

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM403460 (US10335399, Example 2-5 | US10806724, Example 2-5) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human recombinant mGlur2 assessed as inhibition constant by cAMP Glosensor assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02004 BindingDB Entry DOI: 10.7270/Q2TQ65FG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM403460 (US10335399, Example 2-5 | US10806724, Example 2-5) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human recombinant mGlur2 assessed as inhibition constant by cAMP Glosensor assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02004 BindingDB Entry DOI: 10.7270/Q2TQ65FG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM50584759 (CHEMBL5089623) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human recombinant mGlur2 assessed as inhibition constant by cAMP Glosensor assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02004 BindingDB Entry DOI: 10.7270/Q2TQ65FG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM50584759 (CHEMBL5089623) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human recombinant mGlur2 assessed as inhibition constant by cAMP Glosensor assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02004 BindingDB Entry DOI: 10.7270/Q2TQ65FG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50223216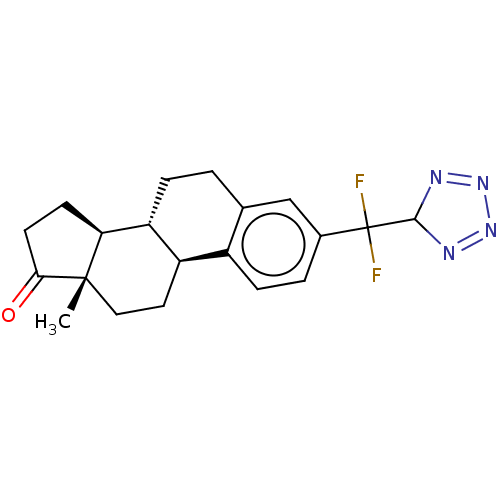 (CHEMBL3348562) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibitory activity against steroid sulfatase (STS) | Bioorg Med Chem Lett 14: 151-5 (2003) BindingDB Entry DOI: 10.7270/Q28K79NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50366887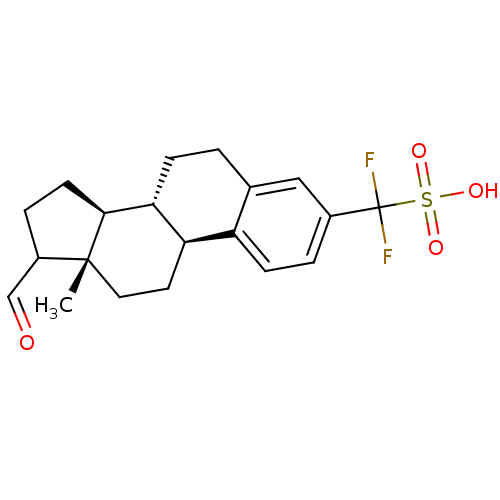 (CHEMBL1627329) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibitory activity against steroid sulfatase (STS) | Bioorg Med Chem Lett 14: 151-5 (2003) BindingDB Entry DOI: 10.7270/Q28K79NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50072590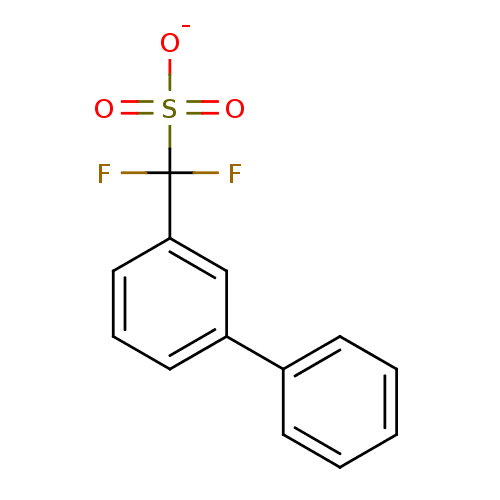 (CHEMBL109531 | Sodium; biphenyl-3-yl-difluoro-meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Binding affinity against Protein tyrosine phosphatase-1B (PTP1B) | Bioorg Med Chem Lett 8: 3275-80 (1999) BindingDB Entry DOI: 10.7270/Q20R9NK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50366886 (CHEMBL1628110) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Competitive inhibitory activity against steroid sulfatase (STS) | Bioorg Med Chem Lett 14: 151-5 (2003) BindingDB Entry DOI: 10.7270/Q28K79NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50366524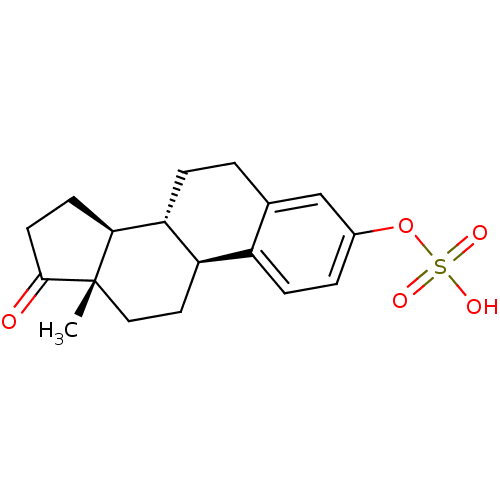 (ESTRONE | ESTROPIPATE | Estrone 3-sulfate | Estron...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibitory activity against steroid sulfatase (STS) desulfation of 4-MUS | Bioorg Med Chem Lett 14: 151-5 (2003) BindingDB Entry DOI: 10.7270/Q28K79NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50366888 (CHEMBL1627836) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibitory activity against steroid sulfatase (STS) | Bioorg Med Chem Lett 14: 151-5 (2003) BindingDB Entry DOI: 10.7270/Q28K79NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50072593 (5-(Biphenyl-3-yl-difluoro-methyl)-1-sodium-1H-tetr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 9.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibitory activity against protein tyrosine phosphatase 1B (PTP1B) using fluorescein diphosphate as substrate at Km concentration (20 uM) | Bioorg Med Chem Lett 8: 3275-80 (1999) BindingDB Entry DOI: 10.7270/Q20R9NK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50366885 (CHEMBL1628114) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibitory activity against steroid sulfatase (STS) | Bioorg Med Chem Lett 14: 151-5 (2003) BindingDB Entry DOI: 10.7270/Q28K79NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50366884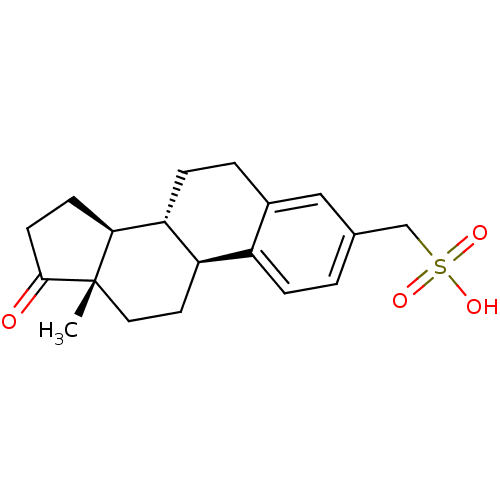 (CHEMBL1628109) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibitory activity against steroid sulfatase (STS) | Bioorg Med Chem Lett 14: 151-5 (2003) BindingDB Entry DOI: 10.7270/Q28K79NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50241361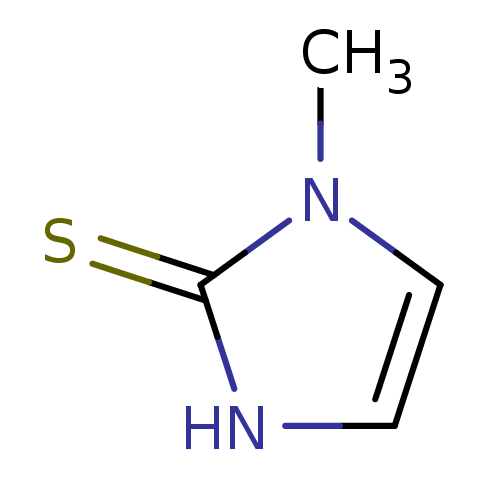 (CHEMBL1515 | METHIMAZOLE | US9138393, Methimazole ...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | 4.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hungkuang University Curated by ChEMBL | Assay Description Mixed-type inhibition of mushroom tyrosinase-mediated L-tyrosine oxidation assessed as enzyme-inhibitor complex after 10 mins by Lineweaver-Burk plot... | Bioorg Med Chem 22: 2809-15 (2014) Article DOI: 10.1016/j.bmc.2014.03.009 BindingDB Entry DOI: 10.7270/Q2417125 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50497083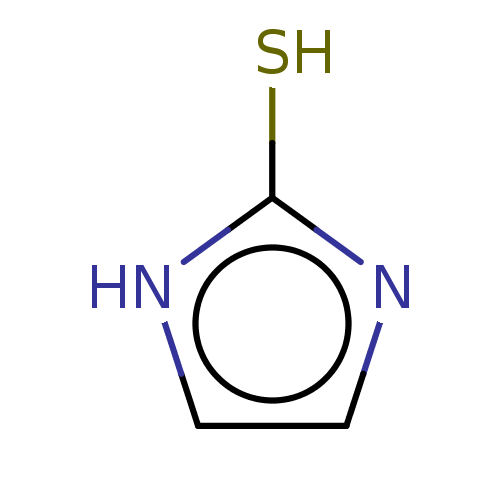 (CHEMBL3260774) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 5.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hungkuang University Curated by ChEMBL | Assay Description Mixed-type inhibition of mushroom tyrosinase-mediated L-tyrosine oxidation assessed as enzyme-inhibitor complex after 10 mins by Lineweaver-Burk plot... | Bioorg Med Chem 22: 2809-15 (2014) Article DOI: 10.1016/j.bmc.2014.03.009 BindingDB Entry DOI: 10.7270/Q2417125 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50366884 (CHEMBL1628109) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 6.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibitory activity against steroid sulfatase (STS) using methodology of Li et al. | Bioorg Med Chem Lett 14: 151-5 (2003) BindingDB Entry DOI: 10.7270/Q28K79NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50497084 (CHEMBL3260775) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.75E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hungkuang University Curated by ChEMBL | Assay Description Noncompetitive inhibition of mushroom tyrosinase-mediated L-tyrosine oxidation assessed as enzyme-inhibitor complex after 10 mins by Lineweaver-Burk ... | Bioorg Med Chem 22: 2809-15 (2014) Article DOI: 10.1016/j.bmc.2014.03.009 BindingDB Entry DOI: 10.7270/Q2417125 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM403460 (US10335399, Example 2-5 | US10806724, Example 2-5) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Negative allosteric modulation activity at human recombinant mGlur2 expressed in CHO cells in presence of cAMP by chemiluminescence based assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02004 BindingDB Entry DOI: 10.7270/Q2TQ65FG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM393885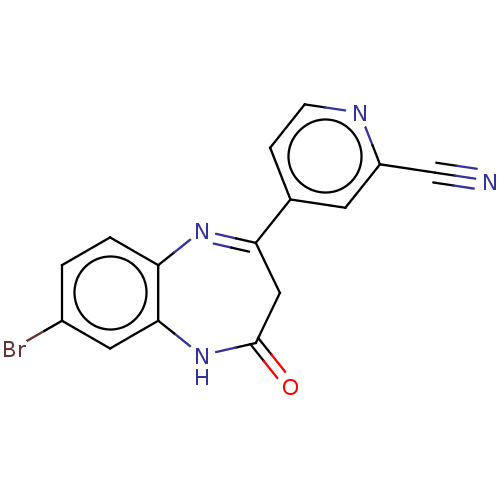 (US10597367, Example 182 | US9969726, Example 182) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Negative allosteric modulator activity at human recombinant mGlur2 expressed in HEK293 cells by calcium assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02004 BindingDB Entry DOI: 10.7270/Q2TQ65FG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM50584759 (CHEMBL5089623) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Negative allosteric modulation activity at human recombinant mGlur2 expressed in CHO cells in presence of cAMP by chemiluminescence based assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02004 BindingDB Entry DOI: 10.7270/Q2TQ65FG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM50584759 (CHEMBL5089623) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Negative allosteric modulation activity at human recombinant mGlur2 expressed in CHO cells in presence of cAMP by chemiluminescence based assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02004 BindingDB Entry DOI: 10.7270/Q2TQ65FG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50353025 (CHEMBL1821987) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibition of iNOS in mouse RAW264.7 cells assessed as anti-inflammatory activity by measuring maximum inhibition of LPS-induced nitric oxide product... | J Nat Prod 74: 1875-80 (2011) Article DOI: 10.1021/np200279r BindingDB Entry DOI: 10.7270/Q2474B72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50072595 ((Biphenyl-3-yl-difluoro-methyl)-phosphonium | CHEM...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibitory activity against PTP1B as a model Protein tyrosine transferase(PTP) using fluorescein diphosphate as substrate at Km concentration (20 uM)... | Bioorg Med Chem Lett 8: 3275-80 (1999) BindingDB Entry DOI: 10.7270/Q20R9NK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50353027 (CHEMBL1821990) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibition of iNOS in mouse RAW264.7 cells assessed as anti-inflammatory activity by measuring maximum inhibition of LPS-induced nitric oxide product... | J Nat Prod 74: 1875-80 (2011) Article DOI: 10.1021/np200279r BindingDB Entry DOI: 10.7270/Q2474B72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50207159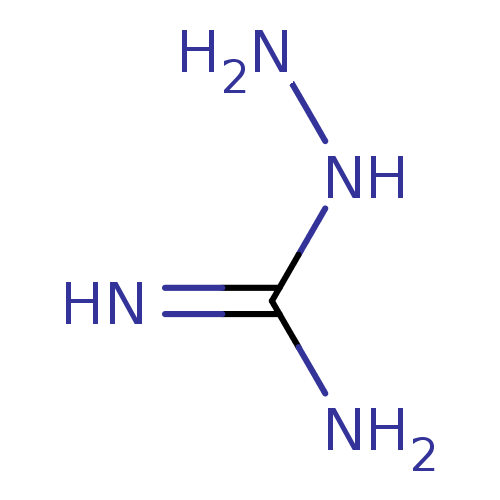 (2-aminoguanidine | 2-azanylguanidine | AMINOGUANID...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents | DrugBank MMDB PDB Article PubMed | n/a | n/a | 2.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibition of iNOS in mouse RAW264.7 cells assessed as anti-inflammatory activity by measuring maximum inhibition of LPS-induced nitric oxide product... | J Nat Prod 74: 1875-80 (2011) Article DOI: 10.1021/np200279r BindingDB Entry DOI: 10.7270/Q2474B72 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50072597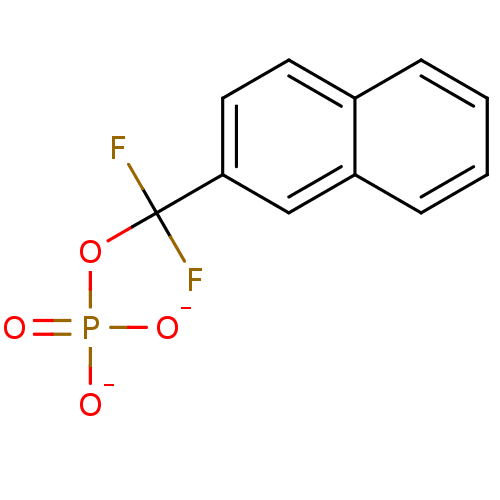 ((Difluoro-naphthalen-2-yl-methyl)-phosphonium | CH...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibitory activity against protein tyrosine phosphatase 1B (PTP1B) using fluorescein diphosphate as substrate at Km concentration (20 uM) | Bioorg Med Chem Lett 8: 3275-80 (1999) BindingDB Entry DOI: 10.7270/Q20R9NK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50353026 (CHEMBL1821989) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibition of iNOS in mouse RAW264.7 cells assessed as anti-inflammatory activity by measuring maximum inhibition of LPS-induced nitric oxide product... | J Nat Prod 74: 1875-80 (2011) Article DOI: 10.1021/np200279r BindingDB Entry DOI: 10.7270/Q2474B72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50353024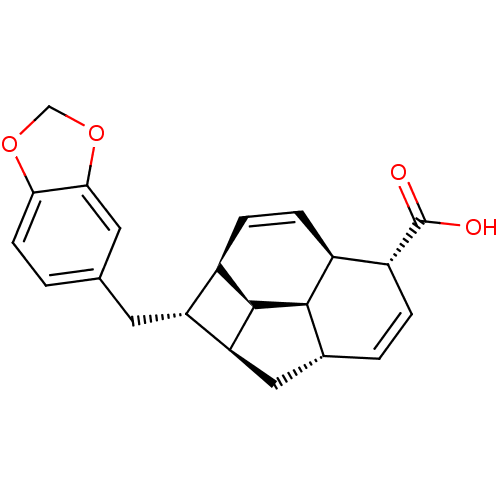 (CHEMBL1821986) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibition of iNOS in mouse RAW264.7 cells assessed as anti-inflammatory activity by measuring maximum inhibition of LPS-induced nitric oxide product... | J Nat Prod 74: 1875-80 (2011) Article DOI: 10.1021/np200279r BindingDB Entry DOI: 10.7270/Q2474B72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50353030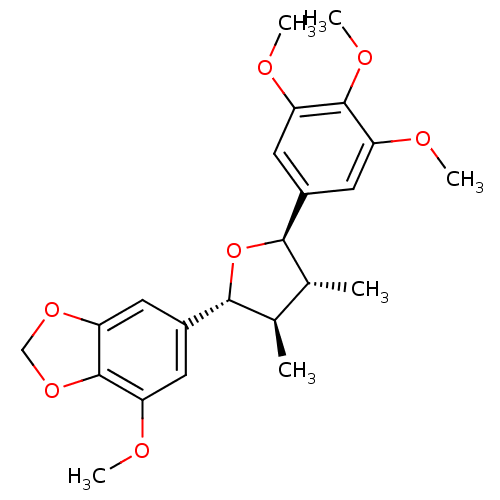 (CHEMBL1821993) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibition of iNOS in mouse RAW264.7 cells assessed as anti-inflammatory activity by measuring maximum inhibition of LPS-induced nitric oxide product... | J Nat Prod 74: 1875-80 (2011) Article DOI: 10.1021/np200279r BindingDB Entry DOI: 10.7270/Q2474B72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50353023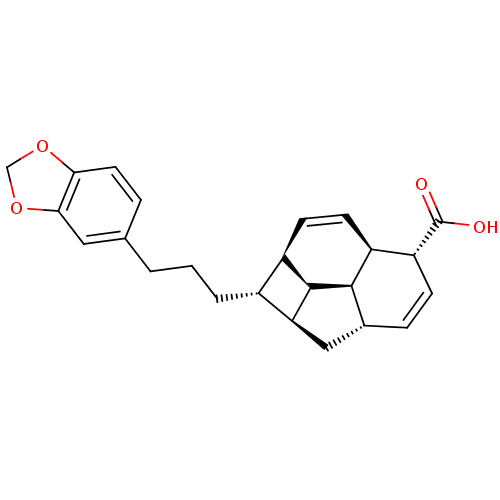 (CHEMBL1821985) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibition of iNOS in mouse RAW264.7 cells assessed as anti-inflammatory activity by measuring maximum inhibition of LPS-induced nitric oxide product... | J Nat Prod 74: 1875-80 (2011) Article DOI: 10.1021/np200279r BindingDB Entry DOI: 10.7270/Q2474B72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50353029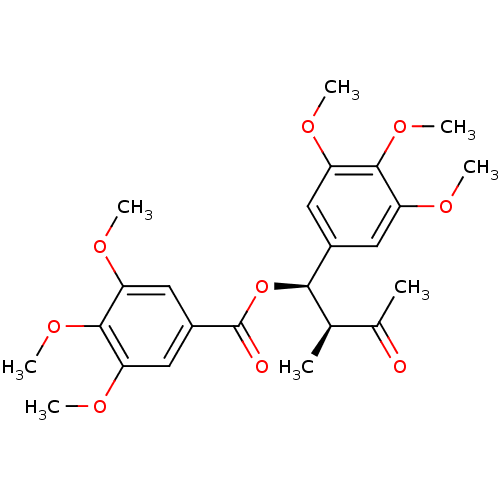 (CHEMBL1821992) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibition of iNOS in mouse RAW264.7 cells assessed as anti-inflammatory activity by measuring maximum inhibition of LPS-induced nitric oxide product... | J Nat Prod 74: 1875-80 (2011) Article DOI: 10.1021/np200279r BindingDB Entry DOI: 10.7270/Q2474B72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50353028 (CHEMBL1821991) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibition of iNOS in mouse RAW264.7 cells assessed as anti-inflammatory activity by measuring maximum inhibition of LPS-induced nitric oxide product... | J Nat Prod 74: 1875-80 (2011) Article DOI: 10.1021/np200279r BindingDB Entry DOI: 10.7270/Q2474B72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50353031 (CHEMBL1821994) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibition of iNOS in mouse RAW264.7 cells assessed as anti-inflammatory activity by measuring maximum inhibition of LPS-induced nitric oxide product... | J Nat Prod 74: 1875-80 (2011) Article DOI: 10.1021/np200279r BindingDB Entry DOI: 10.7270/Q2474B72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50353032 (CHEMBL1821988) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibition of iNOS in mouse RAW264.7 cells assessed as anti-inflammatory activity by measuring maximum inhibition of LPS-induced nitric oxide product... | J Nat Prod 74: 1875-80 (2011) Article DOI: 10.1021/np200279r BindingDB Entry DOI: 10.7270/Q2474B72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50072590 (CHEMBL109531 | Sodium; biphenyl-3-yl-difluoro-meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.15E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Binding affinity against Protein tyrosine phosphatase-1B (PTP1B) | Bioorg Med Chem Lett 8: 3275-80 (1999) BindingDB Entry DOI: 10.7270/Q20R9NK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50225106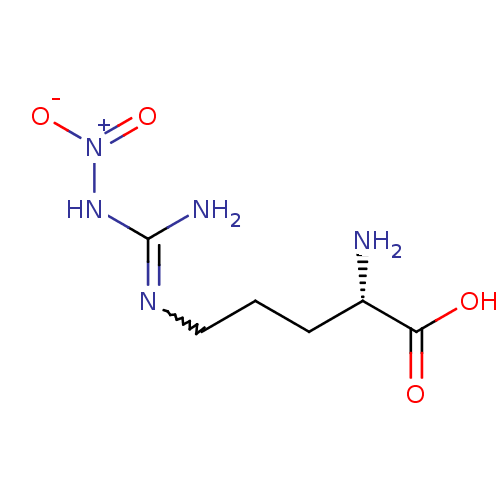 ((2S)-2-amino-5-{[(E)-amino(nitroimino)methyl]amino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.52E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibition of iNOS in mouse RAW264.7 cells assessed as anti-inflammatory activity by measuring maximum inhibition of LPS-induced nitric oxide product... | J Nat Prod 74: 1875-80 (2011) Article DOI: 10.1021/np200279r BindingDB Entry DOI: 10.7270/Q2474B72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50072598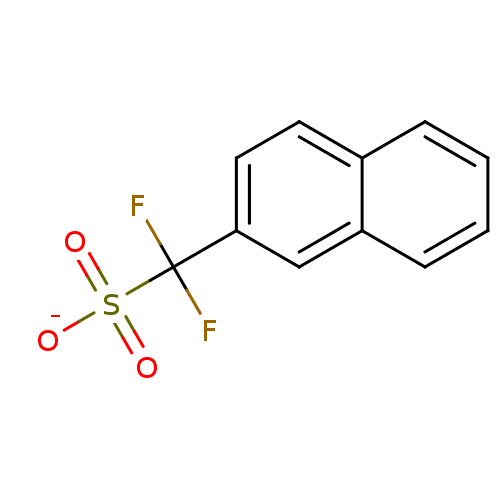 (CHEMBL109646 | Sodium; difluoro-naphthalen-2-yl-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.75E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibitory activity against protein tyrosine phosphatase 1B (PTP1B) using fluorescein diphosphate as substrate at Km concentration (20 uM) | Bioorg Med Chem Lett 8: 3275-80 (1999) BindingDB Entry DOI: 10.7270/Q20R9NK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50072593 (5-(Biphenyl-3-yl-difluoro-methyl)-1-sodium-1H-tetr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.95E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibitory activity against protein tyrosine phosphatase 1B (PTP1B) using fluorescein diphosphate as substrate at Km concentration (20 uM) | Bioorg Med Chem Lett 8: 3275-80 (1999) BindingDB Entry DOI: 10.7270/Q20R9NK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50072592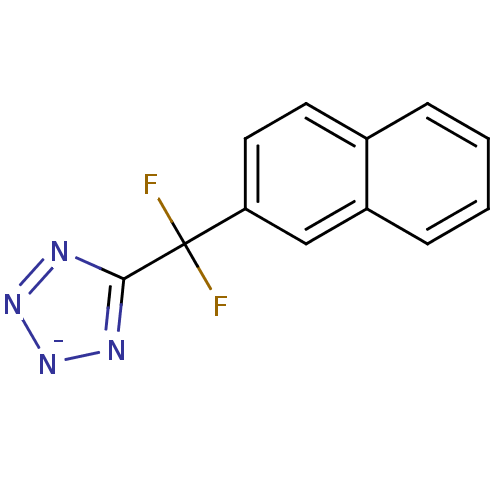 (1-Sodium-5-(difluoro-naphthalen-2-yl-methyl)-1H-te...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibitory activity against protein tyrosine phosphatase 1B (PTP1B) using fluorescein diphosphate as substrate at Km concentration (20 uM) | Bioorg Med Chem Lett 8: 3275-80 (1999) BindingDB Entry DOI: 10.7270/Q20R9NK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50072591 (2-(Biphenyl-3-yloxy)-2-fluoro-malonic acid | CHEMB...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibitory activity against protein tyrosine phosphatase 1B (PTP1B) using fluorescein diphosphate as substrate at Km concentration (20 uM) | Bioorg Med Chem Lett 8: 3275-80 (1999) BindingDB Entry DOI: 10.7270/Q20R9NK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50072596 (2-Fluoro-2-(naphthalen-2-yloxy)-malonic acid | CHE...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibitory activity against protein tyrosine phosphatase 1B (PTP1B) using fluorescein diphosphate as substrate at Km concentration (20 uM) | Bioorg Med Chem Lett 8: 3275-80 (1999) BindingDB Entry DOI: 10.7270/Q20R9NK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50072594 (CHEMBL114030 | Sodium; biphenyl-3-yl-difluoro-acet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 4.35E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibitory activity against protein tyrosine phosphatase 1B (PTP1B) using fluorescein diphosphate as substrate at Km concentration (20 uM) | Bioorg Med Chem Lett 8: 3275-80 (1999) BindingDB Entry DOI: 10.7270/Q20R9NK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50072589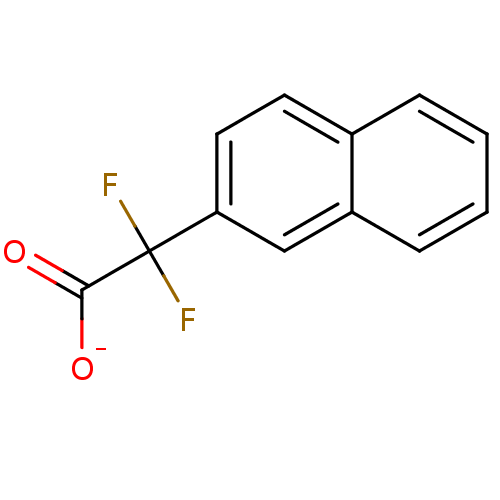 (CHEMBL110361 | Sodium; difluoro-naphthalen-2-yl-ac...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibitory activity against protein tyrosine phosphatase 1B (PTP1B) using fluorescein diphosphate as substrate at Km concentration (20 uM) | Bioorg Med Chem Lett 8: 3275-80 (1999) BindingDB Entry DOI: 10.7270/Q20R9NK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50241361 (CHEMBL1515 | METHIMAZOLE | US9138393, Methimazole ...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 1.43E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hungkuang University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase-mediated L-tyrosine hydroxylation after 30 mins by ELISA | Bioorg Med Chem 22: 2809-15 (2014) Article DOI: 10.1016/j.bmc.2014.03.009 BindingDB Entry DOI: 10.7270/Q2417125 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50497084 (CHEMBL3260775) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.45E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hungkuang University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase-mediated L-tyrosine hydroxylation after 30 mins by ELISA | Bioorg Med Chem 22: 2809-15 (2014) Article DOI: 10.1016/j.bmc.2014.03.009 BindingDB Entry DOI: 10.7270/Q2417125 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50497083 (CHEMBL3260774) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.11E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hungkuang University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase-mediated L-tyrosine hydroxylation after 30 mins by ELISA | Bioorg Med Chem 22: 2809-15 (2014) Article DOI: 10.1016/j.bmc.2014.03.009 BindingDB Entry DOI: 10.7270/Q2417125 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM50194617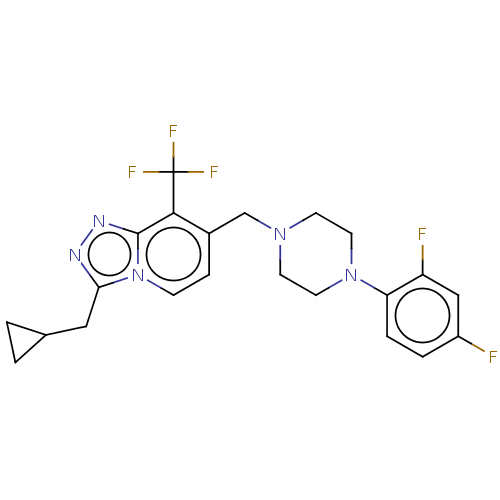 (CHEMBL3926416) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 166 | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00593 BindingDB Entry DOI: 10.7270/Q2FB571B | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM50194707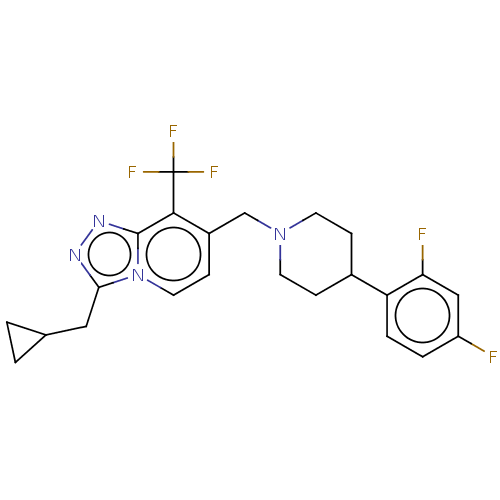 (CHEMBL3938796) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00593 BindingDB Entry DOI: 10.7270/Q2FB571B | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM50601001 (CHEMBL5207431) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00593 BindingDB Entry DOI: 10.7270/Q2FB571B | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM50601002 (CHEMBL5208947) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00593 BindingDB Entry DOI: 10.7270/Q2FB571B | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 101 total ) | Next | Last >> |