 Found 258 hits with Last Name = 'cao' and Initial = 'l'
Found 258 hits with Last Name = 'cao' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gamma-aminobutyric acid receptor subunit alpha-1/beta-3/gamma-2
(Homo sapiens (Human)) | BDBM144227
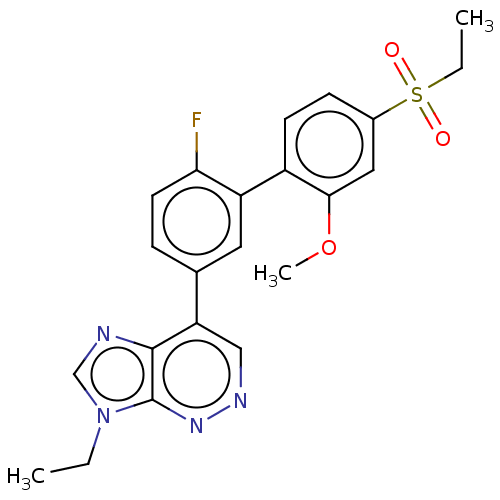
(US8952008, 4)Show SMILES CCn1cnc2c(cnnc12)-c1ccc(F)c(c1)-c1ccc(cc1OC)S(=O)(=O)CC Show InChI InChI=1S/C22H21FN4O3S/c1-4-27-13-24-21-18(12-25-26-22(21)27)14-6-9-19(23)17(10-14)16-8-7-15(11-20(16)30-3)31(28,29)5-2/h6-13H,4-5H2,1-3H3 | UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-flumazenil from human GABAA alpha1beta3gamma2 receptor expressed in HEK293 cell membranes measured after 2 hrs by liquid scintil... |
J Med Chem 62: 5773-5796 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00322
BindingDB Entry DOI: 10.7270/Q2HQ437D |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1/beta-3/gamma-2
(Homo sapiens (Human)) | BDBM50512947

(CHEMBL4545044)Show SMILES CCn1cnc2c(cnnc12)-c1ccc(F)c(c1)-c1cc2CN(C)C(=O)c2cc1OC Show InChI InChI=1S/C23H20FN5O2/c1-4-29-12-25-21-18(10-26-27-22(21)29)13-5-6-19(24)16(7-13)17-8-14-11-28(2)23(30)15(14)9-20(17)31-3/h5-10,12H,4,11H2,1-3H3 | UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-flumazenil from human GABAA alpha1beta3gamma2 receptor expressed in HEK293 cell membranes measured after 2 hrs by liquid scintil... |
J Med Chem 62: 5773-5796 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00322
BindingDB Entry DOI: 10.7270/Q2HQ437D |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-3/beta-3/gamma-2
(Homo sapiens (Human)) | BDBM144227

(US8952008, 4)Show SMILES CCn1cnc2c(cnnc12)-c1ccc(F)c(c1)-c1ccc(cc1OC)S(=O)(=O)CC Show InChI InChI=1S/C22H21FN4O3S/c1-4-27-13-24-21-18(12-25-26-22(21)27)14-6-9-19(23)17(10-14)16-8-7-15(11-20(16)30-3)31(28,29)5-2/h6-13H,4-5H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-flumazenil from human GABAA alpha3beta3gamma2 receptor expressed in HEK293 cell membranes measured after 2 hrs by liquid scintil... |
J Med Chem 62: 5773-5796 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00322
BindingDB Entry DOI: 10.7270/Q2HQ437D |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-3/beta-3/gamma-2
(Homo sapiens (Human)) | BDBM50512947

(CHEMBL4545044)Show SMILES CCn1cnc2c(cnnc12)-c1ccc(F)c(c1)-c1cc2CN(C)C(=O)c2cc1OC Show InChI InChI=1S/C23H20FN5O2/c1-4-29-12-25-21-18(10-26-27-22(21)29)13-5-6-19(24)16(7-13)17-8-14-11-28(2)23(30)15(14)9-20(17)31-3/h5-10,12H,4,11H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-flumazenil from human GABAA alpha3beta3gamma2 receptor expressed in HEK293 cell membranes measured after 2 hrs by liquid scintil... |
J Med Chem 62: 5773-5796 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00322
BindingDB Entry DOI: 10.7270/Q2HQ437D |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5/beta-2/gamma-2
(Homo sapiens (Human)) | BDBM144227

(US8952008, 4)Show SMILES CCn1cnc2c(cnnc12)-c1ccc(F)c(c1)-c1ccc(cc1OC)S(=O)(=O)CC Show InChI InChI=1S/C22H21FN4O3S/c1-4-27-13-24-21-18(12-25-26-22(21)27)14-6-9-19(23)17(10-14)16-8-7-15(11-20(16)30-3)31(28,29)5-2/h6-13H,4-5H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-flumazenil from human GABAA alpha5beta2gamma2 receptor expressed in HEK293 cell membranes measured after 2 hrs by liquid scintil... |
J Med Chem 62: 5773-5796 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00322
BindingDB Entry DOI: 10.7270/Q2HQ437D |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5/beta-2/gamma-2
(Homo sapiens (Human)) | BDBM50512947

(CHEMBL4545044)Show SMILES CCn1cnc2c(cnnc12)-c1ccc(F)c(c1)-c1cc2CN(C)C(=O)c2cc1OC Show InChI InChI=1S/C23H20FN5O2/c1-4-29-12-25-21-18(10-26-27-22(21)29)13-5-6-19(24)16(7-13)17-8-14-11-28(2)23(30)15(14)9-20(17)31-3/h5-10,12H,4,11H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-flumazenil from human GABAA alpha5beta2gamma2 receptor expressed in HEK293 cell membranes measured after 2 hrs by liquid scintil... |
J Med Chem 62: 5773-5796 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00322
BindingDB Entry DOI: 10.7270/Q2HQ437D |
More data for this
Ligand-Target Pair | |
Solute carrier family 15 member 1
(Homo sapiens (Human)) | BDBM50519221
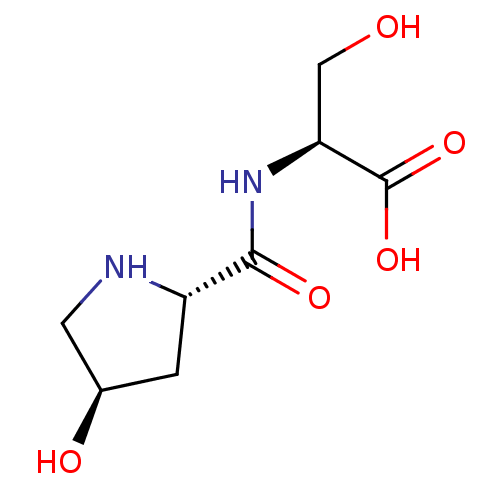
(CHEMBL4460144)Show SMILES OC[C@H](NC(=O)[C@@H]1C[C@@H](O)CN1)C(O)=O |r| Show InChI InChI=1S/C8H14N2O5/c11-3-6(8(14)15)10-7(13)5-1-4(12)2-9-5/h4-6,9,11-12H,1-3H2,(H,10,13)(H,14,15)/t4-,5+,6+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 2.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian Medical University
Curated by ChEMBL
| Assay Description
Substrate activity at PEPT1 in human Caco2 cells assessed as inhibition of Gly-Sar uptake by LC-MS/MS analysis |
J Med Chem 62: 7708-7721 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00358
BindingDB Entry DOI: 10.7270/Q2CZ3BJ4 |
More data for this
Ligand-Target Pair | |
Solute carrier family 15 member 1
(Homo sapiens (Human)) | BDBM50519220

(CHEMBL2229108)Show SMILES OC[C@@H]1NC(=O)[C@@H]2C[C@@H](O)CN2C1=O |r| Show InChI InChI=1S/C8H12N2O4/c11-3-5-8(14)10-2-4(12)1-6(10)7(13)9-5/h4-6,11-12H,1-3H2,(H,9,13)/t4-,5+,6+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 3.60E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian Medical University
Curated by ChEMBL
| Assay Description
Substrate activity at PEPT1 in human Caco2 cells assessed as inhibition of Gly-Sar uptake by LC-MS/MS analysis |
J Med Chem 62: 7708-7721 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00358
BindingDB Entry DOI: 10.7270/Q2CZ3BJ4 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50322823

((S)-N-(4-(3-chloro-4-fluorophenylamino)-7-(tetrahy...)Show SMILES CN(C)C\C=C\C(=O)Nc1cc2c(Nc3ccc(F)c(Cl)c3)ncnc2cc1O[C@H]1CCOC1 |r| Show InChI InChI=1S/C24H25ClFN5O3/c1-31(2)8-3-4-23(32)30-21-11-17-20(12-22(21)34-16-7-9-33-13-16)27-14-28-24(17)29-15-5-6-19(26)18(25)10-15/h3-6,10-12,14,16H,7-9,13H2,1-2H3,(H,30,32)(H,27,28,29)/b4-3+/t16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029668

(AZD-9291 | Osimertinib | US10085983, Compound AZD-...)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1cn(C)c2ccccc12 Show InChI InChI=1S/C28H33N7O2/c1-7-27(36)30-22-16-23(26(37-6)17-25(22)34(4)15-14-33(2)3)32-28-29-13-12-21(31-28)20-18-35(5)24-11-9-8-10-19(20)24/h7-13,16-18H,1,14-15H2,2-6H3,(H,30,36)(H,29,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50580965
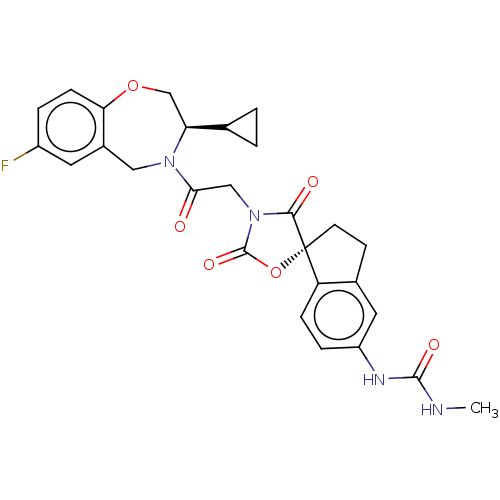
(CHEMBL5090239)Show SMILES CNC(=O)Nc1ccc2c(CC[C@@]22OC(=O)N(CC(=O)N3Cc4cc(F)ccc4OC[C@H]3C3CC3)C2=O)c1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of p300 (unknown origin) preincubated for 15 mins followed by addition of substrate [3H] Ac-CoA and measured after 60 mins by liquid scint... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116512
BindingDB Entry DOI: 10.7270/Q23B640X |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50580965

(CHEMBL5090239)Show SMILES CNC(=O)Nc1ccc2c(CC[C@@]22OC(=O)N(CC(=O)N3Cc4cc(F)ccc4OC[C@H]3C3CC3)C2=O)c1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of p300 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116512
BindingDB Entry DOI: 10.7270/Q23B640X |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50580962
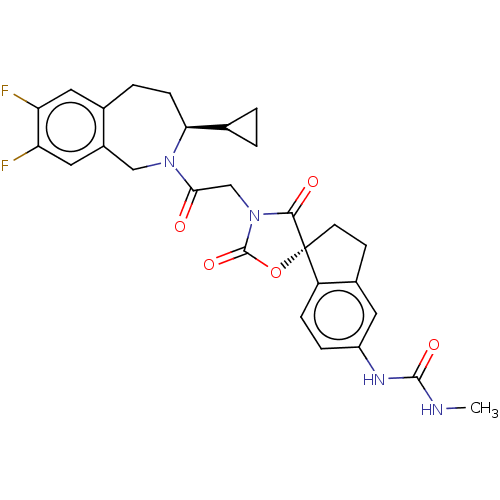
(CHEMBL5082755)Show SMILES CNC(=O)Nc1ccc2c(CC[C@@]22OC(=O)N(CC(=O)N3Cc4cc(F)c(F)cc4CC[C@H]3C3CC3)C2=O)c1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of p300 (unknown origin) preincubated for 15 mins followed by addition of substrate [3H] Ac-CoA and measured after 60 mins by liquid scint... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116512
BindingDB Entry DOI: 10.7270/Q23B640X |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029668

(AZD-9291 | Osimertinib | US10085983, Compound AZD-...)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1cn(C)c2ccccc12 Show InChI InChI=1S/C28H33N7O2/c1-7-27(36)30-22-16-23(26(37-6)17-25(22)34(4)15-14-33(2)3)32-28-29-13-12-21(31-28)20-18-35(5)24-11-9-8-10-19(20)24/h7-13,16-18H,1,14-15H2,2-6H3,(H,30,36)(H,29,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50614754
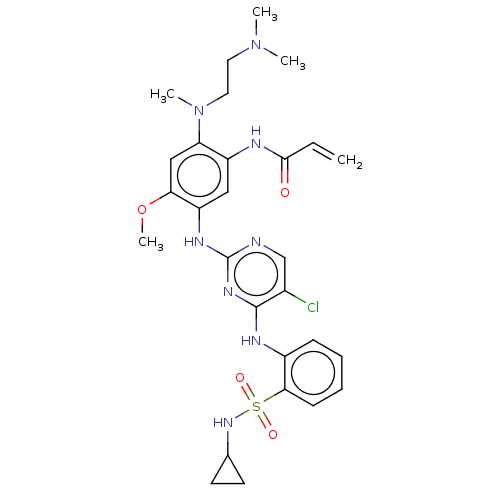
(CHEMBL5284780)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1ncc(Cl)c(Nc2ccccc2S(=O)(=O)NC2CC2)n1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50506372
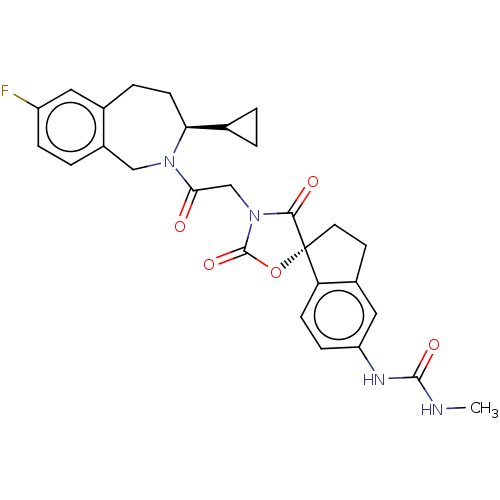
(CHEMBL4475351)Show SMILES CNC(=O)Nc1ccc2c(CC[C@@]22OC(=O)N(CC(=O)N3Cc4ccc(F)cc4CC[C@H]3C3CC3)C2=O)c1 |r| Show InChI InChI=1S/C28H29FN4O5/c1-30-26(36)31-21-7-8-22-18(13-21)10-11-28(22)25(35)33(27(37)38-28)15-24(34)32-14-19-4-6-20(29)12-17(19)5-9-23(32)16-2-3-16/h4,6-8,12-13,16,23H,2-3,5,9-11,14-15H2,1H3,(H2,30,31,36)/t23-,28+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of p300 (unknown origin) preincubated for 15 mins followed by addition of substrate [3H] Ac-CoA and measured after 60 mins by liquid scint... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116512
BindingDB Entry DOI: 10.7270/Q23B640X |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50614754

(CHEMBL5284780)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1ncc(Cl)c(Nc2ccccc2S(=O)(=O)NC2CC2)n1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50580968
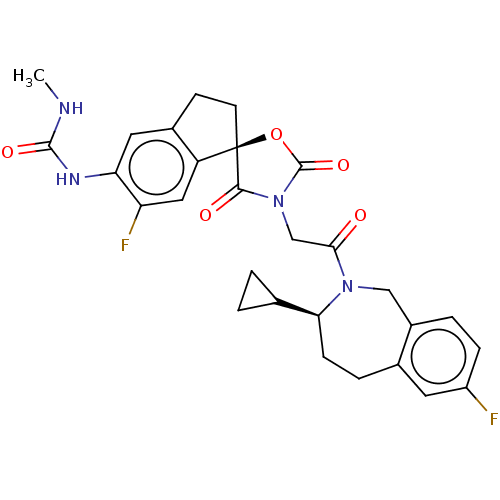
(CHEMBL5083020)Show SMILES CNC(=O)Nc1cc2CC[C@@]3(OC(=O)N(CC(=O)N4Cc5ccc(F)cc5CC[C@H]4C4CC4)C3=O)c2cc1F |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of p300 (unknown origin) preincubated for 15 mins followed by addition of substrate [3H] Ac-CoA and measured after 60 mins by liquid scint... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116512
BindingDB Entry DOI: 10.7270/Q23B640X |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Capital Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human dihydrofolate reductase by spectrophotometric analysis |
Eur J Med Chem 64: 401-9 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.017
BindingDB Entry DOI: 10.7270/Q20P11F4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50580963
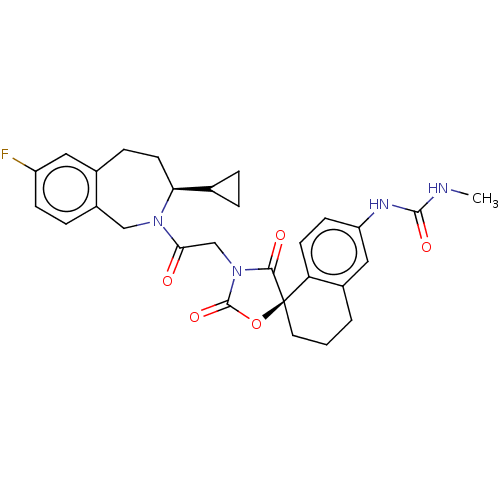
(CHEMBL5077158)Show SMILES CNC(=O)Nc1ccc2c(CCC[C@@]22OC(=O)N(CC(=O)N3Cc4ccc(F)cc4CC[C@H]3C3CC3)C2=O)c1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of p300 (unknown origin) preincubated for 15 mins followed by addition of substrate [3H] Ac-CoA and measured after 60 mins by liquid scint... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116512
BindingDB Entry DOI: 10.7270/Q23B640X |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50506366
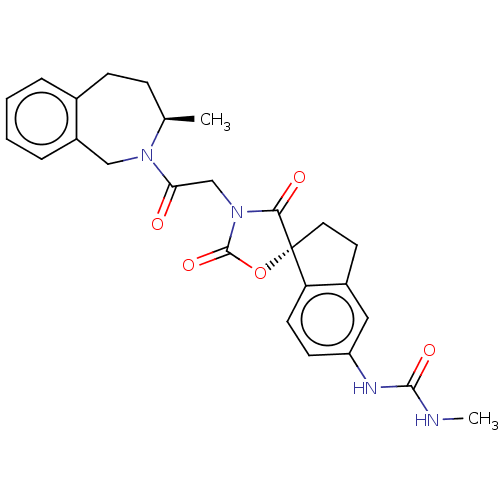
(CHEMBL4524352)Show SMILES CNC(=O)Nc1ccc2c(CC[C@@]22OC(=O)N(CC(=O)N3Cc4ccccc4CC[C@H]3C)C2=O)c1 |r| Show InChI InChI=1S/C26H28N4O5/c1-16-7-8-17-5-3-4-6-19(17)14-29(16)22(31)15-30-23(32)26(35-25(30)34)12-11-18-13-20(9-10-21(18)26)28-24(33)27-2/h3-6,9-10,13,16H,7-8,11-12,14-15H2,1-2H3,(H2,27,28,33)/t16-,26-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of p300 (unknown origin) preincubated for 15 mins followed by addition of substrate [3H] Ac-CoA and measured after 60 mins by liquid scint... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116512
BindingDB Entry DOI: 10.7270/Q23B640X |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D3
(Homo sapiens (Human)) | BDBM50588665
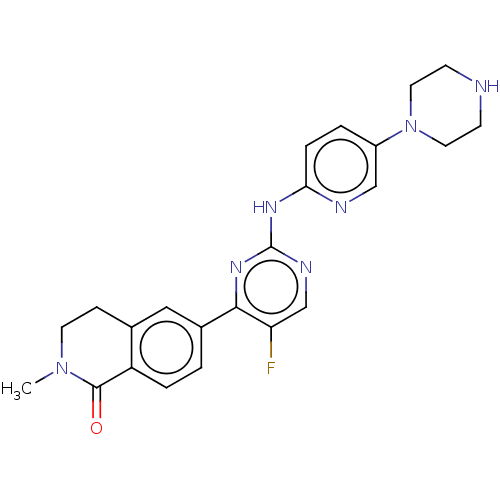
(CHEMBL5171767)Show SMILES CN1CCc2cc(ccc2C1=O)-c1nc(Nc2ccc(cn2)N2CCNCC2)ncc1F | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00947
BindingDB Entry DOI: 10.7270/Q2B56PQD |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50614754

(CHEMBL5284780)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1ncc(Cl)c(Nc2ccccc2S(=O)(=O)NC2CC2)n1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50580964
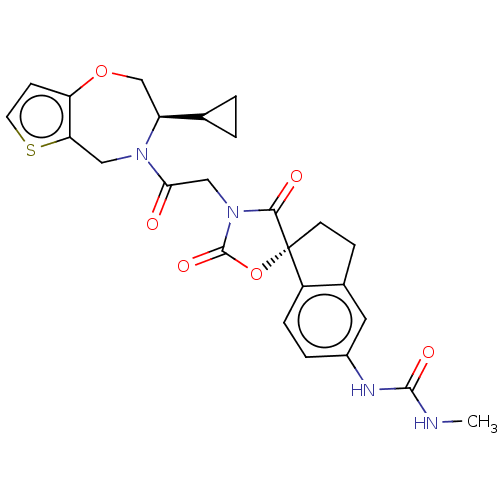
(CHEMBL5094423)Show SMILES CNC(=O)Nc1ccc2c(CC[C@@]22OC(=O)N(CC(=O)N3Cc4sccc4OC[C@H]3C3CC3)C2=O)c1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of p300 (unknown origin) preincubated for 15 mins followed by addition of substrate [3H] Ac-CoA and measured after 60 mins by liquid scint... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116512
BindingDB Entry DOI: 10.7270/Q23B640X |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50108116
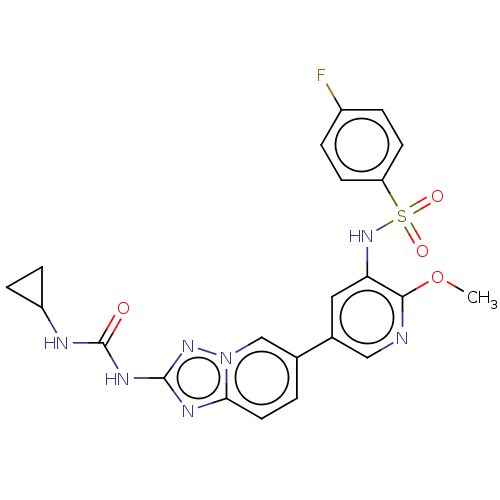
(CHEMBL3601923)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1)-c1ccc2nc(NC(=O)NC3CC3)nn2c1 Show InChI InChI=1S/C22H20FN7O4S/c1-34-20-18(29-35(32,33)17-7-3-15(23)4-8-17)10-14(11-24-20)13-2-9-19-26-21(28-30(19)12-13)27-22(31)25-16-5-6-16/h2-4,7-12,16,29H,5-6H2,1H3,(H2,25,27,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as reduction of ATP level after 40 mins by luciferase based luminescence assay |
Bioorg Med Chem 23: 5662-71 (2015)
Article DOI: 10.1016/j.bmc.2015.07.017
BindingDB Entry DOI: 10.7270/Q2VX0J9B |
More data for this
Ligand-Target Pair | |
CREB-binding protein
(Homo sapiens (Human)) | BDBM50580962

(CHEMBL5082755)Show SMILES CNC(=O)Nc1ccc2c(CC[C@@]22OC(=O)N(CC(=O)N3Cc4cc(F)c(F)cc4CC[C@H]3C3CC3)C2=O)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CBP (unknown origin) preincubated for 15 mins followed by addition of substrate [3H] Ac-CoA and measured after 60 mins by liquid scinti... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116512
BindingDB Entry DOI: 10.7270/Q23B640X |
More data for this
Ligand-Target Pair | |
CREB-binding protein
(Homo sapiens (Human)) | BDBM50580965

(CHEMBL5090239)Show SMILES CNC(=O)Nc1ccc2c(CC[C@@]22OC(=O)N(CC(=O)N3Cc4cc(F)ccc4OC[C@H]3C3CC3)C2=O)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CBP (unknown origin) preincubated for 15 mins followed by addition of substrate [3H] Ac-CoA and measured after 60 mins by liquid scinti... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116512
BindingDB Entry DOI: 10.7270/Q23B640X |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D3
(Homo sapiens (Human)) | BDBM50588663

(CHEMBL5185518)Show SMILES Fc1cnc(Nc2ccc(cn2)N2CCNCC2)nc1-c1ccc2C(=O)NCCc2c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00947
BindingDB Entry DOI: 10.7270/Q2B56PQD |
More data for this
Ligand-Target Pair | |
CREB-binding protein
(Homo sapiens (Human)) | BDBM50506372

(CHEMBL4475351)Show SMILES CNC(=O)Nc1ccc2c(CC[C@@]22OC(=O)N(CC(=O)N3Cc4ccc(F)cc4CC[C@H]3C3CC3)C2=O)c1 |r| Show InChI InChI=1S/C28H29FN4O5/c1-30-26(36)31-21-7-8-22-18(13-21)10-11-28(22)25(35)33(27(37)38-28)15-24(34)32-14-19-4-6-20(29)12-17(19)5-9-23(32)16-2-3-16/h4,6-8,12-13,16,23H,2-3,5,9-11,14-15H2,1H3,(H2,30,31,36)/t23-,28+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CBP (unknown origin) preincubated for 15 mins followed by addition of substrate [3H] Ac-CoA and measured after 60 mins by liquid scinti... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116512
BindingDB Entry DOI: 10.7270/Q23B640X |
More data for this
Ligand-Target Pair | |
CREB-binding protein
(Homo sapiens (Human)) | BDBM50580968

(CHEMBL5083020)Show SMILES CNC(=O)Nc1cc2CC[C@@]3(OC(=O)N(CC(=O)N4Cc5ccc(F)cc5CC[C@H]4C4CC4)C3=O)c2cc1F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CBP (unknown origin) preincubated for 15 mins followed by addition of substrate [3H] Ac-CoA and measured after 60 mins by liquid scinti... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116512
BindingDB Entry DOI: 10.7270/Q23B640X |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50580974
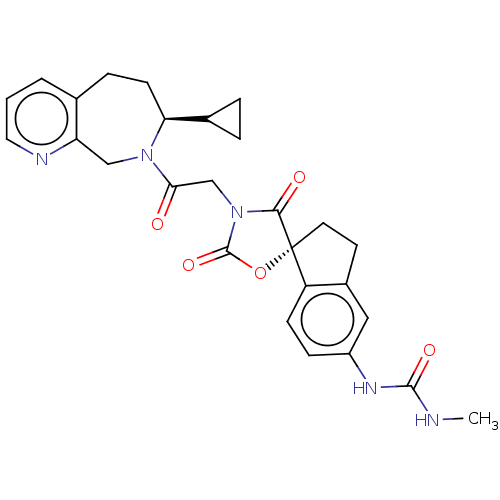
(CHEMBL5078353)Show SMILES CNC(=O)Nc1ccc2c(CC[C@@]22OC(=O)N(CC(=O)N3Cc4ncccc4CC[C@H]3C3CC3)C2=O)c1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of p300 (unknown origin) preincubated for 15 mins followed by addition of substrate [3H] Ac-CoA and measured after 60 mins by liquid scint... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116512
BindingDB Entry DOI: 10.7270/Q23B640X |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50580970
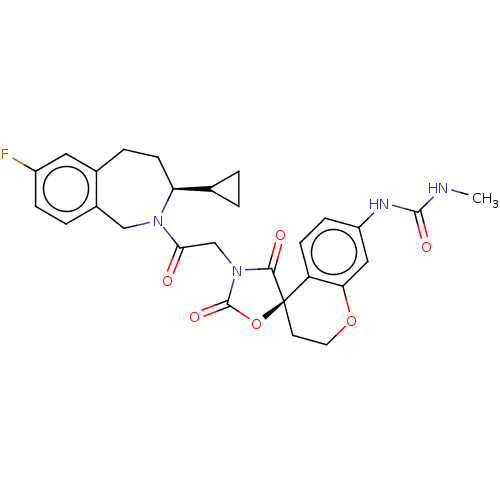
(CHEMBL5081689)Show SMILES CNC(=O)Nc1ccc2c(OCC[C@@]22OC(=O)N(CC(=O)N3Cc4ccc(F)cc4CC[C@H]3C3CC3)C2=O)c1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of p300 (unknown origin) preincubated for 15 mins followed by addition of substrate [3H] Ac-CoA and measured after 60 mins by liquid scint... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116512
BindingDB Entry DOI: 10.7270/Q23B640X |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50580972
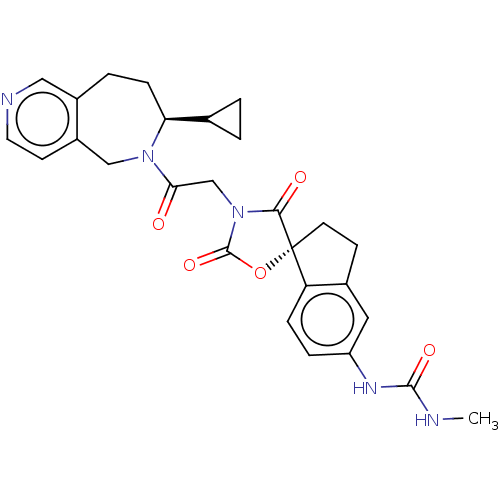
(CHEMBL5078021)Show SMILES CNC(=O)Nc1ccc2c(CC[C@@]22OC(=O)N(CC(=O)N3Cc4ccncc4CC[C@H]3C3CC3)C2=O)c1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of p300 (unknown origin) preincubated for 15 mins followed by addition of substrate [3H] Ac-CoA and measured after 60 mins by liquid scint... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116512
BindingDB Entry DOI: 10.7270/Q23B640X |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50580971
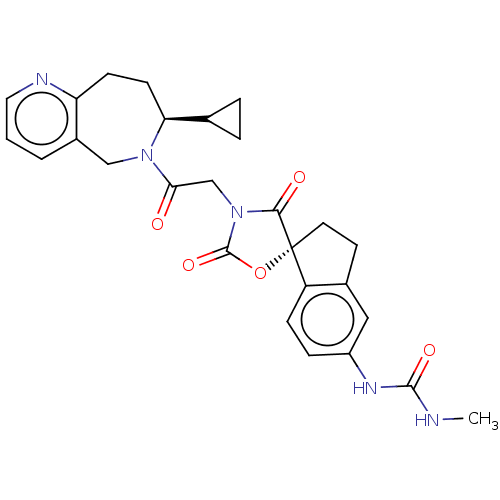
(CHEMBL5085891)Show SMILES CNC(=O)Nc1ccc2c(CC[C@@]22OC(=O)N(CC(=O)N3Cc4cccnc4CC[C@H]3C3CC3)C2=O)c1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of p300 (unknown origin) preincubated for 15 mins followed by addition of substrate [3H] Ac-CoA and measured after 60 mins by liquid scint... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116512
BindingDB Entry DOI: 10.7270/Q23B640X |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D3
(Homo sapiens (Human)) | BDBM50588664
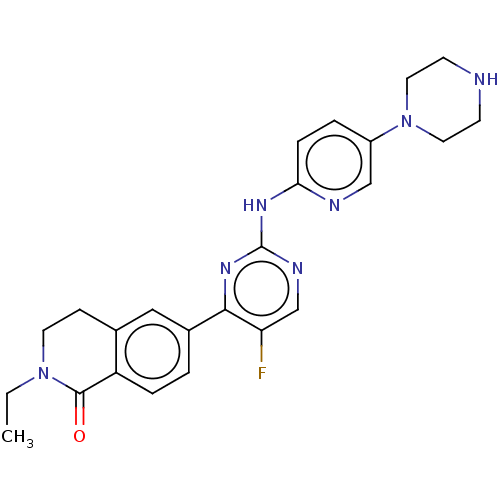
(CHEMBL5170480)Show SMILES CCN1CCc2cc(ccc2C1=O)-c1nc(Nc2ccc(cn2)N2CCNCC2)ncc1F | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00947
BindingDB Entry DOI: 10.7270/Q2B56PQD |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50580967
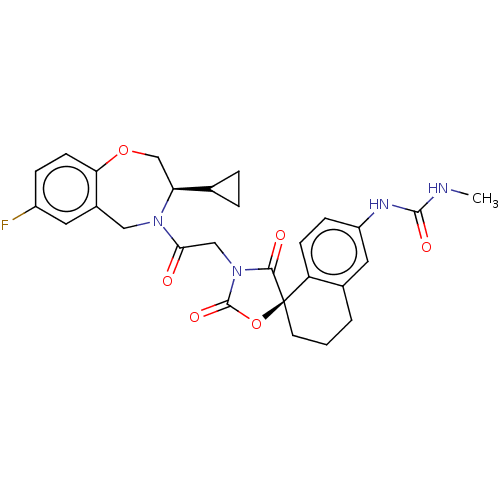
(CHEMBL5083262)Show SMILES CNC(=O)Nc1ccc2c(CCC[C@@]22OC(=O)N(CC(=O)N3Cc4cc(F)ccc4OC[C@H]3C3CC3)C2=O)c1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of p300 (unknown origin) preincubated for 15 mins followed by addition of substrate [3H] Ac-CoA and measured after 60 mins by liquid scint... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116512
BindingDB Entry DOI: 10.7270/Q23B640X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50108117
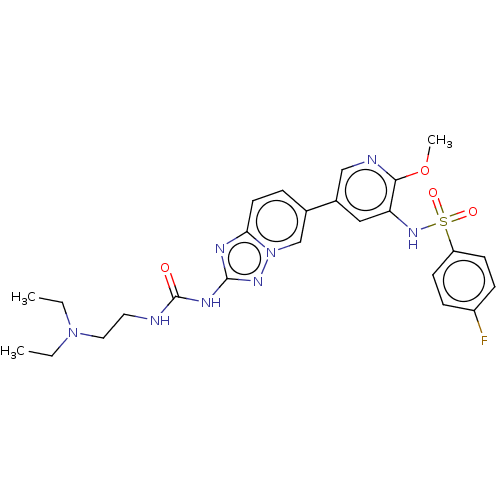
(CHEMBL3601925)Show SMILES CCN(CC)CCNC(=O)Nc1nc2ccc(cn2n1)-c1cnc(OC)c(NS(=O)(=O)c2ccc(F)cc2)c1 Show InChI InChI=1S/C25H29FN8O4S/c1-4-33(5-2)13-12-27-25(35)30-24-29-22-11-6-17(16-34(22)31-24)18-14-21(23(38-3)28-15-18)32-39(36,37)20-9-7-19(26)8-10-20/h6-11,14-16,32H,4-5,12-13H2,1-3H3,(H2,27,30,31,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as reduction of ATP level after 40 mins by luciferase based luminescence assay |
Bioorg Med Chem 23: 5662-71 (2015)
Article DOI: 10.1016/j.bmc.2015.07.017
BindingDB Entry DOI: 10.7270/Q2VX0J9B |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 6/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM6309
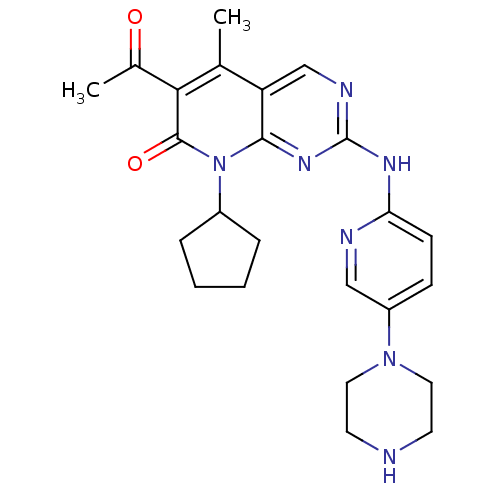
(6-Acetyl-8-cyclopentyl-5-methyl-2-(5-piperazin-1-y...)Show SMILES CC(=O)c1c(C)c2cnc(Nc3ccc(cn3)N3CCNCC3)nc2n(C2CCCC2)c1=O Show InChI InChI=1S/C24H29N7O2/c1-15-19-14-27-24(28-20-8-7-18(13-26-20)30-11-9-25-10-12-30)29-22(19)31(17-5-3-4-6-17)23(33)21(15)16(2)32/h7-8,13-14,17,25H,3-6,9-12H2,1-2H3,(H,26,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00947
BindingDB Entry DOI: 10.7270/Q2B56PQD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
CREB-binding protein
(Homo sapiens (Human)) | BDBM50580964

(CHEMBL5094423)Show SMILES CNC(=O)Nc1ccc2c(CC[C@@]22OC(=O)N(CC(=O)N3Cc4sccc4OC[C@H]3C3CC3)C2=O)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CBP (unknown origin) preincubated for 15 mins followed by addition of substrate [3H] Ac-CoA and measured after 60 mins by liquid scinti... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116512
BindingDB Entry DOI: 10.7270/Q23B640X |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50580969
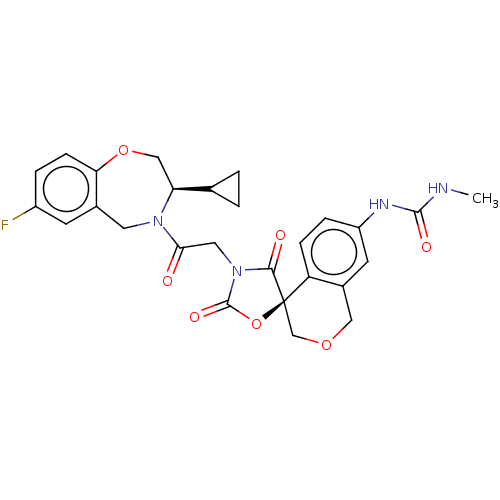
(CHEMBL5073432)Show SMILES CNC(=O)Nc1ccc2c(COC[C@@]22OC(=O)N(CC(=O)N3Cc4cc(F)ccc4OC[C@H]3C3CC3)C2=O)c1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of p300 (unknown origin) preincubated for 15 mins followed by addition of substrate [3H] Ac-CoA and measured after 60 mins by liquid scint... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116512
BindingDB Entry DOI: 10.7270/Q23B640X |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
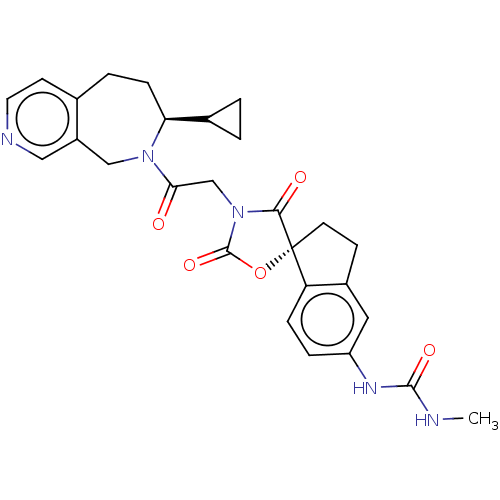
(Homo sapiens (Human)) | BDBM50580973
(CHEMBL5084035)Show SMILES CNC(=O)Nc1ccc2c(CC[C@@]22OC(=O)N(CC(=O)N3Cc4cnccc4CC[C@H]3C3CC3)C2=O)c1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of p300 (unknown origin) preincubated for 15 mins followed by addition of substrate [3H] Ac-CoA and measured after 60 mins by liquid scint... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116512
BindingDB Entry DOI: 10.7270/Q23B640X |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D3
(Homo sapiens (Human)) | BDBM50588661
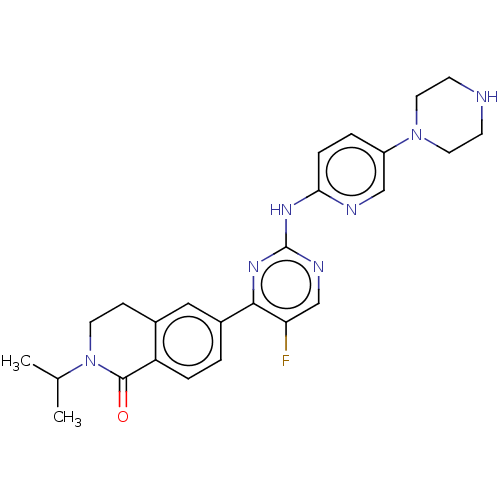
(CHEMBL5205035)Show SMILES CC(C)N1CCc2cc(ccc2C1=O)-c1nc(Nc2ccc(cn2)N2CCNCC2)ncc1F | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00947
BindingDB Entry DOI: 10.7270/Q2B56PQD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50108116

(CHEMBL3601923)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1)-c1ccc2nc(NC(=O)NC3CC3)nn2c1 Show InChI InChI=1S/C22H20FN7O4S/c1-34-20-18(29-35(32,33)17-7-3-15(23)4-8-17)10-14(11-24-20)13-2-9-19-26-21(28-30(19)12-13)27-22(31)25-16-5-6-16/h2-4,7-12,16,29H,5-6H2,1H3,(H2,25,27,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) assessed as reduction of ATP level after 40 mins by luciferase based luminescence assay |
Bioorg Med Chem 23: 5662-71 (2015)
Article DOI: 10.1016/j.bmc.2015.07.017
BindingDB Entry DOI: 10.7270/Q2VX0J9B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50108117

(CHEMBL3601925)Show SMILES CCN(CC)CCNC(=O)Nc1nc2ccc(cn2n1)-c1cnc(OC)c(NS(=O)(=O)c2ccc(F)cc2)c1 Show InChI InChI=1S/C25H29FN8O4S/c1-4-33(5-2)13-12-27-25(35)30-24-29-22-11-6-17(16-34(22)31-24)18-14-21(23(38-3)28-15-18)32-39(36,37)20-9-7-19(26)8-10-20/h6-11,14-16,32H,4-5,12-13H2,1-3H3,(H2,27,30,31,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as reduction of ATP level after 40 mins by luciferase based luminescence assay |
Bioorg Med Chem 23: 5662-71 (2015)
Article DOI: 10.1016/j.bmc.2015.07.017
BindingDB Entry DOI: 10.7270/Q2VX0J9B |
More data for this
Ligand-Target Pair | |
CREB-binding protein
(Homo sapiens (Human)) | BDBM50580963

(CHEMBL5077158)Show SMILES CNC(=O)Nc1ccc2c(CCC[C@@]22OC(=O)N(CC(=O)N3Cc4ccc(F)cc4CC[C@H]3C3CC3)C2=O)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CBP (unknown origin) preincubated for 15 mins followed by addition of substrate [3H] Ac-CoA and measured after 60 mins by liquid scinti... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116512
BindingDB Entry DOI: 10.7270/Q23B640X |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029668

(AZD-9291 | Osimertinib | US10085983, Compound AZD-...)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1cn(C)c2ccccc12 Show InChI InChI=1S/C28H33N7O2/c1-7-27(36)30-22-16-23(26(37-6)17-25(22)34(4)15-14-33(2)3)32-28-29-13-12-21(31-28)20-18-35(5)24-11-9-8-10-19(20)24/h7-13,16-18H,1,14-15H2,2-6H3,(H,30,36)(H,29,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50467836
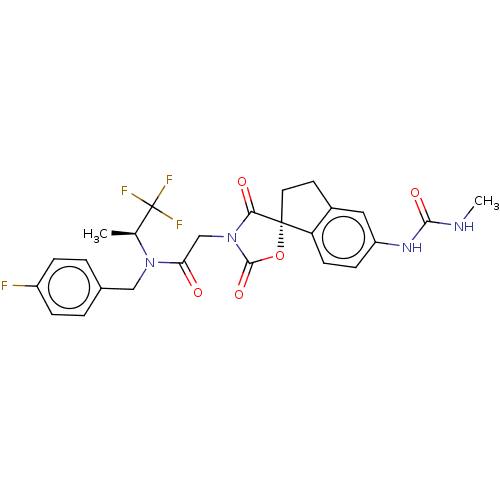
(CHEMBL4282264)Show SMILES CNC(=O)Nc1ccc2c(CC[C@@]22OC(=O)N(CC(=O)N(Cc3ccc(F)cc3)[C@@H](C)C(F)(F)F)C2=O)c1 |r| Show InChI InChI=1S/C25H24F4N4O5/c1-14(25(27,28)29)32(12-15-3-5-17(26)6-4-15)20(34)13-33-21(35)24(38-23(33)37)10-9-16-11-18(7-8-19(16)24)31-22(36)30-2/h3-8,11,14H,9-10,12-13H2,1-2H3,(H2,30,31,36)/t14-,24+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of p300 (unknown origin) preincubated for 15 mins followed by addition of substrate [3H] Ac-CoA and measured after 60 mins by liquid scint... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116512
BindingDB Entry DOI: 10.7270/Q23B640X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50108116

(CHEMBL3601923)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1)-c1ccc2nc(NC(=O)NC3CC3)nn2c1 Show InChI InChI=1S/C22H20FN7O4S/c1-34-20-18(29-35(32,33)17-7-3-15(23)4-8-17)10-14(11-24-20)13-2-9-19-26-21(28-30(19)12-13)27-22(31)25-16-5-6-16/h2-4,7-12,16,29H,5-6H2,1H3,(H2,25,27,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as reduction of ATP level after 40 mins by luciferase based luminescence assay |
Bioorg Med Chem 23: 5662-71 (2015)
Article DOI: 10.1016/j.bmc.2015.07.017
BindingDB Entry DOI: 10.7270/Q2VX0J9B |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50562813
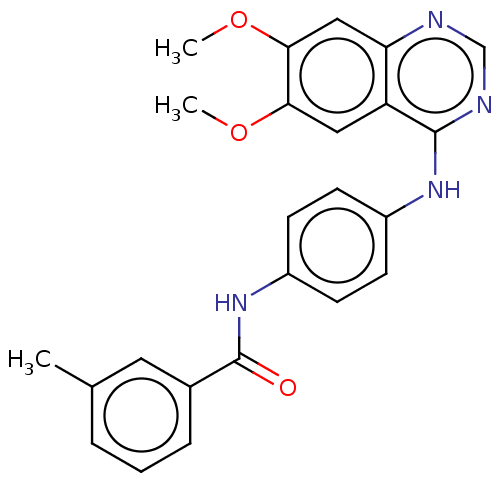
(CHEMBL4765039)Show SMILES COc1cc2ncnc(Nc3ccc(NC(=O)c4cccc(C)c4)cc3)c2cc1OC | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of VEGFR2 (unknown origin) by caliper mobility shift assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127788
BindingDB Entry DOI: 10.7270/Q2S75M2D |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data