 Found 47 hits with Last Name = 'chonan' and Initial = 't'
Found 47 hits with Last Name = 'chonan' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetyl-CoA carboxylase 1/2
(Homo sapiens (Human)) | BDBM50482275
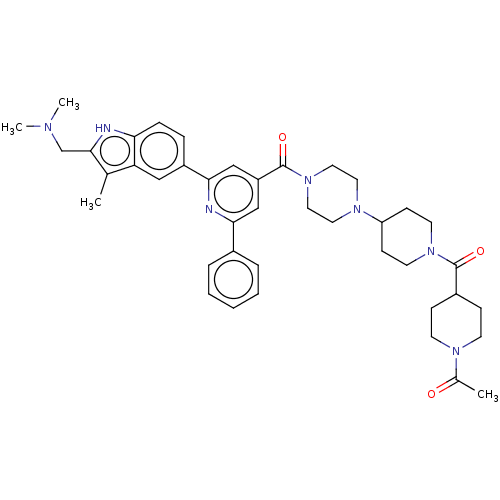
(CHEMBL1170494)Show SMILES CN(C)Cc1[nH]c2ccc(cc2c1C)-c1cc(cc(n1)-c1ccccc1)C(=O)N1CCN(CC1)C1CCN(CC1)C(=O)C1CCN(CC1)C(C)=O Show InChI InChI=1S/C41H51N7O3/c1-28-35-24-32(10-11-36(35)42-39(28)27-44(3)4)38-26-33(25-37(43-38)30-8-6-5-7-9-30)41(51)48-22-20-46(21-23-48)34-14-18-47(19-15-34)40(50)31-12-16-45(17-13-31)29(2)49/h5-11,24-26,31,34,42H,12-23,27H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1/2-mediated malonyl-CoA synthesis |
Bioorg Med Chem Lett 20: 3965-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.134
BindingDB Entry DOI: 10.7270/Q2F76GC2 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1/2
(Homo sapiens (Human)) | BDBM50482279
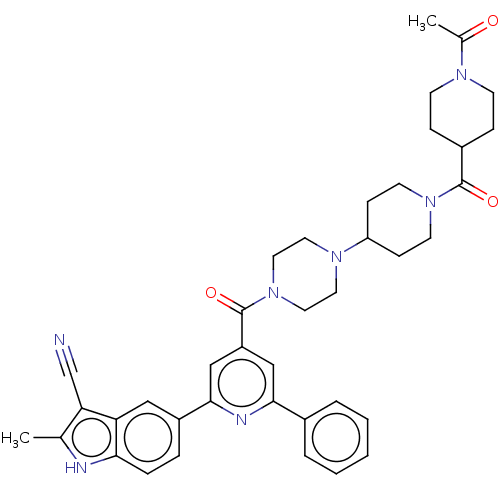
(CHEMBL1170493)Show SMILES CC(=O)N1CCC(CC1)C(=O)N1CCC(CC1)N1CCN(CC1)C(=O)c1cc(nc(c1)-c1ccc2[nH]c(C)c(C#N)c2c1)-c1ccccc1 Show InChI InChI=1S/C39H43N7O3/c1-26-34(25-40)33-22-30(8-9-35(33)41-26)37-24-31(23-36(42-37)28-6-4-3-5-7-28)39(49)46-20-18-44(19-21-46)32-12-16-45(17-13-32)38(48)29-10-14-43(15-11-29)27(2)47/h3-9,22-24,29,32,41H,10-21H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1/2-mediated malonyl-CoA synthesis |
Bioorg Med Chem Lett 20: 3965-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.134
BindingDB Entry DOI: 10.7270/Q2F76GC2 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1/2
(Homo sapiens (Human)) | BDBM50482288
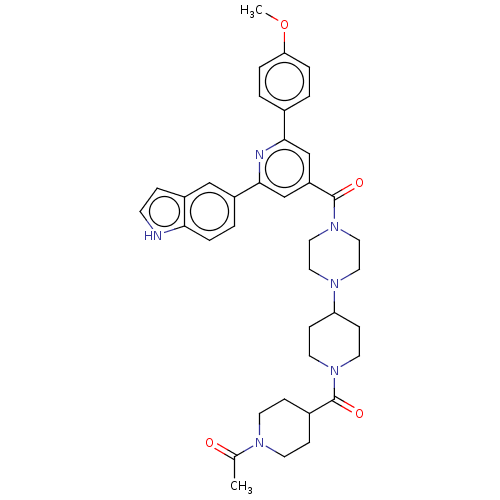
(CHEMBL1170510)Show SMILES COc1ccc(cc1)-c1cc(cc(n1)-c1ccc2[nH]ccc2c1)C(=O)N1CCN(CC1)C1CCN(CC1)C(=O)C1CCN(CC1)C(C)=O Show InChI InChI=1S/C38H44N6O4/c1-26(45)41-15-10-28(11-16-41)37(46)43-17-12-32(13-18-43)42-19-21-44(22-20-42)38(47)31-24-35(27-3-6-33(48-2)7-4-27)40-36(25-31)29-5-8-34-30(23-29)9-14-39-34/h3-9,14,23-25,28,32,39H,10-13,15-22H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1/2-mediated malonyl-CoA synthesis |
Bioorg Med Chem Lett 20: 3965-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.134
BindingDB Entry DOI: 10.7270/Q2F76GC2 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50311816
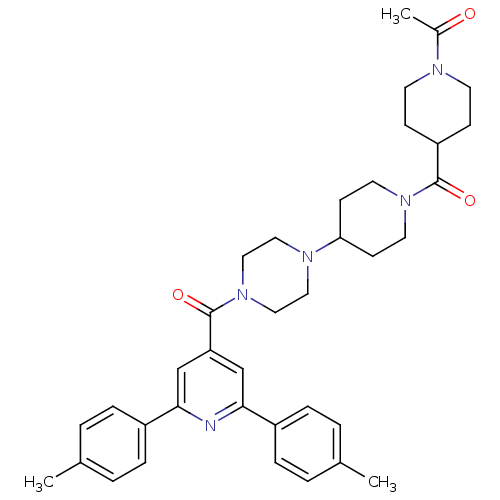
(1-(4-(4-(4-(2,6-dip-tolylisonicotinoyl)piperazin-1...)Show SMILES CC(=O)N1CCC(CC1)C(=O)N1CCC(CC1)N1CCN(CC1)C(=O)c1cc(nc(c1)-c1ccc(C)cc1)-c1ccc(C)cc1 Show InChI InChI=1S/C37H45N5O3/c1-26-4-8-29(9-5-26)34-24-32(25-35(38-34)30-10-6-27(2)7-11-30)37(45)42-22-20-40(21-23-42)33-14-18-41(19-15-33)36(44)31-12-16-39(17-13-31)28(3)43/h4-11,24-25,31,33H,12-23H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACC2 |
Bioorg Med Chem Lett 19: 6645-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.012
BindingDB Entry DOI: 10.7270/Q2ZP468S |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50311816

(1-(4-(4-(4-(2,6-dip-tolylisonicotinoyl)piperazin-1...)Show SMILES CC(=O)N1CCC(CC1)C(=O)N1CCC(CC1)N1CCN(CC1)C(=O)c1cc(nc(c1)-c1ccc(C)cc1)-c1ccc(C)cc1 Show InChI InChI=1S/C37H45N5O3/c1-26-4-8-29(9-5-26)34-24-32(25-35(38-34)30-10-6-27(2)7-11-30)37(45)42-22-20-40(21-23-42)33-14-18-41(19-15-33)36(44)31-12-16-39(17-13-31)28(3)43/h4-11,24-25,31,33H,12-23H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of c-Myc tagged human recombinant ACC2 expressed in baculovirus infected Sf9 cell system assessed as malonyl-CoA synthesis |
Bioorg Med Chem 19: 1580-93 (2011)
Article DOI: 10.1016/j.bmc.2011.01.041
BindingDB Entry DOI: 10.7270/Q2NS0VWF |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1/2
(Homo sapiens (Human)) | BDBM50482276
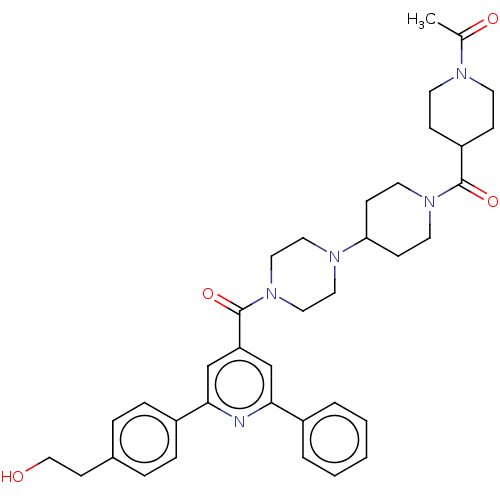
(CHEMBL1170515)Show SMILES CC(=O)N1CCC(CC1)C(=O)N1CCC(CC1)N1CCN(CC1)C(=O)c1cc(nc(c1)-c1ccc(CCO)cc1)-c1ccccc1 Show InChI InChI=1S/C37H45N5O4/c1-27(44)39-16-11-31(12-17-39)36(45)41-18-13-33(14-19-41)40-20-22-42(23-21-40)37(46)32-25-34(29-5-3-2-4-6-29)38-35(26-32)30-9-7-28(8-10-30)15-24-43/h2-10,25-26,31,33,43H,11-24H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1/2-mediated malonyl-CoA synthesis |
Bioorg Med Chem Lett 20: 3965-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.134
BindingDB Entry DOI: 10.7270/Q2F76GC2 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1/2
(Homo sapiens (Human)) | BDBM50482290
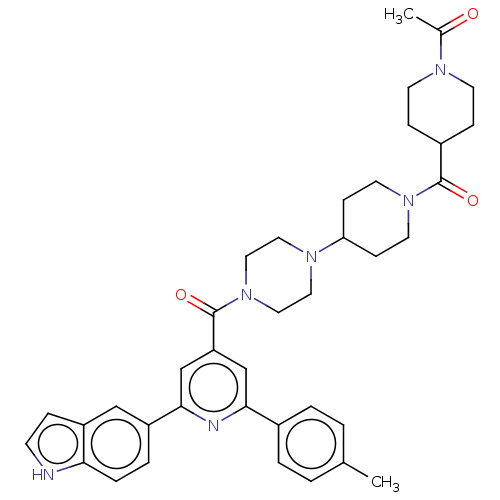
(CHEMBL1170508)Show SMILES CC(=O)N1CCC(CC1)C(=O)N1CCC(CC1)N1CCN(CC1)C(=O)c1cc(nc(c1)-c1ccc2[nH]ccc2c1)-c1ccc(C)cc1 Show InChI InChI=1S/C38H44N6O3/c1-26-3-5-28(6-4-26)35-24-32(25-36(40-35)30-7-8-34-31(23-30)9-14-39-34)38(47)44-21-19-42(20-22-44)33-12-17-43(18-13-33)37(46)29-10-15-41(16-11-29)27(2)45/h3-9,14,23-25,29,33,39H,10-13,15-22H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1/2-mediated malonyl-CoA synthesis |
Bioorg Med Chem Lett 20: 3965-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.134
BindingDB Entry DOI: 10.7270/Q2F76GC2 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1/2
(Homo sapiens (Human)) | BDBM50482278

(CHEMBL1170498)Show SMILES COc1ccc(cc1OC)-c1cc(cc(n1)-c1ccc2[nH]ncc2c1)C(=O)N1CCN(CC1)C1CCN(CC1)C(=O)C1CCN(CC1)C(C)=O Show InChI InChI=1S/C38H45N7O5/c1-25(46)42-12-8-26(9-13-42)37(47)44-14-10-31(11-15-44)43-16-18-45(19-17-43)38(48)29-21-33(27-4-6-32-30(20-27)24-39-41-32)40-34(22-29)28-5-7-35(49-2)36(23-28)50-3/h4-7,20-24,26,31H,8-19H2,1-3H3,(H,39,41) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1/2-mediated malonyl-CoA synthesis |
Bioorg Med Chem Lett 20: 3965-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.134
BindingDB Entry DOI: 10.7270/Q2F76GC2 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1/2
(Homo sapiens (Human)) | BDBM50482266
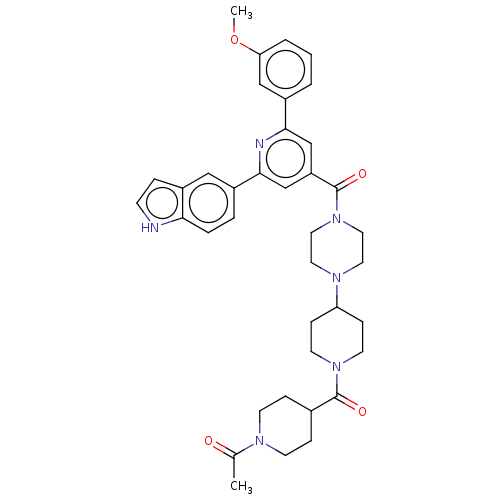
(CHEMBL1170509)Show SMILES COc1cccc(c1)-c1cc(cc(n1)-c1ccc2[nH]ccc2c1)C(=O)N1CCN(CC1)C1CCN(CC1)C(=O)C1CCN(CC1)C(C)=O Show InChI InChI=1S/C38H44N6O4/c1-26(45)41-14-9-27(10-15-41)37(46)43-16-11-32(12-17-43)42-18-20-44(21-19-42)38(47)31-24-35(28-4-3-5-33(23-28)48-2)40-36(25-31)29-6-7-34-30(22-29)8-13-39-34/h3-8,13,22-25,27,32,39H,9-12,14-21H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1/2-mediated malonyl-CoA synthesis |
Bioorg Med Chem Lett 20: 3965-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.134
BindingDB Entry DOI: 10.7270/Q2F76GC2 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1/2
(Homo sapiens (Human)) | BDBM50482273
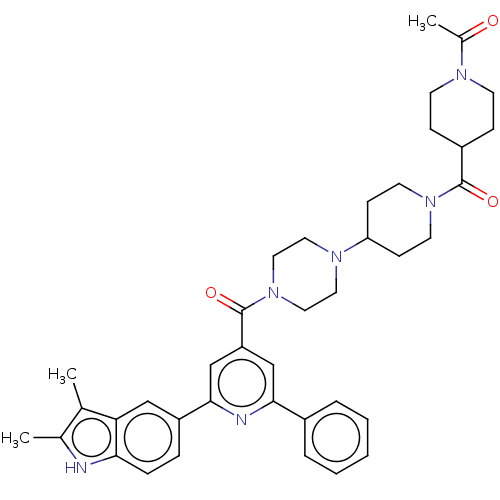
(CHEMBL1170490)Show SMILES CC(=O)N1CCC(CC1)C(=O)N1CCC(CC1)N1CCN(CC1)C(=O)c1cc(nc(c1)-c1ccc2[nH]c(C)c(C)c2c1)-c1ccccc1 Show InChI InChI=1S/C39H46N6O3/c1-26-27(2)40-35-10-9-31(23-34(26)35)37-25-32(24-36(41-37)29-7-5-4-6-8-29)39(48)45-21-19-43(20-22-45)33-13-17-44(18-14-33)38(47)30-11-15-42(16-12-30)28(3)46/h4-10,23-25,30,33,40H,11-22H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1/2-mediated malonyl-CoA synthesis |
Bioorg Med Chem Lett 20: 3965-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.134
BindingDB Entry DOI: 10.7270/Q2F76GC2 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1/2
(Homo sapiens (Human)) | BDBM50482265
(CHEMBL1170505)Show SMILES CC(=O)N1CCC(CC1)C(=O)N1CCC(CC1)N1CCN(CC1)C(=O)c1cc(nc(c1)-c1ccc2[nH]ccc2c1)-c1cccc(F)c1 Show InChI InChI=1S/C37H41FN6O3/c1-25(45)41-13-8-26(9-14-41)36(46)43-15-10-32(11-16-43)42-17-19-44(20-18-42)37(47)30-23-34(27-3-2-4-31(38)22-27)40-35(24-30)28-5-6-33-29(21-28)7-12-39-33/h2-7,12,21-24,26,32,39H,8-11,13-20H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1/2-mediated malonyl-CoA synthesis |
Bioorg Med Chem Lett 20: 3965-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.134
BindingDB Entry DOI: 10.7270/Q2F76GC2 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1/2
(Homo sapiens (Human)) | BDBM50482286
(CHEMBL1170501)Show SMILES CC(=O)N1CCC(CC1)C(=O)N1CCC(CC1)N1CCN(CC1)C(=O)c1cc(nc(c1)-c1ccc2[nH]cc(C)c2c1)-c1ccccc1 Show InChI InChI=1S/C38H44N6O3/c1-26-25-39-34-9-8-30(22-33(26)34)36-24-31(23-35(40-36)28-6-4-3-5-7-28)38(47)44-20-18-42(19-21-44)32-12-16-43(17-13-32)37(46)29-10-14-41(15-11-29)27(2)45/h3-9,22-25,29,32,39H,10-21H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1/2-mediated malonyl-CoA synthesis |
Bioorg Med Chem Lett 20: 3965-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.134
BindingDB Entry DOI: 10.7270/Q2F76GC2 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1/2
(Homo sapiens (Human)) | BDBM50482284
(CHEMBL1170507)Show SMILES CC(=O)N1CCC(CC1)C(=O)N1CCC(CC1)N1CCN(CC1)C(=O)c1cc(nc(c1)-c1ccc2[nH]ccc2c1)-c1cccc(C)c1 Show InChI InChI=1S/C38H44N6O3/c1-26-4-3-5-29(22-26)35-24-32(25-36(40-35)30-6-7-34-31(23-30)8-13-39-34)38(47)44-20-18-42(19-21-44)33-11-16-43(17-12-33)37(46)28-9-14-41(15-10-28)27(2)45/h3-8,13,22-25,28,33,39H,9-12,14-21H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1/2-mediated malonyl-CoA synthesis |
Bioorg Med Chem Lett 20: 3965-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.134
BindingDB Entry DOI: 10.7270/Q2F76GC2 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1/2
(Homo sapiens (Human)) | BDBM50482263

(CHEMBL1170718)Show SMILES CC(=O)N1CCC(CC1)C(=O)N1CCC(CC1)N1CCN(CC1)C(=O)c1cc(nc(c1)-c1ccc2[nH]ncc2c1)-c1ccccc1 Show InChI InChI=1S/C36H41N7O3/c1-25(44)40-13-9-27(10-14-40)35(45)42-15-11-31(12-16-42)41-17-19-43(20-18-41)36(46)29-22-33(26-5-3-2-4-6-26)38-34(23-29)28-7-8-32-30(21-28)24-37-39-32/h2-8,21-24,27,31H,9-20H2,1H3,(H,37,39) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1/2-mediated malonyl-CoA synthesis |
Bioorg Med Chem Lett 20: 3965-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.134
BindingDB Entry DOI: 10.7270/Q2F76GC2 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1/2
(Homo sapiens (Human)) | BDBM50482258
(CHEMBL1170721)Show SMILES CC(=O)N1CCC(CC1)C(=O)N1CCC(CC1)N1CCN(CC1)C(=O)c1cc(nc(c1)-c1ccc(CO)cc1)-c1ccccc1 Show InChI InChI=1S/C36H43N5O4/c1-26(43)38-15-11-30(12-16-38)35(44)40-17-13-32(14-18-40)39-19-21-41(22-20-39)36(45)31-23-33(28-5-3-2-4-6-28)37-34(24-31)29-9-7-27(25-42)8-10-29/h2-10,23-24,30,32,42H,11-22,25H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1/2-mediated malonyl-CoA synthesis |
Bioorg Med Chem Lett 20: 3965-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.134
BindingDB Entry DOI: 10.7270/Q2F76GC2 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1/2
(Homo sapiens (Human)) | BDBM50482281
(CHEMBL1170517)Show SMILES CC(=O)N1CCC(CC1)C(=O)N1CCC(CC1)N1CCN(CC1)C(=O)c1cc(nc(c1)-c1ccc(OCCO)cc1)-c1ccccc1 Show InChI InChI=1S/C37H45N5O5/c1-27(44)39-15-11-30(12-16-39)36(45)41-17-13-32(14-18-41)40-19-21-42(22-20-40)37(46)31-25-34(28-5-3-2-4-6-28)38-35(26-31)29-7-9-33(10-8-29)47-24-23-43/h2-10,25-26,30,32,43H,11-24H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1/2-mediated malonyl-CoA synthesis |
Bioorg Med Chem Lett 20: 3965-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.134
BindingDB Entry DOI: 10.7270/Q2F76GC2 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1/2
(Homo sapiens (Human)) | BDBM50482257
(CHEMBL1170719)Show SMILES CC(=O)N1CCC(CC1)C(=O)N1CCC(CC1)N1CCN(CC1)C(=O)c1cc(nc(c1)-c1ccc(O)cc1)-c1ccccc1 Show InChI InChI=1S/C35H41N5O4/c1-25(41)37-15-11-28(12-16-37)34(43)39-17-13-30(14-18-39)38-19-21-40(22-20-38)35(44)29-23-32(26-5-3-2-4-6-26)36-33(24-29)27-7-9-31(42)10-8-27/h2-10,23-24,28,30,42H,11-22H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1/2-mediated malonyl-CoA synthesis |
Bioorg Med Chem Lett 20: 3965-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.134
BindingDB Entry DOI: 10.7270/Q2F76GC2 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1/2
(Homo sapiens (Human)) | BDBM50482282
(CHEMBL1170717)Show SMILES CC(=O)N1CCC(CC1)C(=O)N1CCC(CC1)N1CCN(CC1)C(=O)c1cc(nc(c1)-c1ccc2cc[nH]c2c1)-c1ccccc1 Show InChI InChI=1S/C37H42N6O3/c1-26(44)40-15-10-29(11-16-40)36(45)42-17-12-32(13-18-42)41-19-21-43(22-20-41)37(46)31-24-34(27-5-3-2-4-6-27)39-35(25-31)30-8-7-28-9-14-38-33(28)23-30/h2-9,14,23-25,29,32,38H,10-13,15-22H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1/2-mediated malonyl-CoA synthesis |
Bioorg Med Chem Lett 20: 3965-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.134
BindingDB Entry DOI: 10.7270/Q2F76GC2 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1/2
(Homo sapiens (Human)) | BDBM50482280
(CHEMBL1170720)Show SMILES CC(=O)N1CCC(CC1)C(=O)N1CCC(CC1)N1CCN(CC1)C(=O)c1cc(nc(c1)-c1cccc(CO)c1)-c1ccccc1 Show InChI InChI=1S/C36H43N5O4/c1-26(43)38-14-10-29(11-15-38)35(44)40-16-12-32(13-17-40)39-18-20-41(21-19-39)36(45)31-23-33(28-7-3-2-4-8-28)37-34(24-31)30-9-5-6-27(22-30)25-42/h2-9,22-24,29,32,42H,10-21,25H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1/2-mediated malonyl-CoA synthesis |
Bioorg Med Chem Lett 20: 3965-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.134
BindingDB Entry DOI: 10.7270/Q2F76GC2 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1/2
(Homo sapiens (Human)) | BDBM50482277
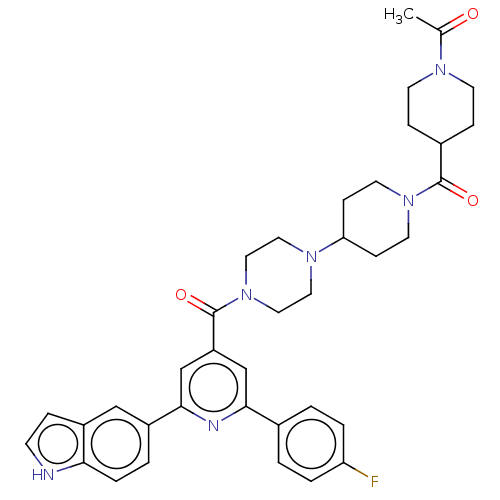
(CHEMBL1170506)Show SMILES CC(=O)N1CCC(CC1)C(=O)N1CCC(CC1)N1CCN(CC1)C(=O)c1cc(nc(c1)-c1ccc2[nH]ccc2c1)-c1ccc(F)cc1 Show InChI InChI=1S/C37H41FN6O3/c1-25(45)41-14-9-27(10-15-41)36(46)43-16-11-32(12-17-43)42-18-20-44(21-19-42)37(47)30-23-34(26-2-5-31(38)6-3-26)40-35(24-30)28-4-7-33-29(22-28)8-13-39-33/h2-8,13,22-24,27,32,39H,9-12,14-21H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1/2-mediated malonyl-CoA synthesis |
Bioorg Med Chem Lett 20: 3965-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.134
BindingDB Entry DOI: 10.7270/Q2F76GC2 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1/2
(Homo sapiens (Human)) | BDBM50482269
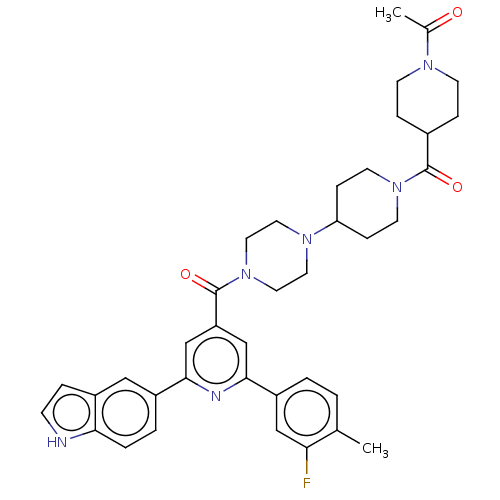
(CHEMBL1170496)Show SMILES CC(=O)N1CCC(CC1)C(=O)N1CCC(CC1)N1CCN(CC1)C(=O)c1cc(nc(c1)-c1ccc2[nH]ccc2c1)-c1ccc(C)c(F)c1 Show InChI InChI=1S/C38H43FN6O3/c1-25-3-4-29(22-33(25)39)36-24-31(23-35(41-36)28-5-6-34-30(21-28)7-12-40-34)38(48)45-19-17-43(18-20-45)32-10-15-44(16-11-32)37(47)27-8-13-42(14-9-27)26(2)46/h3-7,12,21-24,27,32,40H,8-11,13-20H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1/2-mediated malonyl-CoA synthesis |
Bioorg Med Chem Lett 20: 3965-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.134
BindingDB Entry DOI: 10.7270/Q2F76GC2 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1
(Rattus norvegicus (Rat)) | BDBM50189617
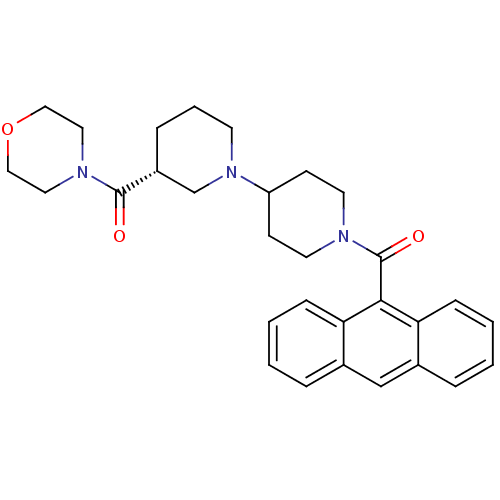
((3R)-1'-(9-anthrylcarbonyl)-3-(morpholin-4-ylcarbo...)Show SMILES O=C([C@@H]1CCCN(C1)C1CCN(CC1)C(=O)c1c2ccccc2cc2ccccc12)N1CCOCC1 |r| Show InChI InChI=1S/C30H35N3O3/c34-29(32-16-18-36-19-17-32)24-8-5-13-33(21-24)25-11-14-31(15-12-25)30(35)28-26-9-3-1-6-22(26)20-23-7-2-4-10-27(23)28/h1-4,6-7,9-10,20,24-25H,5,8,11-19,21H2/t24-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of rat ACC1 |
Bioorg Med Chem Lett 19: 6645-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.012
BindingDB Entry DOI: 10.7270/Q2ZP468S |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1/2
(Homo sapiens (Human)) | BDBM50482283
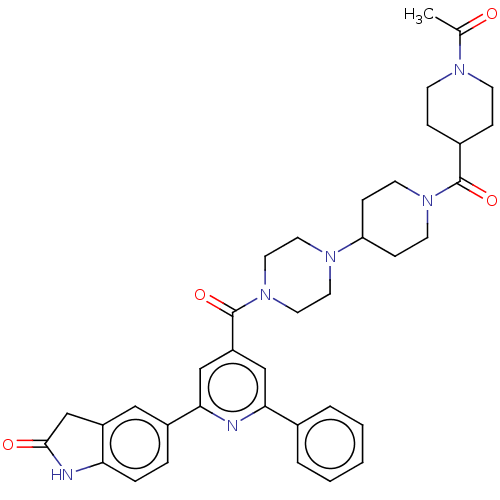
(CHEMBL1170504)Show SMILES CC(=O)N1CCC(CC1)C(=O)N1CCC(CC1)N1CCN(CC1)C(=O)c1cc(nc(c1)-c1ccc2NC(=O)Cc2c1)-c1ccccc1 Show InChI InChI=1S/C37H42N6O4/c1-25(44)40-13-9-27(10-14-40)36(46)42-15-11-31(12-16-42)41-17-19-43(20-18-41)37(47)30-22-33(26-5-3-2-4-6-26)38-34(23-30)28-7-8-32-29(21-28)24-35(45)39-32/h2-8,21-23,27,31H,9-20,24H2,1H3,(H,39,45) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1/2-mediated malonyl-CoA synthesis |
Bioorg Med Chem Lett 20: 3965-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.134
BindingDB Entry DOI: 10.7270/Q2F76GC2 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1/2
(Homo sapiens (Human)) | BDBM50482262
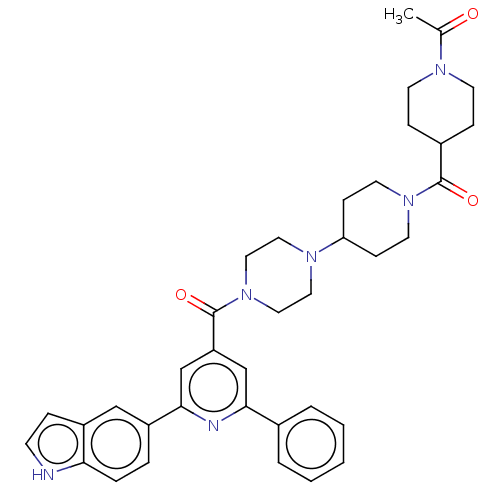
(CHEMBL1170716)Show SMILES CC(=O)N1CCC(CC1)C(=O)N1CCC(CC1)N1CCN(CC1)C(=O)c1cc(nc(c1)-c1ccc2[nH]ccc2c1)-c1ccccc1 Show InChI InChI=1S/C37H42N6O3/c1-26(44)40-15-10-28(11-16-40)36(45)42-17-12-32(13-18-42)41-19-21-43(22-20-41)37(46)31-24-34(27-5-3-2-4-6-27)39-35(25-31)29-7-8-33-30(23-29)9-14-38-33/h2-9,14,23-25,28,32,38H,10-13,15-22H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1/2-mediated malonyl-CoA synthesis |
Bioorg Med Chem Lett 20: 3965-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.134
BindingDB Entry DOI: 10.7270/Q2F76GC2 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1/2
(Homo sapiens (Human)) | BDBM50482264
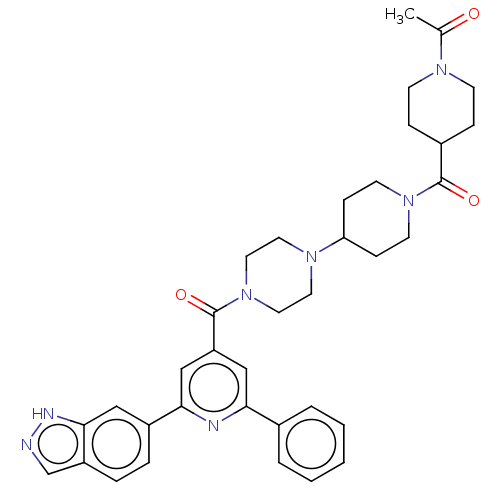
(CHEMBL1170503)Show SMILES CC(=O)N1CCC(CC1)C(=O)N1CCC(CC1)N1CCN(CC1)C(=O)c1cc(nc(c1)-c1ccc2cn[nH]c2c1)-c1ccccc1 Show InChI InChI=1S/C36H41N7O3/c1-25(44)40-13-9-27(10-14-40)35(45)42-15-11-31(12-16-42)41-17-19-43(20-18-41)36(46)30-22-32(26-5-3-2-4-6-26)38-33(23-30)28-7-8-29-24-37-39-34(29)21-28/h2-8,21-24,27,31H,9-20H2,1H3,(H,37,39) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1/2-mediated malonyl-CoA synthesis |
Bioorg Med Chem Lett 20: 3965-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.134
BindingDB Entry DOI: 10.7270/Q2F76GC2 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1/2
(Homo sapiens (Human)) | BDBM50482271
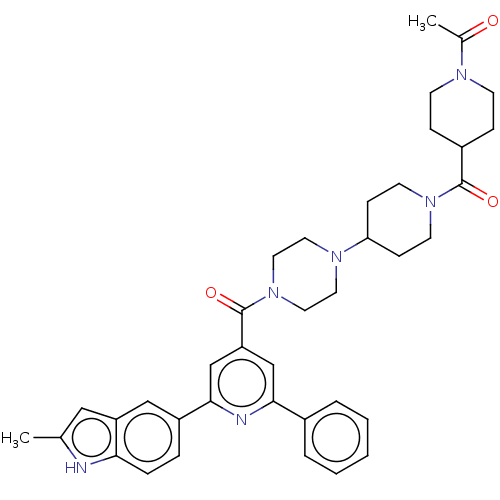
(CHEMBL1170500)Show SMILES CC(=O)N1CCC(CC1)C(=O)N1CCC(CC1)N1CCN(CC1)C(=O)c1cc(nc(c1)-c1ccc2[nH]c(C)cc2c1)-c1ccccc1 Show InChI InChI=1S/C38H44N6O3/c1-26-22-31-23-30(8-9-34(31)39-26)36-25-32(24-35(40-36)28-6-4-3-5-7-28)38(47)44-20-18-42(19-21-44)33-12-16-43(17-13-33)37(46)29-10-14-41(15-11-29)27(2)45/h3-9,22-25,29,33,39H,10-21H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1/2-mediated malonyl-CoA synthesis |
Bioorg Med Chem Lett 20: 3965-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.134
BindingDB Entry DOI: 10.7270/Q2F76GC2 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase
(Rattus norvegicus (Rat)) | BDBM50189617

((3R)-1'-(9-anthrylcarbonyl)-3-(morpholin-4-ylcarbo...)Show SMILES O=C([C@@H]1CCCN(C1)C1CCN(CC1)C(=O)c1c2ccccc2cc2ccccc12)N1CCOCC1 |r| Show InChI InChI=1S/C30H35N3O3/c34-29(32-16-18-36-19-17-32)24-8-5-13-33(21-24)25-11-14-31(15-12-25)30(35)28-26-9-3-1-6-22(26)20-23-7-2-4-10-27(23)28/h1-4,6-7,9-10,20,24-25H,5,8,11-19,21H2/t24-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of rat ACC2 |
Bioorg Med Chem Lett 19: 6645-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.012
BindingDB Entry DOI: 10.7270/Q2ZP468S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetyl-CoA carboxylase 1/2
(Homo sapiens (Human)) | BDBM50482285
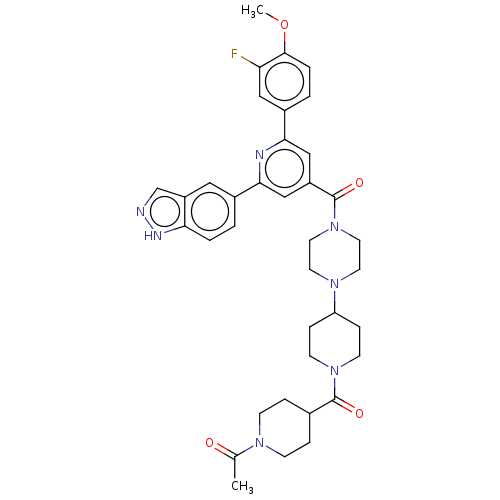
(CHEMBL1170497)Show SMILES COc1ccc(cc1F)-c1cc(cc(n1)-c1ccc2[nH]ncc2c1)C(=O)N1CCN(CC1)C1CCN(CC1)C(=O)C1CCN(CC1)C(C)=O Show InChI InChI=1S/C37H42FN7O4/c1-24(46)42-11-7-25(8-12-42)36(47)44-13-9-30(10-14-44)43-15-17-45(18-16-43)37(48)28-21-33(26-3-5-32-29(19-26)23-39-41-32)40-34(22-28)27-4-6-35(49-2)31(38)20-27/h3-6,19-23,25,30H,7-18H2,1-2H3,(H,39,41) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1/2-mediated malonyl-CoA synthesis |
Bioorg Med Chem Lett 20: 3965-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.134
BindingDB Entry DOI: 10.7270/Q2F76GC2 |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 1
(Homo sapiens (Human)) | BDBM185702
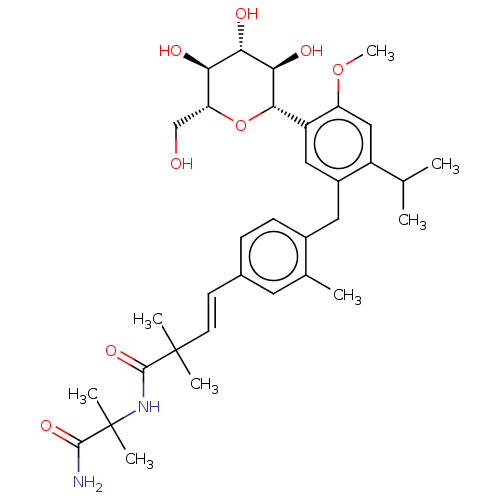
(US9161945, (I))Show SMILES COc1cc(C(C)C)c(Cc2ccc(\C=C\C(C)(C)C(=O)NC(C)(C)C(N)=O)cc2C)cc1[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C34H48N2O8/c1-18(2)23-16-25(43-8)24(30-29(40)28(39)27(38)26(17-37)44-30)15-22(23)14-21-10-9-20(13-19(21)3)11-12-33(4,5)32(42)36-34(6,7)31(35)41/h9-13,15-16,18,26-30,37-40H,14,17H2,1-8H3,(H2,35,41)(H,36,42)/b12-11+/t26-,27-,28+,29-,30+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | 7.4 | 37 |
TAISHO PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
Pretreatment buffer (140 mM choline chloride, 2 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 10 mM HEPES/5 mM Tris, pH 7.4) was added to the stably expressing cel... |
US Patent US9161945 (2015)
BindingDB Entry DOI: 10.7270/Q27M06PF |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1/2
(Homo sapiens (Human)) | BDBM50482270
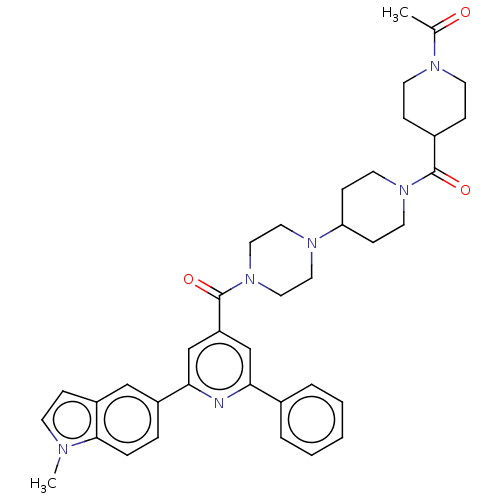
(CHEMBL1170499)Show SMILES CC(=O)N1CCC(CC1)C(=O)N1CCC(CC1)N1CCN(CC1)C(=O)c1cc(nc(c1)-c1ccc2n(C)ccc2c1)-c1ccccc1 Show InChI InChI=1S/C38H44N6O3/c1-27(45)41-16-11-29(12-17-41)37(46)43-18-13-33(14-19-43)42-20-22-44(23-21-42)38(47)32-25-34(28-6-4-3-5-7-28)39-35(26-32)30-8-9-36-31(24-30)10-15-40(36)2/h3-10,15,24-26,29,33H,11-14,16-23H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1/2-mediated malonyl-CoA synthesis |
Bioorg Med Chem Lett 20: 3965-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.134
BindingDB Entry DOI: 10.7270/Q2F76GC2 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1/2
(Homo sapiens (Human)) | BDBM50482261
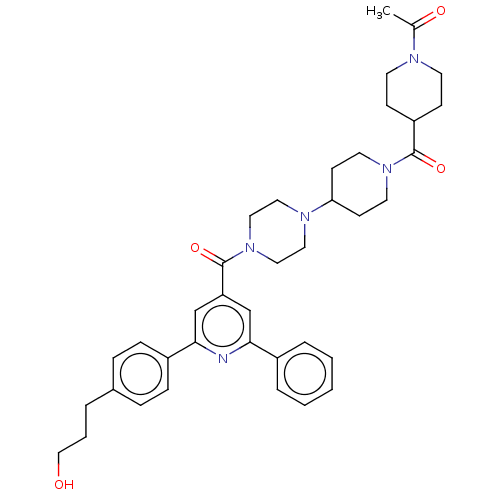
(CHEMBL1170715)Show SMILES CC(=O)N1CCC(CC1)C(=O)N1CCC(CC1)N1CCN(CC1)C(=O)c1cc(nc(c1)-c1ccc(CCCO)cc1)-c1ccccc1 Show InChI InChI=1S/C38H47N5O4/c1-28(45)40-17-13-32(14-18-40)37(46)42-19-15-34(16-20-42)41-21-23-43(24-22-41)38(47)33-26-35(30-7-3-2-4-8-30)39-36(27-33)31-11-9-29(10-12-31)6-5-25-44/h2-4,7-12,26-27,32,34,44H,5-6,13-25H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1/2-mediated malonyl-CoA synthesis |
Bioorg Med Chem Lett 20: 3965-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.134
BindingDB Entry DOI: 10.7270/Q2F76GC2 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1/2
(Homo sapiens (Human)) | BDBM50482268

(CHEMBL1170495)Show SMILES CC(=O)N1CCC(CC1)C(=O)N1CCC(CC1)N1CCN(CC1)C(=O)c1cc(nc(c1)-c1ccc2[nH]ccc2c1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C38H41F3N6O3/c1-25(48)44-14-9-27(10-15-44)36(49)46-16-11-32(12-17-46)45-18-20-47(21-19-45)37(50)30-23-34(26-2-5-31(6-3-26)38(39,40)41)43-35(24-30)28-4-7-33-29(22-28)8-13-42-33/h2-8,13,22-24,27,32,42H,9-12,14-21H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1/2-mediated malonyl-CoA synthesis |
Bioorg Med Chem Lett 20: 3965-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.134
BindingDB Entry DOI: 10.7270/Q2F76GC2 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1/2
(Homo sapiens (Human)) | BDBM50482260
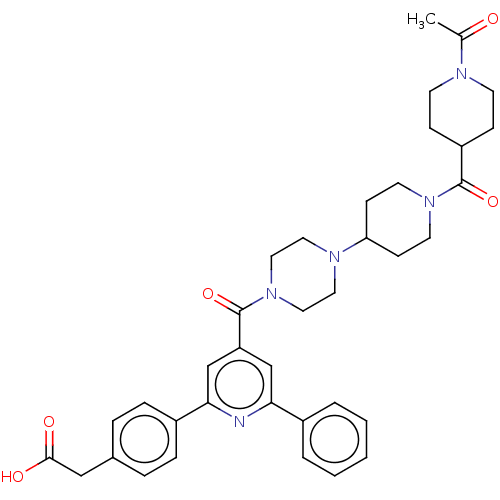
(CHEMBL1170516)Show SMILES CC(=O)N1CCC(CC1)C(=O)N1CCC(CC1)N1CCN(CC1)C(=O)c1cc(nc(c1)-c1ccc(CC(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C37H43N5O5/c1-26(43)39-15-11-30(12-16-39)36(46)41-17-13-32(14-18-41)40-19-21-42(22-20-40)37(47)31-24-33(28-5-3-2-4-6-28)38-34(25-31)29-9-7-27(8-10-29)23-35(44)45/h2-10,24-25,30,32H,11-23H2,1H3,(H,44,45) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1/2-mediated malonyl-CoA synthesis |
Bioorg Med Chem Lett 20: 3965-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.134
BindingDB Entry DOI: 10.7270/Q2F76GC2 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1/2
(Homo sapiens (Human)) | BDBM50482259
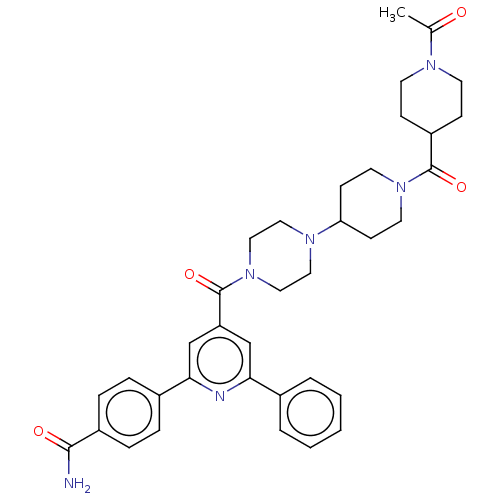
(CHEMBL1170512)Show SMILES CC(=O)N1CCC(CC1)C(=O)N1CCC(CC1)N1CCN(CC1)C(=O)c1cc(nc(c1)-c1ccc(cc1)C(N)=O)-c1ccccc1 Show InChI InChI=1S/C36H42N6O4/c1-25(43)39-15-11-29(12-16-39)35(45)41-17-13-31(14-18-41)40-19-21-42(22-20-40)36(46)30-23-32(26-5-3-2-4-6-26)38-33(24-30)27-7-9-28(10-8-27)34(37)44/h2-10,23-24,29,31H,11-22H2,1H3,(H2,37,44) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1/2-mediated malonyl-CoA synthesis |
Bioorg Med Chem Lett 20: 3965-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.134
BindingDB Entry DOI: 10.7270/Q2F76GC2 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50338559
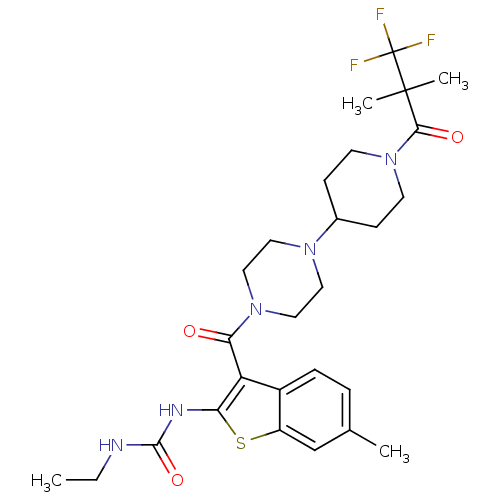
(1,1,1-Trifluoro-2-methylpropan-2-yl 4-[4-({2-[(eth...)Show SMILES CCNC(=O)Nc1sc2cc(C)ccc2c1C(=O)N1CCN(CC1)C1CCN(CC1)C(=O)C(C)(C)C(F)(F)F Show InChI InChI=1S/C27H36F3N5O3S/c1-5-31-25(38)32-22-21(19-7-6-17(2)16-20(19)39-22)23(36)34-14-12-33(13-15-34)18-8-10-35(11-9-18)24(37)26(3,4)27(28,29)30/h6-7,16,18H,5,8-15H2,1-4H3,(H2,31,32,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of c-Myc tagged human recombinant ACC2 expressed in baculovirus infected Sf9 cell system assessed as malonyl-CoA synthesis |
Bioorg Med Chem 19: 1580-93 (2011)
Article DOI: 10.1016/j.bmc.2011.01.041
BindingDB Entry DOI: 10.7270/Q2NS0VWF |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1/2
(Homo sapiens (Human)) | BDBM50482267
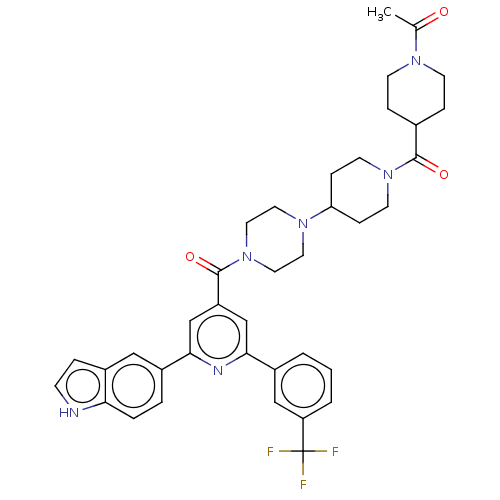
(CHEMBL1170511)Show SMILES CC(=O)N1CCC(CC1)C(=O)N1CCC(CC1)N1CCN(CC1)C(=O)c1cc(nc(c1)-c1ccc2[nH]ccc2c1)-c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C38H41F3N6O3/c1-25(48)44-13-8-26(9-14-44)36(49)46-15-10-32(11-16-46)45-17-19-47(20-18-45)37(50)30-23-34(27-3-2-4-31(22-27)38(39,40)41)43-35(24-30)28-5-6-33-29(21-28)7-12-42-33/h2-7,12,21-24,26,32,42H,8-11,13-20H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1/2-mediated malonyl-CoA synthesis |
Bioorg Med Chem Lett 20: 3965-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.134
BindingDB Entry DOI: 10.7270/Q2F76GC2 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1
(Homo sapiens (Human)) | BDBM50311816

(1-(4-(4-(4-(2,6-dip-tolylisonicotinoyl)piperazin-1...)Show SMILES CC(=O)N1CCC(CC1)C(=O)N1CCC(CC1)N1CCN(CC1)C(=O)c1cc(nc(c1)-c1ccc(C)cc1)-c1ccc(C)cc1 Show InChI InChI=1S/C37H45N5O3/c1-26-4-8-29(9-5-26)34-24-32(25-35(38-34)30-10-6-27(2)7-11-30)37(45)42-22-20-40(21-23-42)33-14-18-41(19-15-33)36(44)31-12-16-39(17-13-31)28(3)43/h4-11,24-25,31,33H,12-23H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of c-Myc tagged human recombinant ACC1 expressed in baculovirus infected Sf9 cell system assessed as malonyl-CoA synthesis |
Bioorg Med Chem 19: 1580-93 (2011)
Article DOI: 10.1016/j.bmc.2011.01.041
BindingDB Entry DOI: 10.7270/Q2NS0VWF |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1
(Homo sapiens (Human)) | BDBM50311816

(1-(4-(4-(4-(2,6-dip-tolylisonicotinoyl)piperazin-1...)Show SMILES CC(=O)N1CCC(CC1)C(=O)N1CCC(CC1)N1CCN(CC1)C(=O)c1cc(nc(c1)-c1ccc(C)cc1)-c1ccc(C)cc1 Show InChI InChI=1S/C37H45N5O3/c1-26-4-8-29(9-5-26)34-24-32(25-35(38-34)30-10-6-27(2)7-11-30)37(45)42-22-20-40(21-23-42)33-14-18-41(19-15-33)36(44)31-12-16-39(17-13-31)28(3)43/h4-11,24-25,31,33H,12-23H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACC1 |
Bioorg Med Chem Lett 19: 6645-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.012
BindingDB Entry DOI: 10.7270/Q2ZP468S |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1/2
(Homo sapiens (Human)) | BDBM50482287
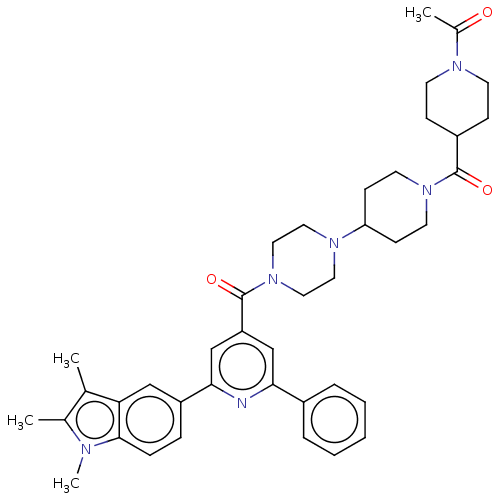
(CHEMBL1170491)Show SMILES CC(=O)N1CCC(CC1)C(=O)N1CCC(CC1)N1CCN(CC1)C(=O)c1cc(nc(c1)-c1ccc2n(C)c(C)c(C)c2c1)-c1ccccc1 Show InChI InChI=1S/C40H48N6O3/c1-27-28(2)42(4)38-11-10-32(24-35(27)38)37-26-33(25-36(41-37)30-8-6-5-7-9-30)40(49)46-22-20-44(21-23-46)34-14-18-45(19-15-34)39(48)31-12-16-43(17-13-31)29(3)47/h5-11,24-26,31,34H,12-23H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1/2-mediated malonyl-CoA synthesis |
Bioorg Med Chem Lett 20: 3965-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.134
BindingDB Entry DOI: 10.7270/Q2F76GC2 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1/2
(Homo sapiens (Human)) | BDBM50482272
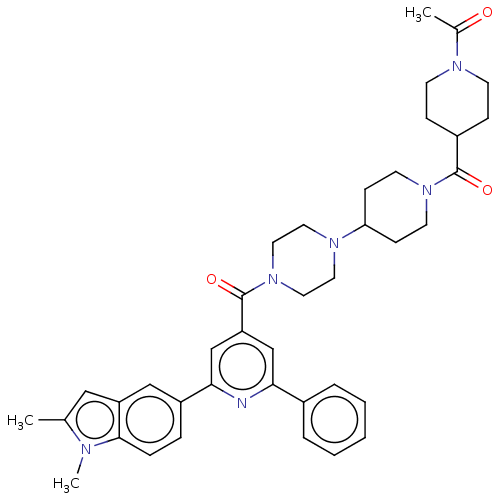
(CHEMBL1170502)Show SMILES CC(=O)N1CCC(CC1)C(=O)N1CCC(CC1)N1CCN(CC1)C(=O)c1cc(nc(c1)-c1ccc2n(C)c(C)cc2c1)-c1ccccc1 Show InChI InChI=1S/C39H46N6O3/c1-27-23-32-24-31(9-10-37(32)41(27)3)36-26-33(25-35(40-36)29-7-5-4-6-8-29)39(48)45-21-19-43(20-22-45)34-13-17-44(18-14-34)38(47)30-11-15-42(16-12-30)28(2)46/h4-10,23-26,30,34H,11-22H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 117 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1/2-mediated malonyl-CoA synthesis |
Bioorg Med Chem Lett 20: 3965-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.134
BindingDB Entry DOI: 10.7270/Q2F76GC2 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1/2
(Homo sapiens (Human)) | BDBM50482289
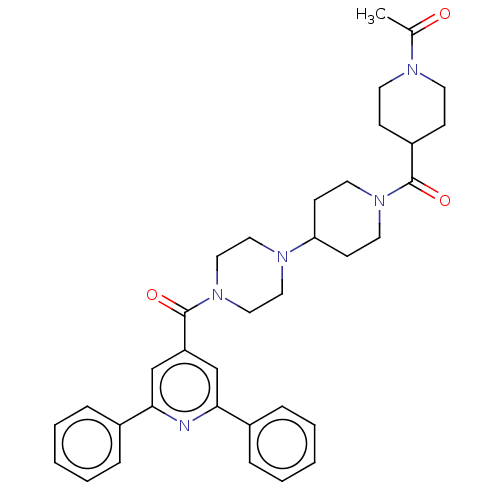
(CHEMBL1076213)Show SMILES CC(=O)N1CCC(CC1)C(=O)N1CCC(CC1)N1CCN(CC1)C(=O)c1cc(nc(c1)-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C35H41N5O3/c1-26(41)37-16-12-29(13-17-37)34(42)39-18-14-31(15-19-39)38-20-22-40(23-21-38)35(43)30-24-32(27-8-4-2-5-9-27)36-33(25-30)28-10-6-3-7-11-28/h2-11,24-25,29,31H,12-23H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1/2-mediated malonyl-CoA synthesis |
Bioorg Med Chem Lett 20: 3965-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.134
BindingDB Entry DOI: 10.7270/Q2F76GC2 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1/2
(Homo sapiens (Human)) | BDBM50482291
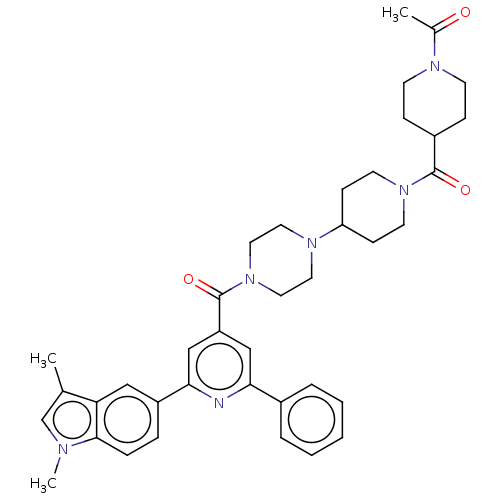
(CHEMBL1170489)Show SMILES CC(=O)N1CCC(CC1)C(=O)N1CCC(CC1)N1CCN(CC1)C(=O)c1cc(nc(c1)-c1ccc2n(C)cc(C)c2c1)-c1ccccc1 Show InChI InChI=1S/C39H46N6O3/c1-27-26-41(3)37-10-9-31(23-34(27)37)36-25-32(24-35(40-36)29-7-5-4-6-8-29)39(48)45-21-19-43(20-22-45)33-13-17-44(18-14-33)38(47)30-11-15-42(16-12-30)28(2)46/h4-10,23-26,30,33H,11-22H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1/2-mediated malonyl-CoA synthesis |
Bioorg Med Chem Lett 20: 3965-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.134
BindingDB Entry DOI: 10.7270/Q2F76GC2 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1/2
(Homo sapiens (Human)) | BDBM50482274
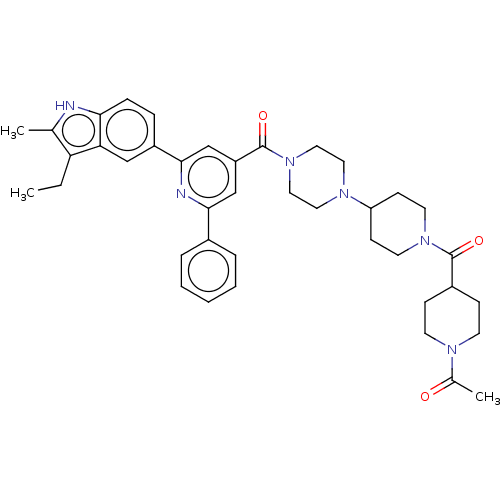
(CHEMBL1170492)Show SMILES CCc1c(C)[nH]c2ccc(cc12)-c1cc(cc(n1)-c1ccccc1)C(=O)N1CCN(CC1)C1CCN(CC1)C(=O)C1CCN(CC1)C(C)=O Show InChI InChI=1S/C40H48N6O3/c1-4-34-27(2)41-36-11-10-31(24-35(34)36)38-26-32(25-37(42-38)29-8-6-5-7-9-29)40(49)46-22-20-44(21-23-46)33-14-18-45(19-15-33)39(48)30-12-16-43(17-13-30)28(3)47/h5-11,24-26,30,33,41H,4,12-23H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 169 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1/2-mediated malonyl-CoA synthesis |
Bioorg Med Chem Lett 20: 3965-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.134
BindingDB Entry DOI: 10.7270/Q2F76GC2 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1
(Homo sapiens (Human)) | BDBM50338559

(1,1,1-Trifluoro-2-methylpropan-2-yl 4-[4-({2-[(eth...)Show SMILES CCNC(=O)Nc1sc2cc(C)ccc2c1C(=O)N1CCN(CC1)C1CCN(CC1)C(=O)C(C)(C)C(F)(F)F Show InChI InChI=1S/C27H36F3N5O3S/c1-5-31-25(38)32-22-21(19-7-6-17(2)16-20(19)39-22)23(36)34-14-12-33(13-15-34)18-8-10-35(11-9-18)24(37)26(3,4)27(28,29)30/h6-7,16,18H,5,8-15H2,1-4H3,(H2,31,32,38) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 192 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of c-Myc tagged human recombinant ACC1 expressed in baculovirus infected Sf9 cell system assessed as malonyl-CoA synthesis |
Bioorg Med Chem 19: 1580-93 (2011)
Article DOI: 10.1016/j.bmc.2011.01.041
BindingDB Entry DOI: 10.7270/Q2NS0VWF |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50189617

((3R)-1'-(9-anthrylcarbonyl)-3-(morpholin-4-ylcarbo...)Show SMILES O=C([C@@H]1CCCN(C1)C1CCN(CC1)C(=O)c1c2ccccc2cc2ccccc12)N1CCOCC1 |r| Show InChI InChI=1S/C30H35N3O3/c34-29(32-16-18-36-19-17-32)24-8-5-13-33(21-24)25-11-14-31(15-12-25)30(35)28-26-9-3-1-6-22(26)20-23-7-2-4-10-27(23)28/h1-4,6-7,9-10,20,24-25H,5,8,11-19,21H2/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 194 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACC2 |
Bioorg Med Chem Lett 19: 6645-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.012
BindingDB Entry DOI: 10.7270/Q2ZP468S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetyl-CoA carboxylase 1
(Homo sapiens (Human)) | BDBM50189617

((3R)-1'-(9-anthrylcarbonyl)-3-(morpholin-4-ylcarbo...)Show SMILES O=C([C@@H]1CCCN(C1)C1CCN(CC1)C(=O)c1c2ccccc2cc2ccccc12)N1CCOCC1 |r| Show InChI InChI=1S/C30H35N3O3/c34-29(32-16-18-36-19-17-32)24-8-5-13-33(21-24)25-11-14-31(15-12-25)30(35)28-26-9-3-1-6-22(26)20-23-7-2-4-10-27(23)28/h1-4,6-7,9-10,20,24-25H,5,8,11-19,21H2/t24-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 456 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACC1 |
Bioorg Med Chem Lett 19: 6645-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.012
BindingDB Entry DOI: 10.7270/Q2ZP468S |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM185702

(US9161945, (I))Show SMILES COc1cc(C(C)C)c(Cc2ccc(\C=C\C(C)(C)C(=O)NC(C)(C)C(N)=O)cc2C)cc1[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C34H48N2O8/c1-18(2)23-16-25(43-8)24(30-29(40)28(39)27(38)26(17-37)44-30)15-22(23)14-21-10-9-20(13-19(21)3)11-12-33(4,5)32(42)36-34(6,7)31(35)41/h9-13,15-16,18,26-30,37-40H,14,17H2,1-8H3,(H2,35,41)(H,36,42)/b12-11+/t26-,27-,28+,29-,30+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 554 | n/a | n/a | n/a | n/a | 7.4 | 37 |
TAISHO PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
Pretreatment buffer (140 mM choline chloride, 2 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 10 mM HEPES/5 mM Tris, pH 7.4) was added to the stably expressing cel... |
US Patent US9161945 (2015)
BindingDB Entry DOI: 10.7270/Q27M06PF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data