 Found 22 hits with Last Name = 'caaveiro' and Initial = 'jm'
Found 22 hits with Last Name = 'caaveiro' and Initial = 'jm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50591389
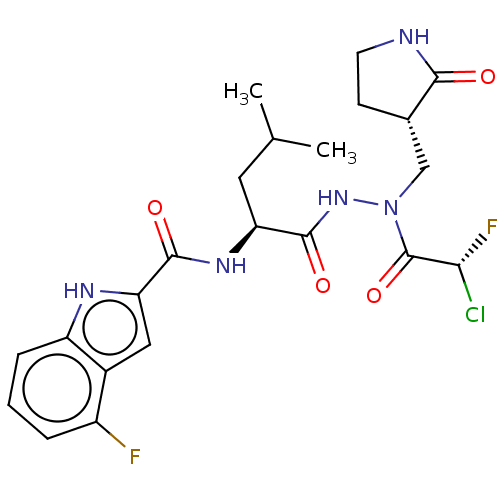
(CHEMBL5179778)Show SMILES CC(C)C[C@H](NC(=O)c1cc2c(F)cccc2[nH]1)C(=O)NN(C[C@@H]1CCNC1=O)C(=O)[C@H](F)Cl |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01081
BindingDB Entry DOI: 10.7270/Q2NK3K0J |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50591390
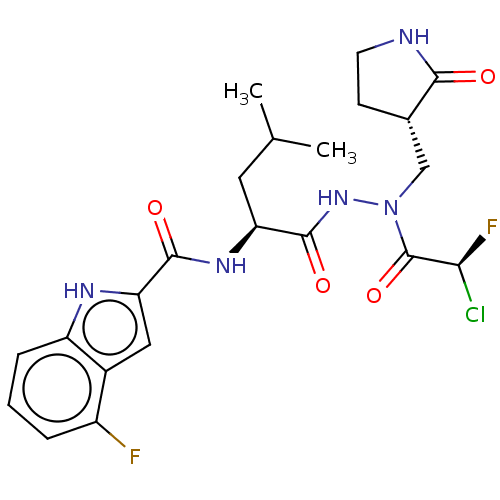
(CHEMBL5190754)Show SMILES CC(C)C[C@H](NC(=O)c1cc2c(F)cccc2[nH]1)C(=O)NN(C[C@@H]1CCNC1=O)C(=O)[C@@H](F)Cl |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 224 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01081
BindingDB Entry DOI: 10.7270/Q2NK3K0J |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM496902

(CVD-0018409 | PF-07321332 | US11351149, Example 13...)Show SMILES CC(C)(C)[C@H](NC(=O)C(F)(F)F)C(=O)N1C[C@H]2[C@@H]([C@H]1C(=O)N[C@@H](C[C@@H]1CCNC1=O)C#N)C2(C)C Show InChI InChI=1S/C23H32F3N5O4/c1-21(2,3)16(30-20(35)23(24,25)26)19(34)31-10-13-14(22(13,4)5)15(31)18(33)29-12(9-27)8-11-6-7-28-17(11)32/h11-16H,6-8,10H2,1-5H3,(H,28,32)(H,29,33)(H,30,35)/t11-,12-,13-,14-,15-,16+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01081
BindingDB Entry DOI: 10.7270/Q2NK3K0J |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM420298

(CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...)Show SMILES COc1cccc2[nH]c(cc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C(=O)CO Show InChI InChI=1S/C24H32N4O6/c1-13(2)9-18(23(32)27-17(20(30)12-29)10-14-7-8-25-22(14)31)28-24(33)19-11-15-16(26-19)5-4-6-21(15)34-3/h4-6,11,13-14,17-18,26,29H,7-10,12H2,1-3H3,(H,25,31)(H,27,32)(H,28,33)/t14-,17-,18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01081
BindingDB Entry DOI: 10.7270/Q2NK3K0J |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50591389

(CHEMBL5179778)Show SMILES CC(C)C[C@H](NC(=O)c1cc2c(F)cccc2[nH]1)C(=O)NN(C[C@@H]1CCNC1=O)C(=O)[C@H](F)Cl |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01081
BindingDB Entry DOI: 10.7270/Q2NK3K0J |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50591391
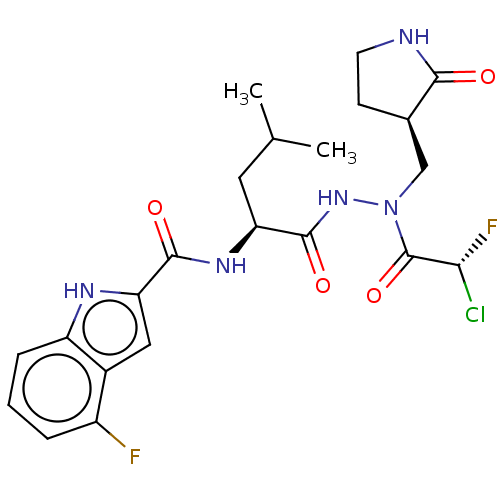
(CHEMBL5179611)Show SMILES CC(C)C[C@H](NC(=O)c1cc2c(F)cccc2[nH]1)C(=O)NN(C[C@H]1CCNC1=O)C(=O)[C@H](F)Cl |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01081
BindingDB Entry DOI: 10.7270/Q2NK3K0J |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50591388
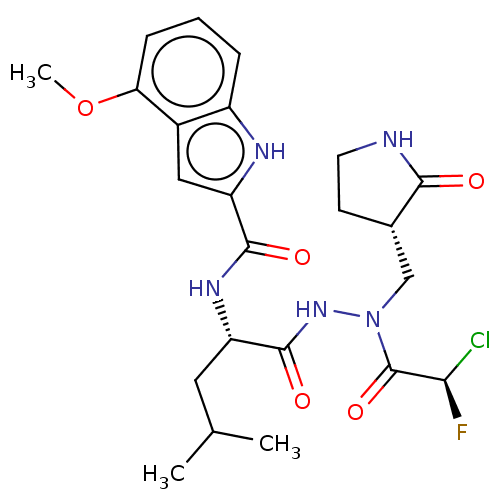
(CHEMBL5198073)Show SMILES COc1cccc2[nH]c(cc12)C(=O)N[C@@H](CC(C)C)C(=O)NN(C[C@@H]1CCNC1=O)C(=O)[C@H](F)Cl |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01081
BindingDB Entry DOI: 10.7270/Q2NK3K0J |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50591392
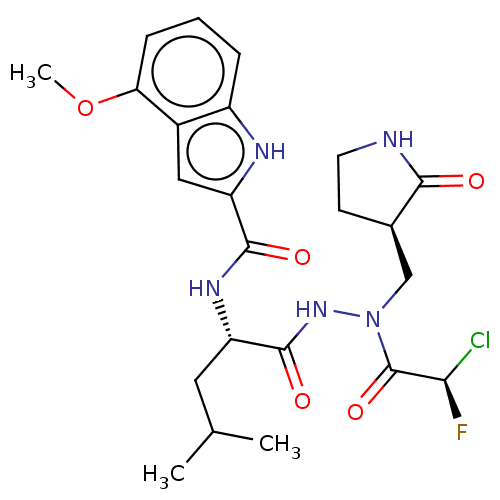
(CHEMBL5195394)Show SMILES COc1cccc2[nH]c(cc12)C(=O)N[C@@H](CC(C)C)C(=O)NN(C[C@H]1CCNC1=O)C(=O)[C@H](F)Cl |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01081
BindingDB Entry DOI: 10.7270/Q2NK3K0J |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50591390

(CHEMBL5190754)Show SMILES CC(C)C[C@H](NC(=O)c1cc2c(F)cccc2[nH]1)C(=O)NN(C[C@@H]1CCNC1=O)C(=O)[C@@H](F)Cl |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01081
BindingDB Entry DOI: 10.7270/Q2NK3K0J |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50591394

(CHEMBL5172924)Show SMILES CC(C)C[C@H](NC(=O)c1cc2c(F)cccc2[nH]1)C(=O)NN(C[C@@H]1CCNC1=O)C(C)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 332 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01081
BindingDB Entry DOI: 10.7270/Q2NK3K0J |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50591393
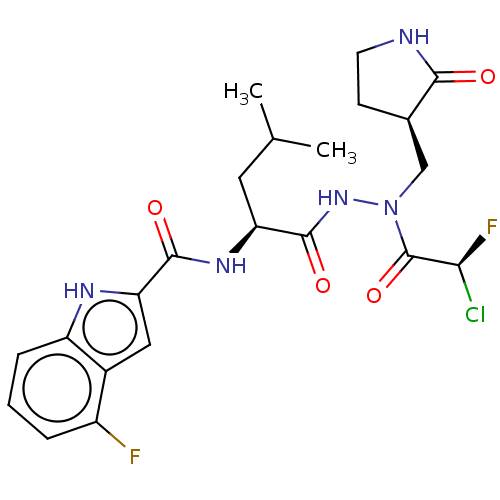
(CHEMBL5197241)Show SMILES CC(C)C[C@H](NC(=O)c1cc2c(F)cccc2[nH]1)C(=O)NN(C[C@H]1CCNC1=O)C(=O)[C@@H](F)Cl |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 464 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01081
BindingDB Entry DOI: 10.7270/Q2NK3K0J |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50591389

(CHEMBL5179778)Show SMILES CC(C)C[C@H](NC(=O)c1cc2c(F)cccc2[nH]1)C(=O)NN(C[C@@H]1CCNC1=O)C(=O)[C@H](F)Cl |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01081
BindingDB Entry DOI: 10.7270/Q2NK3K0J |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50591389

(CHEMBL5179778)Show SMILES CC(C)C[C@H](NC(=O)c1cc2c(F)cccc2[nH]1)C(=O)NN(C[C@@H]1CCNC1=O)C(=O)[C@H](F)Cl |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01081
BindingDB Entry DOI: 10.7270/Q2NK3K0J |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50591389

(CHEMBL5179778)Show SMILES CC(C)C[C@H](NC(=O)c1cc2c(F)cccc2[nH]1)C(=O)NN(C[C@@H]1CCNC1=O)C(=O)[C@H](F)Cl |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01081
BindingDB Entry DOI: 10.7270/Q2NK3K0J |
More data for this
Ligand-Target Pair | |
Steroid Delta-isomerase
(Pseudomonas putida) | BDBM50292538
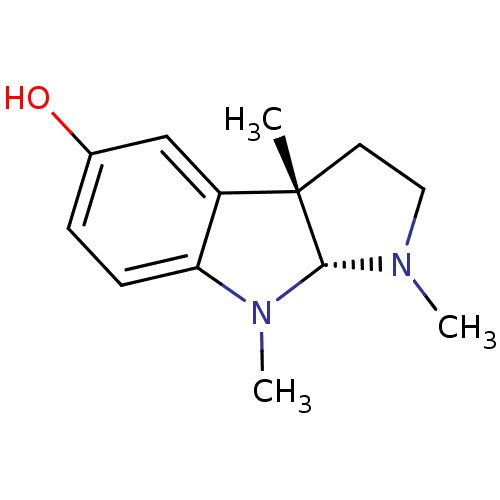
((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show InChI InChI=1S/C13H18N2O/c1-13-6-7-14(2)12(13)15(3)11-5-4-9(16)8-10(11)13/h4-5,8,12,16H,6-7H2,1-3H3/t12-,13+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.80E+5 | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Binding affinity to Pseudomonas putida KSI by isothermal titration calorimetry |
J Med Chem 56: 2155-9 (2013)
Article DOI: 10.1021/jm301603n
BindingDB Entry DOI: 10.7270/Q2PR7X98 |
More data for this
Ligand-Target Pair | |
Steroid Delta-isomerase
(Pseudomonas putida) | BDBM50427552
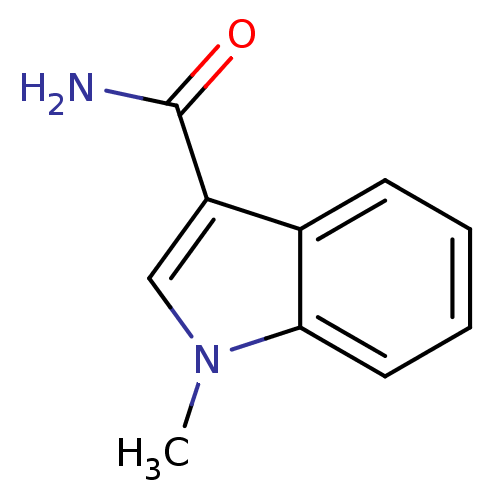
(CHEMBL382602)Show InChI InChI=1S/C10H10N2O/c1-12-6-8(10(11)13)7-4-2-3-5-9(7)12/h2-6H,1H3,(H2,11,13) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 6.30E+4 | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Binding affinity to Pseudomonas putida KSI by isothermal titration calorimetry |
J Med Chem 56: 2155-9 (2013)
Article DOI: 10.1021/jm301603n
BindingDB Entry DOI: 10.7270/Q2PR7X98 |
More data for this
Ligand-Target Pair | |
Steroid Delta-isomerase
(Pseudomonas putida) | BDBM50427551
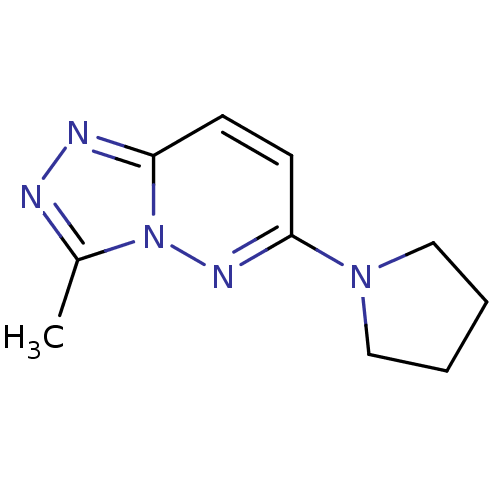
(CHEMBL1499599)Show InChI InChI=1S/C10H13N5/c1-8-11-12-9-4-5-10(13-15(8)9)14-6-2-3-7-14/h4-5H,2-3,6-7H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Binding affinity to Pseudomonas putida KSI by isothermal titration calorimetry |
J Med Chem 56: 2155-9 (2013)
Article DOI: 10.1021/jm301603n
BindingDB Entry DOI: 10.7270/Q2PR7X98 |
More data for this
Ligand-Target Pair | |
Steroid Delta-isomerase
(Pseudomonas putida) | BDBM50375599
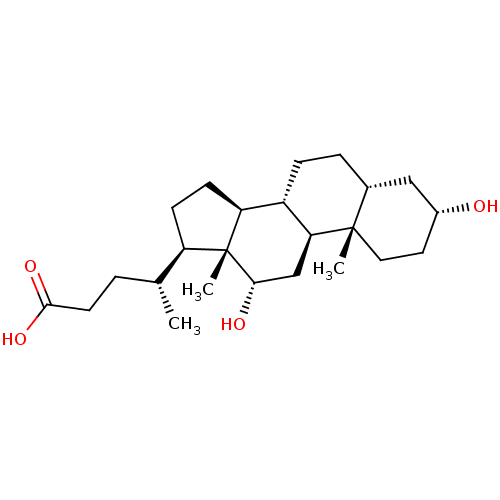
(DEOXYCHOLATE | Deoxycholic Acid | KYBELLA)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C Show InChI InChI=1S/C24H40O4/c1-14(4-9-22(27)28)18-7-8-19-17-6-5-15-12-16(25)10-11-23(15,2)20(17)13-21(26)24(18,19)3/h14-21,25-26H,4-13H2,1-3H3,(H,27,28)/t14-,15-,16-,17+,18-,19+,20+,21+,23+,24-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| n/a | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Binding affinity to Pseudomonas putida KSI by surface plasmon resonance analysis |
J Med Chem 56: 2155-9 (2013)
Article DOI: 10.1021/jm301603n
BindingDB Entry DOI: 10.7270/Q2PR7X98 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50229978
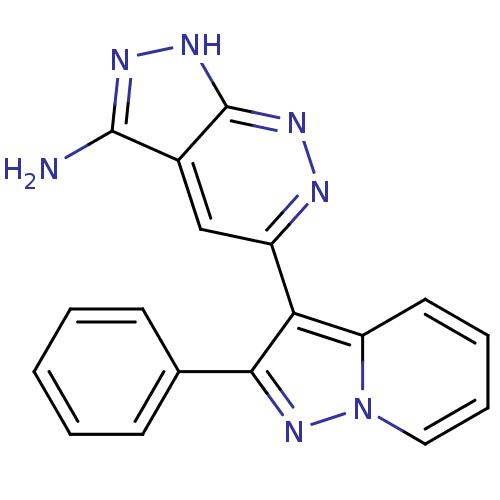
(5-(2-PHENYLPYRAZOLO[1,5-A]PYRIDIN-3-YL)-1H-PYRAZOL...)Show SMILES Nc1n[nH]c2nnc(cc12)-c1c(nn2ccccc12)-c1ccccc1 Show InChI InChI=1S/C18H13N7/c19-17-12-10-13(20-22-18(12)23-21-17)15-14-8-4-5-9-25(14)24-16(15)11-6-2-1-3-7-11/h1-10H,(H3,19,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Binding affinity to ERK2 (unknown origin) by isothermal titration calorimetry |
J Med Chem 56: 2155-9 (2013)
Article DOI: 10.1021/jm301603n
BindingDB Entry DOI: 10.7270/Q2PR7X98 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Steroid Delta-isomerase
(Pseudomonas putida) | BDBM50427553
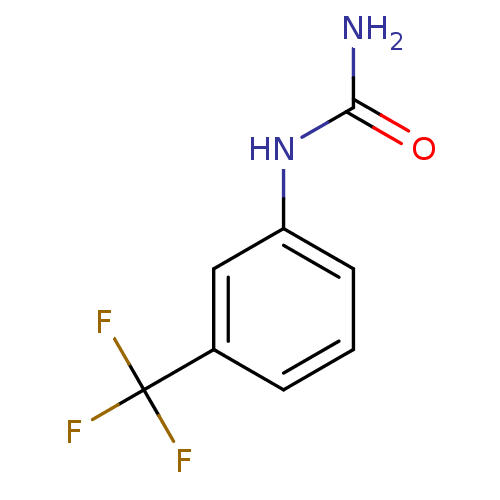
(CHEMBL1338939)Show InChI InChI=1S/C8H7F3N2O/c9-8(10,11)5-2-1-3-6(4-5)13-7(12)14/h1-4H,(H3,12,13,14) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 4.60E+4 | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Binding affinity to Pseudomonas putida KSI by isothermal titration calorimetry |
J Med Chem 56: 2155-9 (2013)
Article DOI: 10.1021/jm301603n
BindingDB Entry DOI: 10.7270/Q2PR7X98 |
More data for this
Ligand-Target Pair | |
Steroid Delta-isomerase
(Pseudomonas putida) | BDBM50427554
(3-(3-(Trifluoromethyl)Phenyl)Acrylamide | CHEMBL10...)Show InChI InChI=1S/C10H8F3NO/c11-10(12,13)8-3-1-2-7(6-8)4-5-9(14)15/h1-6H,(H2,14,15)/b5-4+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Binding affinity to Pseudomonas putida KSI by isothermal titration calorimetry |
J Med Chem 56: 2155-9 (2013)
Article DOI: 10.1021/jm301603n
BindingDB Entry DOI: 10.7270/Q2PR7X98 |
More data for this
Ligand-Target Pair | |
Steroid Delta-isomerase
(Pseudomonas putida) | BDBM50375599

(DEOXYCHOLATE | Deoxycholic Acid | KYBELLA)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C Show InChI InChI=1S/C24H40O4/c1-14(4-9-22(27)28)18-7-8-19-17-6-5-15-12-16(25)10-11-23(15,2)20(17)13-21(26)24(18,19)3/h14-21,25-26H,4-13H2,1-3H3,(H,27,28)/t14-,15-,16-,17+,18-,19+,20+,21+,23+,24-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| n/a | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Binding affinity to Pseudomonas putida KSI by isothermal titration calorimetry |
J Med Chem 56: 2155-9 (2013)
Article DOI: 10.1021/jm301603n
BindingDB Entry DOI: 10.7270/Q2PR7X98 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data