 Found 62 hits with Last Name = 'chan' and Initial = 'yc'
Found 62 hits with Last Name = 'chan' and Initial = 'yc' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM8125

((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...)Show SMILES [H][C@@]1(CO[C@@]2([H])OCC[C@@]12[H])OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C27H37N3O7S/c1-18(2)15-30(38(33,34)21-10-8-20(28)9-11-21)16-24(31)23(14-19-6-4-3-5-7-19)29-27(32)37-25-17-36-26-22(25)12-13-35-26/h3-11,18,22-26,31H,12-17,28H2,1-2H3,(H,29,32)/t22-,23-,24+,25-,26+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
J Med Chem 55: 3387-97 (2012)
Article DOI: 10.1021/jm300072d
BindingDB Entry DOI: 10.7270/Q2T43WZW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM31817

(GRL-02031 | methyl-2-pyrrolidinone, 19b)Show SMILES [H][C@]1(C[C@]2([H])CCO[C@]2([H])C1)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(C[C@H]1CCC(=O)N1)S(=O)(=O)c1ccc(OC)cc1 |r| Show InChI InChI=1S/C30H39N3O8S/c1-39-23-8-10-25(11-9-23)42(37,38)33(18-22-7-12-29(35)31-22)19-27(34)26(15-20-5-3-2-4-6-20)32-30(36)41-24-16-21-13-14-40-28(21)17-24/h2-6,8-11,21-22,24,26-28,34H,7,12-19H2,1H3,(H,31,35)(H,32,36)/t21-,22+,24+,26-,27+,28+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 wild type protease using Abz-Thr-Ile-Nle-p-nitro-Phe-Gln-Arg-NH2 as substrate after 5 mins by spectrophotometry |
J Med Chem 55: 3387-97 (2012)
Article DOI: 10.1021/jm300072d
BindingDB Entry DOI: 10.7270/Q2T43WZW |
More data for this
Ligand-Target Pair | |
HIV-1 protease
(Human immunodeficiency virus) | BDBM31817

(GRL-02031 | methyl-2-pyrrolidinone, 19b)Show SMILES [H][C@]1(C[C@]2([H])CCO[C@]2([H])C1)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(C[C@H]1CCC(=O)N1)S(=O)(=O)c1ccc(OC)cc1 |r| Show InChI InChI=1S/C30H39N3O8S/c1-39-23-8-10-25(11-9-23)42(37,38)33(18-22-7-12-29(35)31-22)19-27(34)26(15-20-5-3-2-4-6-20)32-30(36)41-24-16-21-13-14-40-28(21)17-24/h2-6,8-11,21-22,24,26-28,34H,7,12-19H2,1H3,(H,31,35)(H,32,36)/t21-,22+,24+,26-,27+,28+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease I47V mutant using Abz-Thr-Ile-Nle-p-nitro-Phe-Gln-Arg-NH2 as substrate after 5 mins by spectrophotometry |
J Med Chem 55: 3387-97 (2012)
Article DOI: 10.1021/jm300072d
BindingDB Entry DOI: 10.7270/Q2T43WZW |
More data for this
Ligand-Target Pair | |
HIV-1 protease
(Human immunodeficiency virus) | BDBM31817

(GRL-02031 | methyl-2-pyrrolidinone, 19b)Show SMILES [H][C@]1(C[C@]2([H])CCO[C@]2([H])C1)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(C[C@H]1CCC(=O)N1)S(=O)(=O)c1ccc(OC)cc1 |r| Show InChI InChI=1S/C30H39N3O8S/c1-39-23-8-10-25(11-9-23)42(37,38)33(18-22-7-12-29(35)31-22)19-27(34)26(15-20-5-3-2-4-6-20)32-30(36)41-24-16-21-13-14-40-28(21)17-24/h2-6,8-11,21-22,24,26-28,34H,7,12-19H2,1H3,(H,31,35)(H,32,36)/t21-,22+,24+,26-,27+,28+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease V82A mutant using Abz-Thr-Ile-Nle-p-nitro-Phe-Gln-Arg-NH2 as substrate after 5 mins by spectrophotometry |
J Med Chem 55: 3387-97 (2012)
Article DOI: 10.1021/jm300072d
BindingDB Entry DOI: 10.7270/Q2T43WZW |
More data for this
Ligand-Target Pair | |
HIV-1 protease
(Human immunodeficiency virus) | BDBM31817

(GRL-02031 | methyl-2-pyrrolidinone, 19b)Show SMILES [H][C@]1(C[C@]2([H])CCO[C@]2([H])C1)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(C[C@H]1CCC(=O)N1)S(=O)(=O)c1ccc(OC)cc1 |r| Show InChI InChI=1S/C30H39N3O8S/c1-39-23-8-10-25(11-9-23)42(37,38)33(18-22-7-12-29(35)31-22)19-27(34)26(15-20-5-3-2-4-6-20)32-30(36)41-24-16-21-13-14-40-28(21)17-24/h2-6,8-11,21-22,24,26-28,34H,7,12-19H2,1H3,(H,31,35)(H,32,36)/t21-,22+,24+,26-,27+,28+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease L76V mutant using Abz-Thr-Ile-Nle-p-nitro-Phe-Gln-Arg-NH2 as substrate after 5 mins by spectrophotometry |
J Med Chem 55: 3387-97 (2012)
Article DOI: 10.1021/jm300072d
BindingDB Entry DOI: 10.7270/Q2T43WZW |
More data for this
Ligand-Target Pair | |
HIV-1 protease
(Human immunodeficiency virus) | BDBM31817

(GRL-02031 | methyl-2-pyrrolidinone, 19b)Show SMILES [H][C@]1(C[C@]2([H])CCO[C@]2([H])C1)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(C[C@H]1CCC(=O)N1)S(=O)(=O)c1ccc(OC)cc1 |r| Show InChI InChI=1S/C30H39N3O8S/c1-39-23-8-10-25(11-9-23)42(37,38)33(18-22-7-12-29(35)31-22)19-27(34)26(15-20-5-3-2-4-6-20)32-30(36)41-24-16-21-13-14-40-28(21)17-24/h2-6,8-11,21-22,24,26-28,34H,7,12-19H2,1H3,(H,31,35)(H,32,36)/t21-,22+,24+,26-,27+,28+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease N88D mutant using Abz-Thr-Ile-Nle-p-nitro-Phe-Gln-Arg-NH2 as substrate after 5 mins by spectrophotometry |
J Med Chem 55: 3387-97 (2012)
Article DOI: 10.1021/jm300072d
BindingDB Entry DOI: 10.7270/Q2T43WZW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50288399

(5-(4-Fluoro-phenyl)-4-(4-methanesulfonyl-phenyl)-t...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(N)sc1-c1ccc(F)cc1 Show InChI InChI=1S/C17H14FNO2S2/c1-23(20,21)14-8-4-11(5-9-14)15-10-16(19)22-17(15)12-2-6-13(18)7-3-12/h2-10H,19H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against human Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 2907-2912 (1996)
Article DOI: 10.1016/S0960-894X(96)00513-6
BindingDB Entry DOI: 10.7270/Q2ZW1KWB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50288380
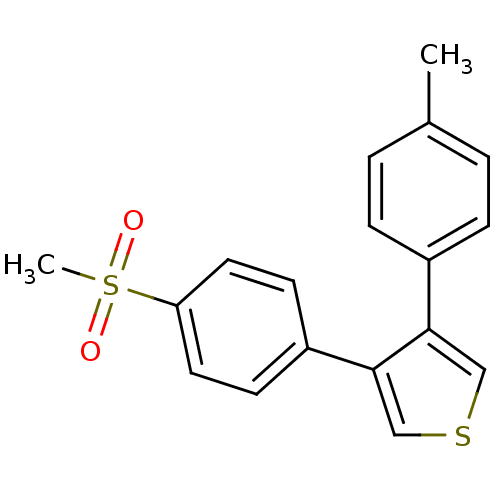
(3-(4-Methanesulfonyl-phenyl)-4-p-tolyl-thiophene |...)Show InChI InChI=1S/C18H16O2S2/c1-13-3-5-14(6-4-13)17-11-21-12-18(17)15-7-9-16(10-8-15)22(2,19)20/h3-12H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against human Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 2907-2912 (1996)
Article DOI: 10.1016/S0960-894X(96)00513-6
BindingDB Entry DOI: 10.7270/Q2ZW1KWB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50288389
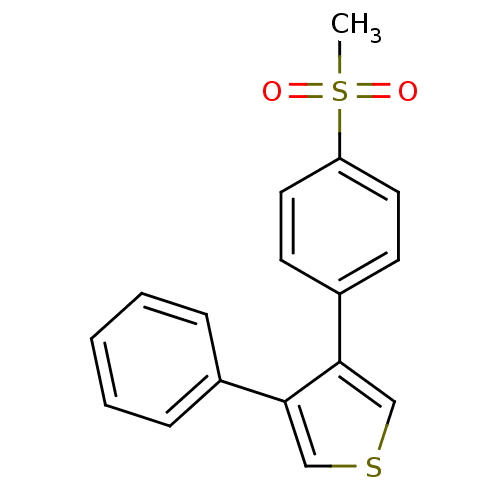
(3-(4-Methanesulfonyl-phenyl)-4-phenyl-thiophene | ...)Show InChI InChI=1S/C17H14O2S2/c1-21(18,19)15-9-7-14(8-10-15)17-12-20-11-16(17)13-5-3-2-4-6-13/h2-12H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against human Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 2907-2912 (1996)
Article DOI: 10.1016/S0960-894X(96)00513-6
BindingDB Entry DOI: 10.7270/Q2ZW1KWB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50288392
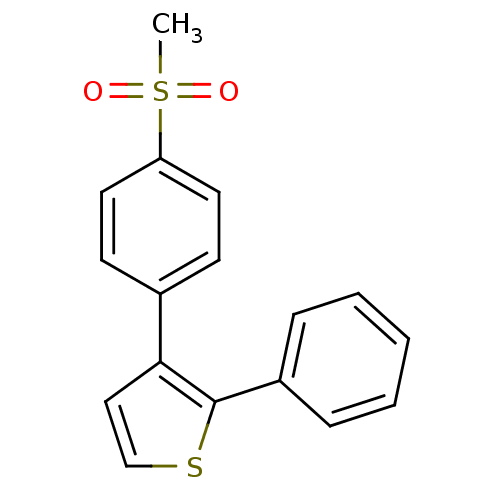
(3-(4-Methanesulfonyl-phenyl)-2-phenyl-thiophene | ...)Show InChI InChI=1S/C17H14O2S2/c1-21(18,19)15-9-7-13(8-10-15)16-11-12-20-17(16)14-5-3-2-4-6-14/h2-12H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against human Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 2907-2912 (1996)
Article DOI: 10.1016/S0960-894X(96)00513-6
BindingDB Entry DOI: 10.7270/Q2ZW1KWB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50286051
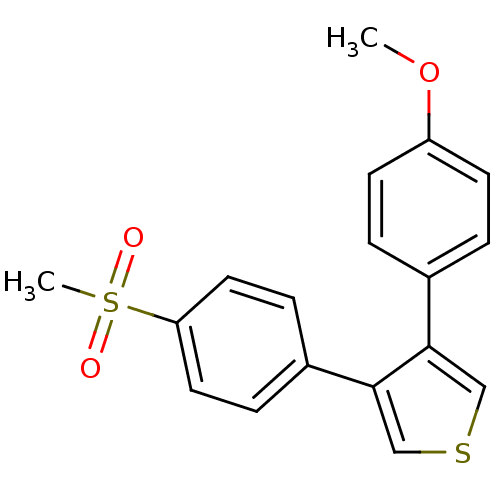
(3-(4-Methanesulfonyl-phenyl)-4-(4-methoxy-phenyl)-...)Show InChI InChI=1S/C18H16O3S2/c1-21-15-7-3-13(4-8-15)17-11-22-12-18(17)14-5-9-16(10-6-14)23(2,19)20/h3-12H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against human Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 2907-2912 (1996)
Article DOI: 10.1016/S0960-894X(96)00513-6
BindingDB Entry DOI: 10.7270/Q2ZW1KWB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50288390
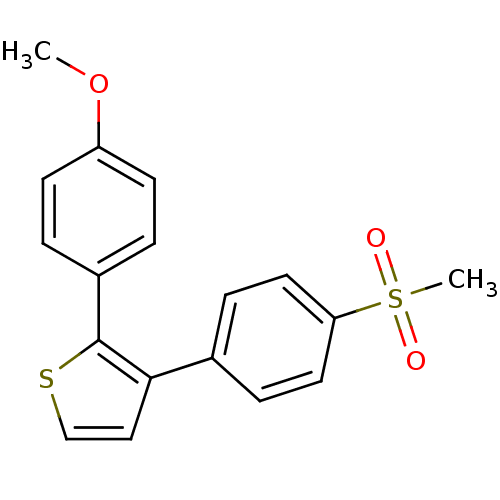
(3-(4-Methanesulfonyl-phenyl)-2-(4-methoxy-phenyl)-...)Show InChI InChI=1S/C18H16O3S2/c1-21-15-7-3-14(4-8-15)18-17(11-12-22-18)13-5-9-16(10-6-13)23(2,19)20/h3-12H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against human Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 2907-2912 (1996)
Article DOI: 10.1016/S0960-894X(96)00513-6
BindingDB Entry DOI: 10.7270/Q2ZW1KWB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50288399

(5-(4-Fluoro-phenyl)-4-(4-methanesulfonyl-phenyl)-t...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(N)sc1-c1ccc(F)cc1 Show InChI InChI=1S/C17H14FNO2S2/c1-23(20,21)14-8-4-11(5-9-14)15-10-16(19)22-17(15)12-2-6-13(18)7-3-12/h2-10H,19H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against ovine Prostaglandin G/H synthase 1 |
Bioorg Med Chem Lett 6: 2907-2912 (1996)
Article DOI: 10.1016/S0960-894X(96)00513-6
BindingDB Entry DOI: 10.7270/Q2ZW1KWB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50288382
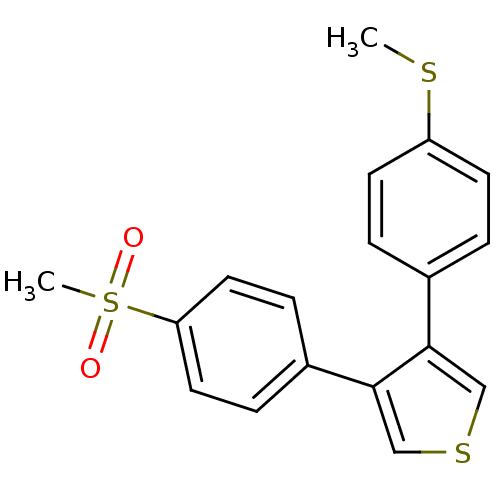
(3-(4-Methanesulfonyl-phenyl)-4-(4-methylsulfanyl-p...)Show InChI InChI=1S/C18H16O2S3/c1-21-15-7-3-13(4-8-15)17-11-22-12-18(17)14-5-9-16(10-6-14)23(2,19)20/h3-12H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against human Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 2907-2912 (1996)
Article DOI: 10.1016/S0960-894X(96)00513-6
BindingDB Entry DOI: 10.7270/Q2ZW1KWB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50029600
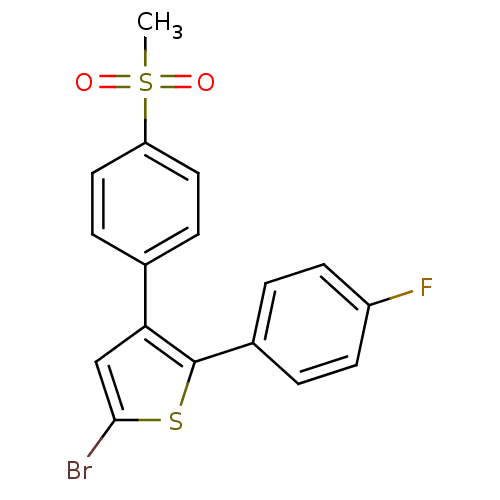
(5-Bromo-2-(4-fluoro-phenyl)-3-(4-methanesulfonyl-p...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(Br)sc1-c1ccc(F)cc1 Show InChI InChI=1S/C17H12BrFO2S2/c1-23(20,21)14-8-4-11(5-9-14)15-10-16(18)22-17(15)12-2-6-13(19)7-3-12/h2-10H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against human Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 2907-2912 (1996)
Article DOI: 10.1016/S0960-894X(96)00513-6
BindingDB Entry DOI: 10.7270/Q2ZW1KWB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50285227
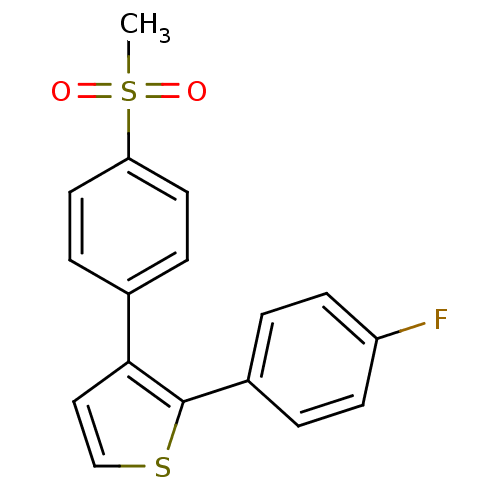
(2-(4-Fluoro-phenyl)-3-(4-methanesulfonyl-phenyl)-t...)Show InChI InChI=1S/C17H13FO2S2/c1-22(19,20)15-8-4-12(5-9-15)16-10-11-21-17(16)13-2-6-14(18)7-3-13/h2-11H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against human Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 2907-2912 (1996)
Article DOI: 10.1016/S0960-894X(96)00513-6
BindingDB Entry DOI: 10.7270/Q2ZW1KWB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50286046
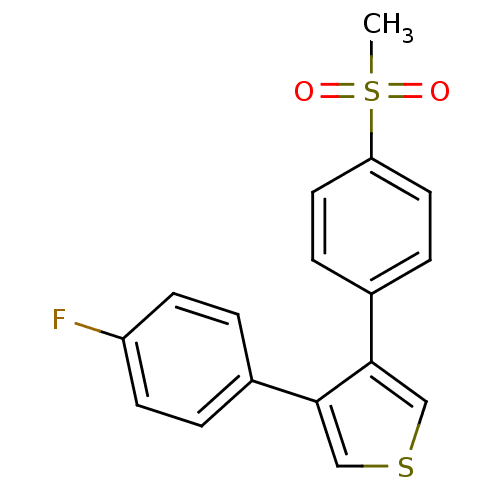
(3-(4-Fluoro-phenyl)-4-(4-methanesulfonyl-phenyl)-t...)Show InChI InChI=1S/C17H13FO2S2/c1-22(19,20)15-8-4-13(5-9-15)17-11-21-10-16(17)12-2-6-14(18)7-3-12/h2-11H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against human Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 2907-2912 (1996)
Article DOI: 10.1016/S0960-894X(96)00513-6
BindingDB Entry DOI: 10.7270/Q2ZW1KWB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50288394

(2-(4-Fluoro-phenyl)-3-(4-methanesulfonyl-phenyl)-5...)Show SMILES Cc1cc(c(s1)-c1ccc(F)cc1)-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C18H15FO2S2/c1-12-11-17(13-5-9-16(10-6-13)23(2,20)21)18(22-12)14-3-7-15(19)8-4-14/h3-11H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against human Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 2907-2912 (1996)
Article DOI: 10.1016/S0960-894X(96)00513-6
BindingDB Entry DOI: 10.7270/Q2ZW1KWB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50076524

(4-Methanesulfonyl-[1,1';2',1'']terphenyl | CHEMBL1...)Show InChI InChI=1S/C19H16O2S/c1-22(20,21)17-13-11-16(12-14-17)19-10-6-5-9-18(19)15-7-3-2-4-8-15/h2-14H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against human Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 2907-2912 (1996)
Article DOI: 10.1016/S0960-894X(96)00513-6
BindingDB Entry DOI: 10.7270/Q2ZW1KWB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50009860
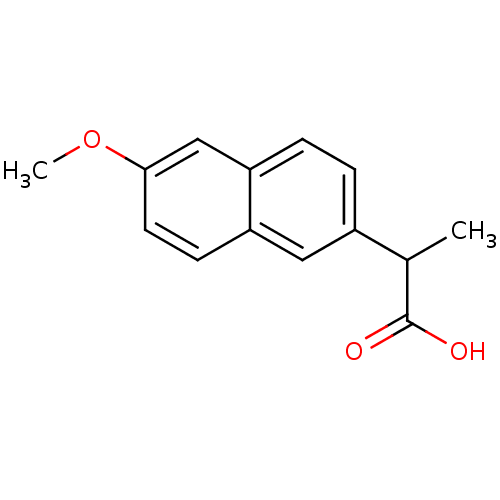
(2-(6-Methoxy-naphthalen-2-yl)-propionic acid | 2-(...)Show InChI InChI=1S/C14H14O3/c1-9(14(15)16)10-3-4-12-8-13(17-2)6-5-11(12)7-10/h3-9H,1-2H3,(H,15,16) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Horticulture and National Food Safety and Toxicology Center
Curated by ChEMBL
| Assay Description
Inhibition of PGHS1 assessed as conversion of arachidonic acid to prostaglandin |
J Nat Prod 62: 294-6 (1999)
Article DOI: 10.1021/np980501m
BindingDB Entry DOI: 10.7270/Q28G8KG8 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50288388

(3-[5-(4-Fluoro-phenyl)-4-(4-methanesulfonyl-phenyl...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(CCC(O)=O)sc1-c1ccc(F)cc1 Show InChI InChI=1S/C20H17FO4S2/c1-27(24,25)17-9-4-13(5-10-17)18-12-16(8-11-19(22)23)26-20(18)14-2-6-15(21)7-3-14/h2-7,9-10,12H,8,11H2,1H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against ovine Prostaglandin G/H synthase 1 |
Bioorg Med Chem Lett 6: 2907-2912 (1996)
Article DOI: 10.1016/S0960-894X(96)00513-6
BindingDB Entry DOI: 10.7270/Q2ZW1KWB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50288391
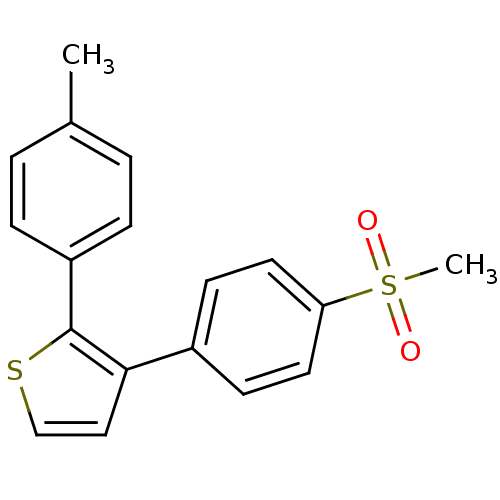
(3-(4-Methanesulfonyl-phenyl)-2-p-tolyl-thiophene |...)Show InChI InChI=1S/C18H16O2S2/c1-13-3-5-15(6-4-13)18-17(11-12-21-18)14-7-9-16(10-8-14)22(2,19)20/h3-12H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against human Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 2907-2912 (1996)
Article DOI: 10.1016/S0960-894X(96)00513-6
BindingDB Entry DOI: 10.7270/Q2ZW1KWB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50288393

(2-(4-Fluoro-phenyl)-3-(4-methanesulfonyl-phenyl)-5...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(sc1-c1ccc(F)cc1)[N+]([O-])=O Show InChI InChI=1S/C17H12FNO4S2/c1-25(22,23)14-8-4-11(5-9-14)15-10-16(19(20)21)24-17(15)12-2-6-13(18)7-3-12/h2-10H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against human Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 2907-2912 (1996)
Article DOI: 10.1016/S0960-894X(96)00513-6
BindingDB Entry DOI: 10.7270/Q2ZW1KWB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50288383
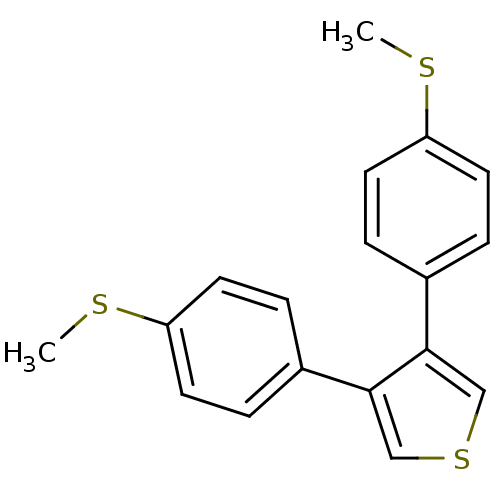
(3,4-Bis-(4-methylsulfanyl-phenyl)-thiophene | CHEM...)Show InChI InChI=1S/C18H16S3/c1-19-15-7-3-13(4-8-15)17-11-21-12-18(17)14-5-9-16(20-2)10-6-14/h3-12H,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against ovine Prostaglandin G/H synthase 1 |
Bioorg Med Chem Lett 6: 2907-2912 (1996)
Article DOI: 10.1016/S0960-894X(96)00513-6
BindingDB Entry DOI: 10.7270/Q2ZW1KWB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50288384
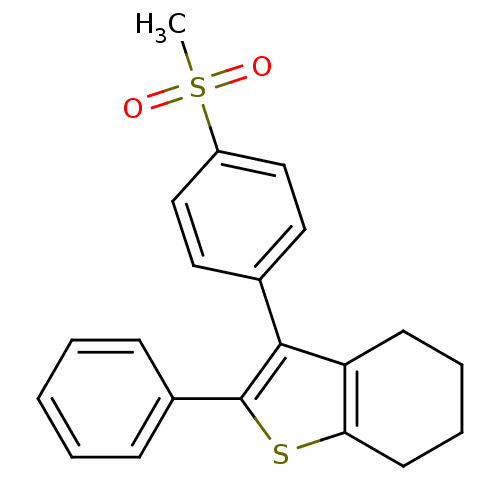
(3-(4-Methanesulfonyl-phenyl)-2-phenyl-4,5,6,7-tetr...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1c2CCCCc2sc1-c1ccccc1 Show InChI InChI=1S/C21H20O2S2/c1-25(22,23)17-13-11-15(12-14-17)20-18-9-5-6-10-19(18)24-21(20)16-7-3-2-4-8-16/h2-4,7-8,11-14H,5-6,9-10H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against human Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 2907-2912 (1996)
Article DOI: 10.1016/S0960-894X(96)00513-6
BindingDB Entry DOI: 10.7270/Q2ZW1KWB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50009859
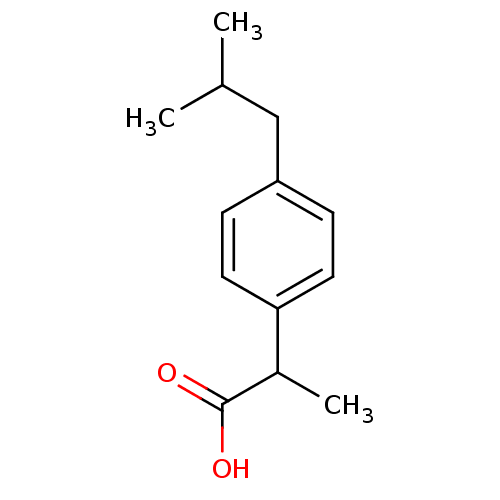
((+-)-2-(p-isobutylphenyl)propionic acid | (+-)-alp...)Show InChI InChI=1S/C13H18O2/c1-9(2)8-11-4-6-12(7-5-11)10(3)13(14)15/h4-7,9-10H,8H2,1-3H3,(H,14,15) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Horticulture and National Food Safety and Toxicology Center
Curated by ChEMBL
| Assay Description
Inhibition of PGHS1 assessed as conversion of arachidonic acid to prostaglandin |
J Nat Prod 62: 294-6 (1999)
Article DOI: 10.1021/np980501m
BindingDB Entry DOI: 10.7270/Q28G8KG8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50288387
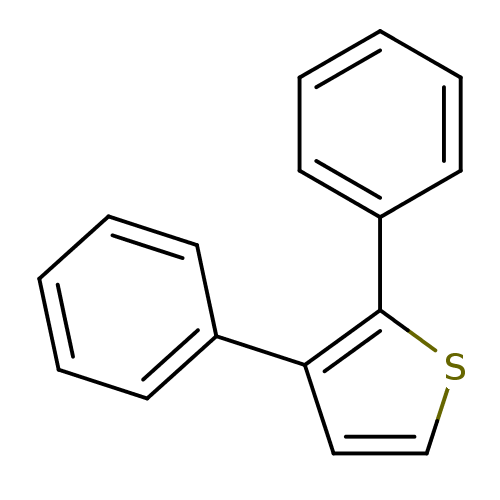
(2,3-Diphenyl-thiophene | CHEMBL318678)Show InChI InChI=1S/C16H12S/c1-3-7-13(8-4-1)15-11-12-17-16(15)14-9-5-2-6-10-14/h1-12H | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against ovine Prostaglandin G/H synthase 1 |
Bioorg Med Chem Lett 6: 2907-2912 (1996)
Article DOI: 10.1016/S0960-894X(96)00513-6
BindingDB Entry DOI: 10.7270/Q2ZW1KWB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50029600

(5-Bromo-2-(4-fluoro-phenyl)-3-(4-methanesulfonyl-p...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(Br)sc1-c1ccc(F)cc1 Show InChI InChI=1S/C17H12BrFO2S2/c1-23(20,21)14-8-4-11(5-9-14)15-10-16(18)22-17(15)12-2-6-13(19)7-3-12/h2-10H,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against ovine Prostaglandin G/H synthase 1 |
Bioorg Med Chem Lett 6: 2907-2912 (1996)
Article DOI: 10.1016/S0960-894X(96)00513-6
BindingDB Entry DOI: 10.7270/Q2ZW1KWB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50288390

(3-(4-Methanesulfonyl-phenyl)-2-(4-methoxy-phenyl)-...)Show InChI InChI=1S/C18H16O3S2/c1-21-15-7-3-14(4-8-15)18-17(11-12-22-18)13-5-9-16(10-6-13)23(2,19)20/h3-12H,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against ovine Prostaglandin G/H synthase 1 |
Bioorg Med Chem Lett 6: 2907-2912 (1996)
Article DOI: 10.1016/S0960-894X(96)00513-6
BindingDB Entry DOI: 10.7270/Q2ZW1KWB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50288395
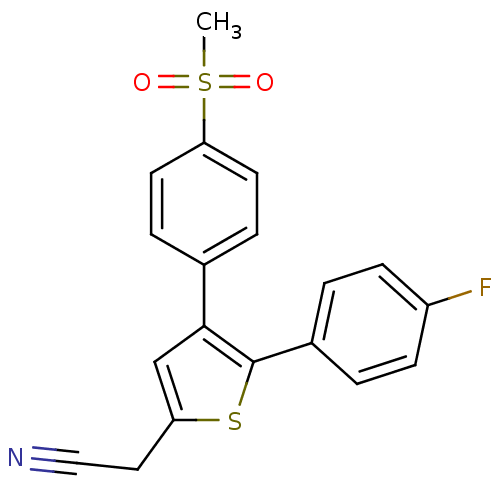
(CHEMBL101459 | [5-(4-Fluoro-phenyl)-4-(4-methanesu...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(CC#N)sc1-c1ccc(F)cc1 Show InChI InChI=1S/C19H14FNO2S2/c1-25(22,23)17-8-4-13(5-9-17)18-12-16(10-11-21)24-19(18)14-2-6-15(20)7-3-14/h2-9,12H,10H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against human Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 2907-2912 (1996)
Article DOI: 10.1016/S0960-894X(96)00513-6
BindingDB Entry DOI: 10.7270/Q2ZW1KWB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50288396
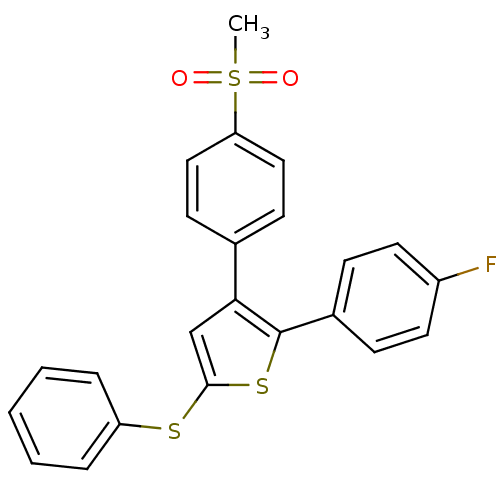
(2-(4-Fluoro-phenyl)-3-(4-methanesulfonyl-phenyl)-5...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(Sc2ccccc2)sc1-c1ccc(F)cc1 Show InChI InChI=1S/C23H17FO2S3/c1-29(25,26)20-13-9-16(10-14-20)21-15-22(27-19-5-3-2-4-6-19)28-23(21)17-7-11-18(24)12-8-17/h2-15H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 6.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against human Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 2907-2912 (1996)
Article DOI: 10.1016/S0960-894X(96)00513-6
BindingDB Entry DOI: 10.7270/Q2ZW1KWB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50288394

(2-(4-Fluoro-phenyl)-3-(4-methanesulfonyl-phenyl)-5...)Show SMILES Cc1cc(c(s1)-c1ccc(F)cc1)-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C18H15FO2S2/c1-12-11-17(13-5-9-16(10-6-13)23(2,20)21)18(22-12)14-3-7-15(19)8-4-14/h3-11H,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against ovine Prostaglandin G/H synthase 1 |
Bioorg Med Chem Lett 6: 2907-2912 (1996)
Article DOI: 10.1016/S0960-894X(96)00513-6
BindingDB Entry DOI: 10.7270/Q2ZW1KWB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50288397
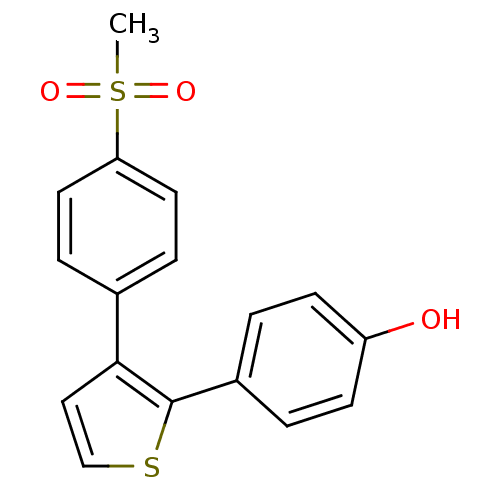
(4-[3-(4-Methanesulfonyl-phenyl)-thiophen-2-yl]-phe...)Show InChI InChI=1S/C17H14O3S2/c1-22(19,20)15-8-4-12(5-9-15)16-10-11-21-17(16)13-2-6-14(18)7-3-13/h2-11,18H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 8.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against human Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 2907-2912 (1996)
Article DOI: 10.1016/S0960-894X(96)00513-6
BindingDB Entry DOI: 10.7270/Q2ZW1KWB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50288382

(3-(4-Methanesulfonyl-phenyl)-4-(4-methylsulfanyl-p...)Show InChI InChI=1S/C18H16O2S3/c1-21-15-7-3-13(4-8-15)17-11-22-12-18(17)14-5-9-16(10-6-14)23(2,19)20/h3-12H,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 9.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against ovine Prostaglandin G/H synthase 1 |
Bioorg Med Chem Lett 6: 2907-2912 (1996)
Article DOI: 10.1016/S0960-894X(96)00513-6
BindingDB Entry DOI: 10.7270/Q2ZW1KWB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50288381

((E)-3-[5-(4-Fluoro-phenyl)-4-(4-methanesulfonyl-ph...)Show SMILES CCOC(=O)\C=C\c1cc(c(s1)-c1ccc(F)cc1)-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C22H19FO4S2/c1-3-27-21(24)13-10-18-14-20(15-6-11-19(12-7-15)29(2,25)26)22(28-18)16-4-8-17(23)9-5-16/h4-14H,3H2,1-2H3/b13-10+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against ovine Prostaglandin G/H synthase 1 |
Bioorg Med Chem Lett 6: 2907-2912 (1996)
Article DOI: 10.1016/S0960-894X(96)00513-6
BindingDB Entry DOI: 10.7270/Q2ZW1KWB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50288383

(3,4-Bis-(4-methylsulfanyl-phenyl)-thiophene | CHEM...)Show InChI InChI=1S/C18H16S3/c1-19-15-7-3-13(4-8-15)17-11-21-12-18(17)14-5-9-16(20-2)10-6-14/h3-12H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.01E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against human Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 2907-2912 (1996)
Article DOI: 10.1016/S0960-894X(96)00513-6
BindingDB Entry DOI: 10.7270/Q2ZW1KWB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50288385

(3-[5-(4-Fluoro-phenyl)-4-(4-methanesulfonyl-phenyl...)Show SMILES CCOC(=O)CCc1cc(c(s1)-c1ccc(F)cc1)-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C22H21FO4S2/c1-3-27-21(24)13-10-18-14-20(15-6-11-19(12-7-15)29(2,25)26)22(28-18)16-4-8-17(23)9-5-16/h4-9,11-12,14H,3,10,13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against human Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 2907-2912 (1996)
Article DOI: 10.1016/S0960-894X(96)00513-6
BindingDB Entry DOI: 10.7270/Q2ZW1KWB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50288385

(3-[5-(4-Fluoro-phenyl)-4-(4-methanesulfonyl-phenyl...)Show SMILES CCOC(=O)CCc1cc(c(s1)-c1ccc(F)cc1)-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C22H21FO4S2/c1-3-27-21(24)13-10-18-14-20(15-6-11-19(12-7-15)29(2,25)26)22(28-18)16-4-8-17(23)9-5-16/h4-9,11-12,14H,3,10,13H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against ovine Prostaglandin G/H synthase 1 |
Bioorg Med Chem Lett 6: 2907-2912 (1996)
Article DOI: 10.1016/S0960-894X(96)00513-6
BindingDB Entry DOI: 10.7270/Q2ZW1KWB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50288398
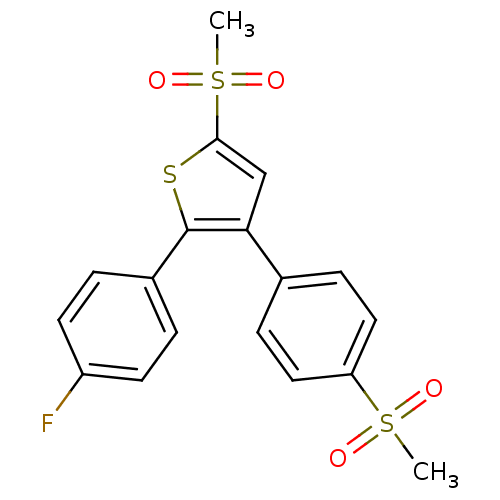
(2-(4-Fluoro-phenyl)-5-methanesulfonyl-3-(4-methane...)Show SMILES CS(=O)(=O)c1cc(c(s1)-c1ccc(F)cc1)-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C18H15FO4S3/c1-25(20,21)15-9-5-12(6-10-15)16-11-17(26(2,22)23)24-18(16)13-3-7-14(19)8-4-13/h3-11H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against human Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 2907-2912 (1996)
Article DOI: 10.1016/S0960-894X(96)00513-6
BindingDB Entry DOI: 10.7270/Q2ZW1KWB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50288397

(4-[3-(4-Methanesulfonyl-phenyl)-thiophen-2-yl]-phe...)Show InChI InChI=1S/C17H14O3S2/c1-22(19,20)15-8-4-12(5-9-15)16-10-11-21-17(16)13-2-6-14(18)7-3-13/h2-11,18H,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 2.93E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against ovine Prostaglandin G/H synthase 1 |
Bioorg Med Chem Lett 6: 2907-2912 (1996)
Article DOI: 10.1016/S0960-894X(96)00513-6
BindingDB Entry DOI: 10.7270/Q2ZW1KWB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50288388

(3-[5-(4-Fluoro-phenyl)-4-(4-methanesulfonyl-phenyl...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(CCC(O)=O)sc1-c1ccc(F)cc1 Show InChI InChI=1S/C20H17FO4S2/c1-27(24,25)17-9-4-13(5-10-17)18-12-16(8-11-19(22)23)26-20(18)14-2-6-15(21)7-3-14/h2-7,9-10,12H,8,11H2,1H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against human Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 2907-2912 (1996)
Article DOI: 10.1016/S0960-894X(96)00513-6
BindingDB Entry DOI: 10.7270/Q2ZW1KWB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50288387

(2,3-Diphenyl-thiophene | CHEMBL318678)Show InChI InChI=1S/C16H12S/c1-3-7-13(8-4-1)15-11-12-17-16(15)14-9-5-2-6-10-14/h1-12H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against human Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 2907-2912 (1996)
Article DOI: 10.1016/S0960-894X(96)00513-6
BindingDB Entry DOI: 10.7270/Q2ZW1KWB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50288386
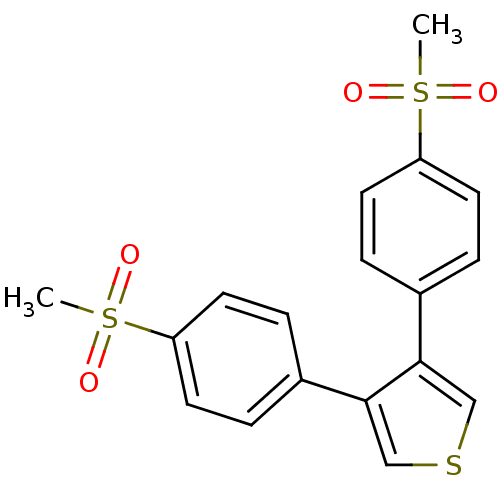
(3,4-Bis-(4-methanesulfonyl-phenyl)-thiophene | CHE...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cscc1-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C18H16O4S3/c1-24(19,20)15-7-3-13(4-8-15)17-11-23-12-18(17)14-5-9-16(10-6-14)25(2,21)22/h3-12H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against human Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 2907-2912 (1996)
Article DOI: 10.1016/S0960-894X(96)00513-6
BindingDB Entry DOI: 10.7270/Q2ZW1KWB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50288381

((E)-3-[5-(4-Fluoro-phenyl)-4-(4-methanesulfonyl-ph...)Show SMILES CCOC(=O)\C=C\c1cc(c(s1)-c1ccc(F)cc1)-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C22H19FO4S2/c1-3-27-21(24)13-10-18-14-20(15-6-11-19(12-7-15)29(2,25)26)22(28-18)16-4-8-17(23)9-5-16/h4-14H,3H2,1-2H3/b13-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against human Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 2907-2912 (1996)
Article DOI: 10.1016/S0960-894X(96)00513-6
BindingDB Entry DOI: 10.7270/Q2ZW1KWB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50288398

(2-(4-Fluoro-phenyl)-5-methanesulfonyl-3-(4-methane...)Show SMILES CS(=O)(=O)c1cc(c(s1)-c1ccc(F)cc1)-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C18H15FO4S3/c1-25(20,21)15-9-5-12(6-10-15)16-11-17(26(2,22)23)24-18(16)13-3-7-14(19)8-4-13/h3-11H,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against ovine Prostaglandin G/H synthase 1 |
Bioorg Med Chem Lett 6: 2907-2912 (1996)
Article DOI: 10.1016/S0960-894X(96)00513-6
BindingDB Entry DOI: 10.7270/Q2ZW1KWB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50288386

(3,4-Bis-(4-methanesulfonyl-phenyl)-thiophene | CHE...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cscc1-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C18H16O4S3/c1-24(19,20)15-7-3-13(4-8-15)17-11-23-12-18(17)14-5-9-16(10-6-14)25(2,21)22/h3-12H,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against ovine Prostaglandin G/H synthase 1 |
Bioorg Med Chem Lett 6: 2907-2912 (1996)
Article DOI: 10.1016/S0960-894X(96)00513-6
BindingDB Entry DOI: 10.7270/Q2ZW1KWB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50288396

(2-(4-Fluoro-phenyl)-3-(4-methanesulfonyl-phenyl)-5...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(Sc2ccccc2)sc1-c1ccc(F)cc1 Show InChI InChI=1S/C23H17FO2S3/c1-29(25,26)20-13-9-16(10-14-20)21-15-22(27-19-5-3-2-4-6-19)28-23(21)17-7-11-18(24)12-8-17/h2-15H,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against ovine Prostaglandin G/H synthase 1 |
Bioorg Med Chem Lett 6: 2907-2912 (1996)
Article DOI: 10.1016/S0960-894X(96)00513-6
BindingDB Entry DOI: 10.7270/Q2ZW1KWB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50288395

(CHEMBL101459 | [5-(4-Fluoro-phenyl)-4-(4-methanesu...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(CC#N)sc1-c1ccc(F)cc1 Show InChI InChI=1S/C19H14FNO2S2/c1-25(22,23)17-8-4-13(5-9-17)18-12-16(10-11-21)24-19(18)14-2-6-15(20)7-3-14/h2-9,12H,10H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against ovine Prostaglandin G/H synthase 1 |
Bioorg Med Chem Lett 6: 2907-2912 (1996)
Article DOI: 10.1016/S0960-894X(96)00513-6
BindingDB Entry DOI: 10.7270/Q2ZW1KWB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50288380

(3-(4-Methanesulfonyl-phenyl)-4-p-tolyl-thiophene |...)Show InChI InChI=1S/C18H16O2S2/c1-13-3-5-14(6-4-13)17-11-21-12-18(17)15-7-9-16(10-8-15)22(2,19)20/h3-12H,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 4.38E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against ovine Prostaglandin G/H synthase 1 |
Bioorg Med Chem Lett 6: 2907-2912 (1996)
Article DOI: 10.1016/S0960-894X(96)00513-6
BindingDB Entry DOI: 10.7270/Q2ZW1KWB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50285227
(2-(4-Fluoro-phenyl)-3-(4-methanesulfonyl-phenyl)-t...)Show InChI InChI=1S/C17H13FO2S2/c1-22(19,20)15-8-4-12(5-9-15)16-10-11-21-17(16)13-2-6-14(18)7-3-13/h2-11H,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 4.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against ovine Prostaglandin G/H synthase 1 |
Bioorg Med Chem Lett 6: 2907-2912 (1996)
Article DOI: 10.1016/S0960-894X(96)00513-6
BindingDB Entry DOI: 10.7270/Q2ZW1KWB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data