

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50336921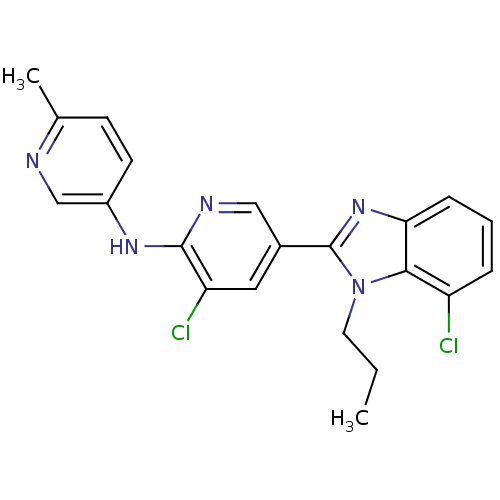 (3-chloro-5-(7-chloro-1-propyl-1H-benzo[d]imidazol-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]ABP688 from human recombinant mGlu5 receptor | ACS Med Chem Lett 2: 58-62 (2011) Article DOI: 10.1021/ml100215b BindingDB Entry DOI: 10.7270/Q22R3RZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50336921 (3-chloro-5-(7-chloro-1-propyl-1H-benzo[d]imidazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]ABP688 from mGlu5 receptor in rat brain tissue | ACS Med Chem Lett 2: 58-62 (2011) Article DOI: 10.1021/ml100215b BindingDB Entry DOI: 10.7270/Q22R3RZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50392323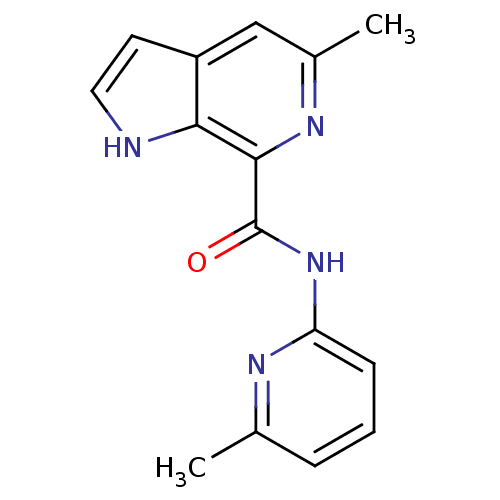 (CHEMBL2153782) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]ABP688 from human mGluR5a transmembrane region expressed in mouse L(tk-) cells | Bioorg Med Chem Lett 22: 6454-9 (2012) Article DOI: 10.1016/j.bmcl.2012.08.053 BindingDB Entry DOI: 10.7270/Q2765GDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 2 (Homo sapiens (Human)) | BDBM86751 (CHEMBL14935 | LY 293558 | LY-293558) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Binding affinity to GluR2 | J Med Chem 53: 5367-82 (2010) Article DOI: 10.1021/jm901688m BindingDB Entry DOI: 10.7270/Q2FX79NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 1 (Homo sapiens (Human)) | BDBM86751 (CHEMBL14935 | LY 293558 | LY-293558) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Binding affinity to GluR5 | J Med Chem 53: 5367-82 (2010) Article DOI: 10.1021/jm901688m BindingDB Entry DOI: 10.7270/Q2FX79NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 1 (Homo sapiens (Human)) | BDBM86751 (CHEMBL14935 | LY 293558 | LY-293558) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Binding affinity to GluR1 | J Med Chem 53: 5367-82 (2010) Article DOI: 10.1021/jm901688m BindingDB Entry DOI: 10.7270/Q2FX79NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 3 (Homo sapiens (Human)) | BDBM86751 (CHEMBL14935 | LY 293558 | LY-293558) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Binding affinity to GluR3 | J Med Chem 53: 5367-82 (2010) Article DOI: 10.1021/jm901688m BindingDB Entry DOI: 10.7270/Q2FX79NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 4 (Homo sapiens (Human)) | BDBM86751 (CHEMBL14935 | LY 293558 | LY-293558) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Binding affinity to GluR4 | J Med Chem 53: 5367-82 (2010) Article DOI: 10.1021/jm901688m BindingDB Entry DOI: 10.7270/Q2FX79NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 2 (Homo sapiens (Human)) | BDBM86751 (CHEMBL14935 | LY 293558 | LY-293558) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Binding affinity to GluR6 | J Med Chem 53: 5367-82 (2010) Article DOI: 10.1021/jm901688m BindingDB Entry DOI: 10.7270/Q2FX79NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 3 (Homo sapiens (Human)) | BDBM86751 (CHEMBL14935 | LY 293558 | LY-293558) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Binding affinity to GluR7 | J Med Chem 53: 5367-82 (2010) Article DOI: 10.1021/jm901688m BindingDB Entry DOI: 10.7270/Q2FX79NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50281849 (CHEMBL4175305) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inverse agonist activity at human His6-tagged RORgammat LBD (264 to 518 residues) assessed as reduction in biotinylated RIP140 co-activator recruitme... | J Med Chem 61: 6724-6735 (2018) Article DOI: 10.1021/acs.jmedchem.8b00529 BindingDB Entry DOI: 10.7270/Q2Z60RK3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM136108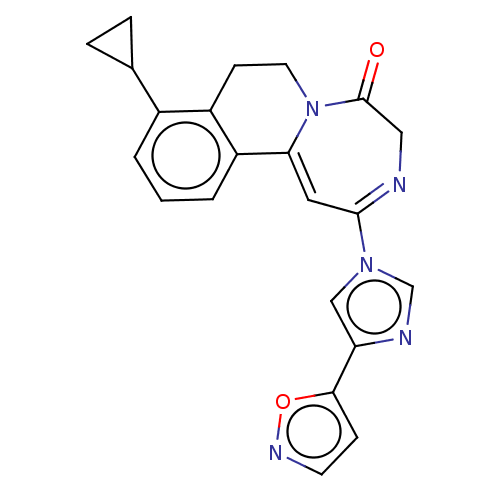 (US8853203, 123b | US9650377, Example 123b) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Activity of compounds of the present invention was examined by determination to what extent the glutamate-induced elevation of the intracellular calc... | US Patent US8853203 (2014) BindingDB Entry DOI: 10.7270/Q2MP520T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM136115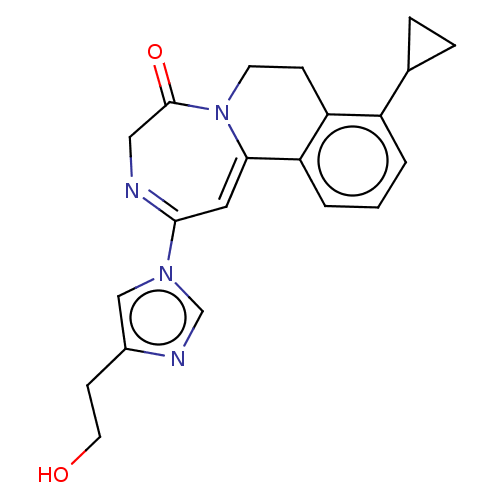 (US8853203, 128 | US9650377, Example 128) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Activity of compounds of the present invention was examined by determination to what extent the glutamate-induced elevation of the intracellular calc... | US Patent US8853203 (2014) BindingDB Entry DOI: 10.7270/Q2MP520T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM136115 (US8853203, 128 | US9650377, Example 128) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Activity of compounds of the present invention with respect to mGluR1 antagonism was examined by an assay based on measurements of L-glutamate induce... | US Patent US9650377 (2017) BindingDB Entry DOI: 10.7270/Q2G73GT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM136048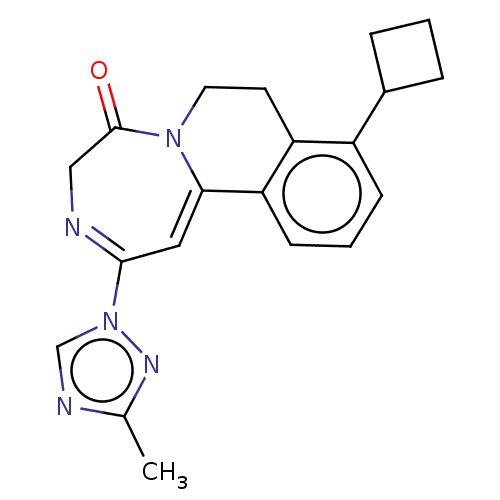 (US8853203, 92 | US9650377, Example 92) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Activity of compounds of the present invention with respect to mGluR1 antagonism was examined by an assay based on measurements of L-glutamate induce... | US Patent US9650377 (2017) BindingDB Entry DOI: 10.7270/Q2G73GT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM136048 (US8853203, 92 | US9650377, Example 92) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Activity of compounds of the present invention was examined by determination to what extent the glutamate-induced elevation of the intracellular calc... | US Patent US8853203 (2014) BindingDB Entry DOI: 10.7270/Q2MP520T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM136108 (US8853203, 123b | US9650377, Example 123b) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Activity of compounds of the present invention with respect to mGluR1 antagonism was examined by an assay based on measurements of L-glutamate induce... | US Patent US9650377 (2017) BindingDB Entry DOI: 10.7270/Q2G73GT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM136132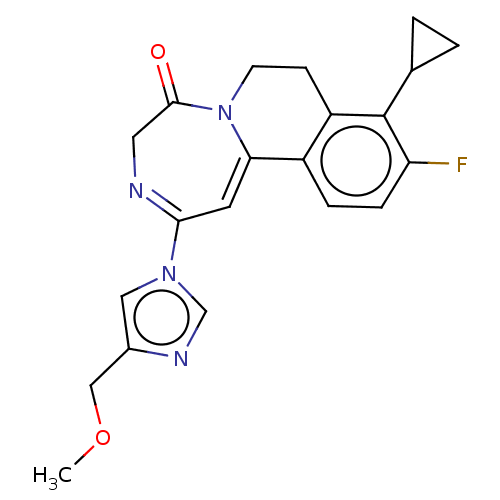 (US8853203, 96-2 | US9650377, Example 96-2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Activity of compounds of the present invention was examined by determination to what extent the glutamate-induced elevation of the intracellular calc... | US Patent US8853203 (2014) BindingDB Entry DOI: 10.7270/Q2MP520T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM136067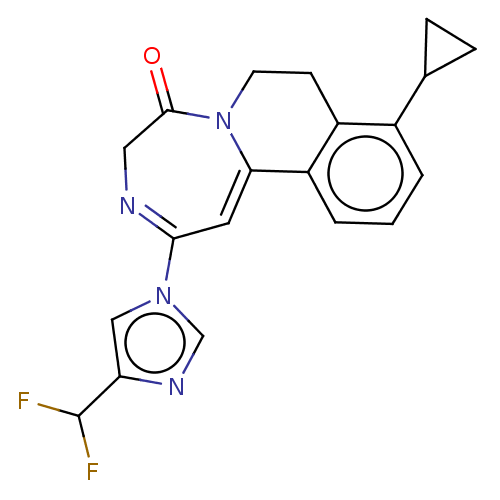 (US8853203, 99l) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Activity of compounds of the present invention was examined by determination to what extent the glutamate-induced elevation of the intracellular calc... | US Patent US8853203 (2014) BindingDB Entry DOI: 10.7270/Q2MP520T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM136068 (US8853203, 99m) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Activity of compounds of the present invention was examined by determination to what extent the glutamate-induced elevation of the intracellular calc... | US Patent US8853203 (2014) BindingDB Entry DOI: 10.7270/Q2MP520T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM136071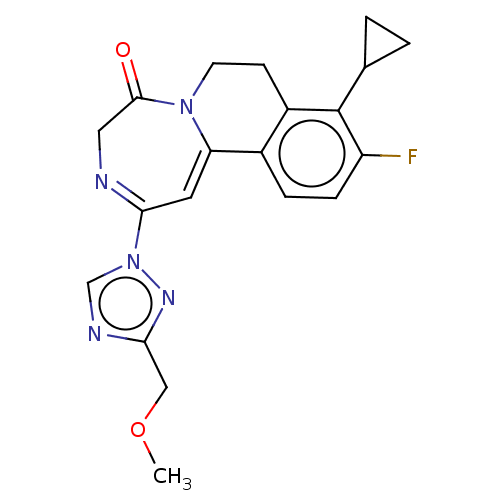 (US8853203, 99p) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Activity of compounds of the present invention was examined by determination to what extent the glutamate-induced elevation of the intracellular calc... | US Patent US8853203 (2014) BindingDB Entry DOI: 10.7270/Q2MP520T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM136055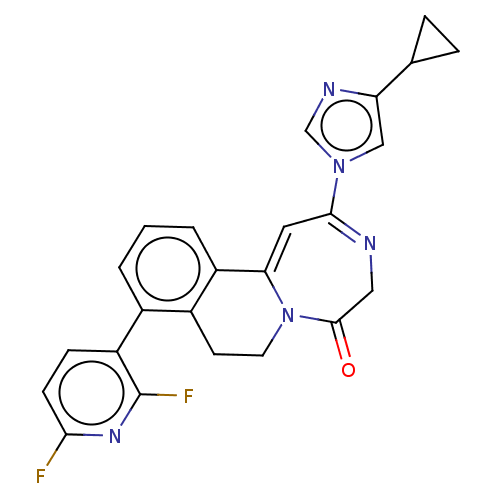 (US8853203, 99 | US9650377, Example 99s) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Activity of compounds of the present invention with respect to mGluR1 antagonism was examined by an assay based on measurements of L-glutamate induce... | US Patent US9650377 (2017) BindingDB Entry DOI: 10.7270/Q2G73GT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM136140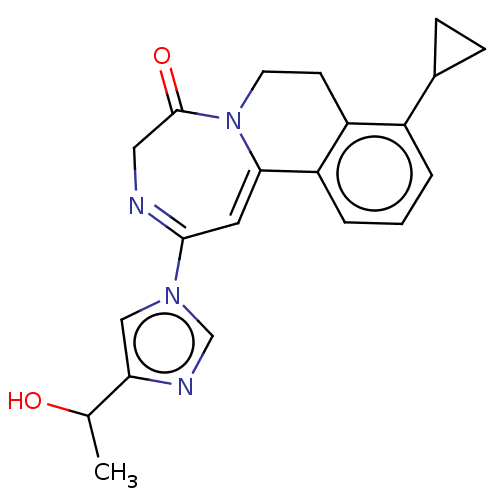 (US8853203, 129 | US9650377, Example 129) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Activity of compounds of the present invention with respect to mGluR1 antagonism was examined by an assay based on measurements of L-glutamate induce... | US Patent US9650377 (2017) BindingDB Entry DOI: 10.7270/Q2G73GT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM136132 (US8853203, 96-2 | US9650377, Example 96-2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Activity of compounds of the present invention with respect to mGluR1 antagonism was examined by an assay based on measurements of L-glutamate induce... | US Patent US9650377 (2017) BindingDB Entry DOI: 10.7270/Q2G73GT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM136055 (US8853203, 99 | US9650377, Example 99s) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Activity of compounds of the present invention with respect to mGluR1 antagonism was examined by an assay based on measurements of L-glutamate induce... | US Patent US9650377 (2017) BindingDB Entry DOI: 10.7270/Q2G73GT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM136055 (US8853203, 99 | US9650377, Example 99s) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Activity of compounds of the present invention with respect to mGluR1 antagonism was examined by an assay based on measurements of L-glutamate induce... | US Patent US9650377 (2017) BindingDB Entry DOI: 10.7270/Q2G73GT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM136140 (US8853203, 129 | US9650377, Example 129) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Activity of compounds of the present invention was examined by determination to what extent the glutamate-induced elevation of the intracellular calc... | US Patent US8853203 (2014) BindingDB Entry DOI: 10.7270/Q2MP520T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM136049 (US8853203, 93 | US9650377, Example 93) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Activity of compounds of the present invention was examined by determination to what extent the glutamate-induced elevation of the intracellular calc... | US Patent US8853203 (2014) BindingDB Entry DOI: 10.7270/Q2MP520T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM136066 (US8853203, 99k) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Activity of compounds of the present invention was examined by determination to what extent the glutamate-induced elevation of the intracellular calc... | US Patent US8853203 (2014) BindingDB Entry DOI: 10.7270/Q2MP520T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM136106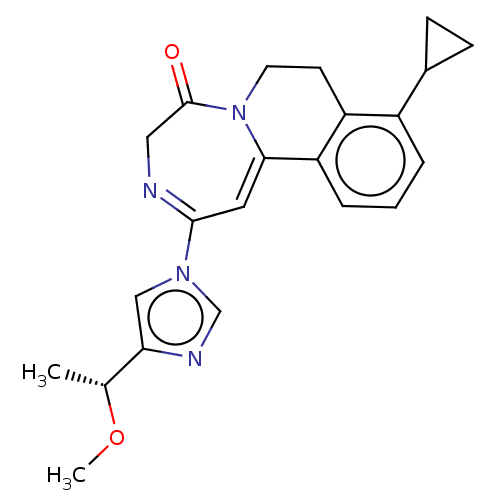 (US8853203, 123 | US9650377, Example 123) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Activity of compounds of the present invention was examined by determination to what extent the glutamate-induced elevation of the intracellular calc... | US Patent US8853203 (2014) BindingDB Entry DOI: 10.7270/Q2MP520T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM136055 (US8853203, 99 | US9650377, Example 99s) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Activity of compounds of the present invention with respect to mGluR1 antagonism was examined by an assay based on measurements of L-glutamate induce... | US Patent US9650377 (2017) BindingDB Entry DOI: 10.7270/Q2G73GT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM136049 (US8853203, 93 | US9650377, Example 93) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Activity of compounds of the present invention with respect to mGluR1 antagonism was examined by an assay based on measurements of L-glutamate induce... | US Patent US9650377 (2017) BindingDB Entry DOI: 10.7270/Q2G73GT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM136106 (US8853203, 123 | US9650377, Example 123) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Activity of compounds of the present invention with respect to mGluR1 antagonism was examined by an assay based on measurements of L-glutamate induce... | US Patent US9650377 (2017) BindingDB Entry DOI: 10.7270/Q2G73GT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50281897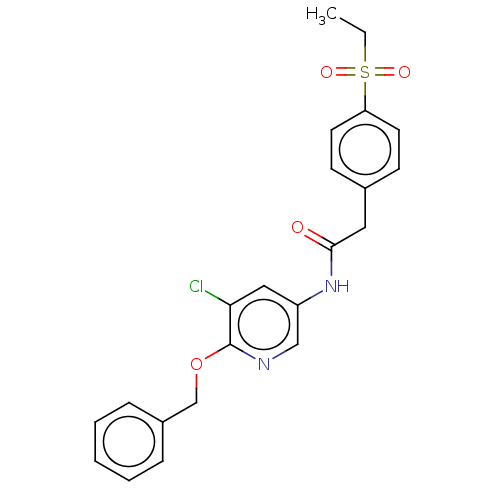 (CHEMBL4171857) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inverse agonist activity at human His6-tagged RORgammat LBD (264 to 518 residues) assessed as reduction in biotinylated RIP140 co-activator recruitme... | J Med Chem 61: 6724-6735 (2018) Article DOI: 10.1021/acs.jmedchem.8b00529 BindingDB Entry DOI: 10.7270/Q2Z60RK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50392324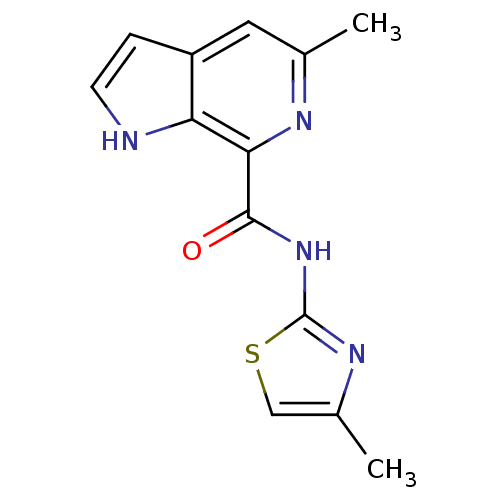 (CHEMBL2153783) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human mGluR5a expressed in mouse L(tk-) cells assessed as inhibition of glutamate-induced Ca2+ influx by FLIPR assay | Bioorg Med Chem Lett 22: 6454-9 (2012) Article DOI: 10.1016/j.bmcl.2012.08.053 BindingDB Entry DOI: 10.7270/Q2765GDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM136105 (US8853203, 122 | US9650377, Example 122) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Activity of compounds of the present invention was examined by determination to what extent the glutamate-induced elevation of the intracellular calc... | US Patent US8853203 (2014) BindingDB Entry DOI: 10.7270/Q2MP520T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM136113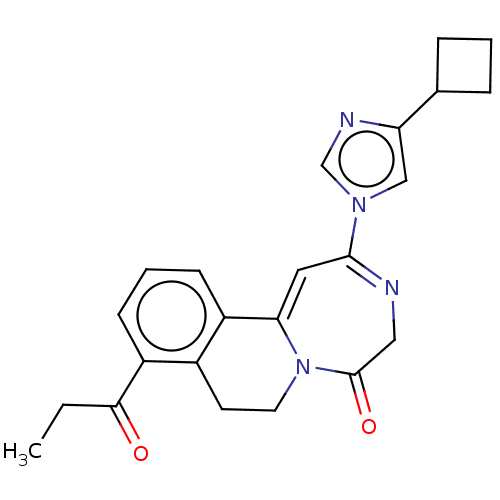 (US8853203, 124 | US9650377, Example 124) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Activity of compounds of the present invention was examined by determination to what extent the glutamate-induced elevation of the intracellular calc... | US Patent US8853203 (2014) BindingDB Entry DOI: 10.7270/Q2MP520T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM136113 (US8853203, 124 | US9650377, Example 124) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Activity of compounds of the present invention with respect to mGluR1 antagonism was examined by an assay based on measurements of L-glutamate induce... | US Patent US9650377 (2017) BindingDB Entry DOI: 10.7270/Q2G73GT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM136105 (US8853203, 122 | US9650377, Example 122) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Activity of compounds of the present invention with respect to mGluR1 antagonism was examined by an assay based on measurements of L-glutamate induce... | US Patent US9650377 (2017) BindingDB Entry DOI: 10.7270/Q2G73GT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM136034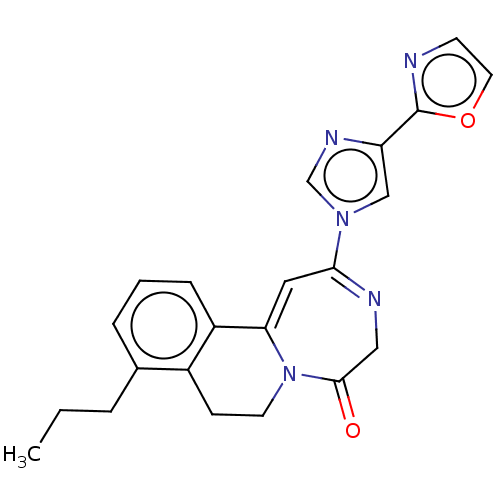 (US8853203, 88a | US9650377, Example 88a) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Activity of compounds of the present invention with respect to mGluR1 antagonism was examined by an assay based on measurements of L-glutamate induce... | US Patent US9650377 (2017) BindingDB Entry DOI: 10.7270/Q2G73GT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM136034 (US8853203, 88a | US9650377, Example 88a) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Activity of compounds of the present invention was examined by determination to what extent the glutamate-induced elevation of the intracellular calc... | US Patent US8853203 (2014) BindingDB Entry DOI: 10.7270/Q2MP520T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM136131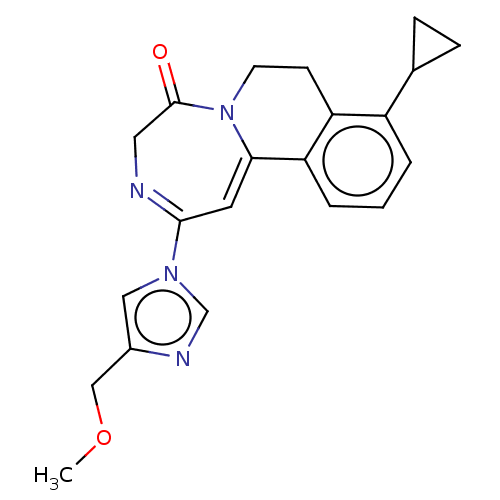 (US8853203, 143 | US8853203, 96-1 | US9650377, Exam...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Activity of compounds of the present invention was examined by determination to what extent the glutamate-induced elevation of the intracellular calc... | US Patent US8853203 (2014) BindingDB Entry DOI: 10.7270/Q2MP520T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM136069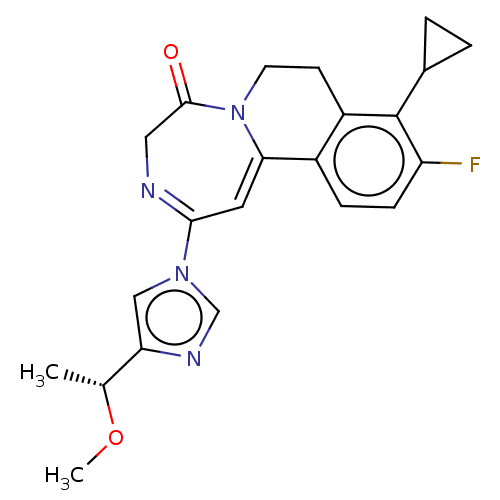 (US8853203, 99n) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Activity of compounds of the present invention was examined by determination to what extent the glutamate-induced elevation of the intracellular calc... | US Patent US8853203 (2014) BindingDB Entry DOI: 10.7270/Q2MP520T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM136055 (US8853203, 99 | US9650377, Example 99s) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Activity of compounds of the present invention with respect to mGluR1 antagonism was examined by an assay based on measurements of L-glutamate induce... | US Patent US9650377 (2017) BindingDB Entry DOI: 10.7270/Q2G73GT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM308239 (US9650377, Example 126) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Activity of compounds of the present invention with respect to mGluR1 antagonism was examined by an assay based on measurements of L-glutamate induce... | US Patent US9650377 (2017) BindingDB Entry DOI: 10.7270/Q2G73GT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM136131 (US8853203, 143 | US8853203, 96-1 | US9650377, Exam...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Activity of compounds of the present invention with respect to mGluR1 antagonism was examined by an assay based on measurements of L-glutamate induce... | US Patent US9650377 (2017) BindingDB Entry DOI: 10.7270/Q2G73GT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM136139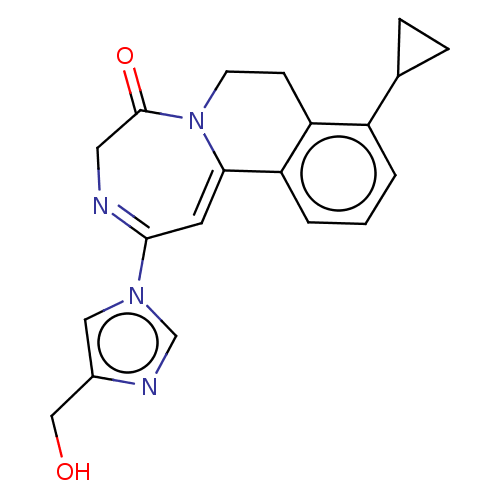 (US8853203, 126) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Activity of compounds of the present invention was examined by determination to what extent the glutamate-induced elevation of the intracellular calc... | US Patent US8853203 (2014) BindingDB Entry DOI: 10.7270/Q2MP520T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM136065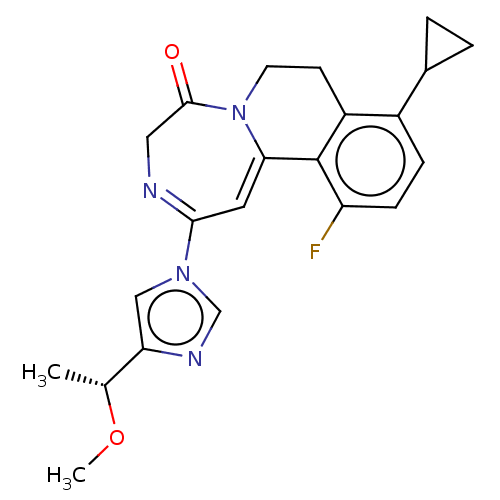 (US8853203, 99j) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Activity of compounds of the present invention was examined by determination to what extent the glutamate-induced elevation of the intracellular calc... | US Patent US8853203 (2014) BindingDB Entry DOI: 10.7270/Q2MP520T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM136078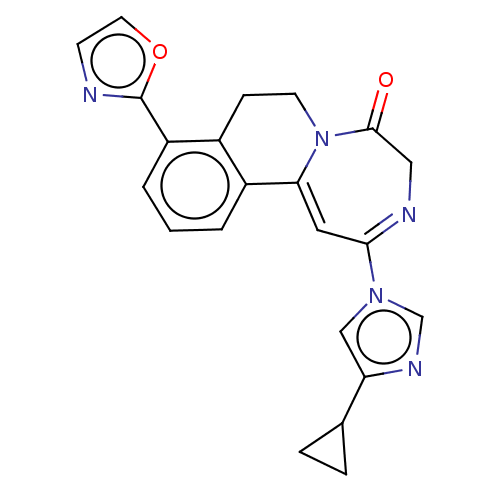 (US8853203, 104 | US9650377, Example 104) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Activity of compounds of the present invention was examined by determination to what extent the glutamate-induced elevation of the intracellular calc... | US Patent US8853203 (2014) BindingDB Entry DOI: 10.7270/Q2MP520T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM135998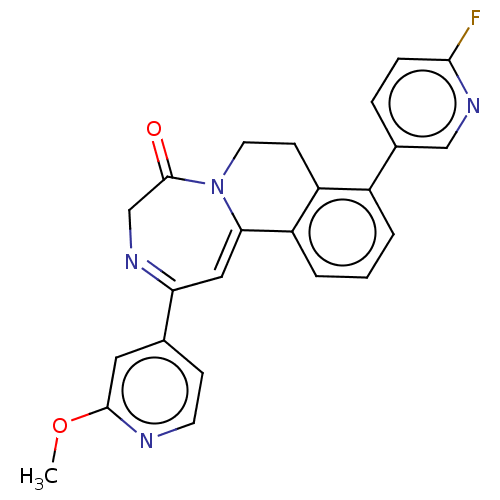 (US8853203, 53 | US9650377, Example 53) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Activity of compounds of the present invention was examined by determination to what extent the glutamate-induced elevation of the intracellular calc... | US Patent US8853203 (2014) BindingDB Entry DOI: 10.7270/Q2MP520T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 854 total ) | Next | Last >> |