 Found 66 hits with Last Name = 'chen' and Initial = 'zl'
Found 66 hits with Last Name = 'chen' and Initial = 'zl' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50421411
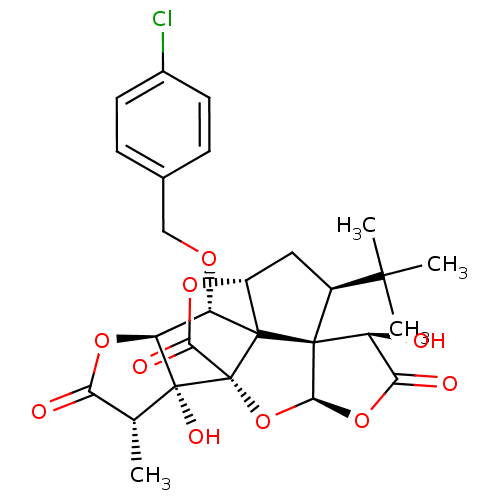
(CHEMBL2304168)Show SMILES C[C@@H]1C(=O)O[C@H]2[C@H](OCc3ccc(Cl)cc3)C34[C@H]5C[C@@H](C(C)(C)C)[C@@]33[C@@H](O)C(=O)O[C@H]3O[C@@]4(C(=O)O5)[C@@]12O Show InChI InChI=1S/C27H29ClO10/c1-11-19(30)36-18-17(34-10-12-5-7-13(28)8-6-12)25-15-9-14(23(2,3)4)24(25)16(29)20(31)37-22(24)38-27(25,21(32)35-15)26(11,18)33/h5-8,11,14-18,22,29,33H,9-10H2,1-4H3/t11-,14+,15-,16+,17+,18+,22+,24+,25?,26-,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Compound was evaluated for anti-platelet activating factor potency |
Bioorg Med Chem Lett 8: 1291-6 (1999)
BindingDB Entry DOI: 10.7270/Q2VH5PB6 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50421412

(CHEMBL2304167)Show SMILES CC1C(=O)O[C@H]2[C@H](O)C34[C@H]5C[C@@H](C(C)(C)C)[C@]33[C@@H](OC(=O)[C@@H]3OCOCc3ccccc3)O[C@@]4(C(=O)O5)[C@@]12O Show InChI InChI=1S/C28H32O11/c1-13-20(30)37-18-17(29)26-16-10-15(24(2,3)4)25(26)19(35-12-34-11-14-8-6-5-7-9-14)21(31)38-23(25)39-28(26,22(32)36-16)27(13,18)33/h5-9,13,15-19,23,29,33H,10-12H2,1-4H3/t13?,15-,16+,17-,18-,19-,23-,25-,26?,27+,28+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Compound was evaluated for anti-platelet activating factor potency |
Bioorg Med Chem Lett 8: 1291-6 (1999)
BindingDB Entry DOI: 10.7270/Q2VH5PB6 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50421410
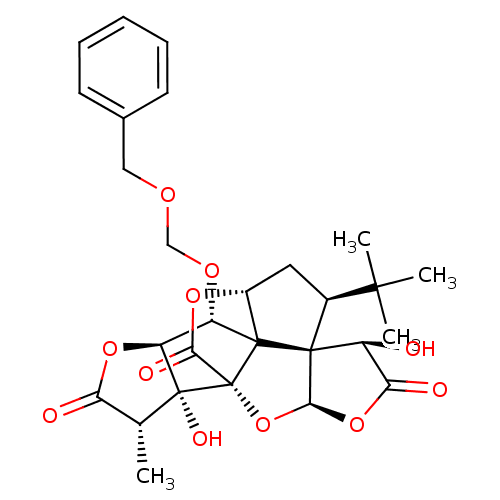
(CHEMBL2304166)Show SMILES C[C@@H]1C(=O)O[C@H]2[C@H](OCOCc3ccccc3)C34[C@H]5C[C@@H](C(C)(C)C)[C@@]33[C@@H](O)C(=O)O[C@H]3O[C@@]4(C(=O)O5)[C@@]12O Show InChI InChI=1S/C28H32O11/c1-13-20(30)37-19-18(35-12-34-11-14-8-6-5-7-9-14)26-16-10-15(24(2,3)4)25(26)17(29)21(31)38-23(25)39-28(26,22(32)36-16)27(13,19)33/h5-9,13,15-19,23,29,33H,10-12H2,1-4H3/t13-,15+,16-,17+,18+,19+,23+,25+,26?,27-,28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Compound was evaluated for anti-platelet activating factor potency |
Bioorg Med Chem Lett 8: 1291-6 (1999)
BindingDB Entry DOI: 10.7270/Q2VH5PB6 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50251276
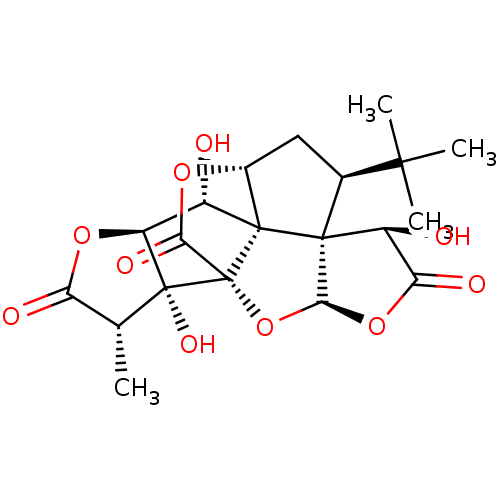
(BN 52021 | CHEMBL514432 | GINKOLIDE B | Gingkolide...)Show SMILES C[C@@H]1C(=O)O[C@H]2[C@H](O)[C@@]34[C@H]5C[C@@H](C(C)(C)C)[C@@]33[C@@H](O)C(=O)O[C@H]3O[C@@]4(C(=O)O5)[C@@]12O |r| Show InChI InChI=1S/C20H24O10/c1-6-12(23)28-11-9(21)18-8-5-7(16(2,3)4)17(18)10(22)13(24)29-15(17)30-20(18,14(25)27-8)19(6,11)26/h6-11,15,21-22,26H,5H2,1-4H3/t6-,7+,8-,9+,10+,11+,15+,17+,18+,19-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 128 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Compound was evaluated for anti-platelet activating factor potency |
Bioorg Med Chem Lett 8: 1291-6 (1999)
BindingDB Entry DOI: 10.7270/Q2VH5PB6 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50259940
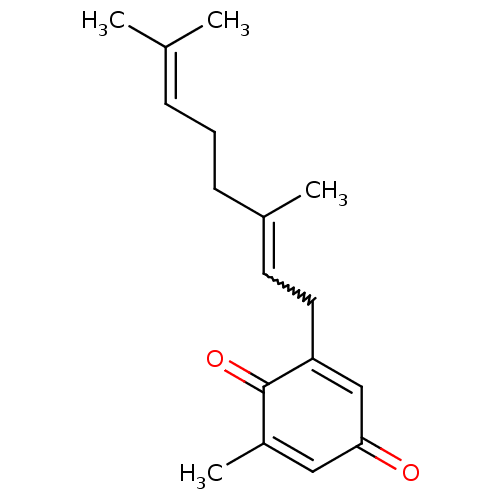
(2-[(2E)-3,7-dimethyl-2,6-octadienyl]-6-methyl-2,5-...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]-[#6](-[#6])=[#6]-[#6]-[#6]-1=[#6]-[#6](=O)-[#6]=[#6](-[#6])-[#6]-1=O |w:8.8,t:10,14| Show InChI InChI=1S/C17H22O2/c1-12(2)6-5-7-13(3)8-9-15-11-16(18)10-14(4)17(15)19/h6,8,10-11H,5,7,9H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich-Heine-Universität Düsseldorf
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase |
J Nat Prod 61: 347-50 (1998)
Article DOI: 10.1021/np970430b
BindingDB Entry DOI: 10.7270/Q2PN95D7 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM32020
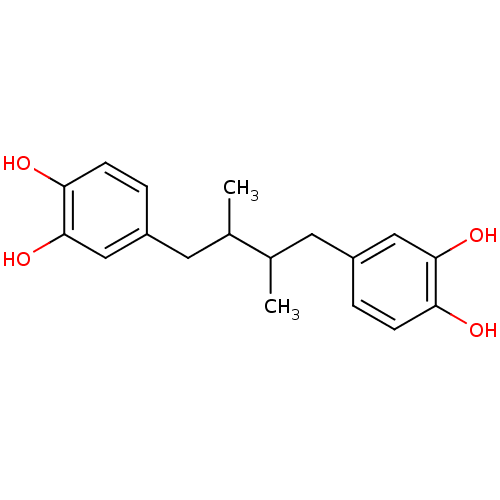
(4-[4-(3,4-dihydroxyphenyl)-2,3-dimethyl-butyl]pyro...)Show InChI InChI=1S/C18H22O4/c1-11(7-13-3-5-15(19)17(21)9-13)12(2)8-14-4-6-16(20)18(22)10-14/h3-6,9-12,19-22H,7-8H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich-Heine-Universität Düsseldorf
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase |
J Nat Prod 61: 347-50 (1998)
Article DOI: 10.1021/np970430b
BindingDB Entry DOI: 10.7270/Q2PN95D7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50259939
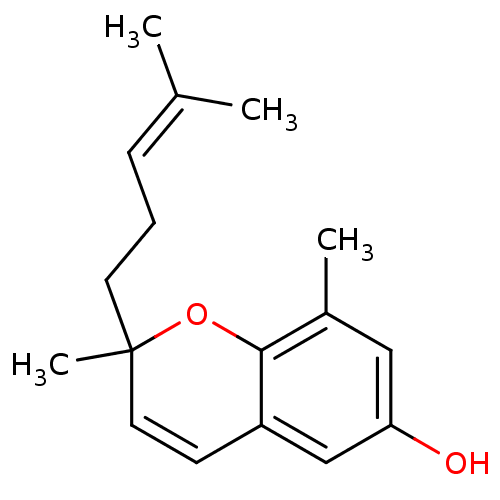
(2,8-dimethyl-6-hydroxy-2-(4-methyl-3-pentenyl)-2h-...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]C1([#6])[#8]-c2c(-[#6])cc(-[#8])cc2-[#6]=[#6]1 |c:18| Show InChI InChI=1S/C17H22O2/c1-12(2)6-5-8-17(4)9-7-14-11-15(18)10-13(3)16(14)19-17/h6-7,9-11,18H,5,8H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich-Heine-Universität Düsseldorf
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase |
J Nat Prod 61: 347-50 (1998)
Article DOI: 10.1021/np970430b
BindingDB Entry DOI: 10.7270/Q2PN95D7 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich-Heine-Universität Düsseldorf
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
J Nat Prod 61: 347-50 (1998)
Article DOI: 10.1021/np970430b
BindingDB Entry DOI: 10.7270/Q2PN95D7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50565345
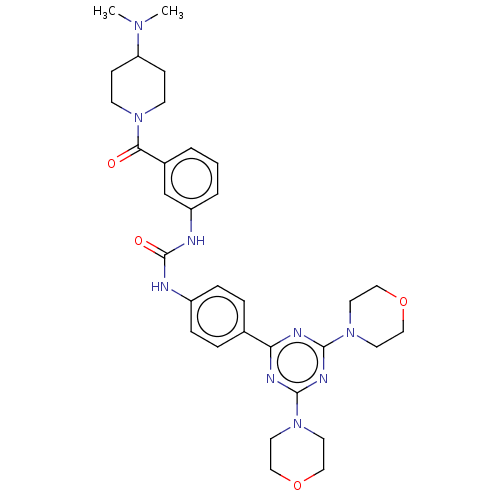
(CHEMBL4778200)Show SMILES CN(C)C1CCN(CC1)C(=O)c1cccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mTOR (unknown origin) using ULight-4E-BP1 as substrate measured after 45 mins in presence of ATP by LANCE Ultra assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112637
BindingDB Entry DOI: 10.7270/Q2MS3XH5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50565338
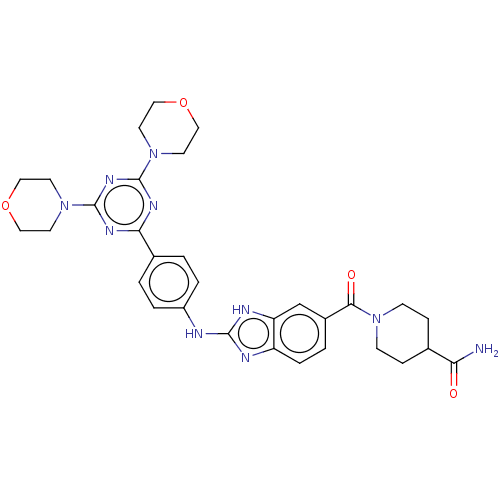
(CHEMBL4787035)Show SMILES NC(=O)C1CCN(CC1)C(=O)c1ccc2nc(Nc3ccc(cc3)-c3nc(nc(n3)N3CCOCC3)N3CCOCC3)[nH]c2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2 as substrate measured after 1 hr in presence of ATP by ADP-Glo reagent based assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112637
BindingDB Entry DOI: 10.7270/Q2MS3XH5 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50259939

(2,8-dimethyl-6-hydroxy-2-(4-methyl-3-pentenyl)-2h-...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]C1([#6])[#8]-c2c(-[#6])cc(-[#8])cc2-[#6]=[#6]1 |c:18| Show InChI InChI=1S/C17H22O2/c1-12(2)6-5-8-17(4)9-7-14-11-15(18)10-13(3)16(14)19-17/h6-7,9-11,18H,5,8H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich-Heine-Universität Düsseldorf
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
J Nat Prod 61: 347-50 (1998)
Article DOI: 10.1021/np970430b
BindingDB Entry DOI: 10.7270/Q2PN95D7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50565340
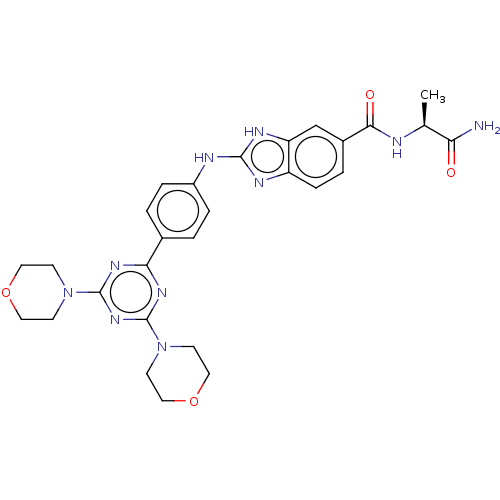
(CHEMBL4794018)Show SMILES C[C@H](NC(=O)c1ccc2nc(Nc3ccc(cc3)-c3nc(nc(n3)N3CCOCC3)N3CCOCC3)[nH]c2c1)C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2 as substrate measured after 1 hr in presence of ATP by ADP-Glo reagent based assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112637
BindingDB Entry DOI: 10.7270/Q2MS3XH5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50565335
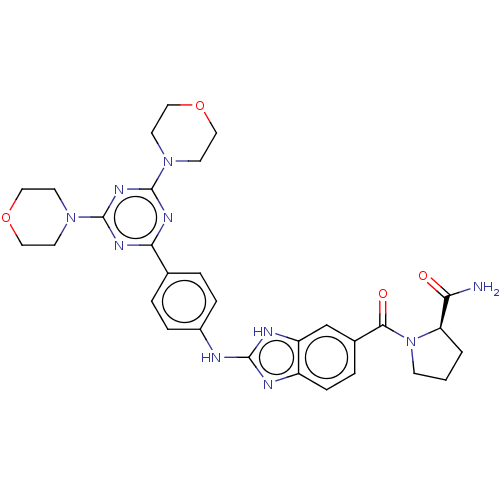
(CHEMBL4790315)Show SMILES NC(=O)[C@H]1CCCN1C(=O)c1ccc2nc(Nc3ccc(cc3)-c3nc(nc(n3)N3CCOCC3)N3CCOCC3)[nH]c2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2 as substrate measured after 1 hr in presence of ATP by ADP-Glo reagent based assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112637
BindingDB Entry DOI: 10.7270/Q2MS3XH5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM25045

(3-(4-morpholin-4-ylpyrido[2,3]furo[2,4-b]pyrimidin...)Show InChI InChI=1S/C19H16N4O3/c24-13-4-1-3-12(11-13)17-21-15-14-5-2-6-20-19(14)26-16(15)18(22-17)23-7-9-25-10-8-23/h1-6,11,24H,7-10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate measured after 1 hr in presence of ATP by ADP-Glo reagent based assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112637
BindingDB Entry DOI: 10.7270/Q2MS3XH5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50565335

(CHEMBL4790315)Show SMILES NC(=O)[C@H]1CCCN1C(=O)c1ccc2nc(Nc3ccc(cc3)-c3nc(nc(n3)N3CCOCC3)N3CCOCC3)[nH]c2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mTOR (unknown origin) using ULight-4E-BP1 as substrate measured after 45 mins in presence of ATP by LANCE Ultra assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112637
BindingDB Entry DOI: 10.7270/Q2MS3XH5 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50565339
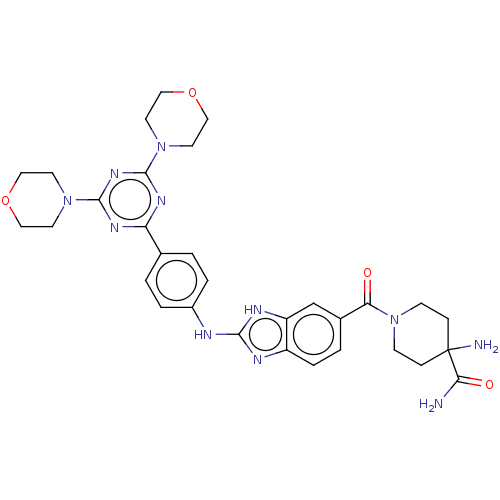
(CHEMBL4788806)Show SMILES NC(=O)C1(N)CCN(CC1)C(=O)c1ccc2nc(Nc3ccc(cc3)-c3nc(nc(n3)N3CCOCC3)N3CCOCC3)[nH]c2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mTOR (unknown origin) using ULight-4E-BP1 as substrate measured after 45 mins in presence of ATP by LANCE Ultra assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112637
BindingDB Entry DOI: 10.7270/Q2MS3XH5 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50565340

(CHEMBL4794018)Show SMILES C[C@H](NC(=O)c1ccc2nc(Nc3ccc(cc3)-c3nc(nc(n3)N3CCOCC3)N3CCOCC3)[nH]c2c1)C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mTOR (unknown origin) using ULight-4E-BP1 as substrate measured after 45 mins in presence of ATP by LANCE Ultra assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112637
BindingDB Entry DOI: 10.7270/Q2MS3XH5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50565345

(CHEMBL4778200)Show SMILES CN(C)C1CCN(CC1)C(=O)c1cccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate measured after 1 hr in presence of ATP by ADP-Glo reagent based assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112637
BindingDB Entry DOI: 10.7270/Q2MS3XH5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50565335

(CHEMBL4790315)Show SMILES NC(=O)[C@H]1CCCN1C(=O)c1ccc2nc(Nc3ccc(cc3)-c3nc(nc(n3)N3CCOCC3)N3CCOCC3)[nH]c2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate measured after 1 hr in presence of ATP by ADP-Glo reagent based assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112637
BindingDB Entry DOI: 10.7270/Q2MS3XH5 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50565336
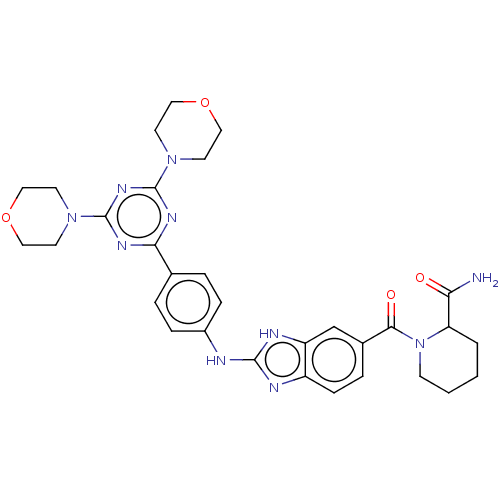
(CHEMBL4793222)Show SMILES NC(=O)C1CCCCN1C(=O)c1ccc2nc(Nc3ccc(cc3)-c3nc(nc(n3)N3CCOCC3)N3CCOCC3)[nH]c2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mTOR (unknown origin) using ULight-4E-BP1 as substrate measured after 45 mins in presence of ATP by LANCE Ultra assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112637
BindingDB Entry DOI: 10.7270/Q2MS3XH5 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM25045

(3-(4-morpholin-4-ylpyrido[2,3]furo[2,4-b]pyrimidin...)Show InChI InChI=1S/C19H16N4O3/c24-13-4-1-3-12(11-13)17-21-15-14-5-2-6-20-19(14)26-16(15)18(22-17)23-7-9-25-10-8-23/h1-6,11,24H,7-10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mTOR (unknown origin) using ULight-4E-BP1 as substrate measured after 45 mins in presence of ATP by LANCE Ultra assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112637
BindingDB Entry DOI: 10.7270/Q2MS3XH5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50565337
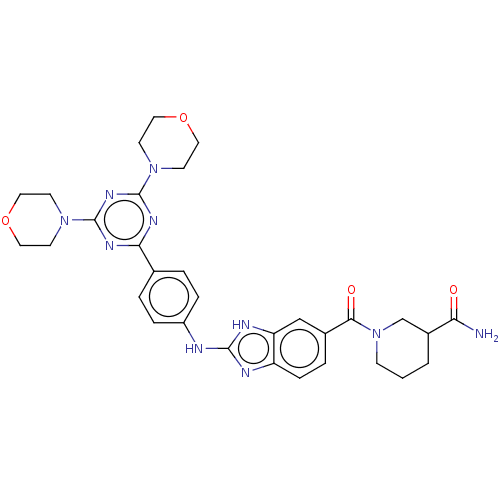
(CHEMBL4777939)Show SMILES NC(=O)C1CCCN(C1)C(=O)c1ccc2nc(Nc3ccc(cc3)-c3nc(nc(n3)N3CCOCC3)N3CCOCC3)[nH]c2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mTOR (unknown origin) using ULight-4E-BP1 as substrate measured after 45 mins in presence of ATP by LANCE Ultra assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112637
BindingDB Entry DOI: 10.7270/Q2MS3XH5 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50565334
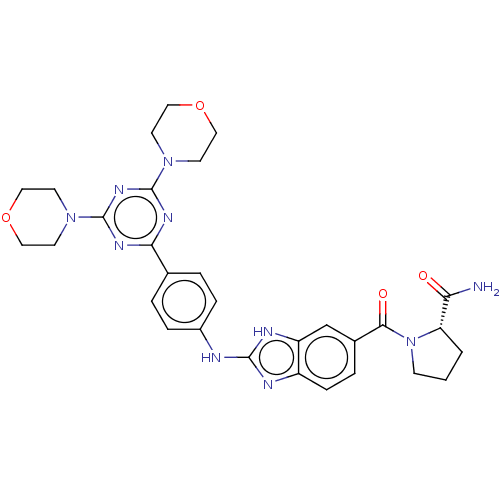
(CHEMBL4786662)Show SMILES NC(=O)[C@@H]1CCCN1C(=O)c1ccc2nc(Nc3ccc(cc3)-c3nc(nc(n3)N3CCOCC3)N3CCOCC3)[nH]c2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mTOR (unknown origin) using ULight-4E-BP1 as substrate measured after 45 mins in presence of ATP by LANCE Ultra assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112637
BindingDB Entry DOI: 10.7270/Q2MS3XH5 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50565343
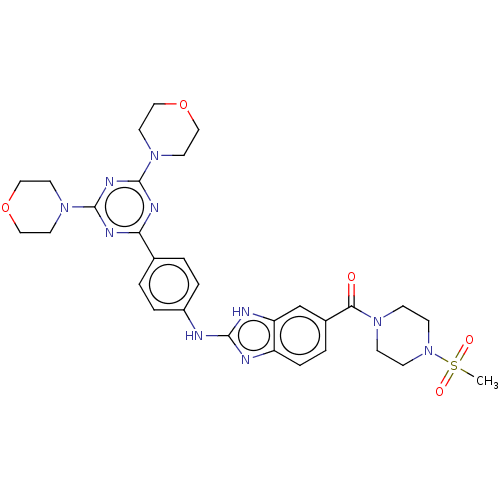
(CHEMBL4782217)Show SMILES CS(=O)(=O)N1CCN(CC1)C(=O)c1ccc2nc(Nc3ccc(cc3)-c3nc(nc(n3)N3CCOCC3)N3CCOCC3)[nH]c2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mTOR (unknown origin) using ULight-4E-BP1 as substrate measured after 45 mins in presence of ATP by LANCE Ultra assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112637
BindingDB Entry DOI: 10.7270/Q2MS3XH5 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50565341
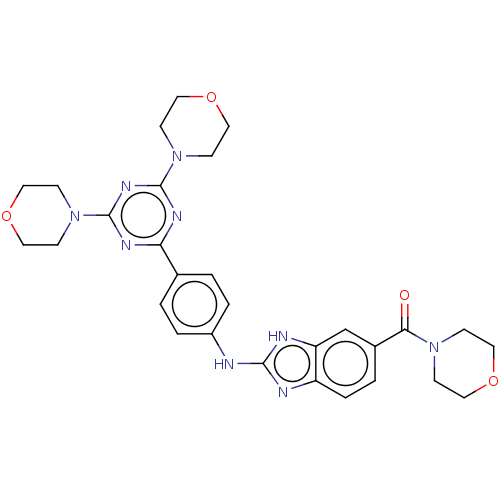
(CHEMBL4778112)Show SMILES O=C(N1CCOCC1)c1ccc2nc(Nc3ccc(cc3)-c3nc(nc(n3)N3CCOCC3)N3CCOCC3)[nH]c2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mTOR (unknown origin) using ULight-4E-BP1 as substrate measured after 45 mins in presence of ATP by LANCE Ultra assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112637
BindingDB Entry DOI: 10.7270/Q2MS3XH5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50565342

(CHEMBL4788677)Show SMILES CN1CCN(CC1)C(=O)c1ccc2nc(Nc3ccc(cc3)-c3nc(nc(n3)N3CCOCC3)N3CCOCC3)[nH]c2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2 as substrate measured after 1 hr in presence of ATP by ADP-Glo reagent based assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112637
BindingDB Entry DOI: 10.7270/Q2MS3XH5 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50565342

(CHEMBL4788677)Show SMILES CN1CCN(CC1)C(=O)c1ccc2nc(Nc3ccc(cc3)-c3nc(nc(n3)N3CCOCC3)N3CCOCC3)[nH]c2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mTOR (unknown origin) using ULight-4E-BP1 as substrate measured after 45 mins in presence of ATP by LANCE Ultra assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112637
BindingDB Entry DOI: 10.7270/Q2MS3XH5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50565340

(CHEMBL4794018)Show SMILES C[C@H](NC(=O)c1ccc2nc(Nc3ccc(cc3)-c3nc(nc(n3)N3CCOCC3)N3CCOCC3)[nH]c2c1)C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate measured after 1 hr in presence of ATP by ADP-Glo reagent based assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112637
BindingDB Entry DOI: 10.7270/Q2MS3XH5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50565334

(CHEMBL4786662)Show SMILES NC(=O)[C@@H]1CCCN1C(=O)c1ccc2nc(Nc3ccc(cc3)-c3nc(nc(n3)N3CCOCC3)N3CCOCC3)[nH]c2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate measured after 1 hr in presence of ATP by ADP-Glo reagent based assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112637
BindingDB Entry DOI: 10.7270/Q2MS3XH5 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50565333
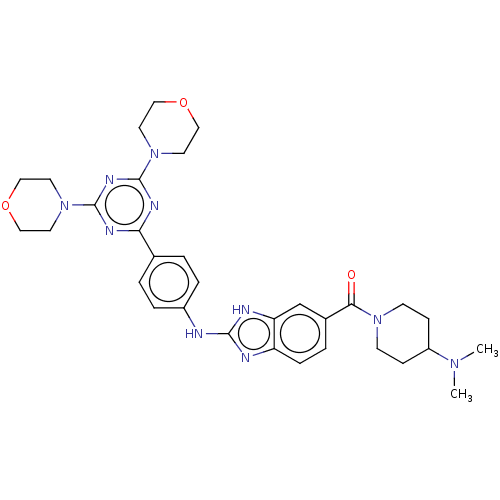
(CHEMBL4793503)Show SMILES CN(C)C1CCN(CC1)C(=O)c1ccc2nc(Nc3ccc(cc3)-c3nc(nc(n3)N3CCOCC3)N3CCOCC3)[nH]c2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mTOR (unknown origin) using ULight-4E-BP1 as substrate measured after 45 mins in presence of ATP by LANCE Ultra assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112637
BindingDB Entry DOI: 10.7270/Q2MS3XH5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50565338

(CHEMBL4787035)Show SMILES NC(=O)C1CCN(CC1)C(=O)c1ccc2nc(Nc3ccc(cc3)-c3nc(nc(n3)N3CCOCC3)N3CCOCC3)[nH]c2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate measured after 1 hr in presence of ATP by ADP-Glo reagent based assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112637
BindingDB Entry DOI: 10.7270/Q2MS3XH5 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50565338

(CHEMBL4787035)Show SMILES NC(=O)C1CCN(CC1)C(=O)c1ccc2nc(Nc3ccc(cc3)-c3nc(nc(n3)N3CCOCC3)N3CCOCC3)[nH]c2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mTOR (unknown origin) using ULight-4E-BP1 as substrate measured after 45 mins in presence of ATP by LANCE Ultra assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112637
BindingDB Entry DOI: 10.7270/Q2MS3XH5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50565341

(CHEMBL4778112)Show SMILES O=C(N1CCOCC1)c1ccc2nc(Nc3ccc(cc3)-c3nc(nc(n3)N3CCOCC3)N3CCOCC3)[nH]c2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate measured after 1 hr in presence of ATP by ADP-Glo reagent based assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112637
BindingDB Entry DOI: 10.7270/Q2MS3XH5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50565335

(CHEMBL4790315)Show SMILES NC(=O)[C@H]1CCCN1C(=O)c1ccc2nc(Nc3ccc(cc3)-c3nc(nc(n3)N3CCOCC3)N3CCOCC3)[nH]c2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kbeta (unknown origin) using PIP2 as substrate measured after 1 hr in presence of ATP by ADP-Glo reagent based assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112637
BindingDB Entry DOI: 10.7270/Q2MS3XH5 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50241938

(Atractylon | CHEMBL486189)Show InChI InChI=1S/C15H20O/c1-10-5-4-6-15(3)8-14-12(7-13(10)15)11(2)9-16-14/h9,13H,1,4-8H2,2-3H3/t13-,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich-Heine-Universität Düsseldorf
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase |
J Nat Prod 61: 347-50 (1998)
Article DOI: 10.1021/np970430b
BindingDB Entry DOI: 10.7270/Q2PN95D7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50565345

(CHEMBL4778200)Show SMILES CN(C)C1CCN(CC1)C(=O)c1cccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kbeta (unknown origin) using PIP2 as substrate measured after 1 hr in presence of ATP by ADP-Glo reagent based assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112637
BindingDB Entry DOI: 10.7270/Q2MS3XH5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50565342

(CHEMBL4788677)Show SMILES CN1CCN(CC1)C(=O)c1ccc2nc(Nc3ccc(cc3)-c3nc(nc(n3)N3CCOCC3)N3CCOCC3)[nH]c2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kbeta (unknown origin) using PIP2 as substrate measured after 1 hr in presence of ATP by ADP-Glo reagent based assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112637
BindingDB Entry DOI: 10.7270/Q2MS3XH5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50565337

(CHEMBL4777939)Show SMILES NC(=O)C1CCCN(C1)C(=O)c1ccc2nc(Nc3ccc(cc3)-c3nc(nc(n3)N3CCOCC3)N3CCOCC3)[nH]c2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate measured after 1 hr in presence of ATP by ADP-Glo reagent based assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112637
BindingDB Entry DOI: 10.7270/Q2MS3XH5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50565339

(CHEMBL4788806)Show SMILES NC(=O)C1(N)CCN(CC1)C(=O)c1ccc2nc(Nc3ccc(cc3)-c3nc(nc(n3)N3CCOCC3)N3CCOCC3)[nH]c2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate measured after 1 hr in presence of ATP by ADP-Glo reagent based assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112637
BindingDB Entry DOI: 10.7270/Q2MS3XH5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50565338

(CHEMBL4787035)Show SMILES NC(=O)C1CCN(CC1)C(=O)c1ccc2nc(Nc3ccc(cc3)-c3nc(nc(n3)N3CCOCC3)N3CCOCC3)[nH]c2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kbeta (unknown origin) using PIP2 as substrate measured after 1 hr in presence of ATP by ADP-Glo reagent based assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112637
BindingDB Entry DOI: 10.7270/Q2MS3XH5 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50240512
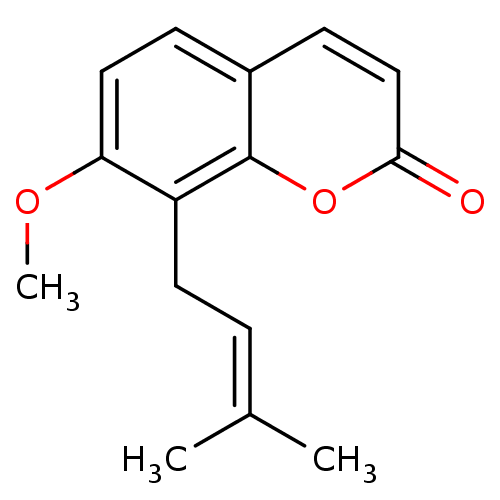
(7-Methoxy-8-(3-methyl-but-2-enyl)-chromen-2-one | ...)Show SMILES [#6]-[#8]-c1ccc2ccc(=O)oc2c1-[#6]\[#6]=[#6](\[#6])-[#6] Show InChI InChI=1S/C15H16O3/c1-10(2)4-7-12-13(17-3)8-5-11-6-9-14(16)18-15(11)12/h4-6,8-9H,7H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.62E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich-Heine-Universität Düsseldorf
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase |
J Nat Prod 61: 347-50 (1998)
Article DOI: 10.1021/np970430b
BindingDB Entry DOI: 10.7270/Q2PN95D7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50565345

(CHEMBL4778200)Show SMILES CN(C)C1CCN(CC1)C(=O)c1cccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate measured after 1 hr in presence of ATP by ADP-Glo reagent based assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112637
BindingDB Entry DOI: 10.7270/Q2MS3XH5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50565341

(CHEMBL4778112)Show SMILES O=C(N1CCOCC1)c1ccc2nc(Nc3ccc(cc3)-c3nc(nc(n3)N3CCOCC3)N3CCOCC3)[nH]c2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2 as substrate measured after 1 hr in presence of ATP by ADP-Glo reagent based assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112637
BindingDB Entry DOI: 10.7270/Q2MS3XH5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50565343

(CHEMBL4782217)Show SMILES CS(=O)(=O)N1CCN(CC1)C(=O)c1ccc2nc(Nc3ccc(cc3)-c3nc(nc(n3)N3CCOCC3)N3CCOCC3)[nH]c2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate measured after 1 hr in presence of ATP by ADP-Glo reagent based assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112637
BindingDB Entry DOI: 10.7270/Q2MS3XH5 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50259940

(2-[(2E)-3,7-dimethyl-2,6-octadienyl]-6-methyl-2,5-...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]-[#6](-[#6])=[#6]-[#6]-[#6]-1=[#6]-[#6](=O)-[#6]=[#6](-[#6])-[#6]-1=O |w:8.8,t:10,14| Show InChI InChI=1S/C17H22O2/c1-12(2)6-5-7-13(3)8-9-15-11-16(18)10-14(4)17(15)19/h6,8,10-11H,5,7,9H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich-Heine-Universität Düsseldorf
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
J Nat Prod 61: 347-50 (1998)
Article DOI: 10.1021/np970430b
BindingDB Entry DOI: 10.7270/Q2PN95D7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50565332
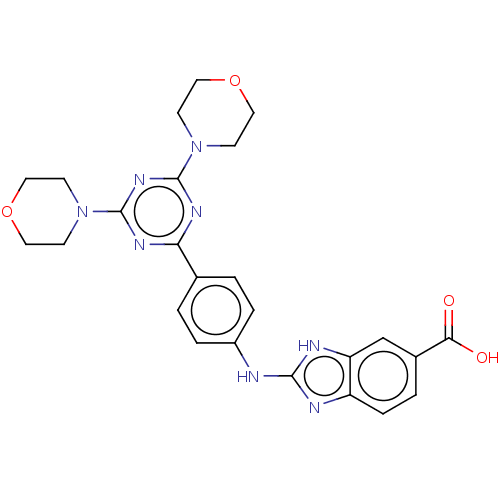
(CHEMBL4783079)Show SMILES OC(=O)c1ccc2nc(Nc3ccc(cc3)-c3nc(nc(n3)N3CCOCC3)N3CCOCC3)[nH]c2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate measured after 1 hr in presence of ATP by ADP-Glo reagent based assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112637
BindingDB Entry DOI: 10.7270/Q2MS3XH5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50565340

(CHEMBL4794018)Show SMILES C[C@H](NC(=O)c1ccc2nc(Nc3ccc(cc3)-c3nc(nc(n3)N3CCOCC3)N3CCOCC3)[nH]c2c1)C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kbeta (unknown origin) using PIP2 as substrate measured after 1 hr in presence of ATP by ADP-Glo reagent based assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112637
BindingDB Entry DOI: 10.7270/Q2MS3XH5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50565336

(CHEMBL4793222)Show SMILES NC(=O)C1CCCCN1C(=O)c1ccc2nc(Nc3ccc(cc3)-c3nc(nc(n3)N3CCOCC3)N3CCOCC3)[nH]c2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate measured after 1 hr in presence of ATP by ADP-Glo reagent based assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112637
BindingDB Entry DOI: 10.7270/Q2MS3XH5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50565333

(CHEMBL4793503)Show SMILES CN(C)C1CCN(CC1)C(=O)c1ccc2nc(Nc3ccc(cc3)-c3nc(nc(n3)N3CCOCC3)N3CCOCC3)[nH]c2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate measured after 1 hr in presence of ATP by ADP-Glo reagent based assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112637
BindingDB Entry DOI: 10.7270/Q2MS3XH5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50565342

(CHEMBL4788677)Show SMILES CN1CCN(CC1)C(=O)c1ccc2nc(Nc3ccc(cc3)-c3nc(nc(n3)N3CCOCC3)N3CCOCC3)[nH]c2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate measured after 1 hr in presence of ATP by ADP-Glo reagent based assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112637
BindingDB Entry DOI: 10.7270/Q2MS3XH5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data