI: 10.7270/Q2BR8TH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50429328 (CHEMBL2334911) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C2 (unknown origin) assessed as decrease in oxidation of 1-acenaphthenol substrate by spectrophotometric analysis | Eur J Med Chem 62: 89-97 (2013) Article DOI: 10.1016/j.ejmech.2012.12.045 BindingDB Entry DOI: 10.7270/Q2BR8TH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50429329 (CHEMBL2334936) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as decrease in oxidation of 1-acenaphthenol substrate by spectrophotometric analysis | Eur J Med Chem 62: 89-97 (2013) Article DOI: 10.1016/j.ejmech.2012.12.045 BindingDB Entry DOI: 10.7270/Q2BR8TH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50429336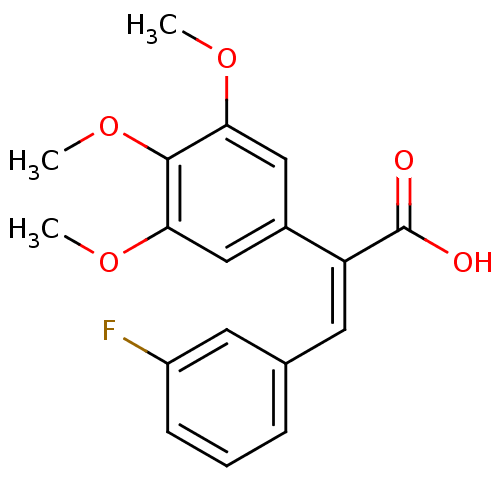 (CHEMBL2334921) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C2 (unknown origin) assessed as decrease in oxidation of 1-acenaphthenol substrate by spectrophotometric analysis | Eur J Med Chem 62: 89-97 (2013) Article DOI: 10.1016/j.ejmech.2012.12.045 BindingDB Entry DOI: 10.7270/Q2BR8TH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50429331 (CHEMBL2334933) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as decrease in oxidation of 1-acenaphthenol substrate by spectrophotometric analysis | Eur J Med Chem 62: 89-97 (2013) Article DOI: 10.1016/j.ejmech.2012.12.045 BindingDB Entry DOI: 10.7270/Q2BR8TH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50429352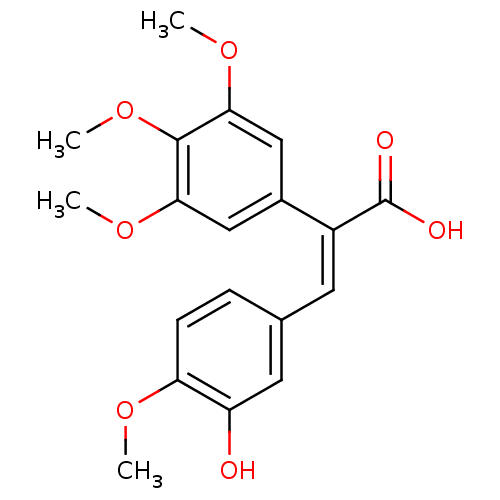 (CHEMBL1642214) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.21E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as decrease in oxidation of 1-acenaphthenol substrate by spectrophotometric analysis | Eur J Med Chem 62: 89-97 (2013) Article DOI: 10.1016/j.ejmech.2012.12.045 BindingDB Entry DOI: 10.7270/Q2BR8TH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50429335 (CHEMBL2334927) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.28E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as decrease in oxidation of 1-acenaphthenol substrate by spectrophotometric analysis | Eur J Med Chem 62: 89-97 (2013) Article DOI: 10.1016/j.ejmech.2012.12.045 BindingDB Entry DOI: 10.7270/Q2BR8TH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50374772 (CHEMBL405127) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.39E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as decrease in oxidation of 1-acenaphthenol substrate by spectrophotometric analysis | Eur J Med Chem 62: 89-97 (2013) Article DOI: 10.1016/j.ejmech.2012.12.045 BindingDB Entry DOI: 10.7270/Q2BR8TH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||