 Found 86 hits with Last Name = 'bussiere' and Initial = 'de'
Found 86 hits with Last Name = 'bussiere' and Initial = 'de' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50185219
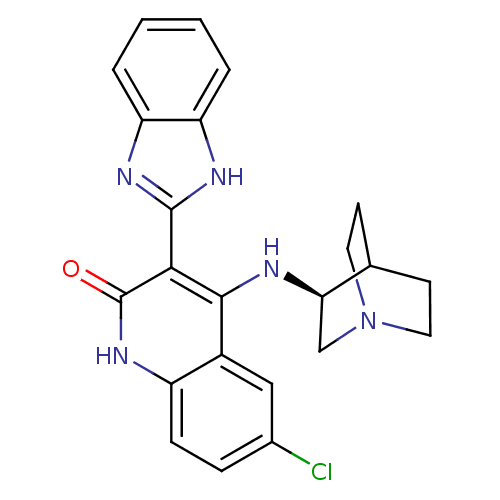
((S)-3-(1H-benzo[d]imidazol-2-yl)-6-chloro-4-(quinu...)Show SMILES Clc1ccc2[nH]c(=O)c(-c3nc4ccccc4[nH]3)c(N[C@@H]3CN4CCC3CC4)c2c1 |wU:20.21,TLB:19:20:24.23:26.27,(4.27,-32.71,;5.6,-33.48,;5.6,-35.02,;6.93,-35.79,;8.27,-35.03,;9.61,-35.79,;10.95,-35.01,;12.28,-35.78,;10.94,-33.46,;12.27,-32.69,;13.68,-33.32,;14.7,-32.18,;16.24,-32.18,;17.02,-30.84,;16.23,-29.5,;14.69,-29.51,;13.93,-30.84,;12.43,-31.16,;9.59,-32.69,;9.59,-31.15,;8.64,-29.94,;8.36,-28.54,;7.01,-27.94,;5.55,-28.58,;5.74,-29.96,;7.27,-29.3,;7.53,-27.41,;7.08,-26.31,;8.26,-33.47,;6.93,-32.71,)| Show InChI InChI=1S/C23H22ClN5O/c24-14-5-6-16-15(11-14)21(25-19-12-29-9-7-13(19)8-10-29)20(23(30)28-16)22-26-17-3-1-2-4-18(17)27-22/h1-6,11,13,19H,7-10,12H2,(H,26,27)(H2,25,28,30)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 |
Bioorg Med Chem Lett 16: 3121-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.059
BindingDB Entry DOI: 10.7270/Q2765DXT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50185221
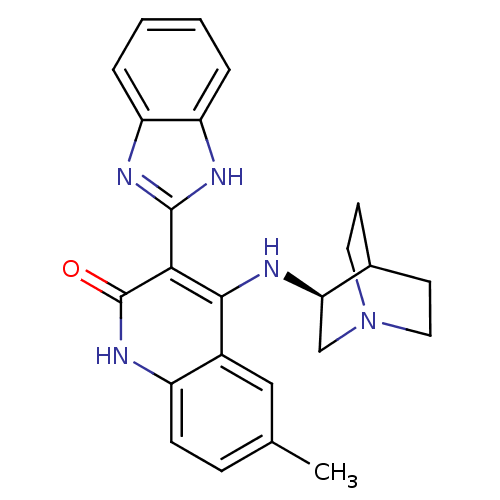
((S)-3-(1H-benzo[d]imidazol-2-yl)-6-methyl-4-(quinu...)Show SMILES Cc1ccc2[nH]c(=O)c(-c3nc4ccccc4[nH]3)c(N[C@@H]3CN4CCC3CC4)c2c1 |wU:20.21,TLB:19:20:24.23:26.27,(17.38,-9.04,;18.71,-9.81,;18.71,-11.36,;20.04,-12.13,;21.38,-11.36,;22.72,-12.13,;24.06,-11.35,;25.39,-12.12,;24.05,-9.8,;25.38,-9.02,;26.79,-9.65,;27.81,-8.51,;29.35,-8.51,;30.13,-7.17,;29.34,-5.83,;27.8,-5.84,;27.04,-7.17,;25.54,-7.5,;22.7,-9.02,;22.7,-7.48,;21.75,-6.27,;21.47,-4.88,;20.12,-4.28,;18.65,-4.92,;18.85,-6.3,;20.38,-5.64,;20.64,-3.75,;20.19,-2.64,;21.37,-9.81,;20.04,-9.04,)| Show InChI InChI=1S/C24H25N5O/c1-14-6-7-17-16(12-14)22(25-20-13-29-10-8-15(20)9-11-29)21(24(30)28-17)23-26-18-4-2-3-5-19(18)27-23/h2-7,12,15,20H,8-11,13H2,1H3,(H,26,27)(H2,25,28,30)/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 |
Bioorg Med Chem Lett 16: 3121-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.059
BindingDB Entry DOI: 10.7270/Q2765DXT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50185218
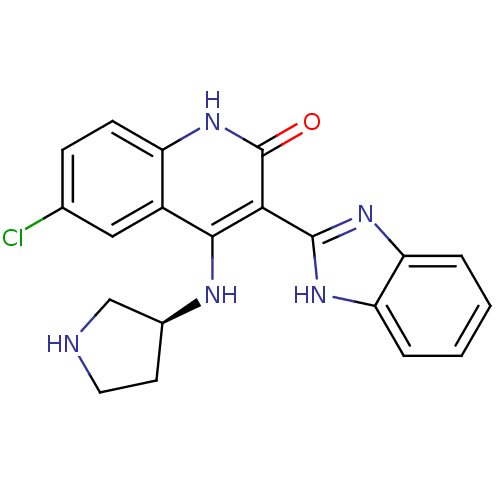
((S)-3-(1H-benzo[d]imidazol-2-yl)-6-chloro-4-(pyrro...)Show SMILES Clc1ccc2[nH]c(=O)c(-c3nc4ccccc4[nH]3)c(N[C@H]3CCNC3)c2c1 Show InChI InChI=1S/C20H18ClN5O/c21-11-5-6-14-13(9-11)18(23-12-7-8-22-10-12)17(20(27)26-14)19-24-15-3-1-2-4-16(15)25-19/h1-6,9,12,22H,7-8,10H2,(H,24,25)(H2,23,26,27)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 |
Bioorg Med Chem Lett 16: 3121-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.059
BindingDB Entry DOI: 10.7270/Q2765DXT |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50313013
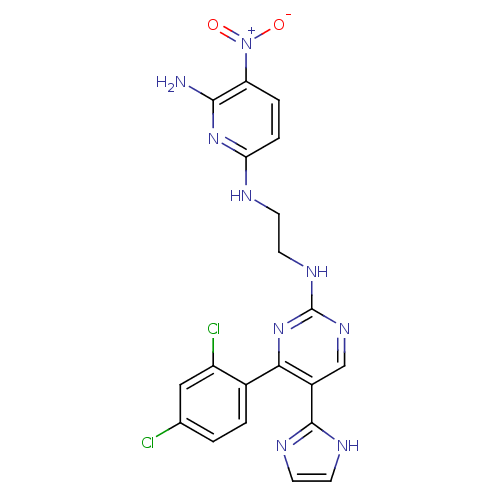
(CHEMBL1080901 | CT-98024 | N2-(2-(4-(2,4-dichlorop...)Show SMILES Nc1nc(NCCNc2ncc(-c3ncc[nH]3)c(n2)-c2ccc(Cl)cc2Cl)ccc1[N+]([O-])=O Show InChI InChI=1S/C20H17Cl2N9O2/c21-11-1-2-12(14(22)9-11)17-13(19-25-6-7-26-19)10-28-20(30-17)27-8-5-24-16-4-3-15(31(32)33)18(23)29-16/h1-4,6-7,9-10H,5,8H2,(H,25,26)(H3,23,24,29)(H,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3beta using biotin-CREB peptide substrate after 1 hr by scintillation counting method |
J Med Chem 60: 8482-8514 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00922
BindingDB Entry DOI: 10.7270/Q25T3NW3 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50243389
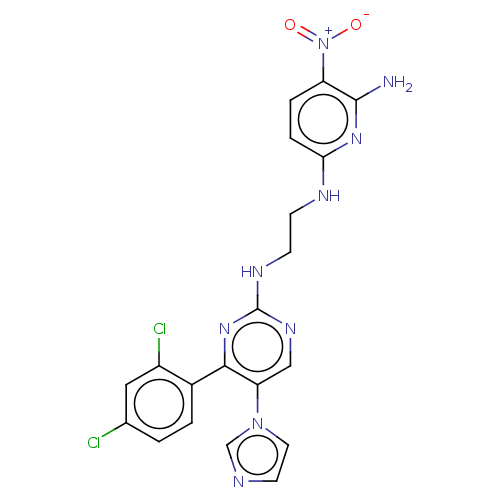
(CHEMBL3185148)Show SMILES Nc1nc(NCCNc2ncc(c(n2)-c2ccc(Cl)cc2Cl)-n2ccnc2)ccc1[N+]([O-])=O Show InChI InChI=1S/C20H17Cl2N9O2/c21-12-1-2-13(14(22)9-12)18-16(30-8-7-24-11-30)10-27-20(29-18)26-6-5-25-17-4-3-15(31(32)33)19(23)28-17/h1-4,7-11H,5-6H2,(H3,23,25,28)(H,26,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3beta using biotin-CREB peptide substrate after 1 hr by scintillation counting method |
J Med Chem 60: 8482-8514 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00922
BindingDB Entry DOI: 10.7270/Q25T3NW3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50185217
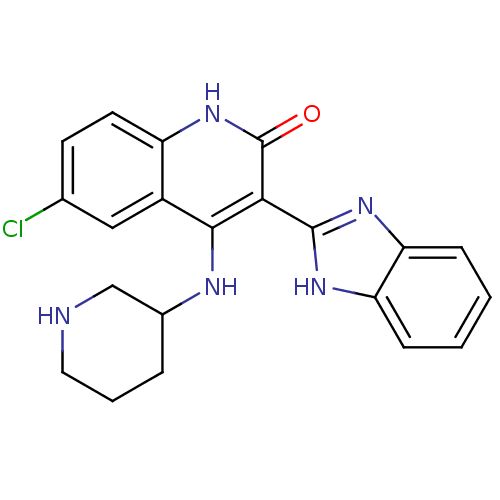
(3-(1H-benzo[d]imidazol-2-yl)-6-chloro-4-(piperidin...)Show SMILES Clc1ccc2[nH]c(=O)c(-c3nc4ccccc4[nH]3)c(NC3CCCNC3)c2c1 Show InChI InChI=1S/C21H20ClN5O/c22-12-7-8-15-14(10-12)19(24-13-4-3-9-23-11-13)18(21(28)27-15)20-25-16-5-1-2-6-17(16)26-20/h1-2,5-8,10,13,23H,3-4,9,11H2,(H,25,26)(H2,24,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 |
Bioorg Med Chem Lett 16: 3121-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.059
BindingDB Entry DOI: 10.7270/Q2765DXT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50185224
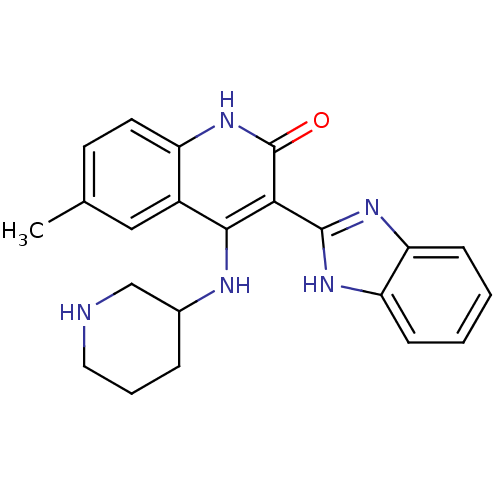
(3-(1H-benzo[d]imidazol-2-yl)-6-methyl-4-(piperidin...)Show SMILES Cc1ccc2[nH]c(=O)c(-c3nc4ccccc4[nH]3)c(NC3CCCNC3)c2c1 Show InChI InChI=1S/C22H23N5O/c1-13-8-9-16-15(11-13)20(24-14-5-4-10-23-12-14)19(22(28)27-16)21-25-17-6-2-3-7-18(17)26-21/h2-3,6-9,11,14,23H,4-5,10,12H2,1H3,(H,25,26)(H2,24,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 |
Bioorg Med Chem Lett 16: 3121-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.059
BindingDB Entry DOI: 10.7270/Q2765DXT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50185216
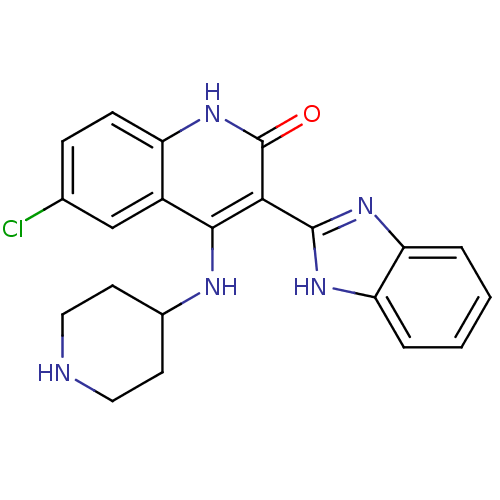
(3-(1H-benzo[d]imidazol-2-yl)-6-chloro-4-(piperidin...)Show SMILES Clc1ccc2[nH]c(=O)c(-c3nc4ccccc4[nH]3)c(NC3CCNCC3)c2c1 Show InChI InChI=1S/C21H20ClN5O/c22-12-5-6-15-14(11-12)19(24-13-7-9-23-10-8-13)18(21(28)27-15)20-25-16-3-1-2-4-17(16)26-20/h1-6,11,13,23H,7-10H2,(H,25,26)(H2,24,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 |
Bioorg Med Chem Lett 16: 3121-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.059
BindingDB Entry DOI: 10.7270/Q2765DXT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50185220

(3-(1H-benzo[d]imidazol-2-yl)-6-methyl-4-(piperidin...)Show SMILES Cc1ccc2[nH]c(=O)c(-c3nc4ccccc4[nH]3)c(NC3CCNCC3)c2c1 Show InChI InChI=1S/C22H23N5O/c1-13-6-7-16-15(12-13)20(24-14-8-10-23-11-9-14)19(22(28)27-16)21-25-17-4-2-3-5-18(17)26-21/h2-7,12,14,23H,8-11H2,1H3,(H,25,26)(H2,24,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 |
Bioorg Med Chem Lett 16: 3121-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.059
BindingDB Entry DOI: 10.7270/Q2765DXT |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50325983

(6-(2-(4-(2,4-dichlorophenyl)-5-(4-methyl-1H-imidaz...)Show SMILES Cc1c[nH]c(n1)-c1cnc(NCCNc2ccc(cn2)C#N)nc1-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C22H18Cl2N8/c1-13-10-29-21(31-13)17-12-30-22(32-20(17)16-4-3-15(23)8-18(16)24)27-7-6-26-19-5-2-14(9-25)11-28-19/h2-5,8,10-12H,6-7H2,1H3,(H,26,28)(H,29,31)(H,27,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3beta using biotin-CREB peptide substrate after 1 hr by scintillation counting method |
J Med Chem 60: 8482-8514 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00922
BindingDB Entry DOI: 10.7270/Q25T3NW3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM50243389

(CHEMBL3185148)Show SMILES Nc1nc(NCCNc2ncc(c(n2)-c2ccc(Cl)cc2Cl)-n2ccnc2)ccc1[N+]([O-])=O Show InChI InChI=1S/C20H17Cl2N9O2/c21-12-1-2-13(14(22)9-12)18-16(30-8-7-24-11-30)10-27-20(29-18)26-6-5-25-17-4-3-15(31(32)33)19(23)28-17/h1-4,7-11H,5-6H2,(H3,23,25,28)(H,26,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3alpha using biotin-CREB peptide substrate after 1 hr by scintillation counting method |
J Med Chem 60: 8482-8514 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00922
BindingDB Entry DOI: 10.7270/Q25T3NW3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50185226

((R)-3-(1H-benzo[d]imidazol-2-yl)-6-chloro-4-(pyrro...)Show SMILES Clc1ccc2[nH]c(=O)c(-c3nc4ccccc4[nH]3)c(N[C@@H]3CCNC3)c2c1 Show InChI InChI=1S/C20H18ClN5O/c21-11-5-6-14-13(9-11)18(23-12-7-8-22-10-12)17(20(27)26-14)19-24-15-3-1-2-4-16(15)25-19/h1-6,9,12,22H,7-8,10H2,(H,24,25)(H2,23,26,27)/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 |
Bioorg Med Chem Lett 16: 3121-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.059
BindingDB Entry DOI: 10.7270/Q2765DXT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50185215
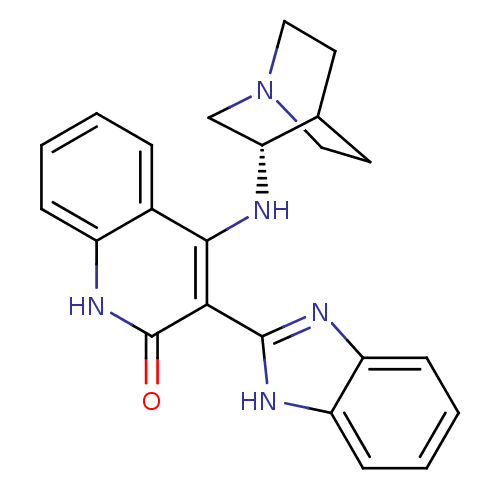
((S)-3-(1H-benzo[d]imidazol-2-yl)-4-(quinuclidin-3-...)Show SMILES O=c1[nH]c2ccccc2c(N[C@@H]2CN3CCC2CC3)c1-c1nc2ccccc2[nH]1 |wU:11.11,TLB:10:11:15.14:17.18,(-2.4,-12.59,;-3.74,-11.82,;-5.08,-12.6,;-6.42,-11.83,;-7.76,-12.6,;-9.09,-11.83,;-9.09,-10.28,;-7.76,-9.51,;-6.43,-10.28,;-5.09,-9.49,;-5.1,-7.95,;-6.05,-6.74,;-6.33,-5.35,;-7.68,-4.75,;-9.14,-5.39,;-8.95,-6.77,;-7.42,-6.11,;-7.16,-4.22,;-7.61,-3.11,;-3.75,-10.27,;-2.41,-9.49,;-1.01,-10.12,;.01,-8.98,;1.56,-8.98,;2.33,-7.64,;1.54,-6.3,;0,-6.31,;-.76,-7.64,;-2.26,-7.97,)| Show InChI InChI=1S/C23H23N5O/c29-23-20(22-25-17-7-3-4-8-18(17)26-22)21(15-5-1-2-6-16(15)27-23)24-19-13-28-11-9-14(19)10-12-28/h1-8,14,19H,9-13H2,(H,25,26)(H2,24,27,29)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 |
Bioorg Med Chem Lett 16: 3121-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.059
BindingDB Entry DOI: 10.7270/Q2765DXT |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM50325983

(6-(2-(4-(2,4-dichlorophenyl)-5-(4-methyl-1H-imidaz...)Show SMILES Cc1c[nH]c(n1)-c1cnc(NCCNc2ccc(cn2)C#N)nc1-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C22H18Cl2N8/c1-13-10-29-21(31-13)17-12-30-22(32-20(17)16-4-3-15(23)8-18(16)24)27-7-6-26-19-5-2-14(9-25)11-28-19/h2-5,8,10-12H,6-7H2,1H3,(H,26,28)(H,29,31)(H,27,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3alpha using biotin-CREB peptide substrate after 1 hr by scintillation counting method |
J Med Chem 60: 8482-8514 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00922
BindingDB Entry DOI: 10.7270/Q25T3NW3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50185223

((S)-3-(1H-benzo[d]imidazol-2-yl)-5,6-dichloro-4-(q...)Show SMILES Clc1ccc2[nH]c(=O)c(-c3nc4ccccc4[nH]3)c(N[C@@H]3CN4CCC3CC4)c2c1Cl |wU:20.21,TLB:19:20:24.23:26.27,(3.37,-44,;4.71,-44.76,;4.71,-46.31,;6.04,-47.08,;7.37,-46.31,;8.71,-47.08,;10.06,-46.3,;11.39,-47.07,;10.05,-44.75,;11.38,-43.97,;12.78,-44.6,;13.81,-43.46,;15.35,-43.46,;16.12,-42.12,;15.34,-40.78,;13.8,-40.79,;13.04,-42.12,;11.53,-42.45,;8.7,-43.97,;8.69,-42.43,;7.74,-41.22,;7.47,-39.83,;6.12,-39.23,;4.65,-39.87,;4.85,-41.25,;6.38,-40.59,;6.63,-38.7,;6.19,-37.59,;7.37,-44.76,;6.03,-43.99,;6.03,-42.46,)| Show InChI InChI=1S/C23H21Cl2N5O/c24-13-5-6-16-18(20(13)25)21(26-17-11-30-9-7-12(17)8-10-30)19(23(31)29-16)22-27-14-3-1-2-4-15(14)28-22/h1-6,12,17H,7-11H2,(H,27,28)(H2,26,29,31)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 |
Bioorg Med Chem Lett 16: 3121-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.059
BindingDB Entry DOI: 10.7270/Q2765DXT |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM50313013

(CHEMBL1080901 | CT-98024 | N2-(2-(4-(2,4-dichlorop...)Show SMILES Nc1nc(NCCNc2ncc(-c3ncc[nH]3)c(n2)-c2ccc(Cl)cc2Cl)ccc1[N+]([O-])=O Show InChI InChI=1S/C20H17Cl2N9O2/c21-11-1-2-12(14(22)9-11)17-13(19-25-6-7-26-19)10-28-20(30-17)27-8-5-24-16-4-3-15(31(32)33)18(23)29-16/h1-4,6-7,9-10H,5,8H2,(H,25,26)(H3,23,24,29)(H,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3alpha using biotin-CREB peptide substrate after 1 hr by scintillation counting method |
J Med Chem 60: 8482-8514 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00922
BindingDB Entry DOI: 10.7270/Q25T3NW3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50185214
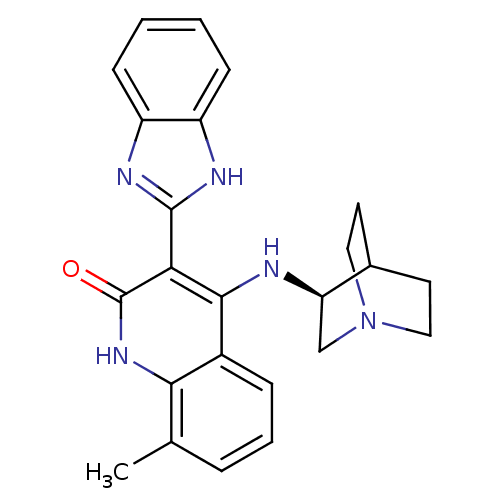
((S)-3-(1H-benzo[d]imidazol-2-yl)-8-methyl-4-(quinu...)Show SMILES Cc1cccc2c(N[C@@H]3CN4CCC3CC4)c(-c3nc4ccccc4[nH]3)c(=O)[nH]c12 |wU:8.7,TLB:7:8:12.11:14.15,(20.4,-48.62,;20.4,-47.08,;19.07,-46.31,;19.07,-44.77,;20.4,-44,;21.73,-44.76,;23.07,-43.98,;23.06,-42.44,;22.11,-41.23,;21.84,-39.83,;20.48,-39.23,;19.02,-39.87,;19.21,-41.25,;20.75,-40.59,;21,-38.7,;20.55,-37.59,;24.42,-44.75,;25.75,-43.98,;27.15,-44.61,;28.18,-43.47,;29.72,-43.46,;30.49,-42.13,;29.71,-40.79,;28.17,-40.79,;27.4,-42.13,;25.9,-42.45,;24.42,-46.3,;25.76,-47.07,;23.08,-47.08,;21.74,-46.31,)| Show InChI InChI=1S/C24H25N5O/c1-14-5-4-6-16-21(14)28-24(30)20(23-26-17-7-2-3-8-18(17)27-23)22(16)25-19-13-29-11-9-15(19)10-12-29/h2-8,15,19H,9-13H2,1H3,(H,26,27)(H2,25,28,30)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 138 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 |
Bioorg Med Chem Lett 16: 3121-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.059
BindingDB Entry DOI: 10.7270/Q2765DXT |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM7266

(14-bromo-8,18-diazatetracyclo[9.7.0.0^{2,7}.0^{12,...)Show InChI InChI=1S/C16H11BrN2O/c17-9-5-6-14-11(7-9)12-8-15(20)18-13-4-2-1-3-10(13)16(12)19-14/h1-7,19H,8H2,(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of GSK3beta (unknown origin) |
J Med Chem 60: 8482-8514 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00922
BindingDB Entry DOI: 10.7270/Q25T3NW3 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50166289
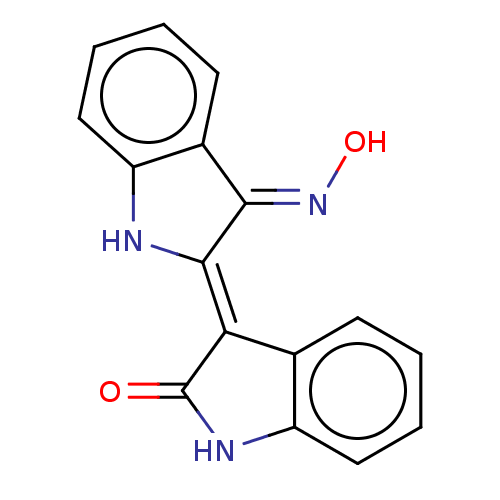
(CHEBI:43645 | CHEMBL216543)Show InChI InChI=1S/C16H11N3O2/c20-16-13(9-5-1-3-7-11(9)18-16)15-14(19-21)10-6-2-4-8-12(10)17-15/h1-8,17,21H,(H,18,20)/b15-13-,19-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of GSK3beta (unknown origin) |
J Med Chem 60: 8482-8514 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00922
BindingDB Entry DOI: 10.7270/Q25T3NW3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50259907
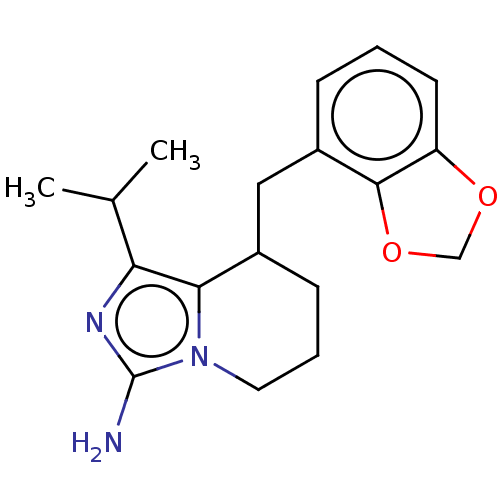
(CHEMBL4101747)Show InChI InChI=1S/C18H23N3O2/c1-11(2)15-16-12(6-4-8-21(16)18(19)20-15)9-13-5-3-7-14-17(13)23-10-22-14/h3,5,7,11-12H,4,6,8-10H2,1-2H3,(H2,19,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Binding affinity to human methionine-13Cepsilon-methyl labeled PRC2-EED (76 to 441 residues) expressed in Escherichia coli BL21 (DE3) by 2D NMR metho... |
J Med Chem 60: 415-427 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01473
BindingDB Entry DOI: 10.7270/Q2DF6TNJ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50185222
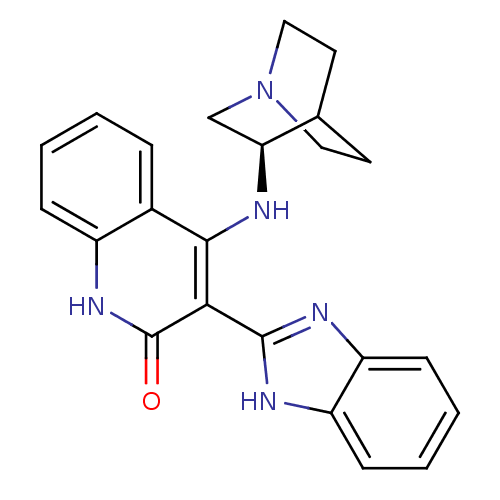
((R)-3-(1H-benzo[d]imidazol-2-yl)-4-(quinuclidin-3-...)Show SMILES O=c1[nH]c2ccccc2c(N[C@H]2CN3CCC2CC3)c1-c1nc2ccccc2[nH]1 |wD:11.11,TLB:10:11:15.14:17.18,(10.4,-12.01,;9.07,-11.25,;7.73,-12.02,;6.39,-11.26,;5.05,-12.02,;3.72,-11.25,;3.72,-9.71,;5.05,-8.94,;6.38,-9.7,;7.71,-8.92,;7.71,-7.38,;6.76,-6.17,;6.48,-4.78,;5.13,-4.17,;3.67,-4.81,;3.86,-6.19,;5.39,-5.54,;5.65,-3.64,;5.2,-2.54,;9.06,-9.69,;10.39,-8.92,;11.8,-9.55,;12.82,-8.41,;14.36,-8.41,;15.13,-7.07,;14.35,-5.73,;12.81,-5.74,;12.05,-7.07,;10.54,-7.4,)| Show InChI InChI=1S/C23H23N5O/c29-23-20(22-25-17-7-3-4-8-18(17)26-22)21(15-5-1-2-6-16(15)27-23)24-19-13-28-11-9-14(19)10-12-28/h1-8,14,19H,9-13H2,(H,25,26)(H2,24,27,29)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 482 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 |
Bioorg Med Chem Lett 16: 3121-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.059
BindingDB Entry DOI: 10.7270/Q2765DXT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50181305
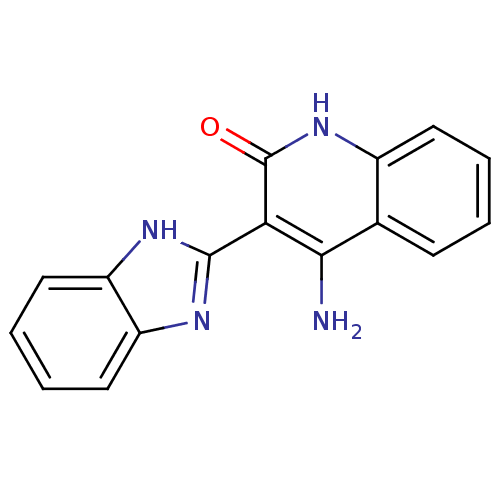
(4-amino-3-(1H-benzo[d]imidazol-2-yl)quinolin-2(1H)...)Show InChI InChI=1S/C16H12N4O/c17-14-9-5-1-2-6-10(9)20-16(21)13(14)15-18-11-7-3-4-8-12(11)19-15/h1-8H,(H,18,19)(H3,17,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 731 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 |
Bioorg Med Chem Lett 16: 3121-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.059
BindingDB Entry DOI: 10.7270/Q2765DXT |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50243389

(CHEMBL3185148)Show SMILES Nc1nc(NCCNc2ncc(c(n2)-c2ccc(Cl)cc2Cl)-n2ccnc2)ccc1[N+]([O-])=O Show InChI InChI=1S/C20H17Cl2N9O2/c21-12-1-2-13(14(22)9-12)18-16(30-8-7-24-11-30)10-27-20(29-18)26-6-5-25-17-4-3-15(31(32)33)19(23)28-17/h1-4,7-11H,5-6H2,(H3,23,25,28)(H,26,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human bFGF receptor tyrosine kinase using KDRY1175 [B91616] biotin-GGGGQDGKDYIVLPI-NH2 peptide substrate after 1 hr by scintillation co... |
J Med Chem 60: 8482-8514 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00922
BindingDB Entry DOI: 10.7270/Q25T3NW3 |
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50259894
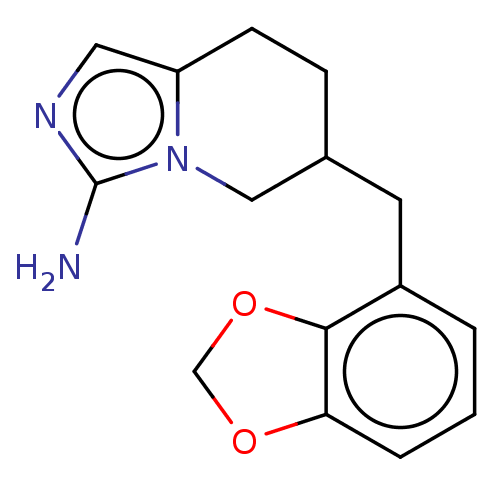
(CHEMBL4066475)Show InChI InChI=1S/C15H17N3O2/c16-15-17-7-12-5-4-10(8-18(12)15)6-11-2-1-3-13-14(11)20-9-19-13/h1-3,7,10H,4-6,8-9H2,(H2,16,17) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of EED in human G401 cells assessed as reduction in H3K27 methylation measured after 48 hrs by ELISA method |
J Med Chem 60: 415-427 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01473
BindingDB Entry DOI: 10.7270/Q2DF6TNJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50259899

(CHEMBL4088291)Show InChI InChI=1S/C18H24FN3O/c1-11(2)17-16-7-4-12(10-22(16)18(20)21-17)8-13-9-14(23-3)5-6-15(13)19/h5-6,9,11-12H,4,7-8,10H2,1-3H3,(H2,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Binding affinity to human methionine-13Cepsilon-methyl labeled PRC2-EED (76 to 441 residues) expressed in Escherichia coli BL21 (DE3) by 2D NMR metho... |
J Med Chem 60: 415-427 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01473
BindingDB Entry DOI: 10.7270/Q2DF6TNJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50259894

(CHEMBL4066475)Show InChI InChI=1S/C15H17N3O2/c16-15-17-7-12-5-4-10(8-18(12)15)6-11-2-1-3-13-14(11)20-9-19-13/h1-3,7,10H,4-6,8-9H2,(H2,16,17) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human PRC2-EED (76 to 441 residues) expressed in Escherichia coli BL21 (DE3) using histone H3[21 to 44, K27MeO] as substrate and SAM as... |
J Med Chem 60: 415-427 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01473
BindingDB Entry DOI: 10.7270/Q2DF6TNJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50259892

(CHEMBL4104157)Show InChI InChI=1S/C14H19N3O2/c15-14(16)17-6-2-3-10(8-17)7-11-4-1-5-12-13(11)19-9-18-12/h1,4-5,10H,2-3,6-9H2,(H3,15,16) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of EED in human G401 cells assessed as reduction in H3K27 methylation measured after 48 hrs by ELISA method |
J Med Chem 60: 415-427 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01473
BindingDB Entry DOI: 10.7270/Q2DF6TNJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50243389

(CHEMBL3185148)Show SMILES Nc1nc(NCCNc2ncc(c(n2)-c2ccc(Cl)cc2Cl)-n2ccnc2)ccc1[N+]([O-])=O Show InChI InChI=1S/C20H17Cl2N9O2/c21-12-1-2-13(14(22)9-12)18-16(30-8-7-24-11-30)10-27-20(29-18)26-6-5-25-17-4-3-15(31(32)33)19(23)28-17/h1-4,7-11H,5-6H2,(H3,23,25,28)(H,26,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human IGF1 receptor tyrosine kinase using biotin-GGGGKKKSPGEYVNIEFG-amide peptide substrate after 1 hr by scintillation counting method |
J Med Chem 60: 8482-8514 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00922
BindingDB Entry DOI: 10.7270/Q25T3NW3 |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50243389

(CHEMBL3185148)Show SMILES Nc1nc(NCCNc2ncc(c(n2)-c2ccc(Cl)cc2Cl)-n2ccnc2)ccc1[N+]([O-])=O Show InChI InChI=1S/C20H17Cl2N9O2/c21-12-1-2-13(14(22)9-12)18-16(30-8-7-24-11-30)10-27-20(29-18)26-6-5-25-17-4-3-15(31(32)33)19(23)28-17/h1-4,7-11H,5-6H2,(H3,23,25,28)(H,26,27,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human insulin receptor tyrosine kinase after 1 hr by scintillation counting method |
J Med Chem 60: 8482-8514 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00922
BindingDB Entry DOI: 10.7270/Q25T3NW3 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50243389

(CHEMBL3185148)Show SMILES Nc1nc(NCCNc2ncc(c(n2)-c2ccc(Cl)cc2Cl)-n2ccnc2)ccc1[N+]([O-])=O Show InChI InChI=1S/C20H17Cl2N9O2/c21-12-1-2-13(14(22)9-12)18-16(30-8-7-24-11-30)10-27-20(29-18)26-6-5-25-17-4-3-15(31(32)33)19(23)28-17/h1-4,7-11H,5-6H2,(H3,23,25,28)(H,26,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human KDR using KDRY1175 [B91616] biotin-GGGGQDGKDYIVLPI-NH2 peptide substrate after 1 hr by scintillation counting method |
J Med Chem 60: 8482-8514 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00922
BindingDB Entry DOI: 10.7270/Q25T3NW3 |
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50259907

(CHEMBL4101747)Show InChI InChI=1S/C18H23N3O2/c1-11(2)15-16-12(6-4-8-21(16)18(19)20-15)9-13-5-3-7-14-17(13)23-10-22-14/h3,5,7,11-12H,4,6,8-10H2,1-2H3,(H2,19,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of EED in human G401 cells assessed as reduction in H3K27 methylation measured after 48 hrs by ELISA method |
J Med Chem 60: 415-427 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01473
BindingDB Entry DOI: 10.7270/Q2DF6TNJ |
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50259891

(CHEMBL4096427)Show InChI InChI=1S/C15H19N3O/c16-15(17)18-7-2-3-11(10-18)9-12-4-1-5-14-13(12)6-8-19-14/h1,4-6,8,11H,2-3,7,9-10H2,(H3,16,17) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human PRC2-EED (76 to 441 residues) expressed in Escherichia coli BL21 (DE3) using histone H3[21 to 44, K27MeO] as substrate and SAM as... |
J Med Chem 60: 415-427 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01473
BindingDB Entry DOI: 10.7270/Q2DF6TNJ |
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50259891

(CHEMBL4096427)Show InChI InChI=1S/C15H19N3O/c16-15(17)18-7-2-3-11(10-18)9-12-4-1-5-14-13(12)6-8-19-14/h1,4-6,8,11H,2-3,7,9-10H2,(H3,16,17) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of EED in human G401 cells assessed as reduction in H3K27 methylation measured after 48 hrs by ELISA method |
J Med Chem 60: 415-427 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01473
BindingDB Entry DOI: 10.7270/Q2DF6TNJ |
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50259910
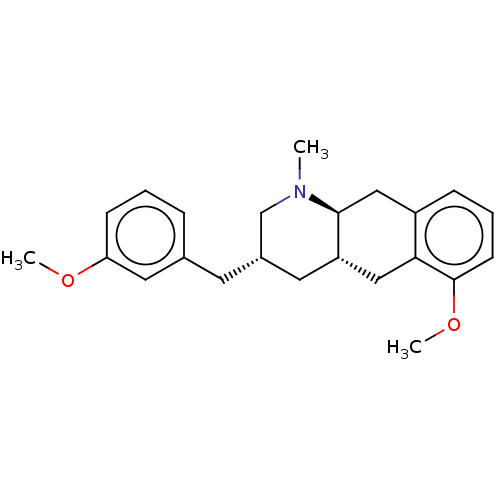
(CHEMBL4097892)Show SMILES [H][C@@]12C[C@H](Cc3cccc(OC)c3)CN(C)[C@@]1([H])Cc1cccc(OC)c1C2 |r| Show InChI InChI=1S/C23H29NO2/c1-24-15-17(10-16-6-4-8-20(12-16)25-2)11-19-13-21-18(14-22(19)24)7-5-9-23(21)26-3/h4-9,12,17,19,22H,10-11,13-15H2,1-3H3/t17-,19-,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Binding affinity to human methionine-13Cepsilon-methyl labeled PRC2-EED (76 to 441 residues) expressed in Escherichia coli BL21 (DE3) by 2D NMR metho... |
J Med Chem 60: 415-427 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01473
BindingDB Entry DOI: 10.7270/Q2DF6TNJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50259890

(CHEMBL4078383)Show InChI InChI=1S/C15H19N3O/c16-15(17)18-7-2-3-11(10-18)9-13-5-1-4-12-6-8-19-14(12)13/h1,4-6,8,11H,2-3,7,9-10H2,(H3,16,17) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of EED in human G401 cells assessed as reduction in H3K27 methylation measured after 48 hrs by ELISA method |
J Med Chem 60: 415-427 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01473
BindingDB Entry DOI: 10.7270/Q2DF6TNJ |
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50259893

(CHEMBL4075055)Show InChI InChI=1S/C16H17N3O/c17-16-18-9-13-5-4-11(10-19(13)16)8-12-2-1-3-15-14(12)6-7-20-15/h1-3,6-7,9,11H,4-5,8,10H2,(H2,17,18) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Binding affinity to human methionine-13Cepsilon-methyl labeled PRC2-EED (76 to 441 residues) expressed in Escherichia coli BL21 (DE3) by 2D NMR metho... |
J Med Chem 60: 415-427 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01473
BindingDB Entry DOI: 10.7270/Q2DF6TNJ |
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50259890

(CHEMBL4078383)Show InChI InChI=1S/C15H19N3O/c16-15(17)18-7-2-3-11(10-18)9-13-5-1-4-12-6-8-19-14(12)13/h1,4-6,8,11H,2-3,7,9-10H2,(H3,16,17) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Binding affinity to human methionine-13Cepsilon-methyl labeled PRC2-EED (76 to 441 residues) expressed in Escherichia coli BL21 (DE3) by 2D NMR metho... |
J Med Chem 60: 415-427 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01473
BindingDB Entry DOI: 10.7270/Q2DF6TNJ |
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50259899

(CHEMBL4088291)Show InChI InChI=1S/C18H24FN3O/c1-11(2)17-16-7-4-12(10-22(16)18(20)21-17)8-13-9-14(23-3)5-6-15(13)19/h5-6,9,11-12H,4,7-8,10H2,1-3H3,(H2,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of EED in human G401 cells assessed as reduction in H3K27 methylation measured after 48 hrs by ELISA method |
J Med Chem 60: 415-427 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01473
BindingDB Entry DOI: 10.7270/Q2DF6TNJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50181317
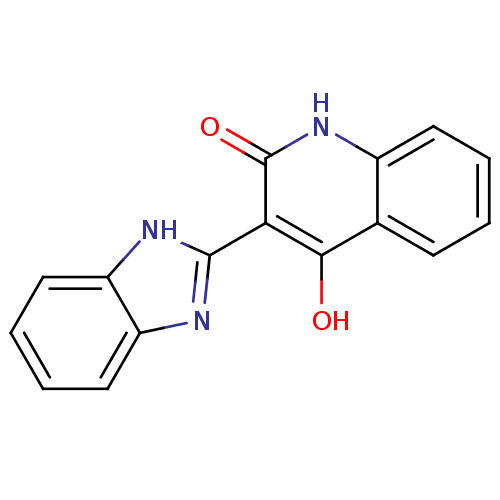
(3-(1H-benzo[d]imidazol-2-yl)-4-hydroxyquinolin-2(1...)Show InChI InChI=1S/C16H11N3O2/c20-14-9-5-1-2-6-10(9)19-16(21)13(14)15-17-11-7-3-4-8-12(11)18-15/h1-8H,(H,17,18)(H2,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 |
Bioorg Med Chem Lett 16: 3121-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.059
BindingDB Entry DOI: 10.7270/Q2765DXT |
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50259892

(CHEMBL4104157)Show InChI InChI=1S/C14H19N3O2/c15-14(16)17-6-2-3-10(8-17)7-11-4-1-5-12-13(11)19-9-18-12/h1,4-5,10H,2-3,6-9H2,(H3,15,16) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human PRC2-EED (76 to 441 residues) expressed in Escherichia coli BL21 (DE3) using histone H3[21 to 44, K27MeO] as substrate and SAM as... |
J Med Chem 60: 415-427 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01473
BindingDB Entry DOI: 10.7270/Q2DF6TNJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50243389

(CHEMBL3185148)Show SMILES Nc1nc(NCCNc2ncc(c(n2)-c2ccc(Cl)cc2Cl)-n2ccnc2)ccc1[N+]([O-])=O Show InChI InChI=1S/C20H17Cl2N9O2/c21-12-1-2-13(14(22)9-12)18-16(30-8-7-24-11-30)10-27-20(29-18)26-6-5-25-17-4-3-15(31(32)33)19(23)28-17/h1-4,7-11H,5-6H2,(H3,23,25,28)(H,26,27,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human CDC2 using biotin histone H1 peptide substrate after 1 hr by scintillation counting method |
J Med Chem 60: 8482-8514 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00922
BindingDB Entry DOI: 10.7270/Q25T3NW3 |
More data for this
Ligand-Target Pair | |
Casein kinase I isoform epsilon
(Homo sapiens (Human)) | BDBM50243389

(CHEMBL3185148)Show SMILES Nc1nc(NCCNc2ncc(c(n2)-c2ccc(Cl)cc2Cl)-n2ccnc2)ccc1[N+]([O-])=O Show InChI InChI=1S/C20H17Cl2N9O2/c21-12-1-2-13(14(22)9-12)18-16(30-8-7-24-11-30)10-27-20(29-18)26-6-5-25-17-4-3-15(31(32)33)19(23)28-17/h1-4,7-11H,5-6H2,(H3,23,25,28)(H,26,27,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human CK1epsilon using biotin peptide substrate after 1 hr by scintillation counting method |
J Med Chem 60: 8482-8514 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00922
BindingDB Entry DOI: 10.7270/Q25T3NW3 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50313013

(CHEMBL1080901 | CT-98024 | N2-(2-(4-(2,4-dichlorop...)Show SMILES Nc1nc(NCCNc2ncc(-c3ncc[nH]3)c(n2)-c2ccc(Cl)cc2Cl)ccc1[N+]([O-])=O Show InChI InChI=1S/C20H17Cl2N9O2/c21-11-1-2-12(14(22)9-11)17-13(19-25-6-7-26-19)10-28-20(30-17)27-8-5-24-16-4-3-15(31(32)33)18(23)29-16/h1-4,6-7,9-10H,5,8H2,(H,25,26)(H3,23,24,29)(H,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human bFGF receptor tyrosine kinase using KDRY1175 [B91616] biotin-GGGGQDGKDYIVLPI-NH2 peptide substrate after 1 hr by scintillation co... |
J Med Chem 60: 8482-8514 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00922
BindingDB Entry DOI: 10.7270/Q25T3NW3 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50243389

(CHEMBL3185148)Show SMILES Nc1nc(NCCNc2ncc(c(n2)-c2ccc(Cl)cc2Cl)-n2ccnc2)ccc1[N+]([O-])=O Show InChI InChI=1S/C20H17Cl2N9O2/c21-12-1-2-13(14(22)9-12)18-16(30-8-7-24-11-30)10-27-20(29-18)26-6-5-25-17-4-3-15(31(32)33)19(23)28-17/h1-4,7-11H,5-6H2,(H3,23,25,28)(H,26,27,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human AKT1/PKB using phospho-AKT peptide substrate after 1 hr by scintillation counting method |
J Med Chem 60: 8482-8514 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00922
BindingDB Entry DOI: 10.7270/Q25T3NW3 |
More data for this
Ligand-Target Pair | |
Angiopoietin-1 receptor
(Homo sapiens (Human)) | BDBM50325983

(6-(2-(4-(2,4-dichlorophenyl)-5-(4-methyl-1H-imidaz...)Show SMILES Cc1c[nH]c(n1)-c1cnc(NCCNc2ccc(cn2)C#N)nc1-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C22H18Cl2N8/c1-13-10-29-21(31-13)17-12-30-22(32-20(17)16-4-3-15(23)8-18(16)24)27-7-6-26-19-5-2-14(9-25)11-28-19/h2-5,8,10-12H,6-7H2,1H3,(H,26,28)(H,29,31)(H,27,30,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human Tie2 using biotin-GGGGAPDLYKDFLT peptide substrate after 1 hr by scintillation counting method |
J Med Chem 60: 8482-8514 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00922
BindingDB Entry DOI: 10.7270/Q25T3NW3 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 1
(Homo sapiens (Human)) | BDBM50325983

(6-(2-(4-(2,4-dichlorophenyl)-5-(4-methyl-1H-imidaz...)Show SMILES Cc1c[nH]c(n1)-c1cnc(NCCNc2ccc(cn2)C#N)nc1-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C22H18Cl2N8/c1-13-10-29-21(31-13)17-12-30-22(32-20(17)16-4-3-15(23)8-18(16)24)27-7-6-26-19-5-2-14(9-25)11-28-19/h2-5,8,10-12H,6-7H2,1H3,(H,26,28)(H,29,31)(H,27,30,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human FLT1 using KDRY1175 [B91616] biotin-GGGGQDGKDYIVLPI-NH2 peptide substrate after 1 hr by scintillation counting method |
J Med Chem 60: 8482-8514 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00922
BindingDB Entry DOI: 10.7270/Q25T3NW3 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50325983

(6-(2-(4-(2,4-dichlorophenyl)-5-(4-methyl-1H-imidaz...)Show SMILES Cc1c[nH]c(n1)-c1cnc(NCCNc2ccc(cn2)C#N)nc1-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C22H18Cl2N8/c1-13-10-29-21(31-13)17-12-30-22(32-20(17)16-4-3-15(23)8-18(16)24)27-7-6-26-19-5-2-14(9-25)11-28-19/h2-5,8,10-12H,6-7H2,1H3,(H,26,28)(H,29,31)(H,27,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human KDR using KDRY1175 [B91616] biotin-GGGGQDGKDYIVLPI-NH2 peptide substrate after 1 hr by scintillation counting method |
J Med Chem 60: 8482-8514 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00922
BindingDB Entry DOI: 10.7270/Q25T3NW3 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50325983

(6-(2-(4-(2,4-dichlorophenyl)-5-(4-methyl-1H-imidaz...)Show SMILES Cc1c[nH]c(n1)-c1cnc(NCCNc2ccc(cn2)C#N)nc1-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C22H18Cl2N8/c1-13-10-29-21(31-13)17-12-30-22(32-20(17)16-4-3-15(23)8-18(16)24)27-7-6-26-19-5-2-14(9-25)11-28-19/h2-5,8,10-12H,6-7H2,1H3,(H,26,28)(H,29,31)(H,27,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human bFGF receptor tyrosine kinase using KDRY1175 [B91616] biotin-GGGGQDGKDYIVLPI-NH2 peptide substrate after 1 hr by scintillation co... |
J Med Chem 60: 8482-8514 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00922
BindingDB Entry DOI: 10.7270/Q25T3NW3 |
More data for this
Ligand-Target Pair | |
Casein kinase I isoform epsilon
(Homo sapiens (Human)) | BDBM50325983

(6-(2-(4-(2,4-dichlorophenyl)-5-(4-methyl-1H-imidaz...)Show SMILES Cc1c[nH]c(n1)-c1cnc(NCCNc2ccc(cn2)C#N)nc1-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C22H18Cl2N8/c1-13-10-29-21(31-13)17-12-30-22(32-20(17)16-4-3-15(23)8-18(16)24)27-7-6-26-19-5-2-14(9-25)11-28-19/h2-5,8,10-12H,6-7H2,1H3,(H,26,28)(H,29,31)(H,27,30,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human CK1epsilon using biotin peptide substrate after 1 hr by scintillation counting method |
J Med Chem 60: 8482-8514 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00922
BindingDB Entry DOI: 10.7270/Q25T3NW3 |
More data for this
Ligand-Target Pair | |
Angiopoietin-1 receptor
(Homo sapiens (Human)) | BDBM50243389

(CHEMBL3185148)Show SMILES Nc1nc(NCCNc2ncc(c(n2)-c2ccc(Cl)cc2Cl)-n2ccnc2)ccc1[N+]([O-])=O Show InChI InChI=1S/C20H17Cl2N9O2/c21-12-1-2-13(14(22)9-12)18-16(30-8-7-24-11-30)10-27-20(29-18)26-6-5-25-17-4-3-15(31(32)33)19(23)28-17/h1-4,7-11H,5-6H2,(H3,23,25,28)(H,26,27,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human Tie2 using biotin-GGGGAPDLYKDFLT peptide substrate after 1 hr by scintillation counting method |
J Med Chem 60: 8482-8514 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00922
BindingDB Entry DOI: 10.7270/Q25T3NW3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data