 Found 114 hits with Last Name = 'cheng' and Initial = 'ms'
Found 114 hits with Last Name = 'cheng' and Initial = 'ms' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50569866

(CHEMBL4860571)Show SMILES O[C@H]1[C@@H](CCc2c(O)c(O)ccc12)NCC1CC(F)(F)C1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to beta2 adrenoceptor (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01195
BindingDB Entry DOI: 10.7270/Q24F1VJ7 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50569864

(CHEMBL4869517)Show SMILES O[C@H]1[C@@H](CCc2c(O)c(O)ccc12)NC1CCCC1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to beta2 adrenoceptor (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01195
BindingDB Entry DOI: 10.7270/Q24F1VJ7 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50000504
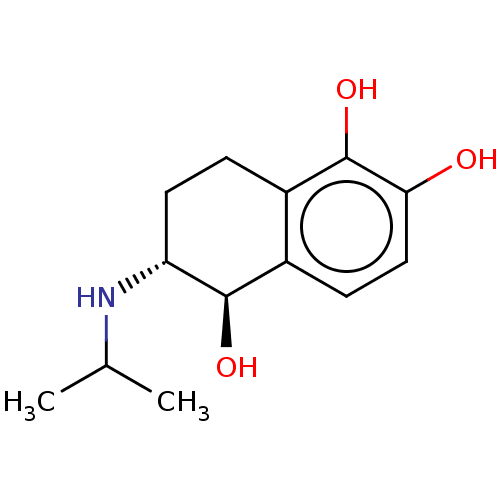
(6-Isopropylamino-5,6,7,8-tetrahydro-naphthalene-1,...)Show InChI InChI=1S/C13H19NO3/c1-7(2)14-10-5-3-9-8(12(10)16)4-6-11(15)13(9)17/h4,6-7,10,12,14-17H,3,5H2,1-2H3/t10-,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to beta2 adrenoceptor (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01195
BindingDB Entry DOI: 10.7270/Q24F1VJ7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50569865

(CHEMBL4871979) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to beta2 adrenoceptor (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01195
BindingDB Entry DOI: 10.7270/Q24F1VJ7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50342128

(3-Imidazol-1-yl-2-methyl-3-[4-(naphthalen-2-ylamin...)Show SMILES COC(=O)C(C)(C)C(c1ccc(Nc2ccc3ccccc3c2)cc1)n1ccnc1 Show InChI InChI=1S/C25H25N3O2/c1-25(2,24(29)30-3)23(28-15-14-26-17-28)19-9-11-21(12-10-19)27-22-13-8-18-6-4-5-7-20(18)16-22/h4-17,23,27H,1-3H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of CYP26A1 (unknown origin) |
Bioorg Med Chem 23: 6763-73 (2015)
Article DOI: 10.1016/j.bmc.2015.08.019
BindingDB Entry DOI: 10.7270/Q2668G0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50377963
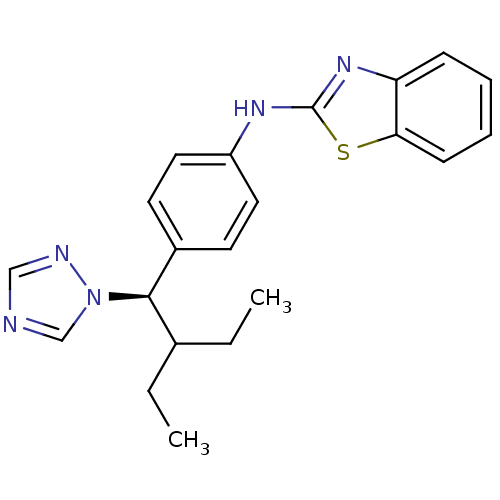
(CHEMBL224305 | R-115866)Show SMILES CCC(CC)[C@H](c1ccc(Nc2nc3ccccc3s2)cc1)n1cncn1 |r| Show InChI InChI=1S/C21H23N5S/c1-3-15(4-2)20(26-14-22-13-23-26)16-9-11-17(12-10-16)24-21-25-18-7-5-6-8-19(18)27-21/h5-15,20H,3-4H2,1-2H3,(H,24,25)/t20-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of CYP26A1 (unknown origin) |
Bioorg Med Chem 23: 6763-73 (2015)
Article DOI: 10.1016/j.bmc.2015.08.019
BindingDB Entry DOI: 10.7270/Q2668G0C |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50248709
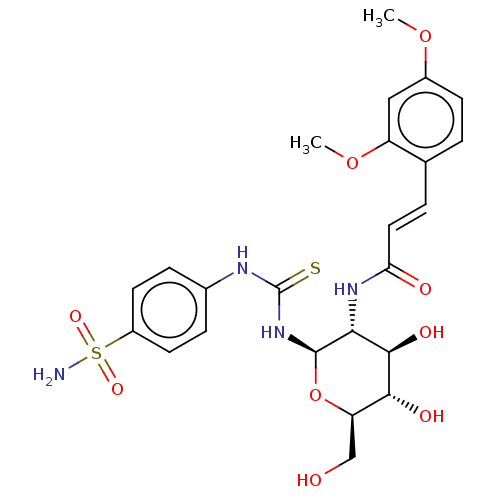
(CHEMBL4103517)Show SMILES COc1ccc(\C=C\C(=O)N[C@@H]2[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]2NC(=S)Nc2ccc(cc2)S(N)(=O)=O)c(OC)c1 |r| Show InChI InChI=1S/C24H30N4O9S2/c1-35-15-7-3-13(17(11-15)36-2)4-10-19(30)27-20-22(32)21(31)18(12-29)37-23(20)28-24(38)26-14-5-8-16(9-6-14)39(25,33)34/h3-11,18,20-23,29,31-32H,12H2,1-2H3,(H,27,30)(H2,25,33,34)(H2,26,28,38)/b10-4+/t18-,20-,21-,22-,23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 9 using 4-nitrophenylacetate as substrate pretreated for 15 mins prior to test by spectrophotometr... |
Eur J Med Chem 132: 1-10 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.023
BindingDB Entry DOI: 10.7270/Q2WS8WNX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50120455

(CHEMBL3617993)Show SMILES CC(C(c1ccc(Nc2nc3ccccc3s2)cc1)n1cncn1)N(C)C Show InChI InChI=1S/C20H22N6S/c1-14(25(2)3)19(26-13-21-12-22-26)15-8-10-16(11-9-15)23-20-24-17-6-4-5-7-18(17)27-20/h4-14,19H,1-3H3,(H,23,24) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of CYP26A1 (unknown origin) |
Bioorg Med Chem 23: 6763-73 (2015)
Article DOI: 10.1016/j.bmc.2015.08.019
BindingDB Entry DOI: 10.7270/Q2668G0C |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50580106

(CHEMBL5080007)Show SMILES CO[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1NC(=O)c1ccc(cc1)S(N)(=O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human carbonic anhydrase 2 using 4-nitrophenylacetate as substrate |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128291
BindingDB Entry DOI: 10.7270/Q2FT8QXR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human carbonic anhydrase 2 using 4-nitrophenylacetate as substrate |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128291
BindingDB Entry DOI: 10.7270/Q2FT8QXR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Binding affinity towards HSV-1 thymidine kinase |
Eur J Med Chem 132: 1-10 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.023
BindingDB Entry DOI: 10.7270/Q2WS8WNX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50580111
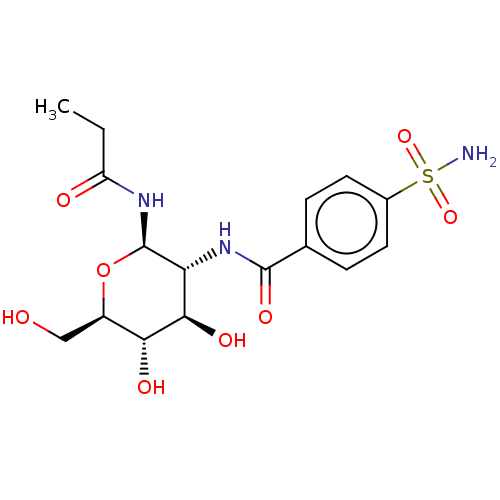
(CHEMBL5087194)Show SMILES CCC(=O)N[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1NC(=O)c1ccc(cc1)S(N)(=O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human carbonic anhydrase 2 using 4-nitrophenylacetate as substrate |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128291
BindingDB Entry DOI: 10.7270/Q2FT8QXR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50248709

(CHEMBL4103517)Show SMILES COc1ccc(\C=C\C(=O)N[C@@H]2[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]2NC(=S)Nc2ccc(cc2)S(N)(=O)=O)c(OC)c1 |r| Show InChI InChI=1S/C24H30N4O9S2/c1-35-15-7-3-13(17(11-15)36-2)4-10-19(30)27-20-22(32)21(31)18(12-29)37-23(20)28-24(38)26-14-5-8-16(9-6-14)39(25,33)34/h3-11,18,20-23,29,31-32H,12H2,1-2H3,(H,27,30)(H2,25,33,34)(H2,26,28,38)/b10-4+/t18-,20-,21-,22-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Binding affinity towards HSV-1 thymidine kinase |
Eur J Med Chem 132: 1-10 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.023
BindingDB Entry DOI: 10.7270/Q2WS8WNX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 9 using 4-nitrophenylacetate as substrate pretreated for 15 mins prior to test by spectrophotometr... |
Eur J Med Chem 132: 1-10 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.023
BindingDB Entry DOI: 10.7270/Q2WS8WNX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human carbonic anhydrase 9 using 4-nitrophenylacetate as substrate |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128291
BindingDB Entry DOI: 10.7270/Q2FT8QXR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50580109
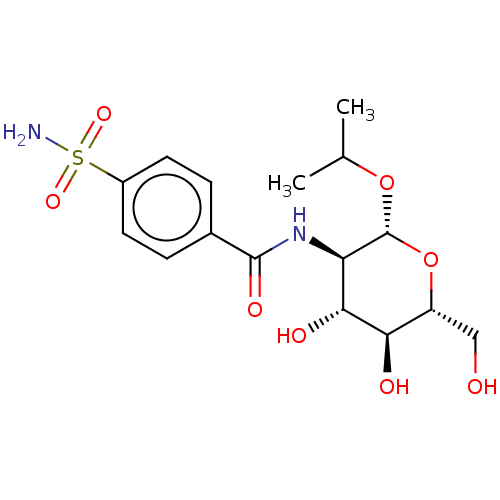
(CHEMBL5082205)Show SMILES CC(C)O[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1NC(=O)c1ccc(cc1)S(N)(=O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human carbonic anhydrase 2 using 4-nitrophenylacetate as substrate |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128291
BindingDB Entry DOI: 10.7270/Q2FT8QXR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50248718

(CHEMBL4075252)Show SMILES COc1cccc(\C=C\C(=O)N[C@@H]2[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]2NC(=S)Nc2ccc(cc2)S(N)(=O)=O)c1OC |r| Show InChI InChI=1S/C24H30N4O9S2/c1-35-16-5-3-4-13(22(16)36-2)6-11-18(30)27-19-21(32)20(31)17(12-29)37-23(19)28-24(38)26-14-7-9-15(10-8-14)39(25,33)34/h3-11,17,19-21,23,29,31-32H,12H2,1-2H3,(H,27,30)(H2,25,33,34)(H2,26,28,38)/b11-6+/t17-,19-,20-,21-,23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 9 using 4-nitrophenylacetate as substrate pretreated for 15 mins prior to test by spectrophotometr... |
Eur J Med Chem 132: 1-10 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.023
BindingDB Entry DOI: 10.7270/Q2WS8WNX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50248718

(CHEMBL4075252)Show SMILES COc1cccc(\C=C\C(=O)N[C@@H]2[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]2NC(=S)Nc2ccc(cc2)S(N)(=O)=O)c1OC |r| Show InChI InChI=1S/C24H30N4O9S2/c1-35-16-5-3-4-13(22(16)36-2)6-11-18(30)27-19-21(32)20(31)17(12-29)37-23(19)28-24(38)26-14-7-9-15(10-8-14)39(25,33)34/h3-11,17,19-21,23,29,31-32H,12H2,1-2H3,(H,27,30)(H2,25,33,34)(H2,26,28,38)/b11-6+/t17-,19-,20-,21-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 2 using 4-nitrophenylacetate as substrate pretreated for 15 mins prior to test by spectrophotometr... |
Eur J Med Chem 132: 1-10 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.023
BindingDB Entry DOI: 10.7270/Q2WS8WNX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50580107
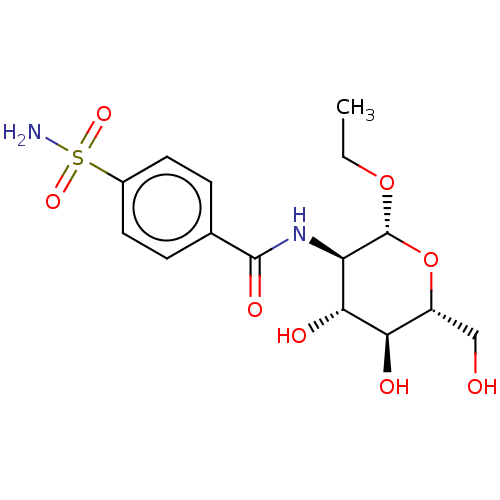
(CHEMBL5082154)Show SMILES CCO[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1NC(=O)c1ccc(cc1)S(N)(=O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human carbonic anhydrase 2 using 4-nitrophenylacetate as substrate |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128291
BindingDB Entry DOI: 10.7270/Q2FT8QXR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50580110
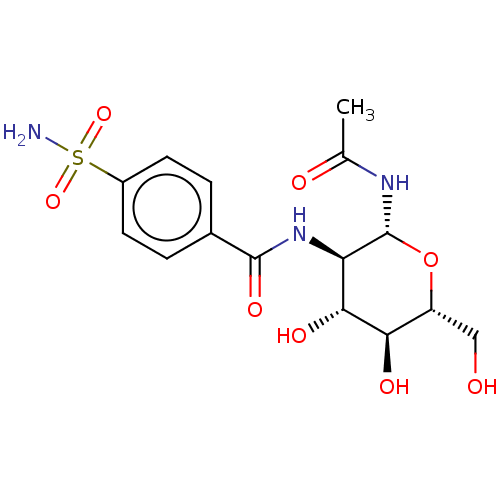
(CHEMBL5074657)Show SMILES CC(=O)N[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1NC(=O)c1ccc(cc1)S(N)(=O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human carbonic anhydrase 2 using 4-nitrophenylacetate as substrate |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128291
BindingDB Entry DOI: 10.7270/Q2FT8QXR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50248710
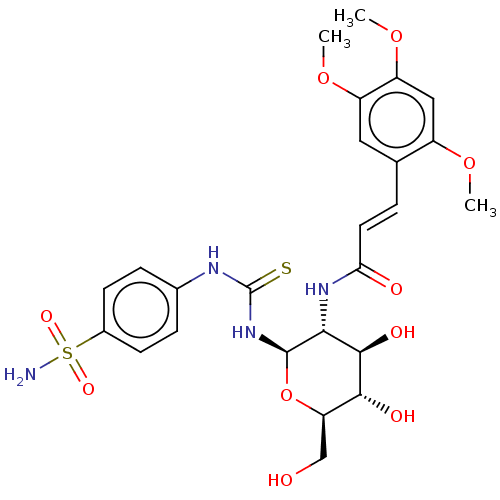
(CHEMBL4070358)Show SMILES COc1cc(OC)c(\C=C\C(=O)N[C@@H]2[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]2NC(=S)Nc2ccc(cc2)S(N)(=O)=O)cc1OC |r| Show InChI InChI=1S/C25H32N4O10S2/c1-36-16-11-18(38-3)17(37-2)10-13(16)4-9-20(31)28-21-23(33)22(32)19(12-30)39-24(21)29-25(40)27-14-5-7-15(8-6-14)41(26,34)35/h4-11,19,21-24,30,32-33H,12H2,1-3H3,(H,28,31)(H2,26,34,35)(H2,27,29,40)/b9-4+/t19-,21-,22-,23-,24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 9 using 4-nitrophenylacetate as substrate pretreated for 15 mins prior to test by spectrophotometr... |
Eur J Med Chem 132: 1-10 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.023
BindingDB Entry DOI: 10.7270/Q2WS8WNX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50580108
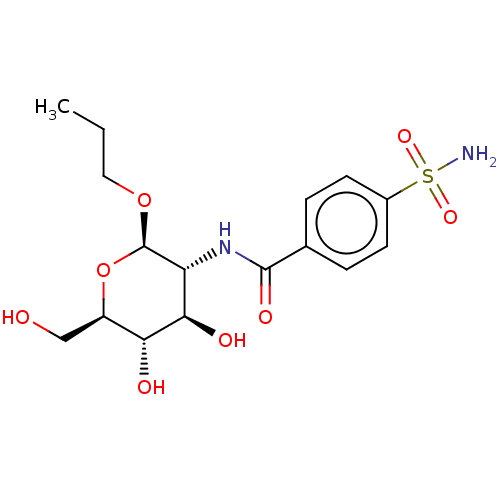
(CHEMBL5076082)Show SMILES CCCO[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1NC(=O)c1ccc(cc1)S(N)(=O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human carbonic anhydrase 2 using 4-nitrophenylacetate as substrate |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128291
BindingDB Entry DOI: 10.7270/Q2FT8QXR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50248707
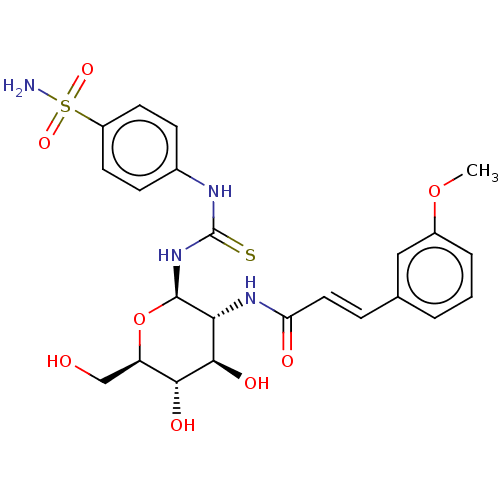
(CHEMBL4075581)Show SMILES COc1cccc(\C=C\C(=O)N[C@@H]2[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]2NC(=S)Nc2ccc(cc2)S(N)(=O)=O)c1 |r| Show InChI InChI=1S/C23H28N4O8S2/c1-34-15-4-2-3-13(11-15)5-10-18(29)26-19-21(31)20(30)17(12-28)35-22(19)27-23(36)25-14-6-8-16(9-7-14)37(24,32)33/h2-11,17,19-22,28,30-31H,12H2,1H3,(H,26,29)(H2,24,32,33)(H2,25,27,36)/b10-5+/t17-,19-,20-,21-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Binding affinity towards HSV-1 thymidine kinase |
Eur J Med Chem 132: 1-10 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.023
BindingDB Entry DOI: 10.7270/Q2WS8WNX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50248719
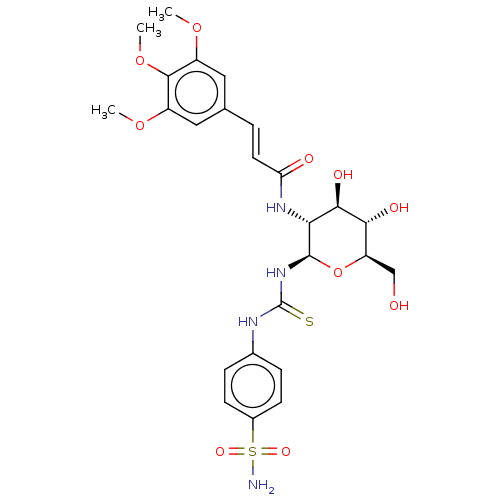
(CHEMBL4096770)Show SMILES COc1cc(\C=C\C(=O)N[C@@H]2[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]2NC(=S)Nc2ccc(cc2)S(N)(=O)=O)cc(OC)c1OC |r| Show InChI InChI=1S/C25H32N4O10S2/c1-36-16-10-13(11-17(37-2)23(16)38-3)4-9-19(31)28-20-22(33)21(32)18(12-30)39-24(20)29-25(40)27-14-5-7-15(8-6-14)41(26,34)35/h4-11,18,20-22,24,30,32-33H,12H2,1-3H3,(H,28,31)(H2,26,34,35)(H2,27,29,40)/b9-4+/t18-,20-,21-,22-,24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 9 using 4-nitrophenylacetate as substrate pretreated for 15 mins prior to test by spectrophotometr... |
Eur J Med Chem 132: 1-10 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.023
BindingDB Entry DOI: 10.7270/Q2WS8WNX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50162789
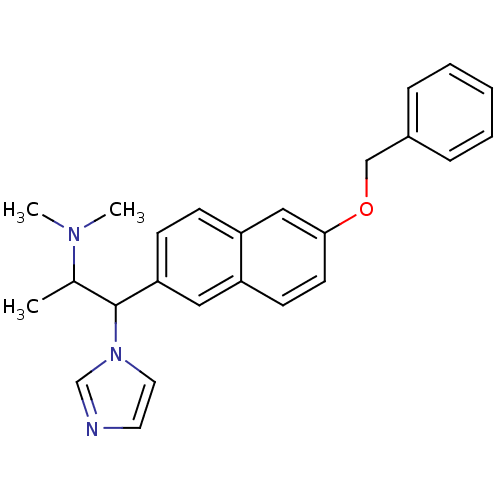
(CHEMBL180856 | [2-(6-Benzyloxy-naphthalen-2-yl)-2-...)Show SMILES CC(C(c1ccc2cc(OCc3ccccc3)ccc2c1)n1ccnc1)N(C)C Show InChI InChI=1S/C25H27N3O/c1-19(27(2)3)25(28-14-13-26-18-28)23-10-9-22-16-24(12-11-21(22)15-23)29-17-20-7-5-4-6-8-20/h4-16,18-19,25H,17H2,1-3H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of CYP26A1 (unknown origin) |
Bioorg Med Chem 23: 6763-73 (2015)
Article DOI: 10.1016/j.bmc.2015.08.019
BindingDB Entry DOI: 10.7270/Q2668G0C |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50248708
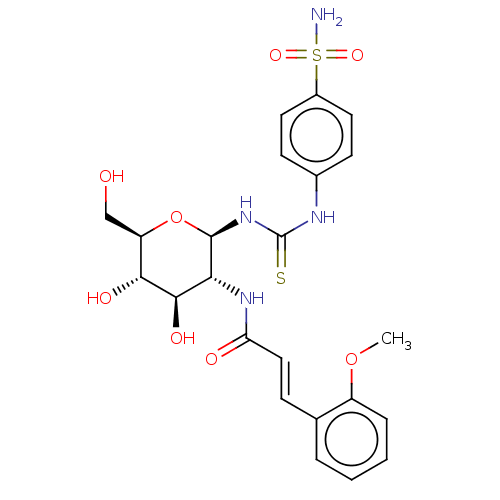
(CHEMBL4090618)Show SMILES COc1ccccc1\C=C\C(=O)N[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1NC(=S)Nc1ccc(cc1)S(N)(=O)=O |r| Show InChI InChI=1S/C23H28N4O8S2/c1-34-16-5-3-2-4-13(16)6-11-18(29)26-19-21(31)20(30)17(12-28)35-22(19)27-23(36)25-14-7-9-15(10-8-14)37(24,32)33/h2-11,17,19-22,28,30-31H,12H2,1H3,(H,26,29)(H2,24,32,33)(H2,25,27,36)/b11-6+/t17-,19-,20-,21-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Binding affinity towards HSV-1 thymidine kinase |
Eur J Med Chem 132: 1-10 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.023
BindingDB Entry DOI: 10.7270/Q2WS8WNX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50248719

(CHEMBL4096770)Show SMILES COc1cc(\C=C\C(=O)N[C@@H]2[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]2NC(=S)Nc2ccc(cc2)S(N)(=O)=O)cc(OC)c1OC |r| Show InChI InChI=1S/C25H32N4O10S2/c1-36-16-10-13(11-17(37-2)23(16)38-3)4-9-19(31)28-20-22(33)21(32)18(12-30)39-24(20)29-25(40)27-14-5-7-15(8-6-14)41(26,34)35/h4-11,18,20-22,24,30,32-33H,12H2,1-3H3,(H,28,31)(H2,26,34,35)(H2,27,29,40)/b9-4+/t18-,20-,21-,22-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 2 using 4-nitrophenylacetate as substrate pretreated for 15 mins prior to test by spectrophotometr... |
Eur J Med Chem 132: 1-10 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.023
BindingDB Entry DOI: 10.7270/Q2WS8WNX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50248708

(CHEMBL4090618)Show SMILES COc1ccccc1\C=C\C(=O)N[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1NC(=S)Nc1ccc(cc1)S(N)(=O)=O |r| Show InChI InChI=1S/C23H28N4O8S2/c1-34-16-5-3-2-4-13(16)6-11-18(29)26-19-21(31)20(30)17(12-28)35-22(19)27-23(36)25-14-7-9-15(10-8-14)37(24,32)33/h2-11,17,19-22,28,30-31H,12H2,1H3,(H,26,29)(H2,24,32,33)(H2,25,27,36)/b11-6+/t17-,19-,20-,21-,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 9 using 4-nitrophenylacetate as substrate pretreated for 15 mins prior to test by spectrophotometr... |
Eur J Med Chem 132: 1-10 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.023
BindingDB Entry DOI: 10.7270/Q2WS8WNX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50248710

(CHEMBL4070358)Show SMILES COc1cc(OC)c(\C=C\C(=O)N[C@@H]2[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]2NC(=S)Nc2ccc(cc2)S(N)(=O)=O)cc1OC |r| Show InChI InChI=1S/C25H32N4O10S2/c1-36-16-11-18(38-3)17(37-2)10-13(16)4-9-20(31)28-21-23(33)22(32)19(12-30)39-24(21)29-25(40)27-14-5-7-15(8-6-14)41(26,34)35/h4-11,19,21-24,30,32-33H,12H2,1-3H3,(H,28,31)(H2,26,34,35)(H2,27,29,40)/b9-4+/t19-,21-,22-,23-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Binding affinity towards HSV-1 thymidine kinase |
Eur J Med Chem 132: 1-10 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.023
BindingDB Entry DOI: 10.7270/Q2WS8WNX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50248706
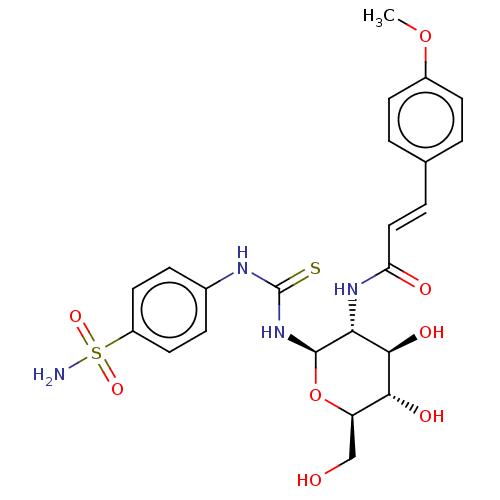
(CHEMBL4063719)Show SMILES COc1ccc(\C=C\C(=O)N[C@@H]2[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]2NC(=S)Nc2ccc(cc2)S(N)(=O)=O)cc1 |r| Show InChI InChI=1S/C23H28N4O8S2/c1-34-15-7-2-13(3-8-15)4-11-18(29)26-19-21(31)20(30)17(12-28)35-22(19)27-23(36)25-14-5-9-16(10-6-14)37(24,32)33/h2-11,17,19-22,28,30-31H,12H2,1H3,(H,26,29)(H2,24,32,33)(H2,25,27,36)/b11-4+/t17-,19-,20-,21-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Binding affinity towards HSV-1 thymidine kinase |
Eur J Med Chem 132: 1-10 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.023
BindingDB Entry DOI: 10.7270/Q2WS8WNX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50580106

(CHEMBL5080007)Show SMILES CO[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1NC(=O)c1ccc(cc1)S(N)(=O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human carbonic anhydrase 9 using 4-nitrophenylacetate as substrate |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128291
BindingDB Entry DOI: 10.7270/Q2FT8QXR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50248707

(CHEMBL4075581)Show SMILES COc1cccc(\C=C\C(=O)N[C@@H]2[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]2NC(=S)Nc2ccc(cc2)S(N)(=O)=O)c1 |r| Show InChI InChI=1S/C23H28N4O8S2/c1-34-15-4-2-3-13(11-15)5-10-18(29)26-19-21(31)20(30)17(12-28)35-22(19)27-23(36)25-14-6-8-16(9-7-14)37(24,32)33/h2-11,17,19-22,28,30-31H,12H2,1H3,(H,26,29)(H2,24,32,33)(H2,25,27,36)/b10-5+/t17-,19-,20-,21-,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 9 using 4-nitrophenylacetate as substrate pretreated for 15 mins prior to test by spectrophotometr... |
Eur J Med Chem 132: 1-10 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.023
BindingDB Entry DOI: 10.7270/Q2WS8WNX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50580109

(CHEMBL5082205)Show SMILES CC(C)O[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1NC(=O)c1ccc(cc1)S(N)(=O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 142 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human carbonic anhydrase 9 using 4-nitrophenylacetate as substrate |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128291
BindingDB Entry DOI: 10.7270/Q2FT8QXR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50580107

(CHEMBL5082154)Show SMILES CCO[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1NC(=O)c1ccc(cc1)S(N)(=O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 152 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human carbonic anhydrase 9 using 4-nitrophenylacetate as substrate |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128291
BindingDB Entry DOI: 10.7270/Q2FT8QXR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50248706

(CHEMBL4063719)Show SMILES COc1ccc(\C=C\C(=O)N[C@@H]2[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]2NC(=S)Nc2ccc(cc2)S(N)(=O)=O)cc1 |r| Show InChI InChI=1S/C23H28N4O8S2/c1-34-15-7-2-13(3-8-15)4-11-18(29)26-19-21(31)20(30)17(12-28)35-22(19)27-23(36)25-14-5-9-16(10-6-14)37(24,32)33/h2-11,17,19-22,28,30-31H,12H2,1H3,(H,26,29)(H2,24,32,33)(H2,25,27,36)/b11-4+/t17-,19-,20-,21-,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 152 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Binding affinity towards HSV-1 thymidine kinase |
Eur J Med Chem 132: 1-10 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.023
BindingDB Entry DOI: 10.7270/Q2WS8WNX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50248717
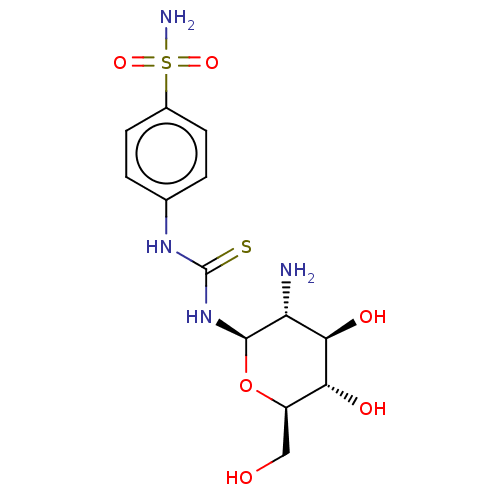
(CHEMBL4085325)Show SMILES N[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1NC(=S)Nc1ccc(cc1)S(N)(=O)=O |r| Show InChI InChI=1S/C13H20N4O6S2/c14-9-11(20)10(19)8(5-18)23-12(9)17-13(24)16-6-1-3-7(4-2-6)25(15,21)22/h1-4,8-12,18-20H,5,14H2,(H2,15,21,22)(H2,16,17,24)/t8-,9-,10-,11-,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 203 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 2 using 4-nitrophenylacetate as substrate pretreated for 15 mins prior to test by spectrophotometr... |
Eur J Med Chem 132: 1-10 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.023
BindingDB Entry DOI: 10.7270/Q2WS8WNX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50120461
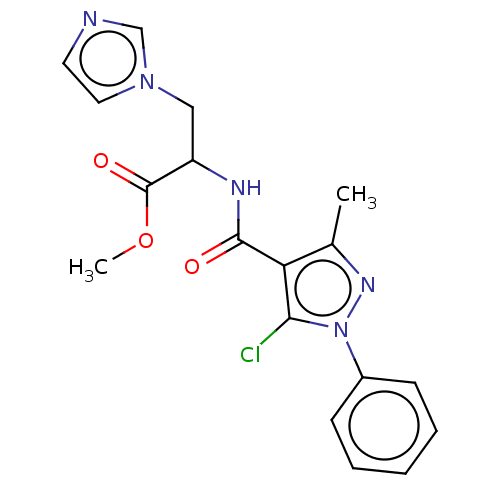
(CHEMBL3617983)Show SMILES COC(=O)C(Cn1ccnc1)NC(=O)c1c(C)nn(c1Cl)-c1ccccc1 Show InChI InChI=1S/C18H18ClN5O3/c1-12-15(16(19)24(22-12)13-6-4-3-5-7-13)17(25)21-14(18(26)27-2)10-23-9-8-20-11-23/h3-9,11,14H,10H2,1-2H3,(H,21,25) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of CYP26A1 in ATRA-induced human HL60 cell microsomes incubated for 30 mins in dark condition with NADPH and ATRA by HPLC method |
Bioorg Med Chem 23: 6763-73 (2015)
Article DOI: 10.1016/j.bmc.2015.08.019
BindingDB Entry DOI: 10.7270/Q2668G0C |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50580110

(CHEMBL5074657)Show SMILES CC(=O)N[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1NC(=O)c1ccc(cc1)S(N)(=O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 227 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human carbonic anhydrase 9 using 4-nitrophenylacetate as substrate |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128291
BindingDB Entry DOI: 10.7270/Q2FT8QXR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50580108

(CHEMBL5076082)Show SMILES CCCO[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1NC(=O)c1ccc(cc1)S(N)(=O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 241 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human carbonic anhydrase 9 using 4-nitrophenylacetate as substrate |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128291
BindingDB Entry DOI: 10.7270/Q2FT8QXR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50580111

(CHEMBL5087194)Show SMILES CCC(=O)N[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1NC(=O)c1ccc(cc1)S(N)(=O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 276 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human carbonic anhydrase 9 using 4-nitrophenylacetate as substrate |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128291
BindingDB Entry DOI: 10.7270/Q2FT8QXR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human carbonic anhydrase 1 using 4-nitrophenylacetate as substrate |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128291
BindingDB Entry DOI: 10.7270/Q2FT8QXR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50120456
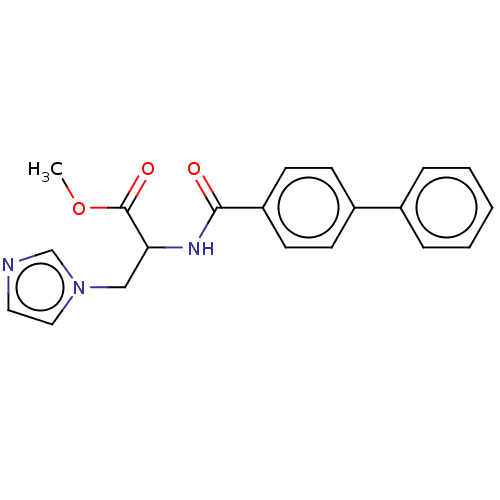
(CHEMBL3617978)Show SMILES COC(=O)C(Cn1ccnc1)NC(=O)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C20H19N3O3/c1-26-20(25)18(13-23-12-11-21-14-23)22-19(24)17-9-7-16(8-10-17)15-5-3-2-4-6-15/h2-12,14,18H,13H2,1H3,(H,22,24) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of CYP26A1 in ATRA-induced human HL60 cell microsomes incubated for 30 mins in dark condition with NADPH and ATRA by HPLC method |
Bioorg Med Chem 23: 6763-73 (2015)
Article DOI: 10.1016/j.bmc.2015.08.019
BindingDB Entry DOI: 10.7270/Q2668G0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50120484
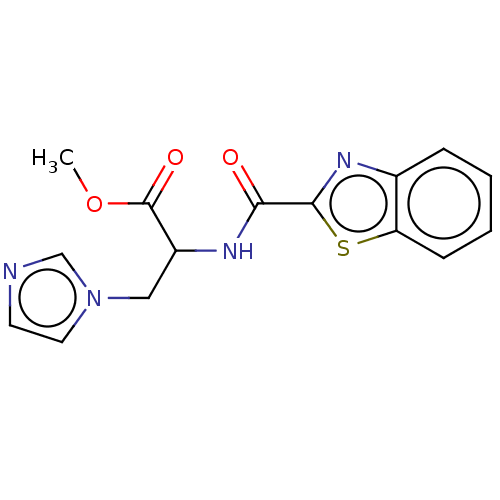
(CHEMBL3617988)Show InChI InChI=1S/C15H14N4O3S/c1-22-15(21)11(8-19-7-6-16-9-19)17-13(20)14-18-10-4-2-3-5-12(10)23-14/h2-7,9,11H,8H2,1H3,(H,17,20) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of CYP26A1 in ATRA-induced human HL60 cell microsomes incubated for 30 mins in dark condition with NADPH and ATRA by HPLC method |
Bioorg Med Chem 23: 6763-73 (2015)
Article DOI: 10.1016/j.bmc.2015.08.019
BindingDB Entry DOI: 10.7270/Q2668G0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50120466
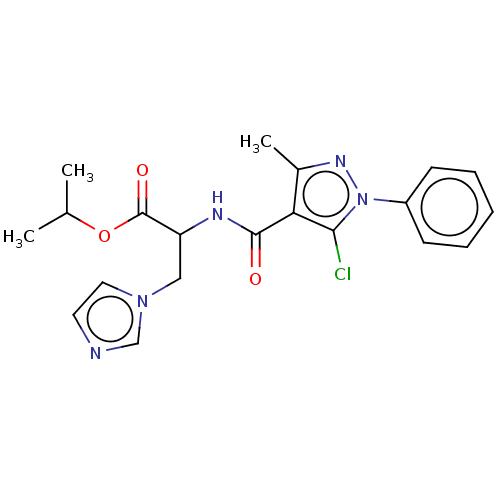
(CHEMBL3617986)Show SMILES CC(C)OC(=O)C(Cn1ccnc1)NC(=O)c1c(C)nn(c1Cl)-c1ccccc1 Show InChI InChI=1S/C20H22ClN5O3/c1-13(2)29-20(28)16(11-25-10-9-22-12-25)23-19(27)17-14(3)24-26(18(17)21)15-7-5-4-6-8-15/h4-10,12-13,16H,11H2,1-3H3,(H,23,27) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of CYP26A1 in ATRA-induced human HL60 cell microsomes incubated for 30 mins in dark condition with NADPH and ATRA by HPLC method |
Bioorg Med Chem 23: 6763-73 (2015)
Article DOI: 10.1016/j.bmc.2015.08.019
BindingDB Entry DOI: 10.7270/Q2668G0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50120459

(CHEMBL3617981)Show SMILES CC(C)OC(=O)C(Cn1ccnc1)NC(=O)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C22H23N3O3/c1-16(2)28-22(27)20(14-25-13-12-23-15-25)24-21(26)19-10-8-18(9-11-19)17-6-4-3-5-7-17/h3-13,15-16,20H,14H2,1-2H3,(H,24,26) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of CYP26A1 in ATRA-induced human HL60 cell microsomes incubated for 30 mins in dark condition with NADPH and ATRA by HPLC method |
Bioorg Med Chem 23: 6763-73 (2015)
Article DOI: 10.1016/j.bmc.2015.08.019
BindingDB Entry DOI: 10.7270/Q2668G0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50120463
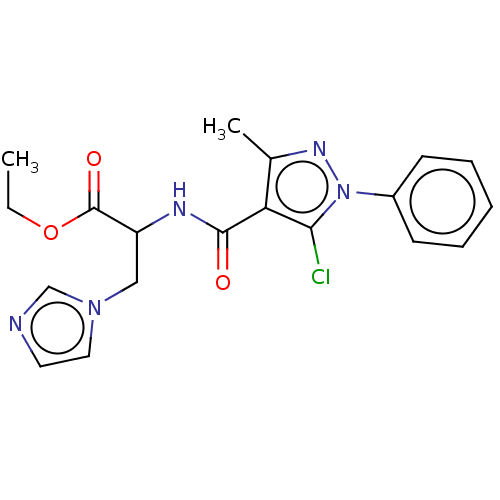
(CHEMBL3617984)Show SMILES CCOC(=O)C(Cn1ccnc1)NC(=O)c1c(C)nn(c1Cl)-c1ccccc1 Show InChI InChI=1S/C19H20ClN5O3/c1-3-28-19(27)15(11-24-10-9-21-12-24)22-18(26)16-13(2)23-25(17(16)20)14-7-5-4-6-8-14/h4-10,12,15H,3,11H2,1-2H3,(H,22,26) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of CYP26A1 in ATRA-induced human HL60 cell microsomes incubated for 30 mins in dark condition with NADPH and ATRA by HPLC method |
Bioorg Med Chem 23: 6763-73 (2015)
Article DOI: 10.1016/j.bmc.2015.08.019
BindingDB Entry DOI: 10.7270/Q2668G0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50120464
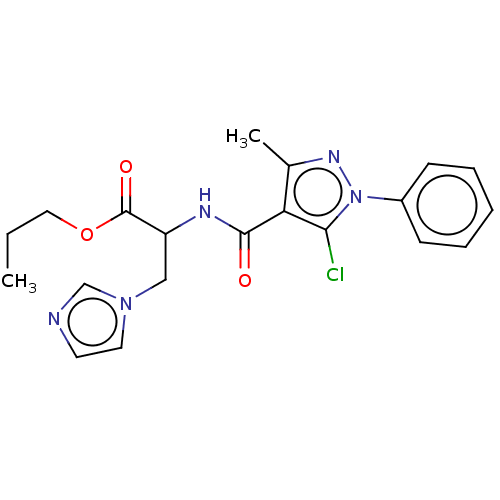
(CHEMBL3617985)Show SMILES CCCOC(=O)C(Cn1ccnc1)NC(=O)c1c(C)nn(c1Cl)-c1ccccc1 Show InChI InChI=1S/C20H22ClN5O3/c1-3-11-29-20(28)16(12-25-10-9-22-13-25)23-19(27)17-14(2)24-26(18(17)21)15-7-5-4-6-8-15/h4-10,13,16H,3,11-12H2,1-2H3,(H,23,27) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of CYP26A1 in ATRA-induced human HL60 cell microsomes incubated for 30 mins in dark condition with NADPH and ATRA by HPLC method |
Bioorg Med Chem 23: 6763-73 (2015)
Article DOI: 10.1016/j.bmc.2015.08.019
BindingDB Entry DOI: 10.7270/Q2668G0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50120487
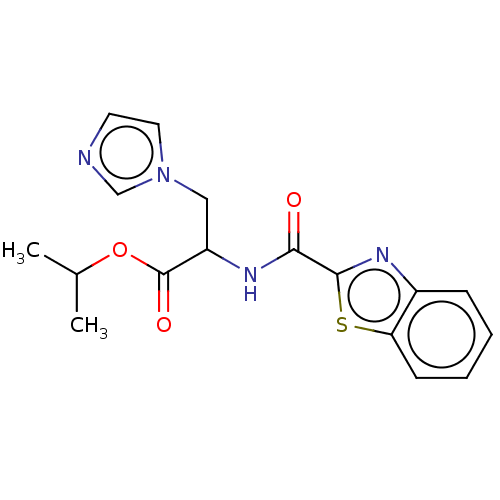
(CHEMBL3617991)Show InChI InChI=1S/C17H18N4O3S/c1-11(2)24-17(23)13(9-21-8-7-18-10-21)19-15(22)16-20-12-5-3-4-6-14(12)25-16/h3-8,10-11,13H,9H2,1-2H3,(H,19,22) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of CYP26A1 in ATRA-induced human HL60 cell microsomes incubated for 30 mins in dark condition with NADPH and ATRA by HPLC method |
Bioorg Med Chem 23: 6763-73 (2015)
Article DOI: 10.1016/j.bmc.2015.08.019
BindingDB Entry DOI: 10.7270/Q2668G0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50120457

(CHEMBL3617979)Show SMILES CCOC(=O)C(Cn1ccnc1)NC(=O)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C21H21N3O3/c1-2-27-21(26)19(14-24-13-12-22-15-24)23-20(25)18-10-8-17(9-11-18)16-6-4-3-5-7-16/h3-13,15,19H,2,14H2,1H3,(H,23,25) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of CYP26A1 in ATRA-induced human HL60 cell microsomes incubated for 30 mins in dark condition with NADPH and ATRA by HPLC method |
Bioorg Med Chem 23: 6763-73 (2015)
Article DOI: 10.1016/j.bmc.2015.08.019
BindingDB Entry DOI: 10.7270/Q2668G0C |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50248717

(CHEMBL4085325)Show SMILES N[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1NC(=S)Nc1ccc(cc1)S(N)(=O)=O |r| Show InChI InChI=1S/C13H20N4O6S2/c14-9-11(20)10(19)8(5-18)23-12(9)17-13(24)16-6-1-3-7(4-2-6)25(15,21)22/h1-4,8-12,18-20H,5,14H2,(H2,15,21,22)(H2,16,17,24)/t8-,9-,10-,11-,12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 667 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Binding affinity towards HSV-1 thymidine kinase |
Eur J Med Chem 132: 1-10 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.023
BindingDB Entry DOI: 10.7270/Q2WS8WNX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data