

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuropeptide Y receptor type 5 (Rat 6B) | BDBM50163525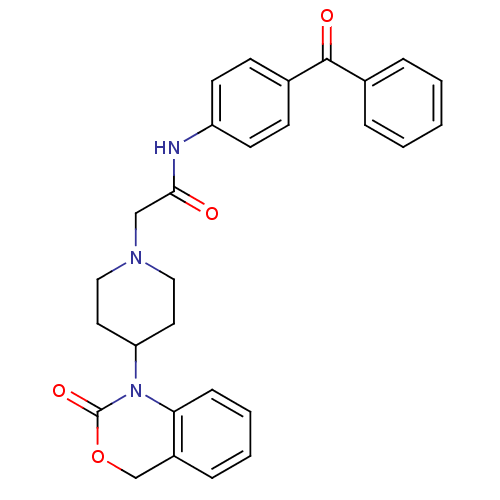 (CHEMBL179972 | N-(4-Benzoyl-phenyl)-2-[4-(2-oxo-4H...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Dr. Esteve, S.A. Curated by ChEMBL | Assay Description Inhibition of [125I]PYY binding to the rat NPY Y5 receptor in C6 cells | J Med Chem 48: 2080-92 (2005) Article DOI: 10.1021/jm049599u BindingDB Entry DOI: 10.7270/Q24X58K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Rat 6B) | BDBM50163516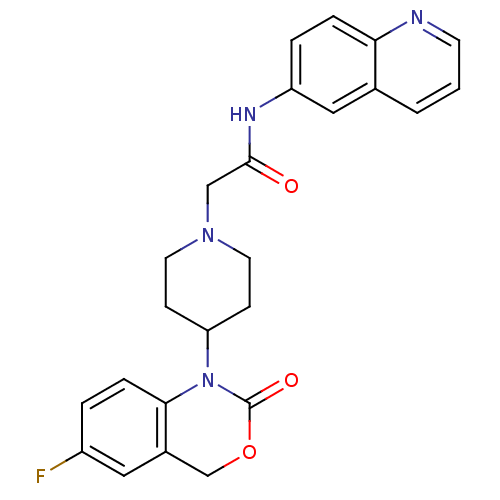 (2-[4-(6-Fluoro-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Dr. Esteve, S.A. Curated by ChEMBL | Assay Description Inhibition of [125I]PYY binding to the rat NPY Y5 receptor in C6 cells | J Med Chem 48: 2080-92 (2005) Article DOI: 10.1021/jm049599u BindingDB Entry DOI: 10.7270/Q24X58K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Rat 6B) | BDBM50163541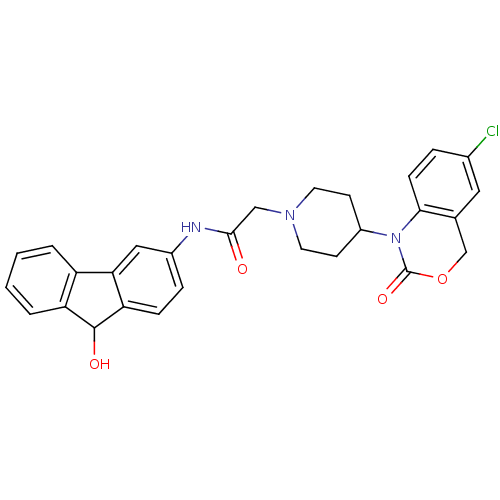 (2-[4-(6-Chloro-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Dr. Esteve, S.A. Curated by ChEMBL | Assay Description Inhibition of [125I]PYY binding to the rat NPY Y5 receptor in C6 cells | J Med Chem 48: 2080-92 (2005) Article DOI: 10.1021/jm049599u BindingDB Entry DOI: 10.7270/Q24X58K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Rat 6B) | BDBM50163539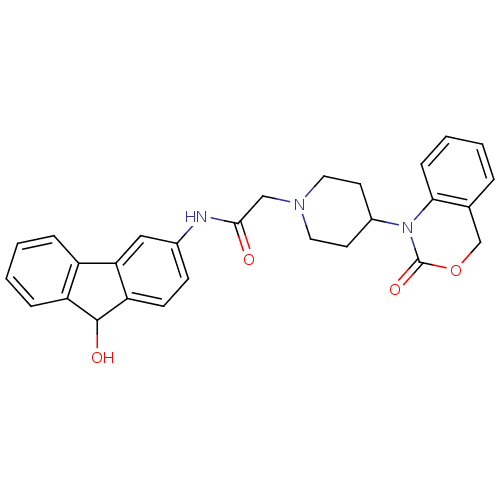 (CHEMBL557802 | N-(9-Hydroxy-9H-fluoren-3-yl)-2-[4-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Dr. Esteve, S.A. Curated by ChEMBL | Assay Description Inhibition of [125I]PYY binding to the rat NPY Y5 receptor in C6 cells | J Med Chem 48: 2080-92 (2005) Article DOI: 10.1021/jm049599u BindingDB Entry DOI: 10.7270/Q24X58K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Rat 6B) | BDBM50163552 (CHEMBL557540 | N-(9-Methyl-9H-carbazol-3-yl)-2-[4-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Dr. Esteve, S.A. Curated by ChEMBL | Assay Description Inhibition of [125I]PYY binding to the rat NPY Y5 receptor in C6 cells | J Med Chem 48: 2080-92 (2005) Article DOI: 10.1021/jm049599u BindingDB Entry DOI: 10.7270/Q24X58K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Rat 6B) | BDBM50163513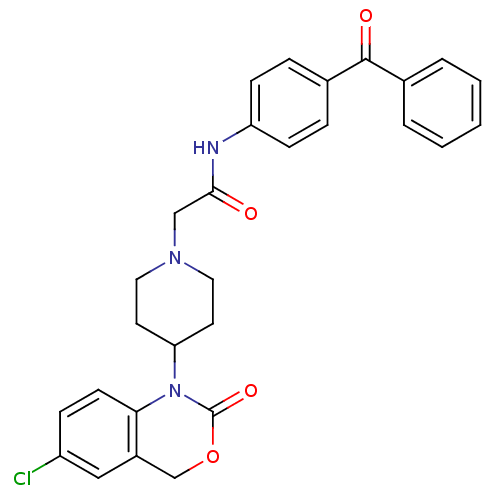 (CHEMBL541121 | N-(4-Benzoyl-phenyl)-2-[4-(6-chloro...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Dr. Esteve, S.A. Curated by ChEMBL | Assay Description Inhibition of [125I]PYY binding to the rat NPY Y5 receptor in C6 cells | J Med Chem 48: 2080-92 (2005) Article DOI: 10.1021/jm049599u BindingDB Entry DOI: 10.7270/Q24X58K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Rat 6B) | BDBM50163545 (CHEMBL537412 | N-(4-Acetyl-phenyl)-2-[4-(2-oxo-4H-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Dr. Esteve, S.A. Curated by ChEMBL | Assay Description Inhibition of [125I]PYY binding to the rat NPY Y5 receptor in C6 cells | J Med Chem 48: 2080-92 (2005) Article DOI: 10.1021/jm049599u BindingDB Entry DOI: 10.7270/Q24X58K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Rat 6B) | BDBM50163535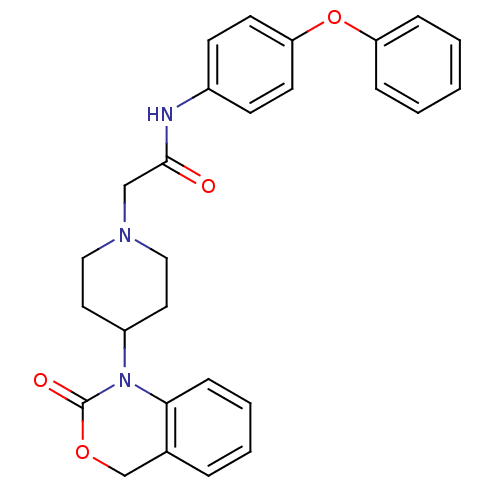 (2-[4-(2-Oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidin...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Dr. Esteve, S.A. Curated by ChEMBL | Assay Description Inhibition of [125I]PYY binding to the rat NPY Y5 receptor in C6 cells | J Med Chem 48: 2080-92 (2005) Article DOI: 10.1021/jm049599u BindingDB Entry DOI: 10.7270/Q24X58K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Rat 6B) | BDBM50163526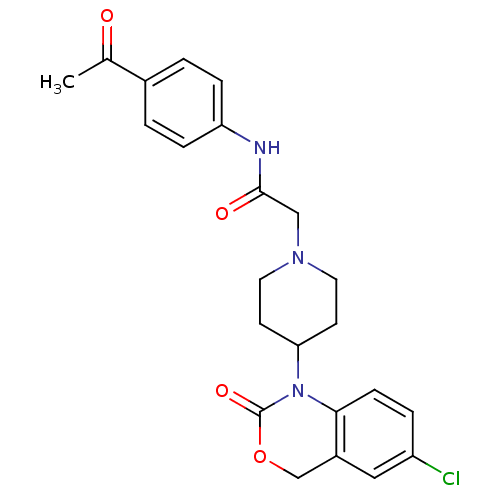 (CHEMBL537191 | N-(4-Acetyl-phenyl)-2-[4-(6-chloro-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Dr. Esteve, S.A. Curated by ChEMBL | Assay Description Inhibition of [125I]PYY binding to the rat NPY Y5 receptor in C6 cells | J Med Chem 48: 2080-92 (2005) Article DOI: 10.1021/jm049599u BindingDB Entry DOI: 10.7270/Q24X58K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM19992 (2-[3-[3-[[2-chloro-3-(trifluoromethyl)phenyl]methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description SPA assays were run in displacement mode using 30 nM [3H]-GW3965. Ligands were tested in 12-point dose-response curves starting at 20 uM. IC50 values... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuropeptide Y receptor type 5 (Rat 6B) | BDBM50163512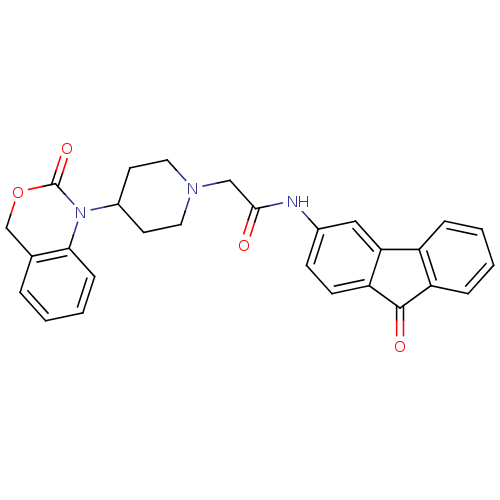 (2-[4-(2-Oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidin...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Dr. Esteve, S.A. Curated by ChEMBL | Assay Description Inhibition of [125I]PYY binding to the rat NPY Y5 receptor in C6 cells | J Med Chem 48: 2080-92 (2005) Article DOI: 10.1021/jm049599u BindingDB Entry DOI: 10.7270/Q24X58K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Rat 6B) | BDBM50163538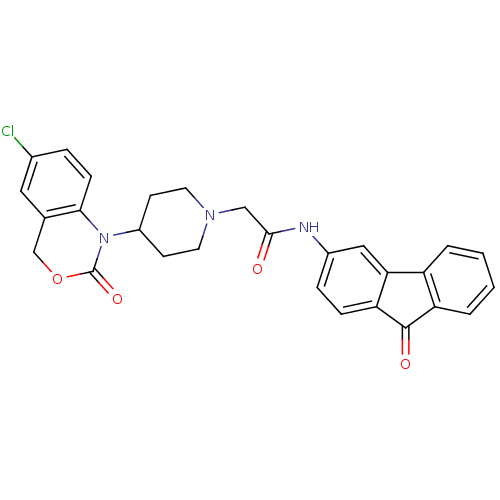 (2-[4-(6-Chloro-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Dr. Esteve, S.A. Curated by ChEMBL | Assay Description Inhibition of [125I]PYY binding to the rat NPY Y5 receptor in C6 cells | J Med Chem 48: 2080-92 (2005) Article DOI: 10.1021/jm049599u BindingDB Entry DOI: 10.7270/Q24X58K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Rat 6B) | BDBM50163548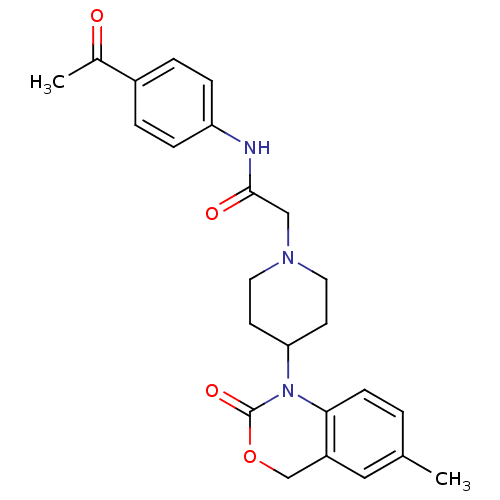 (CHEMBL537187 | N-(4-Acetyl-phenyl)-2-[4-(6-methyl-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Dr. Esteve, S.A. Curated by ChEMBL | Assay Description Inhibition of [125I]PYY binding to the rat NPY Y5 receptor in C6 cells | J Med Chem 48: 2080-92 (2005) Article DOI: 10.1021/jm049599u BindingDB Entry DOI: 10.7270/Q24X58K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Rat 6B) | BDBM50163529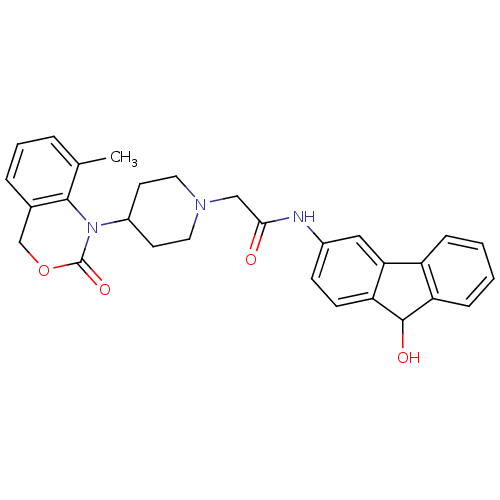 (CHEMBL535824 | N-(9-Hydroxy-9H-fluoren-3-yl)-2-[4-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Dr. Esteve, S.A. Curated by ChEMBL | Assay Description Inhibition of [125I]PYY binding to the rat NPY Y5 receptor in C6 cells | J Med Chem 48: 2080-92 (2005) Article DOI: 10.1021/jm049599u BindingDB Entry DOI: 10.7270/Q24X58K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Rat 6B) | BDBM50163511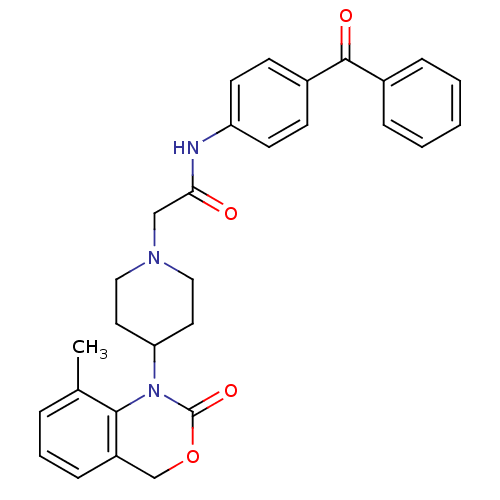 (CHEMBL536276 | N-(4-Benzoyl-phenyl)-2-[4-(8-methyl...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 39.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Dr. Esteve, S.A. Curated by ChEMBL | Assay Description Inhibition of [125I]PYY binding to the rat NPY Y5 receptor in C6 cells | J Med Chem 48: 2080-92 (2005) Article DOI: 10.1021/jm049599u BindingDB Entry DOI: 10.7270/Q24X58K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Rat 6B) | BDBM50163521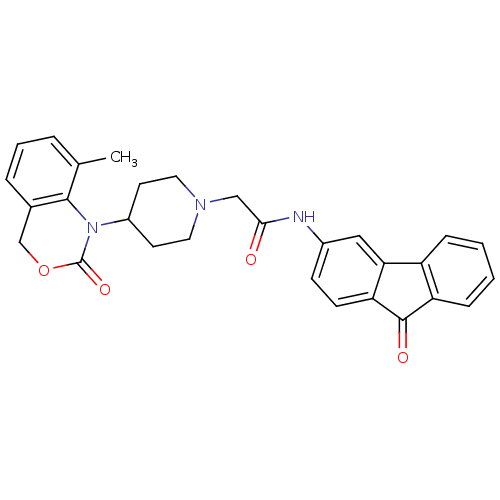 (2-[4-(8-Methyl-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Dr. Esteve, S.A. Curated by ChEMBL | Assay Description Inhibition of [125I]PYY binding to the rat NPY Y5 receptor in C6 cells | J Med Chem 48: 2080-92 (2005) Article DOI: 10.1021/jm049599u BindingDB Entry DOI: 10.7270/Q24X58K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Rat 6B) | BDBM50163514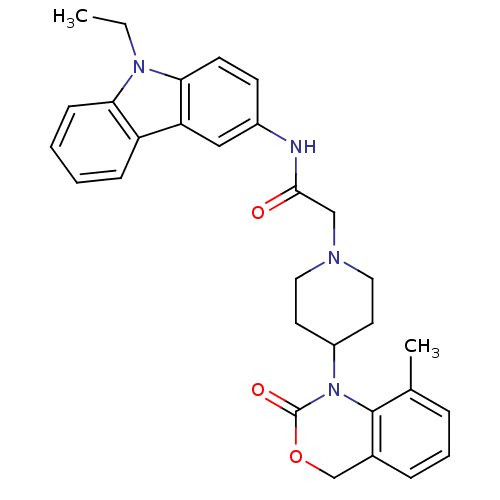 (CHEMBL540365 | N-(9-Ethyl-9H-carbazol-3-yl)-2-[4-(...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Dr. Esteve, S.A. Curated by ChEMBL | Assay Description Inhibition of [125I]PYY binding to the rat NPY Y5 receptor in C6 cells | J Med Chem 48: 2080-92 (2005) Article DOI: 10.1021/jm049599u BindingDB Entry DOI: 10.7270/Q24X58K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Rat 6B) | BDBM50163531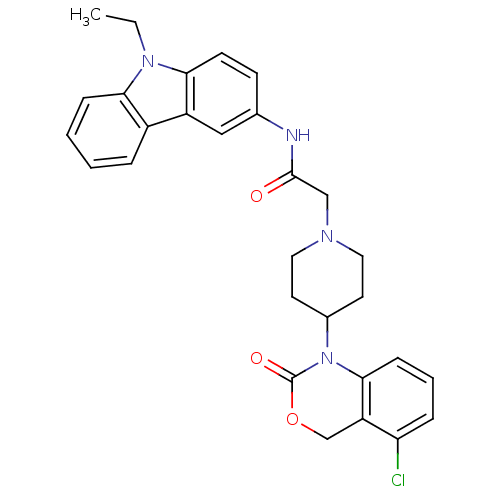 (CHEMBL557817 | N-(9-Ethyl-9H-carbazol-3-yl)-2-[4-(...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 54.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Dr. Esteve, S.A. Curated by ChEMBL | Assay Description Inhibition of [125I]PYY binding to the rat NPY Y5 receptor in C6 cells | J Med Chem 48: 2080-92 (2005) Article DOI: 10.1021/jm049599u BindingDB Entry DOI: 10.7270/Q24X58K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Rat 6B) | BDBM50163540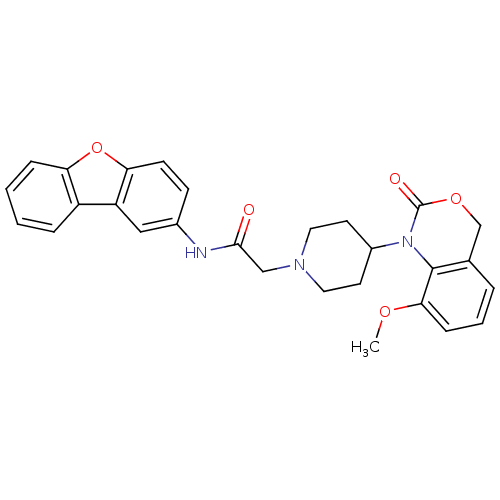 (CHEMBL365777 | N-Dibenzofuran-2-yl-2-[4-(8-methoxy...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 55.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Dr. Esteve, S.A. Curated by ChEMBL | Assay Description Inhibition of [125I]PYY binding to the rat NPY Y5 receptor in C6 cells | J Med Chem 48: 2080-92 (2005) Article DOI: 10.1021/jm049599u BindingDB Entry DOI: 10.7270/Q24X58K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Rat 6B) | BDBM50163550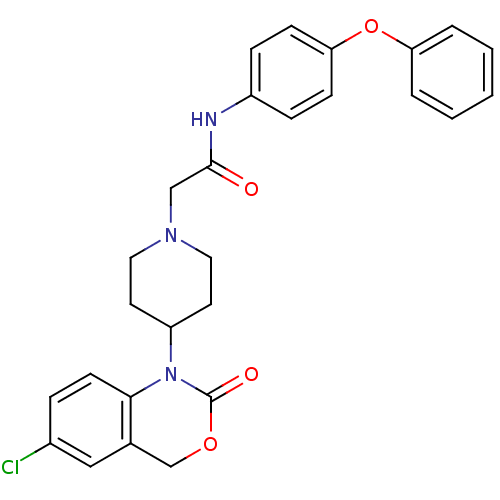 (2-[4-(6-Chloro-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Dr. Esteve, S.A. Curated by ChEMBL | Assay Description Inhibition of [125I]PYY binding to the rat NPY Y5 receptor in C6 cells | J Med Chem 48: 2080-92 (2005) Article DOI: 10.1021/jm049599u BindingDB Entry DOI: 10.7270/Q24X58K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM20132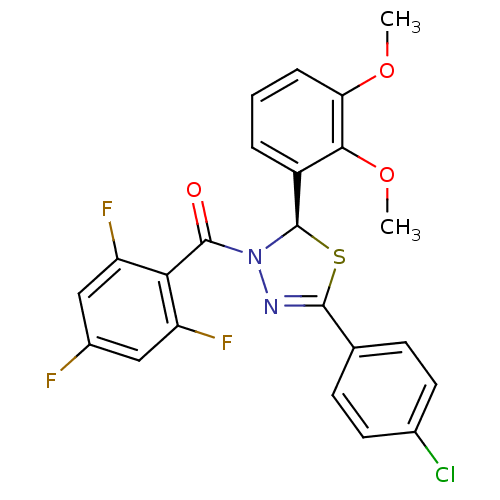 ((2R)-5-(4-chlorophenyl)-2-(2,3-dimethoxyphenyl)-3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description SPA assays were run in displacement mode using 30 nM [3H]-GW3965. Ligands were tested in 12-point dose-response curves starting at 20 uM. IC50 values... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Rat 6B) | BDBM50163530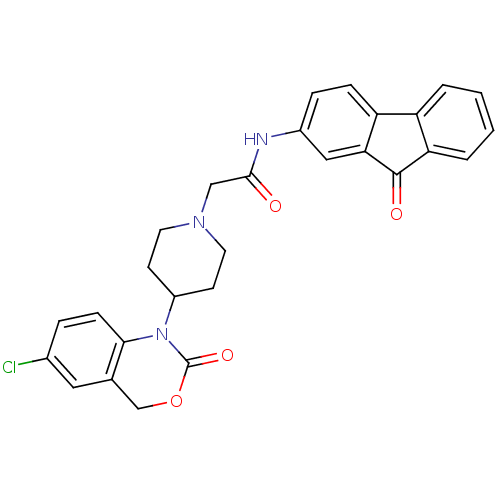 (2-[4-(6-Chloro-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Dr. Esteve, S.A. Curated by ChEMBL | Assay Description Inhibition of [125I]PYY binding to the rat NPY Y5 receptor in C6 cells | J Med Chem 48: 2080-92 (2005) Article DOI: 10.1021/jm049599u BindingDB Entry DOI: 10.7270/Q24X58K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Rat 6B) | BDBM50163533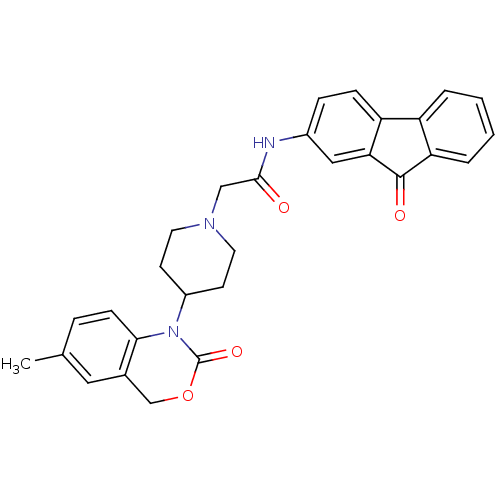 (2-[4-(6-Methyl-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 86.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Dr. Esteve, S.A. Curated by ChEMBL | Assay Description Inhibition of [125I]PYY binding to the rat NPY Y5 receptor in C6 cells | J Med Chem 48: 2080-92 (2005) Article DOI: 10.1021/jm049599u BindingDB Entry DOI: 10.7270/Q24X58K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Rat 6B) | BDBM50163524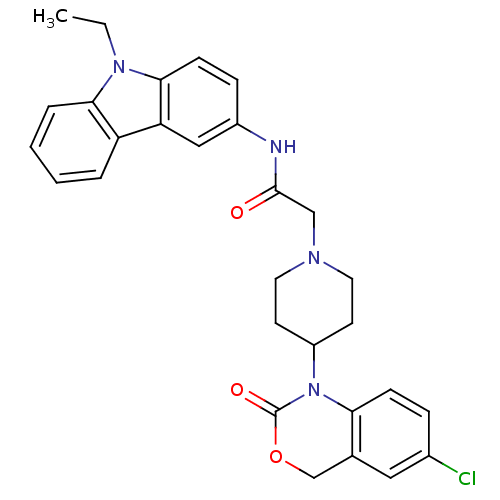 (2-[4-(5-Chloro-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Dr. Esteve, S.A. Curated by ChEMBL | Assay Description Inhibition of [125I]PYY binding to the rat NPY Y5 receptor in C6 cells | J Med Chem 48: 2080-92 (2005) Article DOI: 10.1021/jm049599u BindingDB Entry DOI: 10.7270/Q24X58K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Rat 6B) | BDBM50163519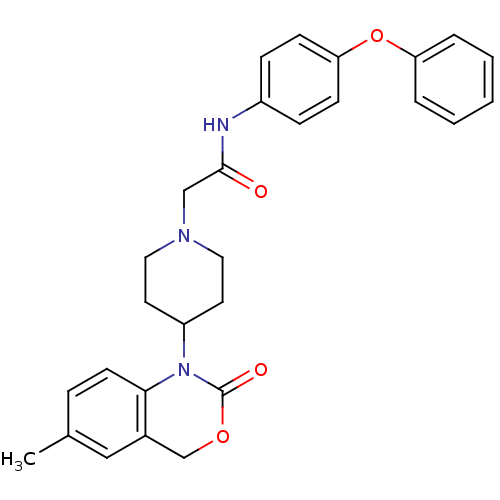 (2-[4-(6-Methyl-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 104 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Dr. Esteve, S.A. Curated by ChEMBL | Assay Description Inhibition of [125I]PYY binding to the rat NPY Y5 receptor in C6 cells | J Med Chem 48: 2080-92 (2005) Article DOI: 10.1021/jm049599u BindingDB Entry DOI: 10.7270/Q24X58K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Rat 6B) | BDBM50163522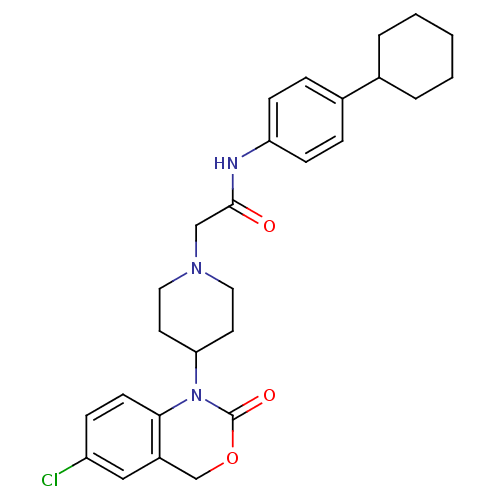 (2-[4-(6-Chloro-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 112 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Dr. Esteve, S.A. Curated by ChEMBL | Assay Description Inhibition of [125I]PYY binding to the rat NPY Y5 receptor in C6 cells | J Med Chem 48: 2080-92 (2005) Article DOI: 10.1021/jm049599u BindingDB Entry DOI: 10.7270/Q24X58K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM19992 (2-[3-[3-[[2-chloro-3-(trifluoromethyl)phenyl]methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 121 | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description SPA assays were run in displacement mode using 30 nM [3H]-GW3965. Ligands were tested in 12-point dose-response curves starting at 20 uM. IC50 values... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuropeptide Y receptor type 5 (Rat 6B) | BDBM50163549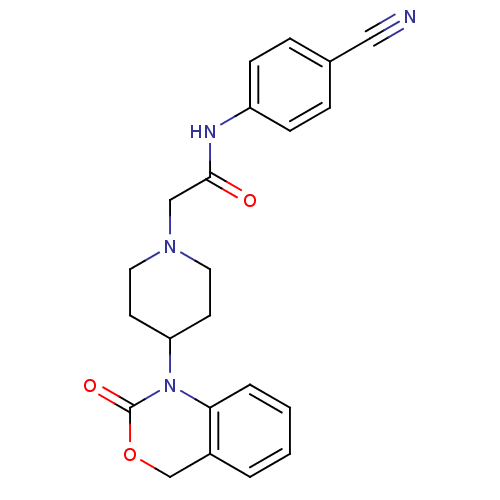 (CHEMBL556999 | N-(4-Cyano-phenyl)-2-[4-(2-oxo-4H-b...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 132 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Dr. Esteve, S.A. Curated by ChEMBL | Assay Description Inhibition of [125I]PYY binding to the rat NPY Y5 receptor in C6 cells | J Med Chem 48: 2080-92 (2005) Article DOI: 10.1021/jm049599u BindingDB Entry DOI: 10.7270/Q24X58K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Rat 6B) | BDBM50163520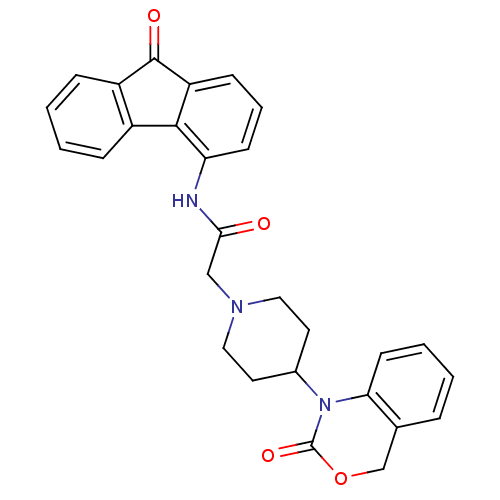 (2-[4-(2-Oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidin...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 138 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Dr. Esteve, S.A. Curated by ChEMBL | Assay Description Inhibition of [125I]PYY binding to the rat NPY Y5 receptor in C6 cells | J Med Chem 48: 2080-92 (2005) Article DOI: 10.1021/jm049599u BindingDB Entry DOI: 10.7270/Q24X58K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Rat 6B) | BDBM50163547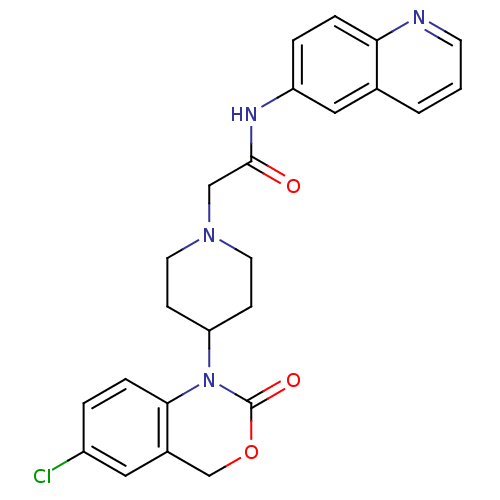 (2-[4-(6-Chloro-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 148 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Dr. Esteve, S.A. Curated by ChEMBL | Assay Description Inhibition of [125I]PYY binding to the rat NPY Y5 receptor in C6 cells | J Med Chem 48: 2080-92 (2005) Article DOI: 10.1021/jm049599u BindingDB Entry DOI: 10.7270/Q24X58K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Rat 6B) | BDBM50163532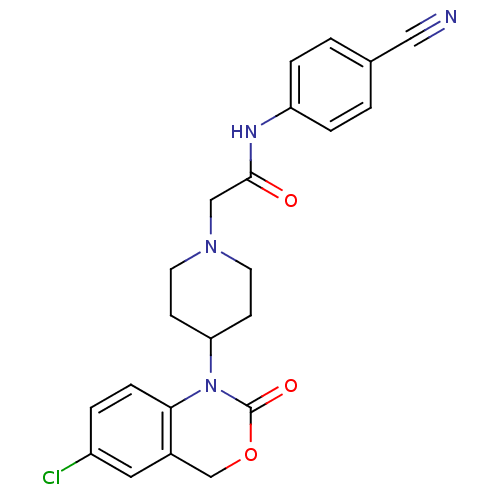 (2-[4-(6-Chloro-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 199 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Dr. Esteve, S.A. Curated by ChEMBL | Assay Description Inhibition of [125I]PYY binding to the rat NPY Y5 receptor in C6 cells | J Med Chem 48: 2080-92 (2005) Article DOI: 10.1021/jm049599u BindingDB Entry DOI: 10.7270/Q24X58K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Rat 6B) | BDBM50163528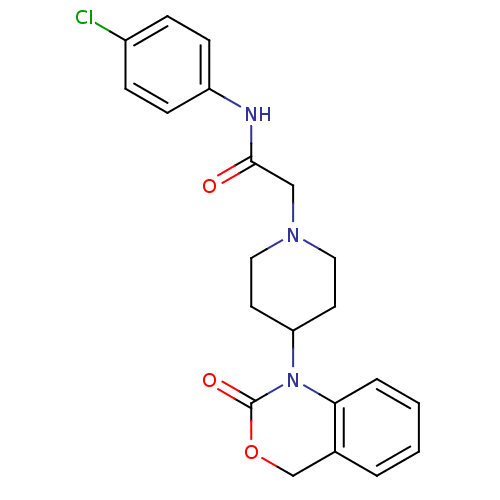 (CHEMBL558393 | N-(4-Chloro-phenyl)-2-[4-(2-oxo-4H-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Dr. Esteve, S.A. Curated by ChEMBL | Assay Description Inhibition of [125I]PYY binding to the rat NPY Y5 receptor in C6 cells | J Med Chem 48: 2080-92 (2005) Article DOI: 10.1021/jm049599u BindingDB Entry DOI: 10.7270/Q24X58K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM20131 (5-(4-chlorophenyl)-2-(2,3-dimethoxyphenyl)-3-[(2,4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description SPA assays were run in displacement mode using 30 nM [3H]-GW3965. Ligands were tested in 12-point dose-response curves starting at 20 uM. IC50 values... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Rat 6B) | BDBM50163542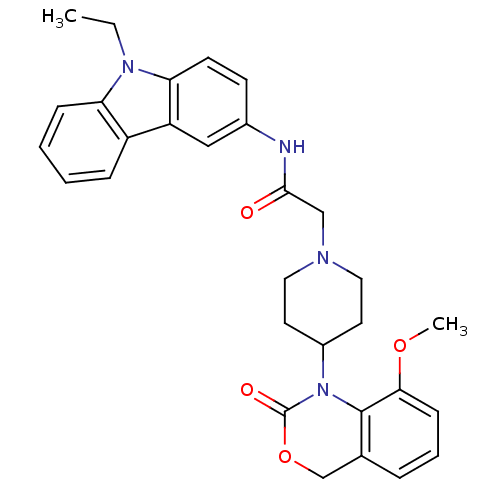 (CHEMBL538569 | N-(9-Ethyl-9H-carbazol-3-yl)-2-[4-(...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 765 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Dr. Esteve, S.A. Curated by ChEMBL | Assay Description Inhibition of [125I]PYY binding to the rat NPY Y5 receptor in C6 cells | J Med Chem 48: 2080-92 (2005) Article DOI: 10.1021/jm049599u BindingDB Entry DOI: 10.7270/Q24X58K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Rat 6B) | BDBM50163551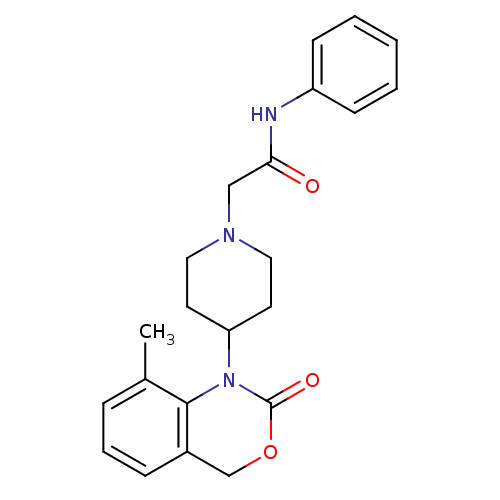 (2-[4-(8-Methyl-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Dr. Esteve, S.A. Curated by ChEMBL | Assay Description Inhibition of [125I]PYY binding to the rat NPY Y5 receptor in C6 cells | J Med Chem 48: 2080-92 (2005) Article DOI: 10.1021/jm049599u BindingDB Entry DOI: 10.7270/Q24X58K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Rat 6B) | BDBM50163515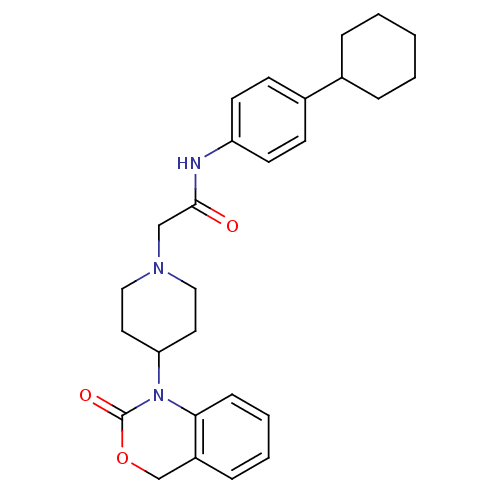 (CHEMBL536496 | N-(4-Cyclohexyl-phenyl)-2-[4-(2-oxo...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Dr. Esteve, S.A. Curated by ChEMBL | Assay Description Inhibition of [125I]PYY binding to the rat NPY Y5 receptor in C6 cells | J Med Chem 48: 2080-92 (2005) Article DOI: 10.1021/jm049599u BindingDB Entry DOI: 10.7270/Q24X58K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Rat 6B) | BDBM50163534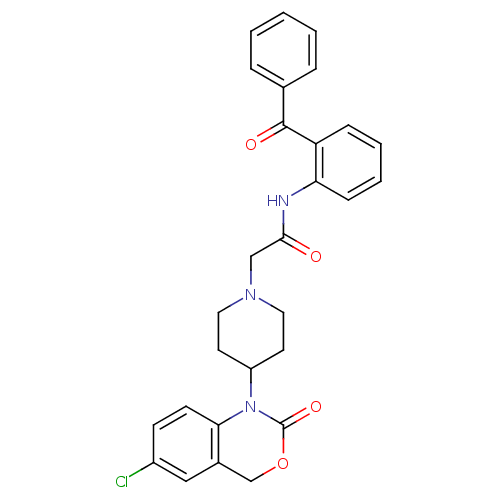 (CHEMBL536729 | N-(2-Benzoyl-phenyl)-2-[4-(6-chloro...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Dr. Esteve, S.A. Curated by ChEMBL | Assay Description Inhibition of [125I]PYY binding to the rat NPY Y5 receptor in C6 cells | J Med Chem 48: 2080-92 (2005) Article DOI: 10.1021/jm049599u BindingDB Entry DOI: 10.7270/Q24X58K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Rat 6B) | BDBM50163536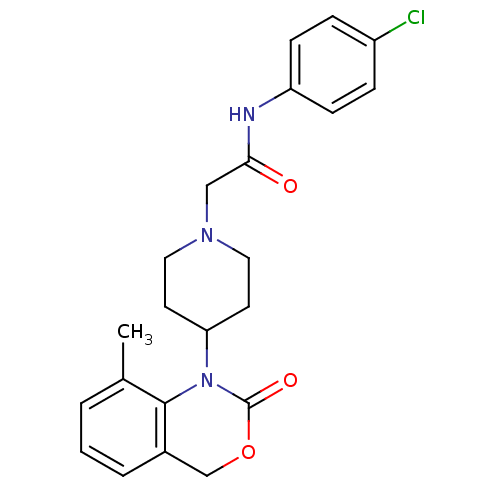 (CHEMBL534483 | N-(4-Chloro-phenyl)-2-[4-(8-methyl-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Dr. Esteve, S.A. Curated by ChEMBL | Assay Description Inhibition of [125I]PYY binding to the rat NPY Y5 receptor in C6 cells | J Med Chem 48: 2080-92 (2005) Article DOI: 10.1021/jm049599u BindingDB Entry DOI: 10.7270/Q24X58K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Rat 6B) | BDBM50163537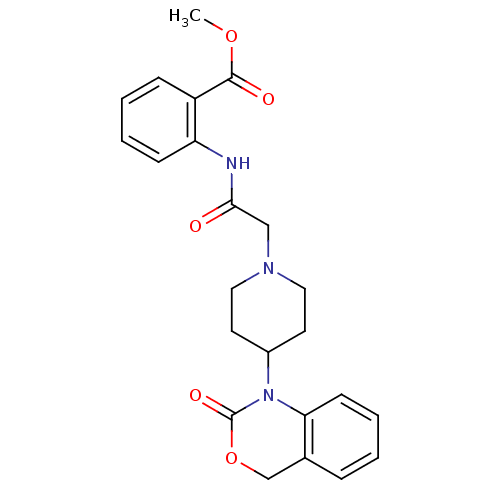 (2-{2-[4-(2-Oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperi...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Dr. Esteve, S.A. Curated by ChEMBL | Assay Description Inhibition of [125I]PYY binding to the rat NPY Y5 receptor in C6 cells | J Med Chem 48: 2080-92 (2005) Article DOI: 10.1021/jm049599u BindingDB Entry DOI: 10.7270/Q24X58K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Rat 6B) | BDBM50163543 (2-{2-[4-(8-Methyl-2-oxo-4H-benzo[d][1,3]oxazin-1-y...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Dr. Esteve, S.A. Curated by ChEMBL | Assay Description Inhibition of [125I]PYY binding to the rat NPY Y5 receptor in C6 cells | J Med Chem 48: 2080-92 (2005) Article DOI: 10.1021/jm049599u BindingDB Entry DOI: 10.7270/Q24X58K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Rat 6B) | BDBM50163544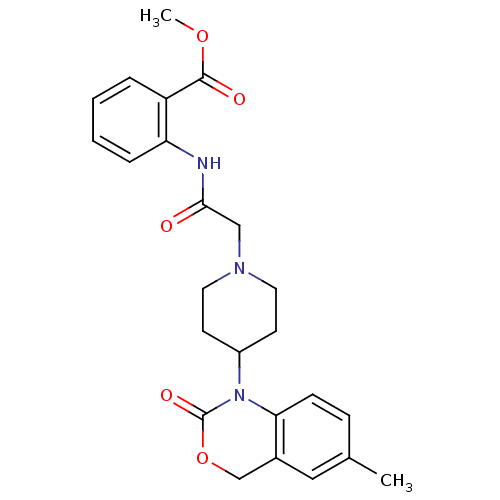 (2-{2-[4-(6-Methyl-2-oxo-4H-benzo[d][1,3]oxazin-1-y...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Dr. Esteve, S.A. Curated by ChEMBL | Assay Description Inhibition of [125I]PYY binding to the rat NPY Y5 receptor in C6 cells | J Med Chem 48: 2080-92 (2005) Article DOI: 10.1021/jm049599u BindingDB Entry DOI: 10.7270/Q24X58K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Rat 6B) | BDBM50163546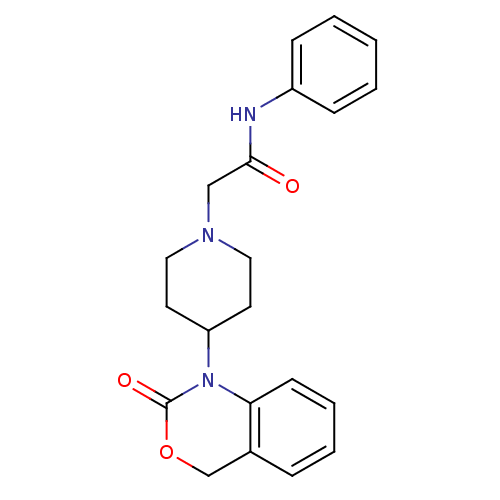 (2-[4-(2-Oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidin...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Dr. Esteve, S.A. Curated by ChEMBL | Assay Description Inhibition of [125I]PYY binding to the rat NPY Y5 receptor in C6 cells | J Med Chem 48: 2080-92 (2005) Article DOI: 10.1021/jm049599u BindingDB Entry DOI: 10.7270/Q24X58K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Rat 6B) | BDBM50163527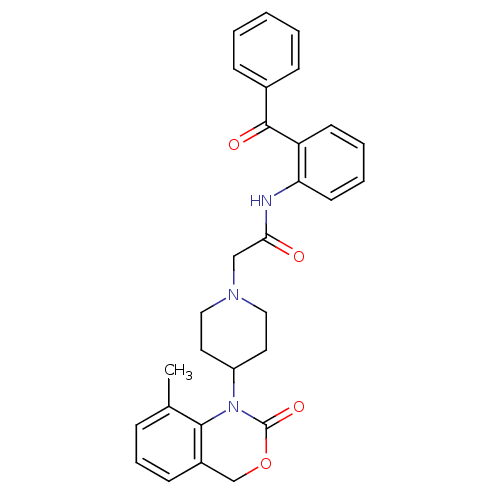 (CHEMBL553250 | N-(2-Benzoyl-phenyl)-2-[4-(8-methyl...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Dr. Esteve, S.A. Curated by ChEMBL | Assay Description Inhibition of [125I]PYY binding to the rat NPY Y5 receptor in C6 cells | J Med Chem 48: 2080-92 (2005) Article DOI: 10.1021/jm049599u BindingDB Entry DOI: 10.7270/Q24X58K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Rat 6B) | BDBM50163518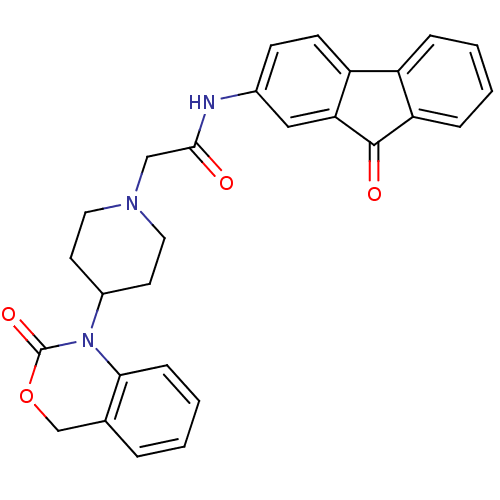 (2-[4-(2-Oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidin...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Dr. Esteve, S.A. Curated by ChEMBL | Assay Description Inhibition of [125I]PYY binding to the rat NPY Y5 receptor in C6 cells | J Med Chem 48: 2080-92 (2005) Article DOI: 10.1021/jm049599u BindingDB Entry DOI: 10.7270/Q24X58K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Rat 6B) | BDBM50163517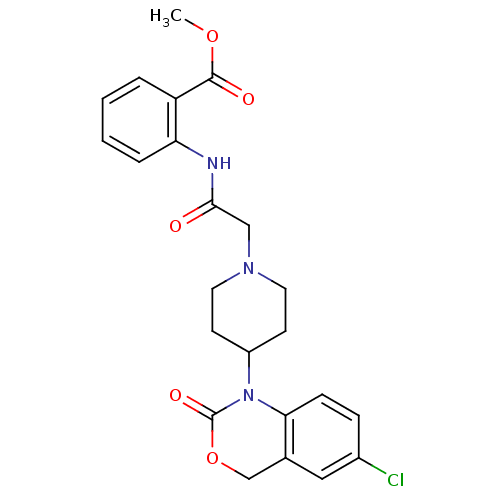 (2-{2-[4-(6-Chloro-2-oxo-4H-benzo[d][1,3]oxazin-1-y...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Dr. Esteve, S.A. Curated by ChEMBL | Assay Description Inhibition of [125I]PYY binding to the rat NPY Y5 receptor in C6 cells | J Med Chem 48: 2080-92 (2005) Article DOI: 10.1021/jm049599u BindingDB Entry DOI: 10.7270/Q24X58K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Rat 6B) | BDBM50163523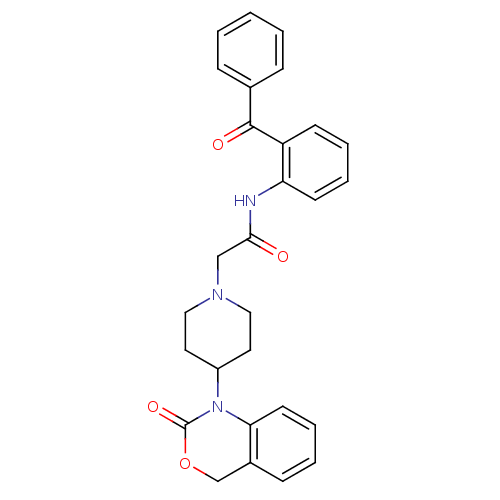 (CHEMBL534710 | N-(2-Benzoyl-phenyl)-2-[4-(2-oxo-4H...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Dr. Esteve, S.A. Curated by ChEMBL | Assay Description Inhibition of [125I]PYY binding to the rat NPY Y5 receptor in C6 cells | J Med Chem 48: 2080-92 (2005) Article DOI: 10.1021/jm049599u BindingDB Entry DOI: 10.7270/Q24X58K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM20132 ((2R)-5-(4-chlorophenyl)-2-(2,3-dimethoxyphenyl)-3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description SPA assays were run in displacement mode using 30 nM [3H]-GW3965. Ligands were tested in 12-point dose-response curves starting at 20 uM. IC50 values... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM20131 (5-(4-chlorophenyl)-2-(2,3-dimethoxyphenyl)-3-[(2,4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description SPA assays were run in displacement mode using 30 nM [3H]-GW3965. Ligands were tested in 12-point dose-response curves starting at 20 uM. IC50 values... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM19992 (2-[3-[3-[[2-chloro-3-(trifluoromethyl)phenyl]methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 97 | n/a | n/a | 7.2 | 37 |
GNF | Assay Description FRET-based co-activator recruitment assay measures agonist activities in a 384-well plate format. Reaction mixture containing His-tagged hLXR, biotin... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM20131 (5-(4-chlorophenyl)-2-(2,3-dimethoxyphenyl)-3-[(2,4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.30E+3 | n/a | n/a | 7.2 | 37 |
GNF | Assay Description FRET-based co-activator recruitment assay measures agonist activities in a 384-well plate format. Reaction mixture containing His-tagged hLXR, biotin... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 94 total ) | Next | Last >> |