 Found 82 hits with Last Name = 'caselli' and Initial = 'a'
Found 82 hits with Last Name = 'caselli' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50367249

(CHEMBL309601)Show SMILES C[C@H](NP(O)(=O)CCc1ccccc1)C(=O)N1CCC[C@H]1C(O)=O Show InChI InChI=1S/C16H23N2O5P/c1-12(15(19)18-10-5-8-14(18)16(20)21)17-24(22,23)11-9-13-6-3-2-4-7-13/h2-4,6-7,12,14H,5,8-11H2,1H3,(H,20,21)(H2,17,22,23)/t12-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Angiotensin I converting enzyme |
J Med Chem 28: 393-9 (1985)
BindingDB Entry DOI: 10.7270/Q28W3DWT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50020843

(2-[(2S)-2-carboxypyrrolidin-1-yl]-1-methyl-2-oxoet...)Show SMILES C[C@@H](NP([O-])([O-])=O)C(=O)N1CCC[C@H]1C(O)=O Show InChI InChI=1S/C8H15N2O6P/c1-5(9-17(14,15)16)7(11)10-4-2-3-6(10)8(12)13/h5-6H,2-4H2,1H3,(H,12,13)(H3,9,14,15,16)/p-2/t5?,6-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Angiotensin I converting enzyme |
J Med Chem 28: 393-9 (1985)
BindingDB Entry DOI: 10.7270/Q28W3DWT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50444695
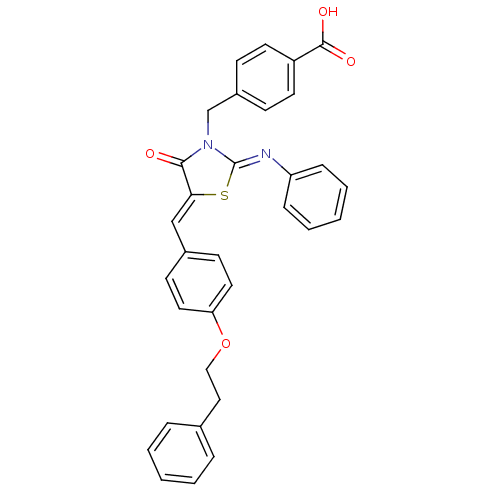
(CHEMBL3098852)Show SMILES OC(=O)c1ccc(CN2\C(S\C(=C/c3ccc(OCCc4ccccc4)cc3)C2=O)=N\c2ccccc2)cc1 Show InChI InChI=1S/C32H26N2O4S/c35-30-29(21-24-13-17-28(18-14-24)38-20-19-23-7-3-1-4-8-23)39-32(33-27-9-5-2-6-10-27)34(30)22-25-11-15-26(16-12-25)31(36)37/h1-18,21H,19-20,22H2,(H,36,37)/b29-21-,33-32- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human recombinant PTP1B expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate by Double reciprocal plo... |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50444693
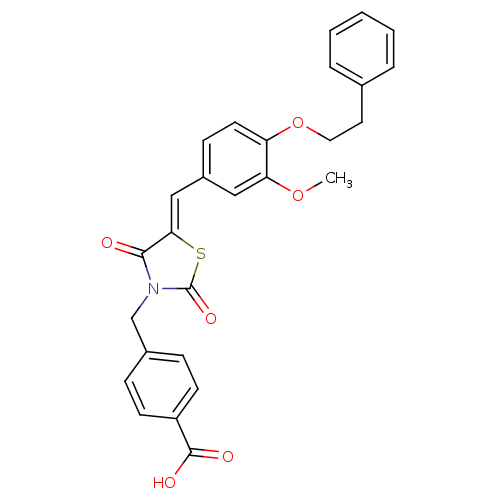
(CHEMBL3098944)Show SMILES COc1cc(\C=C2/SC(=O)N(Cc3ccc(cc3)C(O)=O)C2=O)ccc1OCCc1ccccc1 Show InChI InChI=1S/C27H23NO6S/c1-33-23-15-20(9-12-22(23)34-14-13-18-5-3-2-4-6-18)16-24-25(29)28(27(32)35-24)17-19-7-10-21(11-8-19)26(30)31/h2-12,15-16H,13-14,17H2,1H3,(H,30,31)/b24-16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of human recombinant PTP1B expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate by Double reciprocal... |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50444694

(CHEMBL3098854)Show SMILES COc1cc(\C=C2/S\C(=N/c3ccccc3)N(Cc3ccc(cc3)C(O)=O)C2=O)ccc1OCCc1ccccc1 Show InChI InChI=1S/C33H28N2O5S/c1-39-29-20-25(14-17-28(29)40-19-18-23-8-4-2-5-9-23)21-30-31(36)35(22-24-12-15-26(16-13-24)32(37)38)33(41-30)34-27-10-6-3-7-11-27/h2-17,20-21H,18-19,22H2,1H3,(H,37,38)/b30-21-,34-33- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human recombinant PTP1B expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate by Double reciprocal plo... |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50444692

(CHEMBL3098853)Show SMILES COc1ccc(\C=C2/S\C(=N/c3ccccc3)N(Cc3ccc(cc3)C(O)=O)C2=O)cc1OCCc1ccccc1 Show InChI InChI=1S/C33H28N2O5S/c1-39-28-17-14-25(20-29(28)40-19-18-23-8-4-2-5-9-23)21-30-31(36)35(22-24-12-15-26(16-13-24)32(37)38)33(41-30)34-27-10-6-3-7-11-27/h2-17,20-21H,18-19,22H2,1H3,(H,37,38)/b30-21-,34-33- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant PTP1B expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate by Double reciprocal pl... |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50444696
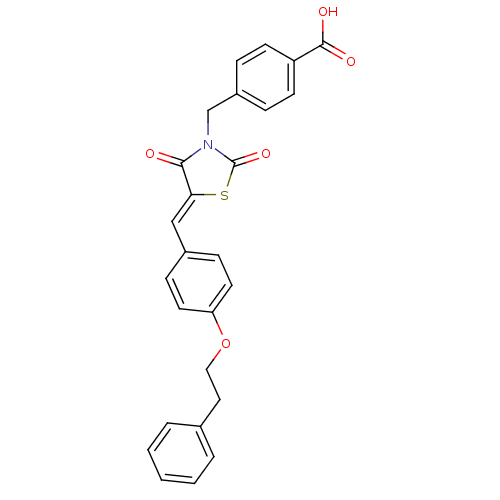
(CHEMBL3098942)Show SMILES OC(=O)c1ccc(CN2C(=O)S\C(=C/c3ccc(OCCc4ccccc4)cc3)C2=O)cc1 Show InChI InChI=1S/C26H21NO5S/c28-24-23(33-26(31)27(24)17-20-6-10-21(11-7-20)25(29)30)16-19-8-12-22(13-9-19)32-15-14-18-4-2-1-3-5-18/h1-13,16H,14-15,17H2,(H,29,30)/b23-16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human recombinant PTP1B expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate by Double reciprocal plo... |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50022582

(CHEMBL53139 | Diastereomer-5-[2-[2-Benzyl-3-(2-met...)Show SMILES CCCCNC(=O)[C@@H](CC(O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)C(Cc1ccccc1)CS(=O)(=O)C(C)(C)C)C(C)C Show InChI InChI=1S/C39H63N5O6S/c1-7-8-19-41-37(47)32(27(2)3)23-35(45)33(21-29-17-13-10-14-18-29)43-38(48)34(22-31-24-40-26-42-31)44-36(46)30(20-28-15-11-9-12-16-28)25-51(49,50)39(4,5)6/h9,11-12,15-16,24,26-27,29-30,32-35,45H,7-8,10,13-14,17-23,25H2,1-6H3,(H,40,42)(H,41,47)(H,43,48)(H,44,46)/t30?,32-,33-,34-,35?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy Limited
Curated by ChEMBL
| Assay Description
In vitro inhibition of Human kideny renin |
J Med Chem 31: 1839-46 (1988)
BindingDB Entry DOI: 10.7270/Q2Z03744 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Renin
(Homo sapiens (Human)) | BDBM50022582

(CHEMBL53139 | Diastereomer-5-[2-[2-Benzyl-3-(2-met...)Show SMILES CCCCNC(=O)[C@@H](CC(O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)C(Cc1ccccc1)CS(=O)(=O)C(C)(C)C)C(C)C Show InChI InChI=1S/C39H63N5O6S/c1-7-8-19-41-37(47)32(27(2)3)23-35(45)33(21-29-17-13-10-14-18-29)43-38(48)34(22-31-24-40-26-42-31)44-36(46)30(20-28-15-11-9-12-16-28)25-51(49,50)39(4,5)6/h9,11-12,15-16,24,26-27,29-30,32-35,45H,7-8,10,13-14,17-23,25H2,1-6H3,(H,40,42)(H,41,47)(H,43,48)(H,44,46)/t30?,32-,33-,34-,35?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy Limited
Curated by ChEMBL
| Assay Description
In vitro inhibition of marmoset plasma renin |
J Med Chem 31: 1839-46 (1988)
BindingDB Entry DOI: 10.7270/Q2Z03744 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Renin
(Homo sapiens (Human)) | BDBM50022582

(CHEMBL53139 | Diastereomer-5-[2-[2-Benzyl-3-(2-met...)Show SMILES CCCCNC(=O)[C@@H](CC(O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)C(Cc1ccccc1)CS(=O)(=O)C(C)(C)C)C(C)C Show InChI InChI=1S/C39H63N5O6S/c1-7-8-19-41-37(47)32(27(2)3)23-35(45)33(21-29-17-13-10-14-18-29)43-38(48)34(22-31-24-40-26-42-31)44-36(46)30(20-28-15-11-9-12-16-28)25-51(49,50)39(4,5)6/h9,11-12,15-16,24,26-27,29-30,32-35,45H,7-8,10,13-14,17-23,25H2,1-6H3,(H,40,42)(H,41,47)(H,43,48)(H,44,46)/t30?,32-,33-,34-,35?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy Limited
Curated by ChEMBL
| Assay Description
In vitro inhibition of Human plasma renin |
J Med Chem 31: 1839-46 (1988)
BindingDB Entry DOI: 10.7270/Q2Z03744 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Renin
(Homo sapiens (Human)) | BDBM50022582

(CHEMBL53139 | Diastereomer-5-[2-[2-Benzyl-3-(2-met...)Show SMILES CCCCNC(=O)[C@@H](CC(O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)C(Cc1ccccc1)CS(=O)(=O)C(C)(C)C)C(C)C Show InChI InChI=1S/C39H63N5O6S/c1-7-8-19-41-37(47)32(27(2)3)23-35(45)33(21-29-17-13-10-14-18-29)43-38(48)34(22-31-24-40-26-42-31)44-36(46)30(20-28-15-11-9-12-16-28)25-51(49,50)39(4,5)6/h9,11-12,15-16,24,26-27,29-30,32-35,45H,7-8,10,13-14,17-23,25H2,1-6H3,(H,40,42)(H,41,47)(H,43,48)(H,44,46)/t30?,32-,33-,34-,35?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Ciba-Geigy Limited
Curated by ChEMBL
| Assay Description
In vitro inhibition of porcine plasma renin |
J Med Chem 31: 1839-46 (1988)
BindingDB Entry DOI: 10.7270/Q2Z03744 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Renin
(Homo sapiens (Human)) | BDBM50022582

(CHEMBL53139 | Diastereomer-5-[2-[2-Benzyl-3-(2-met...)Show SMILES CCCCNC(=O)[C@@H](CC(O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)C(Cc1ccccc1)CS(=O)(=O)C(C)(C)C)C(C)C Show InChI InChI=1S/C39H63N5O6S/c1-7-8-19-41-37(47)32(27(2)3)23-35(45)33(21-29-17-13-10-14-18-29)43-38(48)34(22-31-24-40-26-42-31)44-36(46)30(20-28-15-11-9-12-16-28)25-51(49,50)39(4,5)6/h9,11-12,15-16,24,26-27,29-30,32-35,45H,7-8,10,13-14,17-23,25H2,1-6H3,(H,40,42)(H,41,47)(H,43,48)(H,44,46)/t30?,32-,33-,34-,35?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Ciba-Geigy Limited
Curated by ChEMBL
| Assay Description
In vitro inhibition of marmoset plasma renin |
J Med Chem 31: 1839-46 (1988)
BindingDB Entry DOI: 10.7270/Q2Z03744 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Renin
(Homo sapiens (Human)) | BDBM50022586
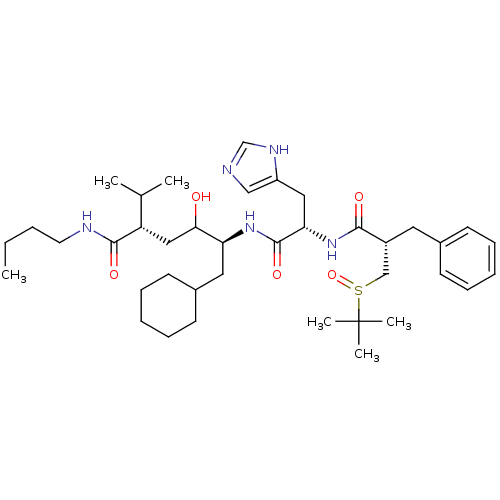
(5-[2-[2-Benzyl-3-(2-methyl-propane-2-sulfinyl)-pro...)Show SMILES CCCCNC(=O)[C@@H](CC(O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)CS(=O)C(C)(C)C)C(C)C Show InChI InChI=1S/C39H63N5O5S/c1-7-8-19-41-37(47)32(27(2)3)23-35(45)33(21-29-17-13-10-14-18-29)43-38(48)34(22-31-24-40-26-42-31)44-36(46)30(25-50(49)39(4,5)6)20-28-15-11-9-12-16-28/h9,11-12,15-16,24,26-27,29-30,32-35,45H,7-8,10,13-14,17-23,25H2,1-6H3,(H,40,42)(H,41,47)(H,43,48)(H,44,46)/t30-,32+,33+,34+,35?,50?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy Limited
Curated by ChEMBL
| Assay Description
In vitro inhibition of purified Human kidney renin (250 pg/mL) incubated with angiotensinogen at pH 7.2 |
J Med Chem 31: 1839-46 (1988)
BindingDB Entry DOI: 10.7270/Q2Z03744 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Renin
(Homo sapiens (Human)) | BDBM50022582

(CHEMBL53139 | Diastereomer-5-[2-[2-Benzyl-3-(2-met...)Show SMILES CCCCNC(=O)[C@@H](CC(O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)C(Cc1ccccc1)CS(=O)(=O)C(C)(C)C)C(C)C Show InChI InChI=1S/C39H63N5O6S/c1-7-8-19-41-37(47)32(27(2)3)23-35(45)33(21-29-17-13-10-14-18-29)43-38(48)34(22-31-24-40-26-42-31)44-36(46)30(20-28-15-11-9-12-16-28)25-51(49,50)39(4,5)6/h9,11-12,15-16,24,26-27,29-30,32-35,45H,7-8,10,13-14,17-23,25H2,1-6H3,(H,40,42)(H,41,47)(H,43,48)(H,44,46)/t30?,32-,33-,34-,35?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy Limited
Curated by ChEMBL
| Assay Description
In vitro inhibition of purified Human kidney renin (250 pg/mL) incubated with angiotensinogen at pH 7.2 |
J Med Chem 31: 1839-46 (1988)
BindingDB Entry DOI: 10.7270/Q2Z03744 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Renin
(Homo sapiens (Human)) | BDBM50022587

(5-[2-(2-tert-Butylsulfanylmethyl-3-phenyl-propiony...)Show SMILES CCCCNC(=O)[C@@H](CC(O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H](CSC(C)(C)C)Cc1ccccc1)C(C)C Show InChI InChI=1S/C39H63N5O4S/c1-7-8-19-41-37(47)32(27(2)3)23-35(45)33(21-29-17-13-10-14-18-29)43-38(48)34(22-31-24-40-26-42-31)44-36(46)30(25-49-39(4,5)6)20-28-15-11-9-12-16-28/h9,11-12,15-16,24,26-27,29-30,32-35,45H,7-8,10,13-14,17-23,25H2,1-6H3,(H,40,42)(H,41,47)(H,43,48)(H,44,46)/t30-,32+,33+,34+,35?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy Limited
Curated by ChEMBL
| Assay Description
In vitro inhibition of purified Human kidney renin (250 pg/mL) incubated with angiotensinogen at pH 7.2 |
J Med Chem 31: 1839-46 (1988)
BindingDB Entry DOI: 10.7270/Q2Z03744 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Renin
(Homo sapiens (Human)) | BDBM50022588
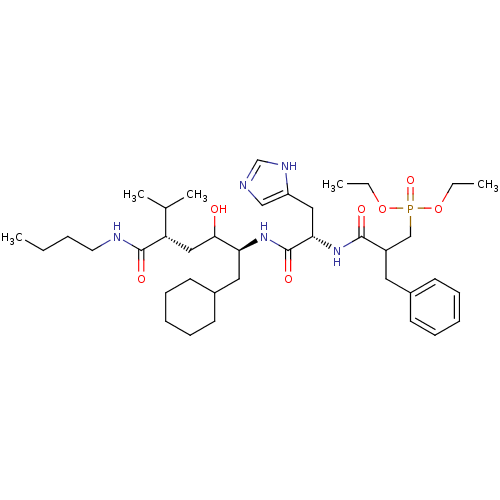
(CHEMBL291787 | {2-[1-(4-Butylcarbamoyl-1-cyclohexy...)Show SMILES CCCCNC(=O)[C@@H](CC(O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)C(Cc1ccccc1)CP(=O)(OCC)OCC)C(C)C Show InChI InChI=1S/C39H64N5O7P/c1-6-9-20-41-38(47)33(28(4)5)24-36(45)34(22-30-18-14-11-15-19-30)43-39(48)35(23-32-25-40-27-42-32)44-37(46)31(21-29-16-12-10-13-17-29)26-52(49,50-7-2)51-8-3/h10,12-13,16-17,25,27-28,30-31,33-36,45H,6-9,11,14-15,18-24,26H2,1-5H3,(H,40,42)(H,41,47)(H,43,48)(H,44,46)/t31?,33-,34-,35-,36?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy Limited
Curated by ChEMBL
| Assay Description
In vitro inhibition of purified Human kidney renin (250 pg/mL) incubated with angiotensinogen at pH 7.2 |
J Med Chem 31: 1839-46 (1988)
BindingDB Entry DOI: 10.7270/Q2Z03744 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50022584

(2-Benzyl-5,5-dimethyl-4-oxo-hexanoic acid [1-(4-bu...)Show SMILES CCCCNC(=O)[C@@H](CC(O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)C(CC(=O)C(C)(C)C)Cc1ccccc1)C(C)C Show InChI InChI=1S/C40H63N5O5/c1-7-8-19-42-38(49)32(27(2)3)24-35(46)33(21-29-17-13-10-14-18-29)44-39(50)34(23-31-25-41-26-43-31)45-37(48)30(22-36(47)40(4,5)6)20-28-15-11-9-12-16-28/h9,11-12,15-16,25-27,29-30,32-35,46H,7-8,10,13-14,17-24H2,1-6H3,(H,41,43)(H,42,49)(H,44,50)(H,45,48)/t30?,32-,33-,34-,35?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy Limited
Curated by ChEMBL
| Assay Description
In vitro inhibition of purified Human kidney renin (250 pg/mL) incubated with angiotensinogen at pH 7.2 |
J Med Chem 31: 1839-46 (1988)
BindingDB Entry DOI: 10.7270/Q2Z03744 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50022590
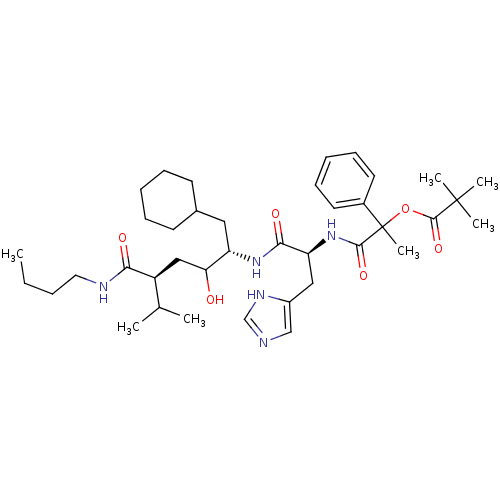
(2,2-Dimethyl-propionic acid 1-[1-(4-butylcarbamoyl...)Show SMILES CCCCNC(=O)[C@@H](CC(O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)C(C)(OC(=O)C(C)(C)C)c1ccccc1)C(C)C Show InChI InChI=1S/C39H61N5O6/c1-8-9-20-41-34(46)30(26(2)3)23-33(45)31(21-27-16-12-10-13-17-27)43-35(47)32(22-29-24-40-25-42-29)44-36(48)39(7,28-18-14-11-15-19-28)50-37(49)38(4,5)6/h11,14-15,18-19,24-27,30-33,45H,8-10,12-13,16-17,20-23H2,1-7H3,(H,40,42)(H,41,46)(H,43,47)(H,44,48)/t30-,31-,32-,33?,39?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy Limited
Curated by ChEMBL
| Assay Description
In vitro inhibition of purified Human kidney renin (250 pg/mL) incubated with angiotensinogen at pH 7.2 |
J Med Chem 31: 1839-46 (1988)
BindingDB Entry DOI: 10.7270/Q2Z03744 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50022859
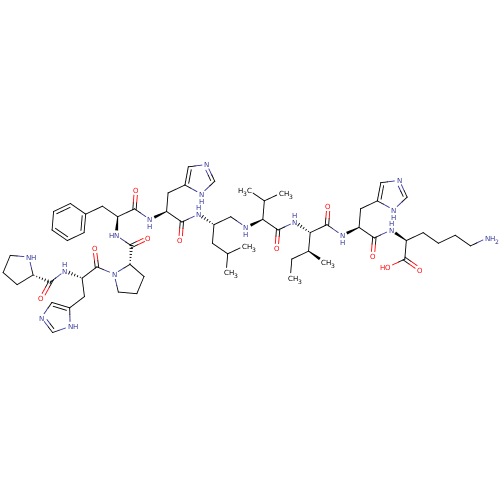
(CHEMBL407670 | Pro-His-Pro-Phe-His-Leu[CH2NH]Val-I...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CCCN1)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CCCCN)C(O)=O Show InChI InChI=1S/C60H91N17O10/c1-7-37(6)51(58(84)74-47(26-40-29-63-33-68-40)55(81)71-44(60(86)87)17-11-12-20-61)76-57(83)50(36(4)5)66-31-42(23-35(2)3)70-53(79)46(25-39-28-62-32-67-39)72-54(80)45(24-38-15-9-8-10-16-38)73-56(82)49-19-14-22-77(49)59(85)48(27-41-30-64-34-69-41)75-52(78)43-18-13-21-65-43/h8-10,15-16,28-30,32-37,42-51,65-66H,7,11-14,17-27,31,61H2,1-6H3,(H,62,67)(H,63,68)(H,64,69)(H,70,79)(H,71,81)(H,72,80)(H,73,82)(H,74,84)(H,75,78)(H,76,83)(H,86,87)/t37-,42-,43-,44-,45-,46-,47-,48-,49-,50-,51-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy Limited
Curated by ChEMBL
| Assay Description
In vitro inhibition of purified Human kidney renin (250 pg/mL) incubated with angiotensinogen at pH 7.2 |
J Med Chem 31: 1839-46 (1988)
BindingDB Entry DOI: 10.7270/Q2Z03744 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50022589
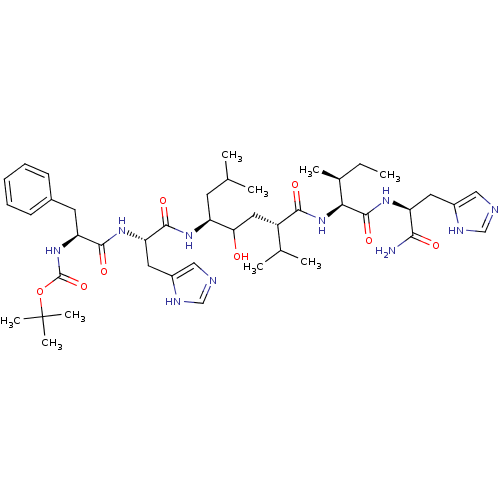
(CHEMBL435178 | {1-[1-(4-{1-[1-Carbamoyl-2-(3H-imid...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](CC(O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(N)=O Show InChI InChI=1S/C44H68N10O8/c1-10-27(6)37(42(60)51-33(38(45)56)18-29-21-46-23-48-29)54-39(57)31(26(4)5)20-36(55)32(16-25(2)3)50-41(59)35(19-30-22-47-24-49-30)52-40(58)34(17-28-14-12-11-13-15-28)53-43(61)62-44(7,8)9/h11-15,21-27,31-37,55H,10,16-20H2,1-9H3,(H2,45,56)(H,46,48)(H,47,49)(H,50,59)(H,51,60)(H,52,58)(H,53,61)(H,54,57)/t27-,31-,32-,33-,34-,35-,36?,37-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy Limited
Curated by ChEMBL
| Assay Description
In vitro inhibition of purified Human kidney renin (250 pg/mL) incubated with angiotensinogen at pH 7.2 |
J Med Chem 31: 1839-46 (1988)
BindingDB Entry DOI: 10.7270/Q2Z03744 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50022585
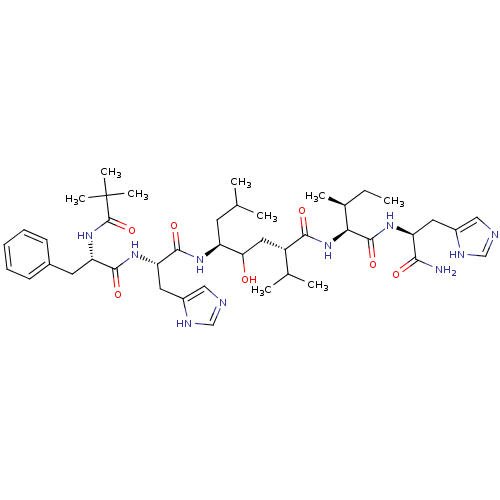
(5-[2-[2-(2,2-Dimethyl-propionylamino)-3-phenyl-pro...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](CC(O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)C(C)(C)C)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(N)=O Show InChI InChI=1S/C44H68N10O7/c1-10-27(6)37(42(60)51-33(38(45)56)18-29-21-46-23-48-29)54-39(57)31(26(4)5)20-36(55)32(16-25(2)3)50-41(59)35(19-30-22-47-24-49-30)52-40(58)34(53-43(61)44(7,8)9)17-28-14-12-11-13-15-28/h11-15,21-27,31-37,55H,10,16-20H2,1-9H3,(H2,45,56)(H,46,48)(H,47,49)(H,50,59)(H,51,60)(H,52,58)(H,53,61)(H,54,57)/t27-,31-,32-,33-,34-,35-,36?,37-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy Limited
Curated by ChEMBL
| Assay Description
In vitro inhibition of purified Human kidney renin (250 pg/mL) incubated with angiotensinogen at pH 7.2 |
J Med Chem 31: 1839-46 (1988)
BindingDB Entry DOI: 10.7270/Q2Z03744 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50022591
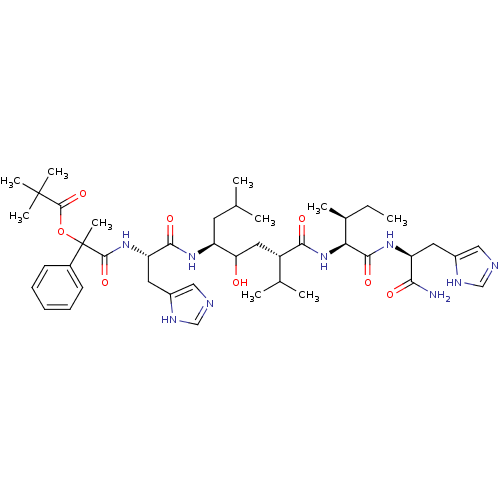
(2,2-Dimethyl-propionic acid 1-[1-(4-{1-[1-carbamoy...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](CC(O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)C(C)(OC(=O)C(C)(C)C)c1ccccc1)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(N)=O Show InChI InChI=1S/C44H67N9O8/c1-11-27(6)36(40(58)51-33(37(45)55)18-29-21-46-23-48-29)53-38(56)31(26(4)5)20-35(54)32(17-25(2)3)50-39(57)34(19-30-22-47-24-49-30)52-41(59)44(10,28-15-13-12-14-16-28)61-42(60)43(7,8)9/h12-16,21-27,31-36,54H,11,17-20H2,1-10H3,(H2,45,55)(H,46,48)(H,47,49)(H,50,57)(H,51,58)(H,52,59)(H,53,56)/t27-,31-,32-,33-,34-,35?,36-,44?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy Limited
Curated by ChEMBL
| Assay Description
In vitro inhibition of purified Human kidney renin (250 pg/mL) incubated with angiotensinogen at pH 7.2 |
J Med Chem 31: 1839-46 (1988)
BindingDB Entry DOI: 10.7270/Q2Z03744 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50022581
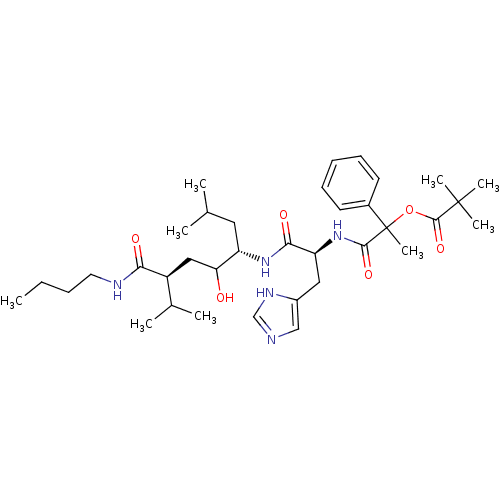
(2,2-Dimethyl-propionic acid 1-[1-(4-butylcarbamoyl...)Show SMILES CCCCNC(=O)[C@@H](CC(O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)C(C)(OC(=O)C(C)(C)C)c1ccccc1)C(C)C Show InChI InChI=1S/C36H57N5O6/c1-10-11-17-38-31(43)27(24(4)5)20-30(42)28(18-23(2)3)40-32(44)29(19-26-21-37-22-39-26)41-33(45)36(9,25-15-13-12-14-16-25)47-34(46)35(6,7)8/h12-16,21-24,27-30,42H,10-11,17-20H2,1-9H3,(H,37,39)(H,38,43)(H,40,44)(H,41,45)/t27-,28-,29-,30?,36?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy Limited
Curated by ChEMBL
| Assay Description
In vitro inhibition of purified Human kidney renin (250 pg/mL) incubated with angiotensinogen at pH 7.2 |
J Med Chem 31: 1839-46 (1988)
BindingDB Entry DOI: 10.7270/Q2Z03744 |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50022582

(CHEMBL53139 | Diastereomer-5-[2-[2-Benzyl-3-(2-met...)Show SMILES CCCCNC(=O)[C@@H](CC(O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)C(Cc1ccccc1)CS(=O)(=O)C(C)(C)C)C(C)C Show InChI InChI=1S/C39H63N5O6S/c1-7-8-19-41-37(47)32(27(2)3)23-35(45)33(21-29-17-13-10-14-18-29)43-38(48)34(22-31-24-40-26-42-31)44-36(46)30(20-28-15-11-9-12-16-28)25-51(49,50)39(4,5)6/h9,11-12,15-16,24,26-27,29-30,32-35,45H,7-8,10,13-14,17-23,25H2,1-6H3,(H,40,42)(H,41,47)(H,43,48)(H,44,46)/t30?,32-,33-,34-,35?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy Limited
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat plasma renin |
J Med Chem 31: 1839-46 (1988)
BindingDB Entry DOI: 10.7270/Q2Z03744 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50444695

(CHEMBL3098852)Show SMILES OC(=O)c1ccc(CN2\C(S\C(=C/c3ccc(OCCc4ccccc4)cc3)C2=O)=N\c2ccccc2)cc1 Show InChI InChI=1S/C32H26N2O4S/c35-30-29(21-24-13-17-28(18-14-24)38-20-19-23-7-3-1-4-8-23)39-32(33-27-9-5-2-6-10-27)34(30)22-25-11-15-26(16-12-25)31(36)37/h1-18,21H,19-20,22H2,(H,36,37)/b29-21-,33-32- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of TC-PTP (unknown origin) expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50444697
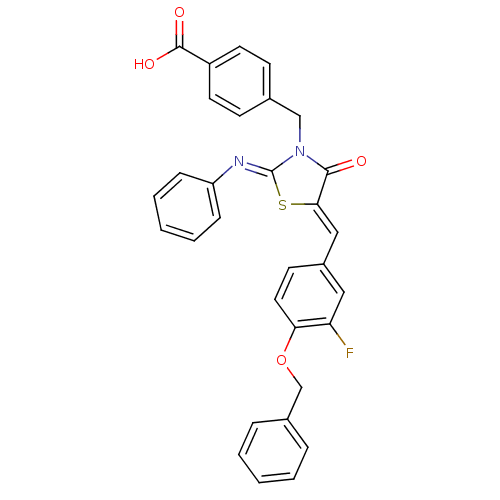
(CHEMBL3098857)Show SMILES OC(=O)c1ccc(CN2C(=O)\C(S\C2=N/c2ccccc2)=C\c2ccc(OCc3ccccc3)c(F)c2)cc1 Show InChI InChI=1S/C31H23FN2O4S/c32-26-17-23(13-16-27(26)38-20-22-7-3-1-4-8-22)18-28-29(35)34(19-21-11-14-24(15-12-21)30(36)37)31(39-28)33-25-9-5-2-6-10-25/h1-18H,19-20H2,(H,36,37)/b28-18-,33-31- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of TC-PTP (unknown origin) expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50444693

(CHEMBL3098944)Show SMILES COc1cc(\C=C2/SC(=O)N(Cc3ccc(cc3)C(O)=O)C2=O)ccc1OCCc1ccccc1 Show InChI InChI=1S/C27H23NO6S/c1-33-23-15-20(9-12-22(23)34-14-13-18-5-3-2-4-6-18)16-24-25(29)28(27(32)35-24)17-19-7-10-21(11-8-19)26(30)31/h2-12,15-16H,13-14,17H2,1H3,(H,30,31)/b24-16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50444695

(CHEMBL3098852)Show SMILES OC(=O)c1ccc(CN2\C(S\C(=C/c3ccc(OCCc4ccccc4)cc3)C2=O)=N\c2ccccc2)cc1 Show InChI InChI=1S/C32H26N2O4S/c35-30-29(21-24-13-17-28(18-14-24)38-20-19-23-7-3-1-4-8-23)39-32(33-27-9-5-2-6-10-27)34(30)22-25-11-15-26(16-12-25)31(36)37/h1-18,21H,19-20,22H2,(H,36,37)/b29-21-,33-32- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50444698
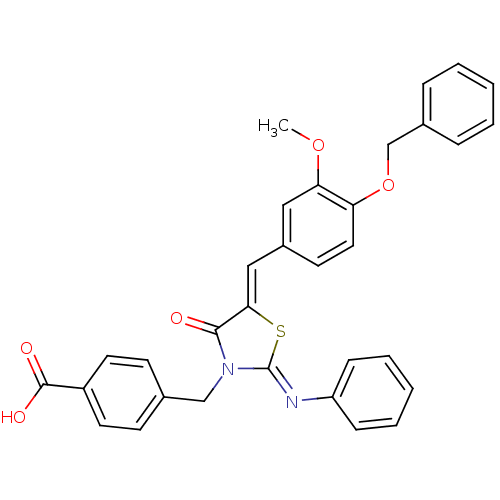
(CHEMBL3098856)Show SMILES COc1cc(\C=C2/S\C(=N/c3ccccc3)N(Cc3ccc(cc3)C(O)=O)C2=O)ccc1OCc1ccccc1 Show InChI InChI=1S/C32H26N2O5S/c1-38-28-18-24(14-17-27(28)39-21-23-8-4-2-5-9-23)19-29-30(35)34(20-22-12-15-25(16-13-22)31(36)37)32(40-29)33-26-10-6-3-7-11-26/h2-19H,20-21H2,1H3,(H,36,37)/b29-19-,33-32- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50444692

(CHEMBL3098853)Show SMILES COc1ccc(\C=C2/S\C(=N/c3ccccc3)N(Cc3ccc(cc3)C(O)=O)C2=O)cc1OCCc1ccccc1 Show InChI InChI=1S/C33H28N2O5S/c1-39-28-17-14-25(20-29(28)40-19-18-23-8-4-2-5-9-23)21-30-31(36)35(22-24-12-15-26(16-13-24)32(37)38)33(41-30)34-27-10-6-3-7-11-27/h2-17,20-21H,18-19,22H2,1H3,(H,37,38)/b30-21-,34-33- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50444700
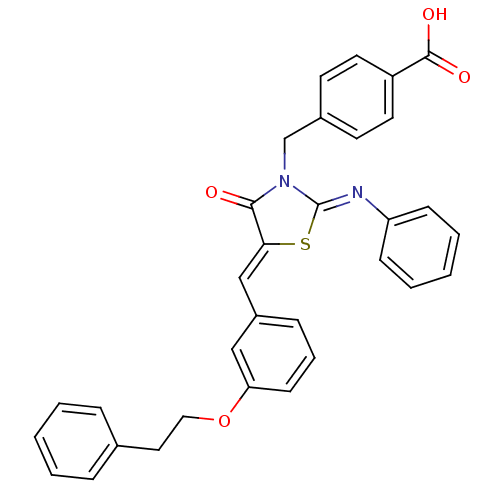
(CHEMBL3098851)Show SMILES OC(=O)c1ccc(CN2\C(S\C(=C/c3cccc(OCCc4ccccc4)c3)C2=O)=N\c2ccccc2)cc1 Show InChI InChI=1S/C32H26N2O4S/c35-30-29(21-25-10-7-13-28(20-25)38-19-18-23-8-3-1-4-9-23)39-32(33-27-11-5-2-6-12-27)34(30)22-24-14-16-26(17-15-24)31(36)37/h1-17,20-21H,18-19,22H2,(H,36,37)/b29-21-,33-32- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of TC-PTP (unknown origin) expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50444697

(CHEMBL3098857)Show SMILES OC(=O)c1ccc(CN2C(=O)\C(S\C2=N/c2ccccc2)=C\c2ccc(OCc3ccccc3)c(F)c2)cc1 Show InChI InChI=1S/C31H23FN2O4S/c32-26-17-23(13-16-27(26)38-20-22-7-3-1-4-8-22)18-28-29(35)34(19-21-11-14-24(15-12-21)30(36)37)31(39-28)33-25-9-5-2-6-10-25/h1-18H,19-20H2,(H,36,37)/b28-18-,33-31- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50444696

(CHEMBL3098942)Show SMILES OC(=O)c1ccc(CN2C(=O)S\C(=C/c3ccc(OCCc4ccccc4)cc3)C2=O)cc1 Show InChI InChI=1S/C26H21NO5S/c28-24-23(33-26(31)27(24)17-20-6-10-21(11-7-20)25(29)30)16-19-8-12-22(13-9-19)32-15-14-18-4-2-1-3-5-18/h1-13,16H,14-15,17H2,(H,29,30)/b23-16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50444694

(CHEMBL3098854)Show SMILES COc1cc(\C=C2/S\C(=N/c3ccccc3)N(Cc3ccc(cc3)C(O)=O)C2=O)ccc1OCCc1ccccc1 Show InChI InChI=1S/C33H28N2O5S/c1-39-29-20-25(14-17-28(29)40-19-18-23-8-4-2-5-9-23)21-30-31(36)35(22-24-12-15-26(16-13-24)32(37)38)33(41-30)34-27-10-6-3-7-11-27/h2-17,20-21H,18-19,22H2,1H3,(H,37,38)/b30-21-,34-33- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Bos taurus) | BDBM50022582

(CHEMBL53139 | Diastereomer-5-[2-[2-Benzyl-3-(2-met...)Show SMILES CCCCNC(=O)[C@@H](CC(O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)C(Cc1ccccc1)CS(=O)(=O)C(C)(C)C)C(C)C Show InChI InChI=1S/C39H63N5O6S/c1-7-8-19-41-37(47)32(27(2)3)23-35(45)33(21-29-17-13-10-14-18-29)43-38(48)34(22-31-24-40-26-42-31)44-36(46)30(20-28-15-11-9-12-16-28)25-51(49,50)39(4,5)6/h9,11-12,15-16,24,26-27,29-30,32-35,45H,7-8,10,13-14,17-23,25H2,1-6H3,(H,40,42)(H,41,47)(H,43,48)(H,44,46)/t30?,32-,33-,34-,35?/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy Limited
Curated by ChEMBL
| Assay Description
In vitro inhibition of bovine cathepsin D |
J Med Chem 31: 1839-46 (1988)
BindingDB Entry DOI: 10.7270/Q2Z03744 |
More data for this
Ligand-Target Pair | |
Low molecular weight phosphotyrosine protein phosphatase
(Homo sapiens (Human)) | BDBM50444697

(CHEMBL3098857)Show SMILES OC(=O)c1ccc(CN2C(=O)\C(S\C2=N/c2ccccc2)=C\c2ccc(OCc3ccccc3)c(F)c2)cc1 Show InChI InChI=1S/C31H23FN2O4S/c32-26-17-23(13-16-27(26)38-20-22-7-3-1-4-8-22)18-28-29(35)34(19-21-11-14-24(15-12-21)30(36)37)31(39-28)33-25-9-5-2-6-10-25/h1-18H,19-20H2,(H,36,37)/b28-18-,33-31- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LMW-PTP IF1 expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50444694

(CHEMBL3098854)Show SMILES COc1cc(\C=C2/S\C(=N/c3ccccc3)N(Cc3ccc(cc3)C(O)=O)C2=O)ccc1OCCc1ccccc1 Show InChI InChI=1S/C33H28N2O5S/c1-39-29-20-25(14-17-28(29)40-19-18-23-8-4-2-5-9-23)21-30-31(36)35(22-24-12-15-26(16-13-24)32(37)38)33(41-30)34-27-10-6-3-7-11-27/h2-17,20-21H,18-19,22H2,1H3,(H,37,38)/b30-21-,34-33- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of TC-PTP (unknown origin) expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50444702
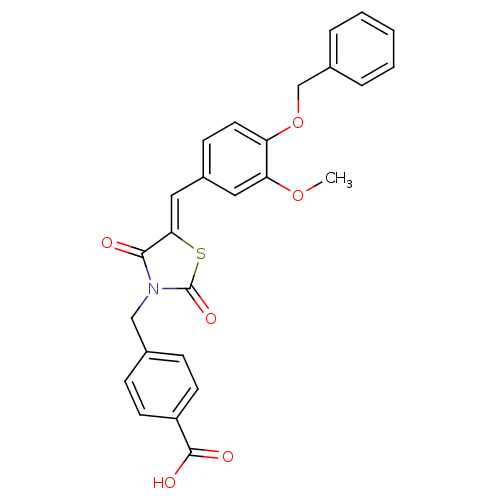
(CHEMBL3098946)Show SMILES COc1cc(\C=C2/SC(=O)N(Cc3ccc(cc3)C(O)=O)C2=O)ccc1OCc1ccccc1 Show InChI InChI=1S/C26H21NO6S/c1-32-22-13-19(9-12-21(22)33-16-18-5-3-2-4-6-18)14-23-24(28)27(26(31)34-23)15-17-7-10-20(11-8-17)25(29)30/h2-14H,15-16H2,1H3,(H,29,30)/b23-14- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of TC-PTP (unknown origin) expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50444693

(CHEMBL3098944)Show SMILES COc1cc(\C=C2/SC(=O)N(Cc3ccc(cc3)C(O)=O)C2=O)ccc1OCCc1ccccc1 Show InChI InChI=1S/C27H23NO6S/c1-33-23-15-20(9-12-22(23)34-14-13-18-5-3-2-4-6-18)16-24-25(29)28(27(32)35-24)17-19-7-10-21(11-8-19)26(30)31/h2-12,15-16H,13-14,17H2,1H3,(H,30,31)/b24-16- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of TC-PTP (unknown origin) expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50444699
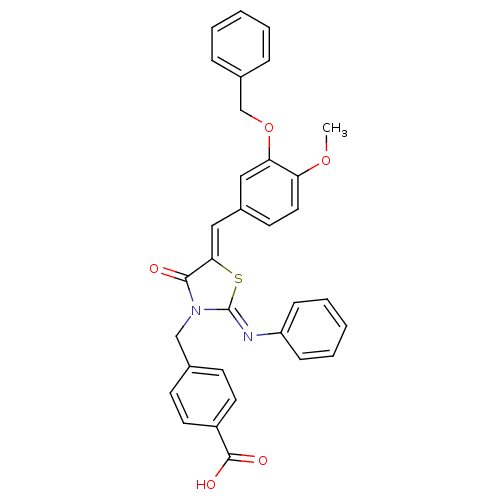
(CHEMBL3098855)Show SMILES COc1ccc(\C=C2/S\C(=N/c3ccccc3)N(Cc3ccc(cc3)C(O)=O)C2=O)cc1OCc1ccccc1 Show InChI InChI=1S/C32H26N2O5S/c1-38-27-17-14-24(18-28(27)39-21-23-8-4-2-5-9-23)19-29-30(35)34(20-22-12-15-25(16-13-22)31(36)37)32(40-29)33-26-10-6-3-7-11-26/h2-19H,20-21H2,1H3,(H,36,37)/b29-19-,33-32- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50444703
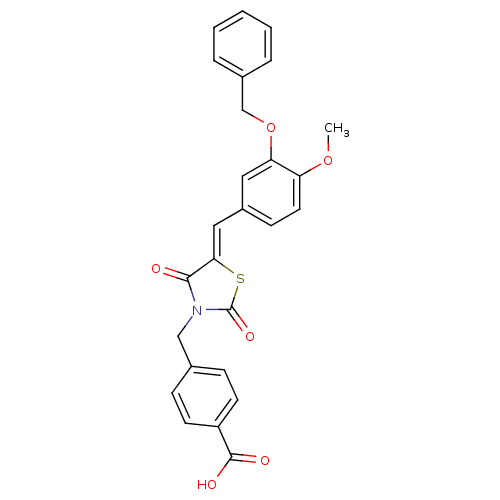
(CHEMBL3098945)Show SMILES COc1ccc(\C=C2/SC(=O)N(Cc3ccc(cc3)C(O)=O)C2=O)cc1OCc1ccccc1 Show InChI InChI=1S/C26H21NO6S/c1-32-21-12-9-19(13-22(21)33-16-18-5-3-2-4-6-18)14-23-24(28)27(26(31)34-23)15-17-7-10-20(11-8-17)25(29)30/h2-14H,15-16H2,1H3,(H,29,30)/b23-14- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Low molecular weight phosphotyrosine protein phosphatase
(Homo sapiens (Human)) | BDBM50444695

(CHEMBL3098852)Show SMILES OC(=O)c1ccc(CN2\C(S\C(=C/c3ccc(OCCc4ccccc4)cc3)C2=O)=N\c2ccccc2)cc1 Show InChI InChI=1S/C32H26N2O4S/c35-30-29(21-24-13-17-28(18-14-24)38-20-19-23-7-3-1-4-8-23)39-32(33-27-9-5-2-6-10-27)34(30)22-25-11-15-26(16-12-25)31(36)37/h1-18,21H,19-20,22H2,(H,36,37)/b29-21-,33-32- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LMW-PTP IF1 expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50444699

(CHEMBL3098855)Show SMILES COc1ccc(\C=C2/S\C(=N/c3ccccc3)N(Cc3ccc(cc3)C(O)=O)C2=O)cc1OCc1ccccc1 Show InChI InChI=1S/C32H26N2O5S/c1-38-27-17-14-24(18-28(27)39-21-23-8-4-2-5-9-23)19-29-30(35)34(20-22-12-15-25(16-13-22)31(36)37)32(40-29)33-26-10-6-3-7-11-26/h2-19H,20-21H2,1H3,(H,36,37)/b29-19-,33-32- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of TC-PTP (unknown origin) expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50444692

(CHEMBL3098853)Show SMILES COc1ccc(\C=C2/S\C(=N/c3ccccc3)N(Cc3ccc(cc3)C(O)=O)C2=O)cc1OCCc1ccccc1 Show InChI InChI=1S/C33H28N2O5S/c1-39-28-17-14-25(20-29(28)40-19-18-23-8-4-2-5-9-23)21-30-31(36)35(22-24-12-15-26(16-13-24)32(37)38)33(41-30)34-27-10-6-3-7-11-27/h2-17,20-21H,18-19,22H2,1H3,(H,37,38)/b30-21-,34-33- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of TC-PTP (unknown origin) expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Low molecular weight phosphotyrosine protein phosphatase
(Homo sapiens (Human)) | BDBM50444698

(CHEMBL3098856)Show SMILES COc1cc(\C=C2/S\C(=N/c3ccccc3)N(Cc3ccc(cc3)C(O)=O)C2=O)ccc1OCc1ccccc1 Show InChI InChI=1S/C32H26N2O5S/c1-38-28-18-24(14-17-27(28)39-21-23-8-4-2-5-9-23)19-29-30(35)34(20-22-12-15-25(16-13-22)31(36)37)32(40-29)33-26-10-6-3-7-11-26/h2-19H,20-21H2,1H3,(H,36,37)/b29-19-,33-32- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LMW-PTP IF1 expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50444700

(CHEMBL3098851)Show SMILES OC(=O)c1ccc(CN2\C(S\C(=C/c3cccc(OCCc4ccccc4)c3)C2=O)=N\c2ccccc2)cc1 Show InChI InChI=1S/C32H26N2O4S/c35-30-29(21-25-10-7-13-28(20-25)38-19-18-23-8-3-1-4-9-23)39-32(33-27-11-5-2-6-12-27)34(30)22-24-14-16-26(17-15-24)31(36)37/h1-17,20-21H,18-19,22H2,(H,36,37)/b29-21-,33-32- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50444702

(CHEMBL3098946)Show SMILES COc1cc(\C=C2/SC(=O)N(Cc3ccc(cc3)C(O)=O)C2=O)ccc1OCc1ccccc1 Show InChI InChI=1S/C26H21NO6S/c1-32-22-13-19(9-12-21(22)33-16-18-5-3-2-4-6-18)14-23-24(28)27(26(31)34-23)15-17-7-10-20(11-8-17)25(29)30/h2-14H,15-16H2,1H3,(H,29,30)/b23-14- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50444698

(CHEMBL3098856)Show SMILES COc1cc(\C=C2/S\C(=N/c3ccccc3)N(Cc3ccc(cc3)C(O)=O)C2=O)ccc1OCc1ccccc1 Show InChI InChI=1S/C32H26N2O5S/c1-38-28-18-24(14-17-27(28)39-21-23-8-4-2-5-9-23)19-29-30(35)34(20-22-12-15-25(16-13-22)31(36)37)32(40-29)33-26-10-6-3-7-11-26/h2-19H,20-21H2,1H3,(H,36,37)/b29-19-,33-32- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of TC-PTP (unknown origin) expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50444696

(CHEMBL3098942)Show SMILES OC(=O)c1ccc(CN2C(=O)S\C(=C/c3ccc(OCCc4ccccc4)cc3)C2=O)cc1 Show InChI InChI=1S/C26H21NO5S/c28-24-23(33-26(31)27(24)17-20-6-10-21(11-7-20)25(29)30)16-19-8-12-22(13-9-19)32-15-14-18-4-2-1-3-5-18/h1-13,16H,14-15,17H2,(H,29,30)/b23-16- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of TC-PTP (unknown origin) expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50444705
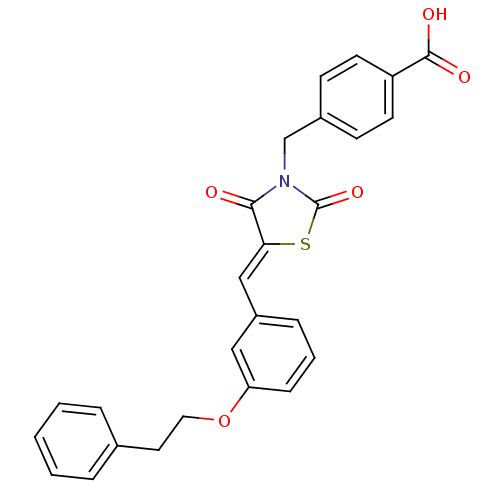
(CHEMBL3098941)Show SMILES OC(=O)c1ccc(CN2C(=O)S\C(=C/c3cccc(OCCc4ccccc4)c3)C2=O)cc1 Show InChI InChI=1S/C26H21NO5S/c28-24-23(33-26(31)27(24)17-19-9-11-21(12-10-19)25(29)30)16-20-7-4-8-22(15-20)32-14-13-18-5-2-1-3-6-18/h1-12,15-16H,13-14,17H2,(H,29,30)/b23-16- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of TC-PTP (unknown origin) expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data