

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50462179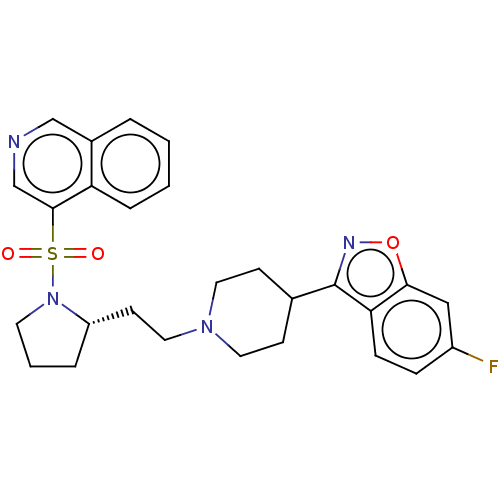 (CHEMBL4245263) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from human D2LR expressed in HEK293 cell membranes after 1 hr at 37 degC by microbeta counting method | Eur J Med Chem 145: 790-804 (2018) Article DOI: 10.1016/j.ejmech.2018.01.002 BindingDB Entry DOI: 10.7270/Q27947BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50462156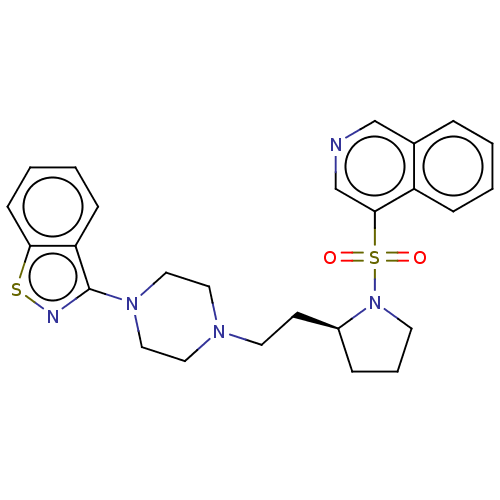 (CHEMBL4246655) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from human D2LR expressed in HEK293 cell membranes after 1 hr at 37 degC by microbeta counting method | Eur J Med Chem 145: 790-804 (2018) Article DOI: 10.1016/j.ejmech.2018.01.002 BindingDB Entry DOI: 10.7270/Q27947BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50291284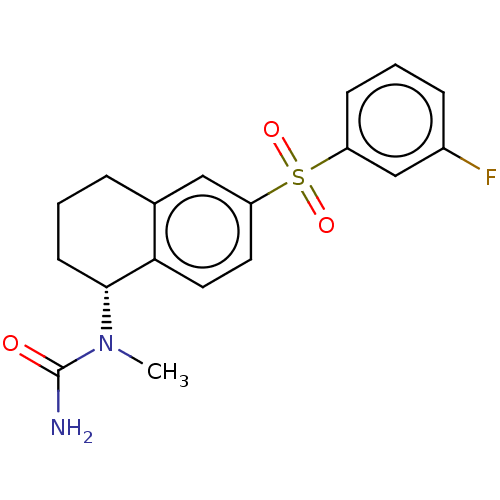 (CHEMBL4170220) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University Curated by ChEMBL | Assay Description Binding affinity to 5HT6R (unknown origin) | Eur J Med Chem 144: 716-729 (2018) Article DOI: 10.1016/j.ejmech.2017.12.053 BindingDB Entry DOI: 10.7270/Q2ZK5K6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM21367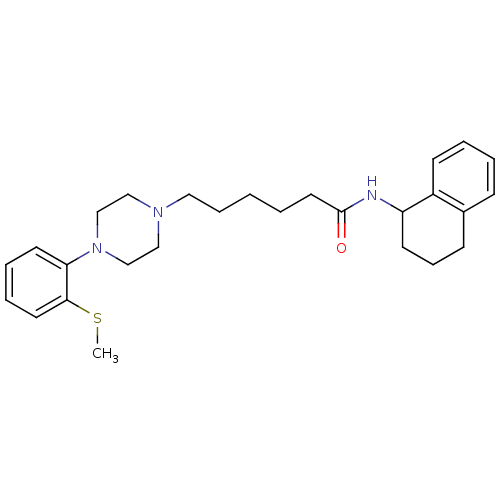 (6-{4-[2-(methylsulfanyl)phenyl]piperazin-1-yl}-N-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-5-CT from human 5-HT7 receptor expressed in HEK293 cells after 1 hr by beta-counting | Eur J Med Chem 78: 10-22 (2014) Article DOI: 10.1016/j.ejmech.2014.03.005 BindingDB Entry DOI: 10.7270/Q2QN6899 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM21367 (6-{4-[2-(methylsulfanyl)phenyl]piperazin-1-yl}-N-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-5-CT from human 5HT7 receptor expressed in HEK293 cells after 1 hr | Eur J Med Chem 92: 202-11 (2015) Article DOI: 10.1016/j.ejmech.2014.12.041 BindingDB Entry DOI: 10.7270/Q2TT4SNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (RAT) | BDBM50318633 (3-benzenesulfonyl-8-piperazin-1-ylquinoline | CHEM...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.234 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]Lu AE60157 from rat brain 5-HT6 receptor | ACS Med Chem Lett 7: 618-22 (2016) Article DOI: 10.1021/acsmedchemlett.6b00056 BindingDB Entry DOI: 10.7270/Q2ST7T9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (RAT) | BDBM50318633 (3-benzenesulfonyl-8-piperazin-1-ylquinoline | CHEM...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.234 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]Lu AE60157 from rat brain 5-HT6 receptor | ACS Med Chem Lett 7: 618-22 (2016) Article DOI: 10.1021/acsmedchemlett.6b00056 BindingDB Entry DOI: 10.7270/Q2ST7T9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50318633 (3-benzenesulfonyl-8-piperazin-1-ylquinoline | CHEM...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University Curated by ChEMBL | Assay Description Binding affinity to 5HT6R (unknown origin) | Eur J Med Chem 144: 716-729 (2018) Article DOI: 10.1016/j.ejmech.2017.12.053 BindingDB Entry DOI: 10.7270/Q2ZK5K6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50174269 (1-(phenylsulfonyl)-4-(piperazin-1-yl)-1H-indole | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6 receptor expressed in HEK293 cells measured after 1 hr by microbeta plate reader method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112765 BindingDB Entry DOI: 10.7270/Q2ZC86K7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50291286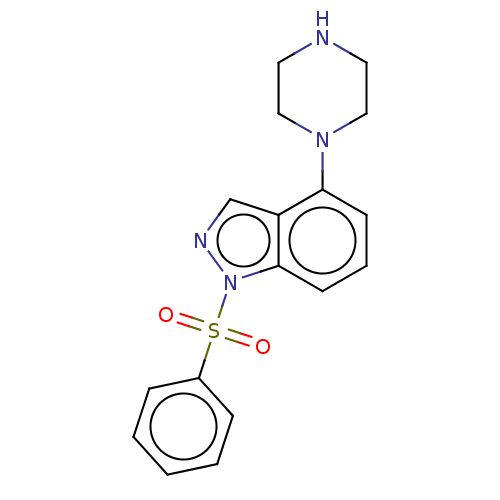 (CHEMBL4169827) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University Curated by ChEMBL | Assay Description Binding affinity to 5HT6R (unknown origin) | Eur J Med Chem 144: 716-729 (2018) Article DOI: 10.1016/j.ejmech.2017.12.053 BindingDB Entry DOI: 10.7270/Q2ZK5K6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50462155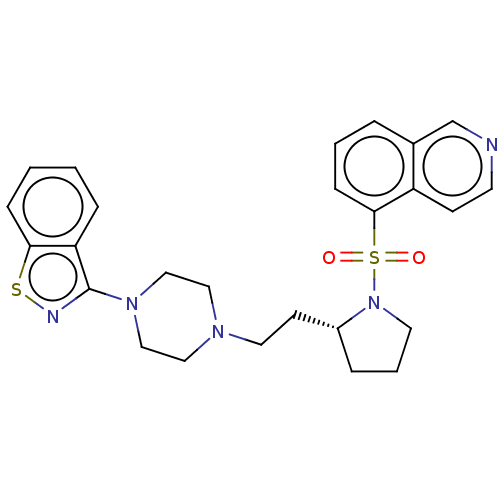 (CHEMBL4239091) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from human D2LR expressed in HEK293 cell membranes after 1 hr at 37 degC by microbeta counting method | Eur J Med Chem 145: 790-804 (2018) Article DOI: 10.1016/j.ejmech.2018.01.002 BindingDB Entry DOI: 10.7270/Q27947BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50001885 ((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-5-CT from human 5-HT7R expressed in HEK293 cell membranes after 1 hr at 37 degC by microbeta counting method | Eur J Med Chem 145: 790-804 (2018) Article DOI: 10.1016/j.ejmech.2018.01.002 BindingDB Entry DOI: 10.7270/Q27947BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50462179 (CHEMBL4245263) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-5-CT from human 5-HT7R expressed in HEK293 cell membranes after 1 hr at 37 degC by microbeta counting method | Eur J Med Chem 145: 790-804 (2018) Article DOI: 10.1016/j.ejmech.2018.01.002 BindingDB Entry DOI: 10.7270/Q27947BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50462148 (CHEMBL4242392) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from human D2LR expressed in HEK293 cell membranes after 1 hr at 37 degC by microbeta counting method | Eur J Med Chem 145: 790-804 (2018) Article DOI: 10.1016/j.ejmech.2018.01.002 BindingDB Entry DOI: 10.7270/Q27947BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50044615 (CHEMBL3329435) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to 5HT6R (unknown origin) | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02009 BindingDB Entry DOI: 10.7270/Q2JQ14RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50001885 ((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-ketanserin from human 5-HT2AR expressed in CHO-K1 cell membranes after 1.5 hrs by microbeta counting method | Eur J Med Chem 145: 790-804 (2018) Article DOI: 10.1016/j.ejmech.2018.01.002 BindingDB Entry DOI: 10.7270/Q27947BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50253281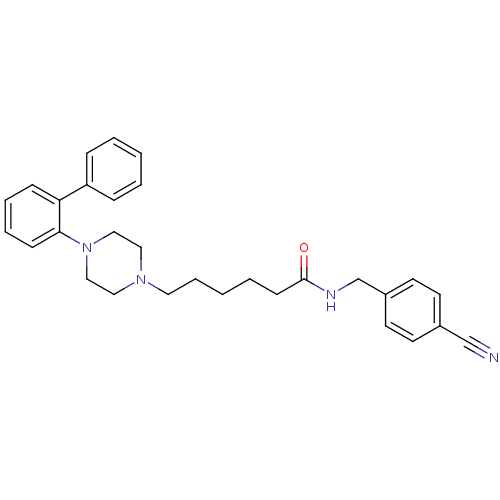 (CHEMBL522691 | N-(4-Cyanophenylmethyl)-4-(2-diphen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-5-CT from human 5-HT7 receptor expressed in HEK293 cells after 1 hr by beta-counting | Eur J Med Chem 78: 10-22 (2014) Article DOI: 10.1016/j.ejmech.2014.03.005 BindingDB Entry DOI: 10.7270/Q2QN6899 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50001885 ((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from human D2LR expressed in HEK293 cell membranes after 1 hr at 37 degC by microbeta counting method | Eur J Med Chem 145: 790-804 (2018) Article DOI: 10.1016/j.ejmech.2018.01.002 BindingDB Entry DOI: 10.7270/Q27947BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50462142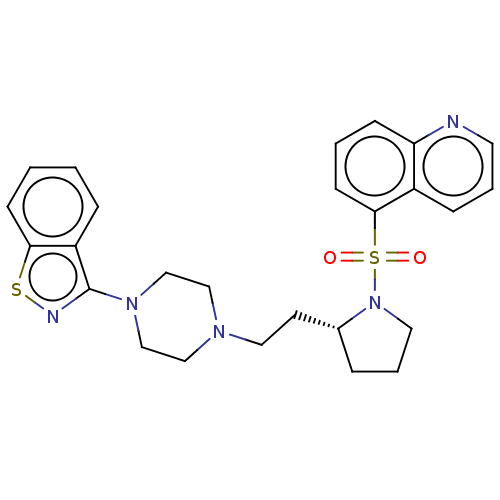 (CHEMBL4242858) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from human D2LR expressed in HEK293 cell membranes after 1 hr at 37 degC by microbeta counting method | Eur J Med Chem 145: 790-804 (2018) Article DOI: 10.1016/j.ejmech.2018.01.002 BindingDB Entry DOI: 10.7270/Q27947BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50048803 (5-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from human D2LR expressed in HEK293 cell membranes after 1 hr at 37 degC by microbeta counting method | Eur J Med Chem 145: 790-804 (2018) Article DOI: 10.1016/j.ejmech.2018.01.002 BindingDB Entry DOI: 10.7270/Q27947BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50130293 (7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from human D2LR expressed in HEK293 cell membranes after 1 hr at 37 degC by microbeta counting method | Eur J Med Chem 145: 790-804 (2018) Article DOI: 10.1016/j.ejmech.2018.01.002 BindingDB Entry DOI: 10.7270/Q27947BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (RAT) | BDBM50019754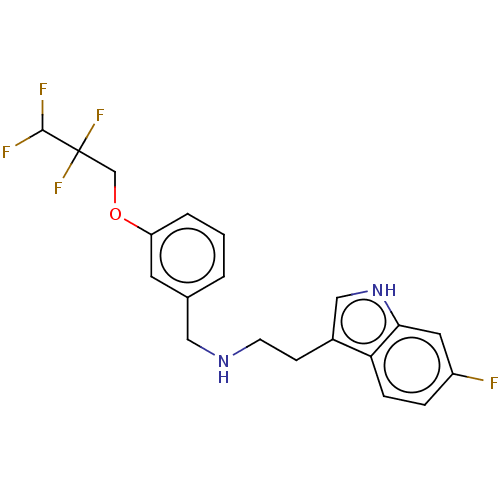 (IDALOPIRDINE | LU-AE58054) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]Lu AE60157 from rat brain 5-HT6 receptor | ACS Med Chem Lett 7: 618-22 (2016) Article DOI: 10.1021/acsmedchemlett.6b00056 BindingDB Entry DOI: 10.7270/Q2ST7T9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (RAT) | BDBM50019754 (IDALOPIRDINE | LU-AE58054) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]Lu AE60157 from rat brain 5-HT6 receptor | ACS Med Chem Lett 7: 618-22 (2016) Article DOI: 10.1021/acsmedchemlett.6b00056 BindingDB Entry DOI: 10.7270/Q2ST7T9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50019754 (IDALOPIRDINE | LU-AE58054) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University Curated by ChEMBL | Assay Description Binding affinity to 5HT6R (unknown origin) | Eur J Med Chem 144: 716-729 (2018) Article DOI: 10.1016/j.ejmech.2017.12.053 BindingDB Entry DOI: 10.7270/Q2ZK5K6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50462152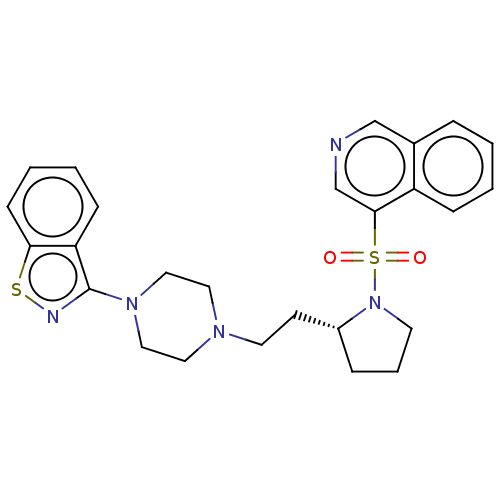 (CHEMBL4238679) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from human D2LR expressed in HEK293 cell membranes after 1 hr at 37 degC by microbeta counting method | Eur J Med Chem 145: 790-804 (2018) Article DOI: 10.1016/j.ejmech.2018.01.002 BindingDB Entry DOI: 10.7270/Q27947BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50174269 (1-(phenylsulfonyl)-4-(piperazin-1-yl)-1H-indole | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University Curated by ChEMBL | Assay Description Binding affinity to 5HT6R (unknown origin) | Eur J Med Chem 144: 716-729 (2018) Article DOI: 10.1016/j.ejmech.2017.12.053 BindingDB Entry DOI: 10.7270/Q2ZK5K6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50003915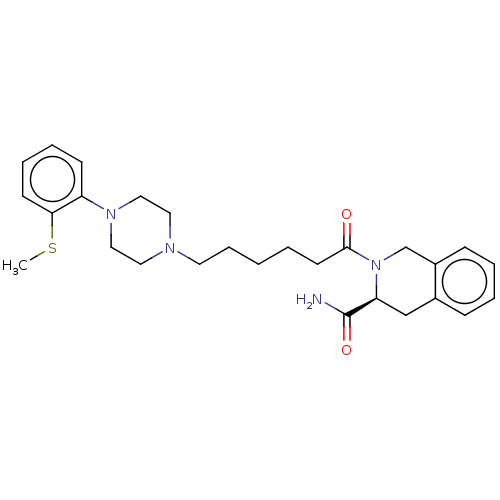 (CHEMBL3235744) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5HT1A receptor expressed in HEK293 cells after 1 hr | Eur J Med Chem 92: 202-11 (2015) Article DOI: 10.1016/j.ejmech.2014.12.041 BindingDB Entry DOI: 10.7270/Q2TT4SNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50562751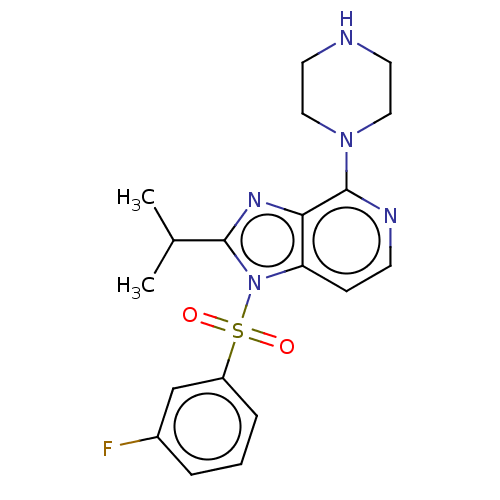 (CHEMBL4760545) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6R expressed in HEK293 cell membranes incubated for 1 hr by micro-beta plate reader based analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02009 BindingDB Entry DOI: 10.7270/Q2JQ14RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50562747 (CHEMBL4756814) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6R expressed in HEK293 cell membranes incubated for 1 hr by micro-beta plate reader based analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02009 BindingDB Entry DOI: 10.7270/Q2JQ14RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50562748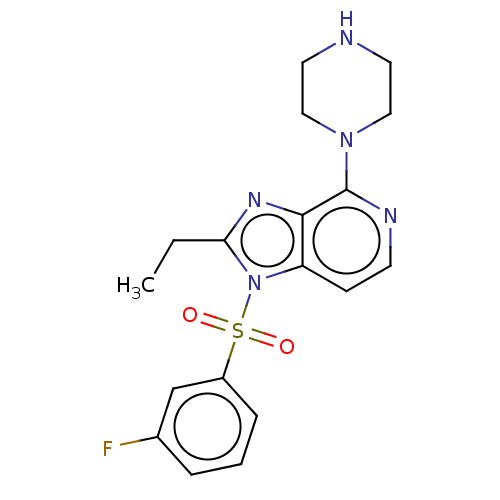 (CHEMBL4760105) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6R expressed in HEK293 cell membranes incubated for 1 hr by micro-beta plate reader based analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02009 BindingDB Entry DOI: 10.7270/Q2JQ14RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50143280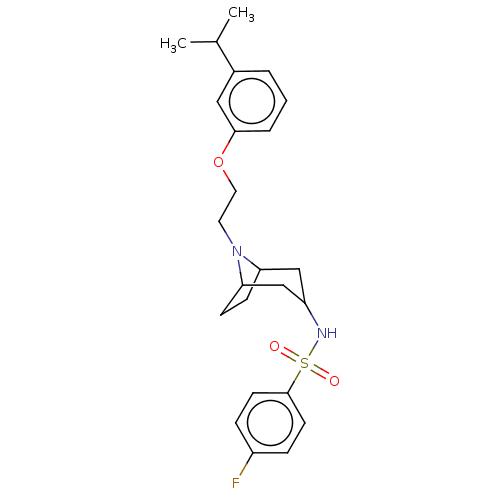 (CHEMBL3759205) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-5-CT from human 5-HT7B receptor expressed in HEK293 cells after 1 hr | Eur J Med Chem 108: 334-46 (2016) Article DOI: 10.1016/j.ejmech.2015.11.040 BindingDB Entry DOI: 10.7270/Q28S4RRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50462142 (CHEMBL4242858) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-5-CT from human 5-HT7R expressed in HEK293 cell membranes after 1 hr at 37 degC by microbeta counting method | Eur J Med Chem 145: 790-804 (2018) Article DOI: 10.1016/j.ejmech.2018.01.002 BindingDB Entry DOI: 10.7270/Q27947BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50462138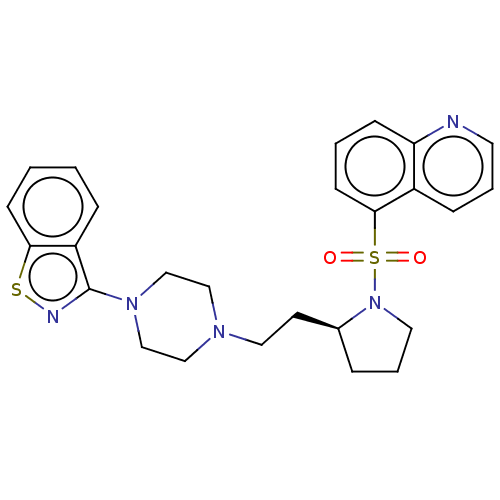 (CHEMBL4246202) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from human D2LR expressed in HEK293 cell membranes after 1 hr at 37 degC by microbeta counting method | Eur J Med Chem 145: 790-804 (2018) Article DOI: 10.1016/j.ejmech.2018.01.002 BindingDB Entry DOI: 10.7270/Q27947BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50070614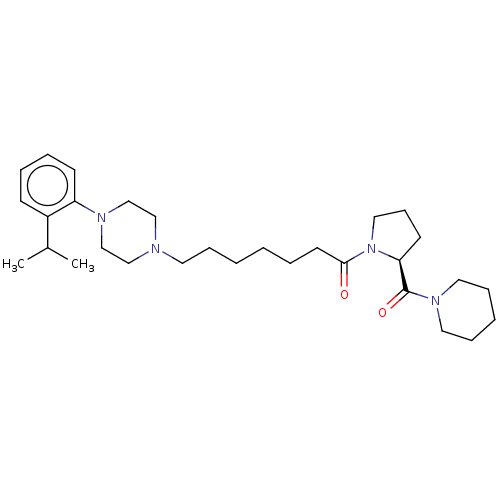 (CHEMBL3409037) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5HT1A receptor expressed in HEK293 cells after 1 hr | Eur J Med Chem 92: 202-11 (2015) Article DOI: 10.1016/j.ejmech.2014.12.041 BindingDB Entry DOI: 10.7270/Q2TT4SNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50462155 (CHEMBL4239091) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-5-CT from human 5-HT7R expressed in HEK293 cell membranes after 1 hr at 37 degC by microbeta counting method | Eur J Med Chem 145: 790-804 (2018) Article DOI: 10.1016/j.ejmech.2018.01.002 BindingDB Entry DOI: 10.7270/Q27947BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50003915 (CHEMBL3235744) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor expressed in HEK293 cells after 1 hr by beta-counting | Eur J Med Chem 78: 10-22 (2014) Article DOI: 10.1016/j.ejmech.2014.03.005 BindingDB Entry DOI: 10.7270/Q2QN6899 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50462168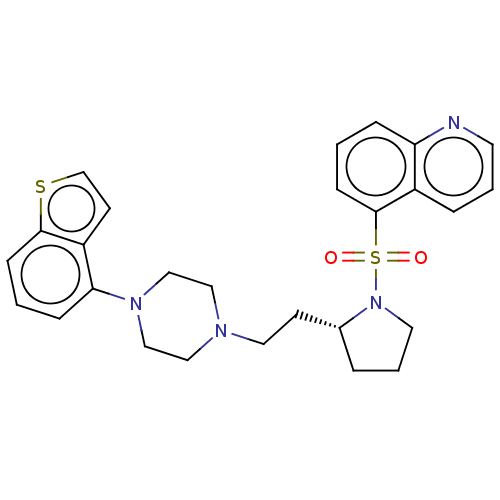 (CHEMBL4243848) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-5-CT from human 5-HT7R expressed in HEK293 cell membranes after 1 hr at 37 degC by microbeta counting method | Eur J Med Chem 145: 790-804 (2018) Article DOI: 10.1016/j.ejmech.2018.01.002 BindingDB Entry DOI: 10.7270/Q27947BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50318633 (3-benzenesulfonyl-8-piperazin-1-ylquinoline | CHEM...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University Curated by ChEMBL | Assay Description Displacement of [3H]-LSD from human 5HT6R expressed in HEK293 cell membranes after 1 hr by microbeta counting method | Eur J Med Chem 144: 716-729 (2018) Article DOI: 10.1016/j.ejmech.2017.12.053 BindingDB Entry DOI: 10.7270/Q2ZK5K6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM10989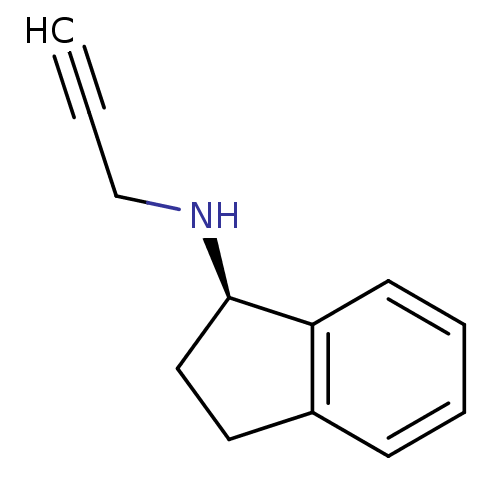 ((1R)-N-(prop-2-yn-1-yl)-2,3-dihydro-1H-inden-1-ami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6 receptor expressed in HEK293 cells measured after 1 hr by microbeta plate reader method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112765 BindingDB Entry DOI: 10.7270/Q2ZC86K7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50318633 (3-benzenesulfonyl-8-piperazin-1-ylquinoline | CHEM...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6R expressed in HEK293 cell membranes incubated for 1 hr by micro-beta plate reader based analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02009 BindingDB Entry DOI: 10.7270/Q2JQ14RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50048803 (5-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)et...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-ketanserin from human 5-HT2AR expressed in CHO-K1 cell membranes after 1.5 hrs by microbeta counting method | Eur J Med Chem 145: 790-804 (2018) Article DOI: 10.1016/j.ejmech.2018.01.002 BindingDB Entry DOI: 10.7270/Q27947BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50462169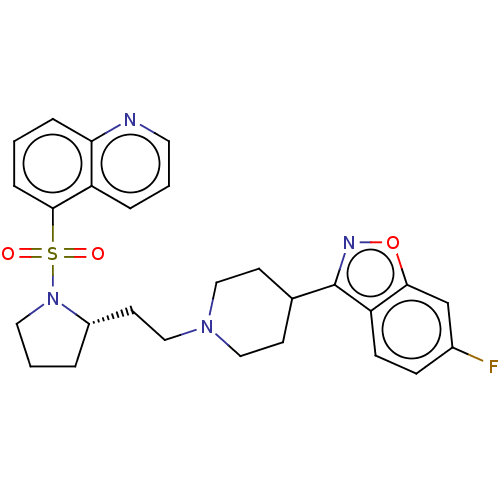 (CHEMBL4241050) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from human D2LR expressed in HEK293 cell membranes after 1 hr at 37 degC by microbeta counting method | Eur J Med Chem 145: 790-804 (2018) Article DOI: 10.1016/j.ejmech.2018.01.002 BindingDB Entry DOI: 10.7270/Q27947BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50532639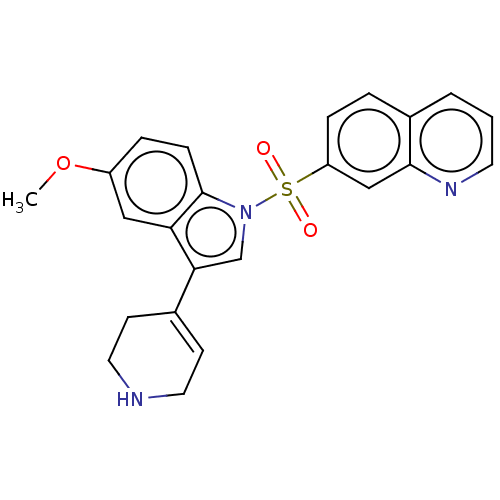 (CHEMBL4447846) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-LSD from human 5-HT6 receptor expressed in HEK293 cells by microbeta liquid scintillation counting | ACS Med Chem Lett 7: 618-22 (2016) Article DOI: 10.1021/acsmedchemlett.6b00056 BindingDB Entry DOI: 10.7270/Q2ST7T9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50532639 (CHEMBL4447846) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-LSD from human 5-HT6 receptor expressed in HEK293 cells by microbeta liquid scintillation counting | ACS Med Chem Lett 7: 618-22 (2016) Article DOI: 10.1021/acsmedchemlett.6b00056 BindingDB Entry DOI: 10.7270/Q2ST7T9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50562749 (CHEMBL4756098) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6R expressed in HEK293 cell membranes incubated for 1 hr by micro-beta plate reader based analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02009 BindingDB Entry DOI: 10.7270/Q2JQ14RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50143372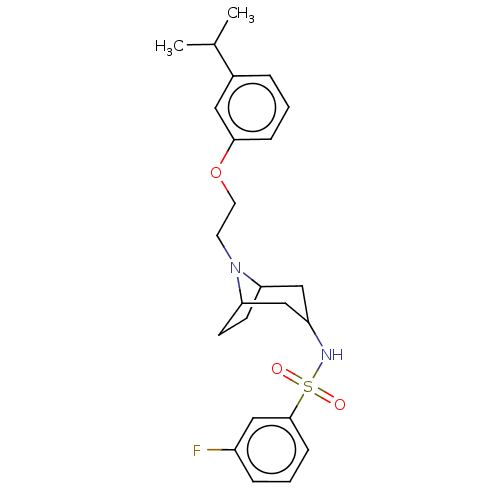 (CHEMBL3759617) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-5-CT from human 5-HT7B receptor expressed in HEK293 cells after 1 hr | Eur J Med Chem 108: 334-46 (2016) Article DOI: 10.1016/j.ejmech.2015.11.040 BindingDB Entry DOI: 10.7270/Q28S4RRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50562744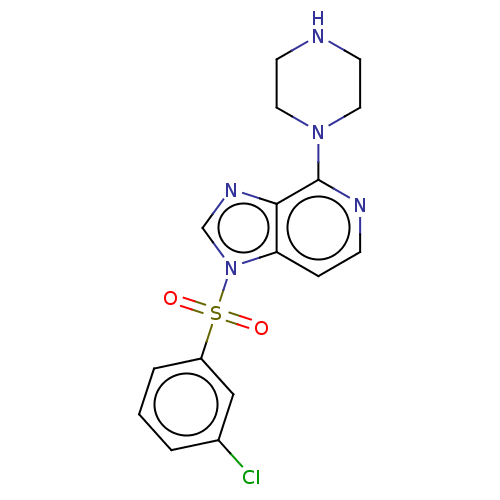 (CHEMBL4777550) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6R expressed in HEK293 cell membranes incubated for 1 hr by micro-beta plate reader based analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02009 BindingDB Entry DOI: 10.7270/Q2JQ14RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50003916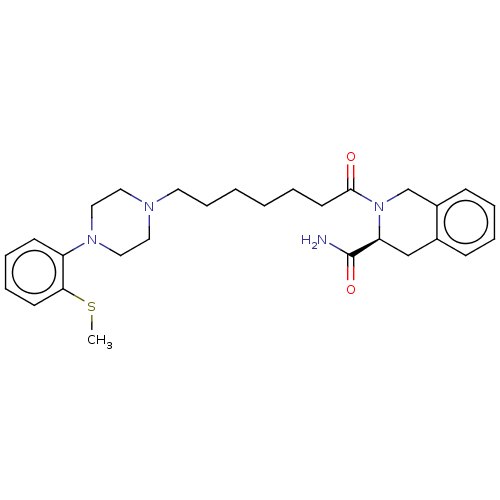 (CHEMBL3235745) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor expressed in HEK293 cells after 1 hr by beta-counting | Eur J Med Chem 78: 10-22 (2014) Article DOI: 10.1016/j.ejmech.2014.03.005 BindingDB Entry DOI: 10.7270/Q2QN6899 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50048803 (5-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)et...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1AR expressed in HEK293 cell membranes after 1 hr by microbeta counting method | Eur J Med Chem 145: 790-804 (2018) Article DOI: 10.1016/j.ejmech.2018.01.002 BindingDB Entry DOI: 10.7270/Q27947BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50462177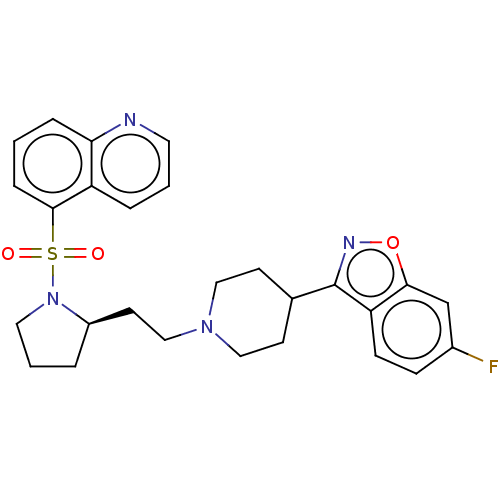 (CHEMBL4250475) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from human D2LR expressed in HEK293 cell membranes after 1 hr at 37 degC by microbeta counting method | Eur J Med Chem 145: 790-804 (2018) Article DOI: 10.1016/j.ejmech.2018.01.002 BindingDB Entry DOI: 10.7270/Q27947BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1074 total ) | Next | Last >> |