 Found 253 hits with Last Name = 'chucholowski' and Initial = 'a'
Found 253 hits with Last Name = 'chucholowski' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50181669
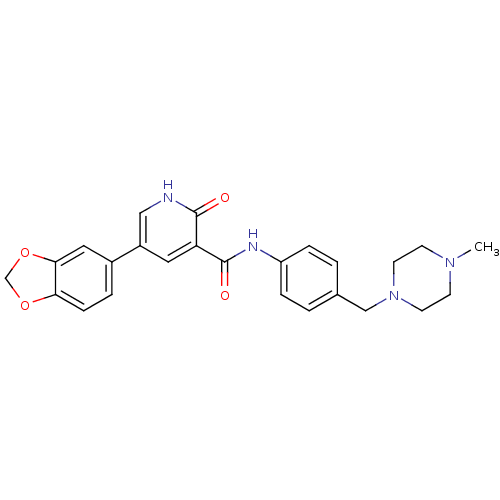
(5-benzo[1,3]dioxol-5-yl-2-oxo-1,2-dihydro-pyridine...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3cc(c[nH]c3=O)-c3ccc4OCOc4c3)cc2)CC1 Show InChI InChI=1S/C25H26N4O4/c1-28-8-10-29(11-9-28)15-17-2-5-20(6-3-17)27-25(31)21-12-19(14-26-24(21)30)18-4-7-22-23(13-18)33-16-32-22/h2-7,12-14H,8-11,15-16H2,1H3,(H,26,30)(H,27,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ChemBridge Research Laboratories and ChemBridge Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against ALK |
J Med Chem 49: 1006-15 (2006)
Article DOI: 10.1021/jm050824x
BindingDB Entry DOI: 10.7270/Q2H994T6 |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50181675

(5-benzo[1,3]dioxol-5-yl-2-oxo-1,2-dihydro-pyridine...)Show SMILES CN1CCN(CCCc2ccc(NC(=O)c3cc(c[nH]c3=O)-c3ccc4OCOc4c3)cc2)CC1 Show InChI InChI=1S/C27H30N4O4/c1-30-11-13-31(14-12-30)10-2-3-19-4-7-22(8-5-19)29-27(33)23-15-21(17-28-26(23)32)20-6-9-24-25(16-20)35-18-34-24/h4-9,15-17H,2-3,10-14,18H2,1H3,(H,28,32)(H,29,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ChemBridge Research Laboratories and ChemBridge Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against ALK |
J Med Chem 49: 1006-15 (2006)
Article DOI: 10.1021/jm050824x
BindingDB Entry DOI: 10.7270/Q2H994T6 |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50181669

(5-benzo[1,3]dioxol-5-yl-2-oxo-1,2-dihydro-pyridine...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3cc(c[nH]c3=O)-c3ccc4OCOc4c3)cc2)CC1 Show InChI InChI=1S/C25H26N4O4/c1-28-8-10-29(11-9-28)15-17-2-5-20(6-3-17)27-25(31)21-12-19(14-26-24(21)30)18-4-7-22-23(13-18)33-16-32-22/h2-7,12-14H,8-11,15-16H2,1H3,(H,26,30)(H,27,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ChemBridge Research Laboratories and ChemBridge Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against IRK |
J Med Chem 49: 1006-15 (2006)
Article DOI: 10.1021/jm050824x
BindingDB Entry DOI: 10.7270/Q2H994T6 |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50181670

(5-benzo[1,3]dioxol-5-yl-2-oxo-1,2-dihydro-pyridine...)Show SMILES CN1CCN(CC1)c1ccc(NC(=O)c2cc(c[nH]c2=O)-c2ccc3OCOc3c2)cc1 Show InChI InChI=1S/C24H24N4O4/c1-27-8-10-28(11-9-27)19-5-3-18(4-6-19)26-24(30)20-12-17(14-25-23(20)29)16-2-7-21-22(13-16)32-15-31-21/h2-7,12-14H,8-11,15H2,1H3,(H,25,29)(H,26,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ChemBridge Research Laboratories and ChemBridge Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against IRK |
J Med Chem 49: 1006-15 (2006)
Article DOI: 10.1021/jm050824x
BindingDB Entry DOI: 10.7270/Q2H994T6 |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50181675

(5-benzo[1,3]dioxol-5-yl-2-oxo-1,2-dihydro-pyridine...)Show SMILES CN1CCN(CCCc2ccc(NC(=O)c3cc(c[nH]c3=O)-c3ccc4OCOc4c3)cc2)CC1 Show InChI InChI=1S/C27H30N4O4/c1-30-11-13-31(14-12-30)10-2-3-19-4-7-22(8-5-19)29-27(33)23-15-21(17-28-26(23)32)20-6-9-24-25(16-20)35-18-34-24/h4-9,15-17H,2-3,10-14,18H2,1H3,(H,28,32)(H,29,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ChemBridge Research Laboratories and ChemBridge Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against IRK |
J Med Chem 49: 1006-15 (2006)
Article DOI: 10.1021/jm050824x
BindingDB Entry DOI: 10.7270/Q2H994T6 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50030815
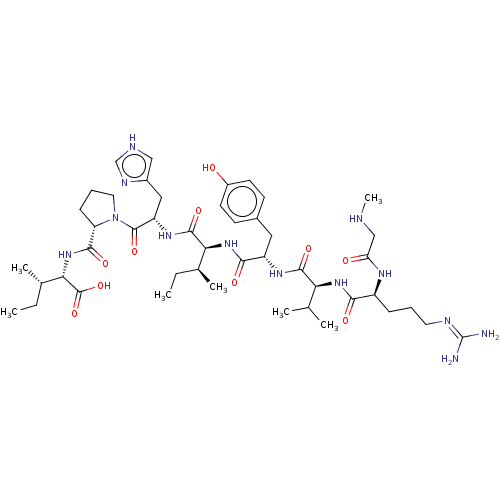
(CHEMBL404594)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)CNC)C(C)C)[C@@H](C)CC)C(O)=O |wU:8.7,25.27,wD:41.44,4.4,15.16,29.31,45.48,2.2,62.65,(8.44,-18.06,;9.36,-17.24,;9.05,-15.73,;7.88,-15.34,;10.19,-14.7,;9.88,-13.19,;11.03,-12.16,;12.2,-12.55,;10.71,-10.66,;11.74,-9.51,;10.97,-8.18,;9.46,-8.5,;9.33,-10.02,;7.99,-10.79,;7.99,-12.02,;6.66,-10.02,;5.32,-10.79,;5.32,-12.33,;4.07,-13.22,;4.55,-14.68,;6.09,-14.68,;6.56,-13.21,;6.66,-8.48,;5.33,-7.71,;4.43,-8.22,;5.33,-6.17,;3.99,-5.39,;4,-3.85,;5.06,-3.24,;2.66,-3.08,;1.33,-3.85,;-.01,-3.08,;-1.34,-3.84,;-2.67,-3.07,;-2.67,-1.53,;-3.73,-.91,;-1.33,-.76,;,-1.54,;2.67,-1.54,;4,-.77,;5.07,-1.39,;4.01,.77,;5.35,1.53,;5.36,3.07,;4.29,3.69,;6.69,3.84,;6.7,5.37,;8.04,6.14,;8.04,7.68,;9.38,8.44,;9.39,9.99,;10.45,10.6,;8.32,10.61,;8.03,3.06,;8.02,1.52,;6.94,.91,;9.35,.74,;9.34,-.8,;10.22,-1.32,;2.68,1.54,;1.61,.93,;2.69,2.78,;6.66,-5.4,;7.73,-6.01,;6.66,-3.86,;7.73,-3.24,;11.66,-15.17,;11.91,-16.38,;12.58,-14.35,)| Show InChI InChI=1S/C46H73N13O10/c1-8-26(5)37(43(66)55-33(21-29-22-50-24-52-29)44(67)59-19-11-13-34(59)41(64)58-38(45(68)69)27(6)9-2)57-40(63)32(20-28-14-16-30(60)17-15-28)54-42(65)36(25(3)4)56-39(62)31(53-35(61)23-49-7)12-10-18-51-46(47)48/h14-17,22,24-27,31-34,36-38,49,60H,8-13,18-21,23H2,1-7H3,(H,50,52)(H,53,61)(H,54,65)(H,55,66)(H,56,62)(H,57,63)(H,58,64)(H,68,69)(H4,47,48,51)/t26-,27-,31-,32-,33-,34-,36-,37-,38-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner Lambert Company
Curated by ChEMBL
| Assay Description
Binding affinity for rat brain Angiotensin II receptor |
J Med Chem 34: 3248-60 (1991)
BindingDB Entry DOI: 10.7270/Q24X5B06 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50030815

(CHEMBL404594)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)CNC)C(C)C)[C@@H](C)CC)C(O)=O |wU:8.7,25.27,wD:41.44,4.4,15.16,29.31,45.48,2.2,62.65,(8.44,-18.06,;9.36,-17.24,;9.05,-15.73,;7.88,-15.34,;10.19,-14.7,;9.88,-13.19,;11.03,-12.16,;12.2,-12.55,;10.71,-10.66,;11.74,-9.51,;10.97,-8.18,;9.46,-8.5,;9.33,-10.02,;7.99,-10.79,;7.99,-12.02,;6.66,-10.02,;5.32,-10.79,;5.32,-12.33,;4.07,-13.22,;4.55,-14.68,;6.09,-14.68,;6.56,-13.21,;6.66,-8.48,;5.33,-7.71,;4.43,-8.22,;5.33,-6.17,;3.99,-5.39,;4,-3.85,;5.06,-3.24,;2.66,-3.08,;1.33,-3.85,;-.01,-3.08,;-1.34,-3.84,;-2.67,-3.07,;-2.67,-1.53,;-3.73,-.91,;-1.33,-.76,;,-1.54,;2.67,-1.54,;4,-.77,;5.07,-1.39,;4.01,.77,;5.35,1.53,;5.36,3.07,;4.29,3.69,;6.69,3.84,;6.7,5.37,;8.04,6.14,;8.04,7.68,;9.38,8.44,;9.39,9.99,;10.45,10.6,;8.32,10.61,;8.03,3.06,;8.02,1.52,;6.94,.91,;9.35,.74,;9.34,-.8,;10.22,-1.32,;2.68,1.54,;1.61,.93,;2.69,2.78,;6.66,-5.4,;7.73,-6.01,;6.66,-3.86,;7.73,-3.24,;11.66,-15.17,;11.91,-16.38,;12.58,-14.35,)| Show InChI InChI=1S/C46H73N13O10/c1-8-26(5)37(43(66)55-33(21-29-22-50-24-52-29)44(67)59-19-11-13-34(59)41(64)58-38(45(68)69)27(6)9-2)57-40(63)32(20-28-14-16-30(60)17-15-28)54-42(65)36(25(3)4)56-39(62)31(53-35(61)23-49-7)12-10-18-51-46(47)48/h14-17,22,24-27,31-34,36-38,49,60H,8-13,18-21,23H2,1-7H3,(H,50,52)(H,53,61)(H,54,65)(H,55,66)(H,56,62)(H,57,63)(H,58,64)(H,68,69)(H4,47,48,51)/t26-,27-,31-,32-,33-,34-,36-,37-,38-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner Lambert Company
Curated by ChEMBL
| Assay Description
Binding affinity against Angiotensin II receptor, from rat adrenal gland |
J Med Chem 34: 3248-60 (1991)
BindingDB Entry DOI: 10.7270/Q24X5B06 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50228195

(Angiotensin Ii | CHEBI:2719)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CC(O)=O)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C50H71N13O12/c1-5-28(4)41(47(72)59-36(23-31-25-54-26-56-31)48(73)63-20-10-14-38(63)45(70)60-37(49(74)75)22-29-11-7-6-8-12-29)62-44(69)35(21-30-15-17-32(64)18-16-30)58-46(71)40(27(2)3)61-43(68)34(13-9-19-55-50(52)53)57-42(67)33(51)24-39(65)66/h6-8,11-12,15-18,25-28,33-38,40-41,64H,5,9-10,13-14,19-24,51H2,1-4H3,(H,54,56)(H,57,67)(H,58,71)(H,59,72)(H,60,70)(H,61,68)(H,62,69)(H,65,66)(H,74,75)(H4,52,53,55)/t28-,33-,34-,35-,36-,37-,38-,40-,41-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner Lambert Company
Curated by ChEMBL
| Assay Description
Binding affinity against Angiotensin II receptor, from rat adrenal gland |
J Med Chem 34: 3248-60 (1991)
BindingDB Entry DOI: 10.7270/Q24X5B06 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50228894

(CHEMBL312754)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)CNC)C(C)C)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(O)=O |wU:57.60,4.4,44.44,24.25,wD:20.21,8.8,61.64,2.2,(10.41,1.37,;9.34,.76,;9.34,-.78,;10.41,-1.4,;8.01,-1.55,;6.67,-.78,;5.34,-1.55,;5.33,-2.78,;4,-.77,;2.67,-1.54,;1.33,-.77,;1.33,.77,;,1.54,;-1.34,.77,;-2.41,1.39,;-1.34,-.77,;,-1.54,;4.01,.77,;5.35,1.53,;6.41,.91,;5.35,3.07,;6.69,3.84,;8.02,3.06,;8.02,1.83,;9.36,3.83,;10.7,3.06,;12.03,3.82,;13.37,3.05,;14.71,3.81,;16.04,3.04,;17.11,3.65,;16.03,1.81,;9.36,5.37,;8.03,6.14,;7.14,5.63,;8.03,7.68,;6.7,8.46,;6.7,9.48,;4.02,3.85,;2.95,3.24,;4.03,5.08,;8,-3.09,;6.94,-3.71,;9.34,-3.87,;9.33,-5.41,;10.67,-6.18,;12,-5.42,;13.4,-6.06,;14.43,-4.91,;13.66,-3.58,;12.15,-3.89,;8,-6.18,;7.11,-5.67,;8,-7.72,;9.25,-8.61,;8.77,-10.07,;7.23,-10.07,;6.76,-8.61,;5.3,-8.12,;5.05,-6.92,;4.14,-9.14,;2.68,-8.65,;2.44,-7.45,;1.52,-9.68,;1.73,-10.68,;.36,-9.29,)| Show InChI InChI=1S/C43H67N13O10/c1-7-24(4)35(40(63)53-31(19-27-20-47-22-49-27)41(64)56-17-9-11-32(56)38(61)50-25(5)42(65)66)55-37(60)30(18-26-12-14-28(57)15-13-26)52-39(62)34(23(2)3)54-36(59)29(51-33(58)21-46-6)10-8-16-48-43(44)45/h12-15,20,22-25,29-32,34-35,46,57H,7-11,16-19,21H2,1-6H3,(H,47,49)(H,50,61)(H,51,58)(H,52,62)(H,53,63)(H,54,59)(H,55,60)(H,65,66)(H4,44,45,48)/t24-,25-,29-,30-,31-,32-,34-,35-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner Lambert Company
Curated by ChEMBL
| Assay Description
Binding affinity against Angiotensin II receptor, from rat adrenal gland |
J Med Chem 34: 3248-60 (1991)
BindingDB Entry DOI: 10.7270/Q24X5B06 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50011204
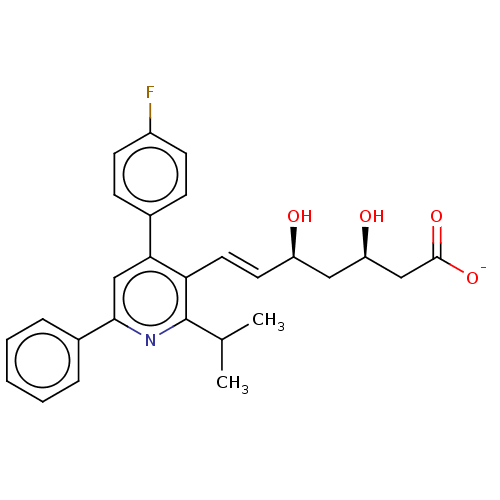
(CHEMBL2368094 | Sodium; 7-[4-(4-fluoro-phenyl)-2-i...)Show SMILES [Na+].CC(C)c1nc(cc(-c2ccc(F)cc2)c1\C=C\[C@@H](O)C[C@@H](O)CC([O-])=O)-c1ccccc1 Show InChI InChI=1S/C27H28FNO4.Na/c1-17(2)27-23(13-12-21(30)14-22(31)15-26(32)33)24(18-8-10-20(28)11-9-18)16-25(29-27)19-6-4-3-5-7-19;/h3-13,16-17,21-22,30-31H,14-15H2,1-2H3,(H,32,33);/q;+1/p-1/b13-12+;/t21-,22-;/m1./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Ability to inhibit microsomal preparation of HMG-CoA reductase in rat liver. |
J Med Chem 34: 463-6 (1991)
BindingDB Entry DOI: 10.7270/Q2MS3TC4 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50228195

(Angiotensin Ii | CHEBI:2719)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CC(O)=O)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C50H71N13O12/c1-5-28(4)41(47(72)59-36(23-31-25-54-26-56-31)48(73)63-20-10-14-38(63)45(70)60-37(49(74)75)22-29-11-7-6-8-12-29)62-44(69)35(21-30-15-17-32(64)18-16-30)58-46(71)40(27(2)3)61-43(68)34(13-9-19-55-50(52)53)57-42(67)33(51)24-39(65)66/h6-8,11-12,15-18,25-28,33-38,40-41,64H,5,9-10,13-14,19-24,51H2,1-4H3,(H,54,56)(H,57,67)(H,58,71)(H,59,72)(H,60,70)(H,61,68)(H,62,69)(H,65,66)(H,74,75)(H4,52,53,55)/t28-,33-,34-,35-,36-,37-,38-,40-,41-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner Lambert Company
Curated by ChEMBL
| Assay Description
Binding affinity for rat brain Angiotensin II receptor |
J Med Chem 34: 3248-60 (1991)
BindingDB Entry DOI: 10.7270/Q24X5B06 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50228894

(CHEMBL312754)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)CNC)C(C)C)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(O)=O |wU:57.60,4.4,44.44,24.25,wD:20.21,8.8,61.64,2.2,(10.41,1.37,;9.34,.76,;9.34,-.78,;10.41,-1.4,;8.01,-1.55,;6.67,-.78,;5.34,-1.55,;5.33,-2.78,;4,-.77,;2.67,-1.54,;1.33,-.77,;1.33,.77,;,1.54,;-1.34,.77,;-2.41,1.39,;-1.34,-.77,;,-1.54,;4.01,.77,;5.35,1.53,;6.41,.91,;5.35,3.07,;6.69,3.84,;8.02,3.06,;8.02,1.83,;9.36,3.83,;10.7,3.06,;12.03,3.82,;13.37,3.05,;14.71,3.81,;16.04,3.04,;17.11,3.65,;16.03,1.81,;9.36,5.37,;8.03,6.14,;7.14,5.63,;8.03,7.68,;6.7,8.46,;6.7,9.48,;4.02,3.85,;2.95,3.24,;4.03,5.08,;8,-3.09,;6.94,-3.71,;9.34,-3.87,;9.33,-5.41,;10.67,-6.18,;12,-5.42,;13.4,-6.06,;14.43,-4.91,;13.66,-3.58,;12.15,-3.89,;8,-6.18,;7.11,-5.67,;8,-7.72,;9.25,-8.61,;8.77,-10.07,;7.23,-10.07,;6.76,-8.61,;5.3,-8.12,;5.05,-6.92,;4.14,-9.14,;2.68,-8.65,;2.44,-7.45,;1.52,-9.68,;1.73,-10.68,;.36,-9.29,)| Show InChI InChI=1S/C43H67N13O10/c1-7-24(4)35(40(63)53-31(19-27-20-47-22-49-27)41(64)56-17-9-11-32(56)38(61)50-25(5)42(65)66)55-37(60)30(18-26-12-14-28(57)15-13-26)52-39(62)34(23(2)3)54-36(59)29(51-33(58)21-46-6)10-8-16-48-43(44)45/h12-15,20,22-25,29-32,34-35,46,57H,7-11,16-19,21H2,1-6H3,(H,47,49)(H,50,61)(H,51,58)(H,52,62)(H,53,63)(H,54,59)(H,55,60)(H,65,66)(H4,44,45,48)/t24-,25-,29-,30-,31-,32-,34-,35-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner Lambert Company
Curated by ChEMBL
| Assay Description
Binding affinity for rat brain Angiotensin II receptor |
J Med Chem 34: 3248-60 (1991)
BindingDB Entry DOI: 10.7270/Q24X5B06 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50006407
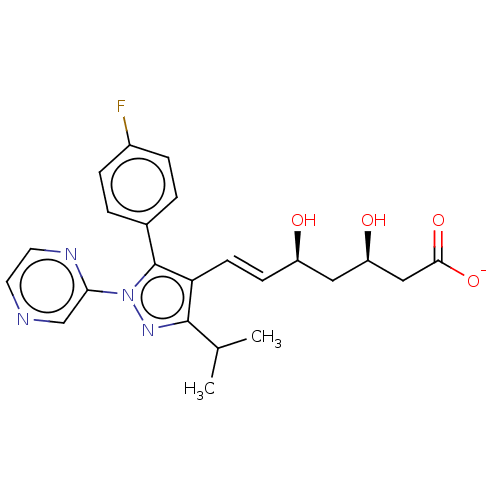
(CHEMBL2367472 | Sodium; 7-[5-(4-fluoro-phenyl)-3-i...)Show SMILES [Na+].CC(C)c1nn(c(c1\C=C\[C@@H](O)C[C@@H](O)CC([O-])=O)-c1ccc(F)cc1)-c1cnccn1 Show InChI InChI=1S/C23H25FN4O4.Na/c1-14(2)22-19(8-7-17(29)11-18(30)12-21(31)32)23(15-3-5-16(24)6-4-15)28(27-22)20-13-25-9-10-26-20;/h3-10,13-14,17-18,29-30H,11-12H2,1-2H3,(H,31,32);/q;+1/p-1/b8-7+;/t17-,18-;/m1./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Ability to inhibit microsomal preparation of HMG-CoA reductase in rat liver. |
J Med Chem 34: 463-6 (1991)
BindingDB Entry DOI: 10.7270/Q2MS3TC4 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50011032
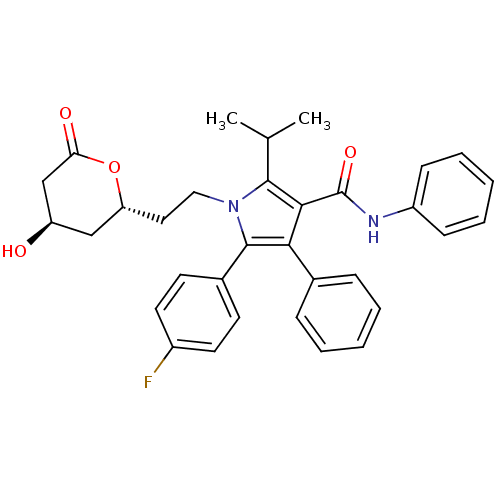
(5-(4-Fluoro-phenyl)-1-[2-(4-hydroxy-6-oxo-tetrahyd...)Show SMILES CC(C)c1c(C(=O)Nc2ccccc2)c(c(-c2ccc(F)cc2)n1CC[C@@H]1C[C@@H](O)CC(=O)O1)-c1ccccc1 Show InChI InChI=1S/C33H33FN2O4/c1-21(2)31-30(33(39)35-25-11-7-4-8-12-25)29(22-9-5-3-6-10-22)32(23-13-15-24(34)16-14-23)36(31)18-17-27-19-26(37)20-28(38)40-27/h3-16,21,26-27,37H,17-20H2,1-2H3,(H,35,39)/t26-,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase activity in partially purified rat liver |
J Med Chem 34: 357-66 (1991)
BindingDB Entry DOI: 10.7270/Q2NV9H6F |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50006409
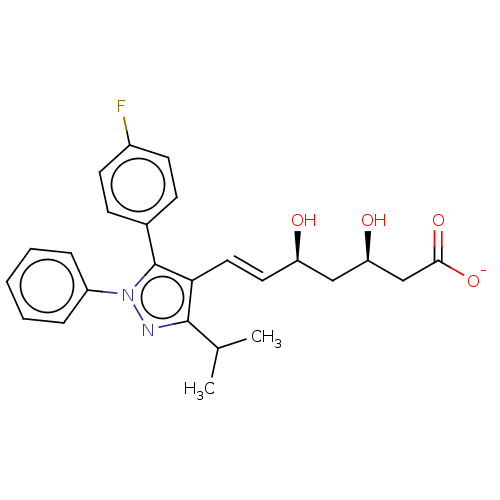
(CHEMBL2367478 | Sodium; 7-[5-(4-fluoro-phenyl)-3-i...)Show SMILES [Na+].CC(C)c1nn(c(c1\C=C\[C@@H](O)C[C@@H](O)CC([O-])=O)-c1ccc(F)cc1)-c1ccccc1 Show InChI InChI=1S/C25H27FN2O4.Na/c1-16(2)24-22(13-12-20(29)14-21(30)15-23(31)32)25(17-8-10-18(26)11-9-17)28(27-24)19-6-4-3-5-7-19;/h3-13,16,20-21,29-30H,14-15H2,1-2H3,(H,31,32);/q;+1/p-1/b13-12+;/t20-,21-;/m1./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Ability to inhibit microsomal preparation of HMG-CoA reductase in rat liver. |
J Med Chem 34: 463-6 (1991)
BindingDB Entry DOI: 10.7270/Q2MS3TC4 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50011213
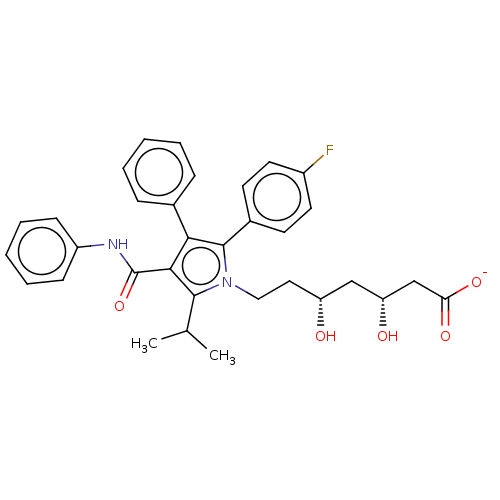
(CHEMBL3349878 | Sodium; 7-[2-(4-fluoro-phenyl)-5-i...)Show SMILES [Na+].CC(C)c1c(C(=O)Nc2ccccc2)c(c(-c2ccc(F)cc2)n1CC[C@@H](O)C[C@@H](O)CC([O-])=O)-c1ccccc1 Show InChI InChI=1S/C33H35FN2O5/c1-21(2)31-30(33(41)35-25-11-7-4-8-12-25)29(22-9-5-3-6-10-22)32(23-13-15-24(34)16-14-23)36(31)18-17-26(37)19-27(38)20-28(39)40/h3-16,21,26-27,37-38H,17-20H2,1-2H3,(H,35,41)(H,39,40)/p-1/t26?,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Ability to inhibit microsomal preparation of HMG-CoA reductase in rat liver. |
J Med Chem 34: 463-6 (1991)
BindingDB Entry DOI: 10.7270/Q2MS3TC4 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50011211

(CHEMBL2368089 | Sodium; 7-[4-(4-fluoro-phenyl)-2-i...)Show SMILES [Na+].CC(C)c1nc(C)cc(-c2ccc(F)cc2)c1\C=C\[C@@H](O)C[C@@H](O)CC([O-])=O Show InChI InChI=1S/C22H26FNO4.Na/c1-13(2)22-19(9-8-17(25)11-18(26)12-21(27)28)20(10-14(3)24-22)15-4-6-16(23)7-5-15;/h4-10,13,17-18,25-26H,11-12H2,1-3H3,(H,27,28);/q;+1/p-1/b9-8+;/t17-,18-;/m1./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Ability to inhibit microsomal preparation of HMG-CoA reductase in rat liver. |
J Med Chem 34: 463-6 (1991)
BindingDB Entry DOI: 10.7270/Q2MS3TC4 |
More data for this
Ligand-Target Pair | |
Acyl-CoA:cholesterol acyltransferase
(Oryctolagus cuniculus) | BDBM50054300
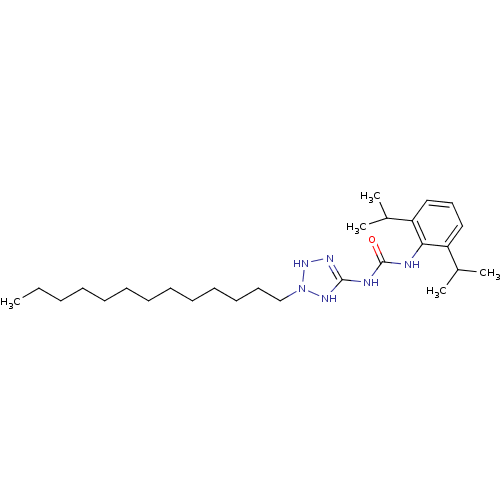
(1-(2,6-Diisopropyl-phenyl)-3-(2-tridecyl-2,3-dihyd...)Show SMILES CCCCCCCCCCCCCN1NN=C(NC(=O)Nc2c(cccc2C(C)C)C(C)C)N1 |t:15| Show InChI InChI=1S/C27H48N6O/c1-6-7-8-9-10-11-12-13-14-15-16-20-33-31-26(30-32-33)29-27(34)28-25-23(21(2)3)18-17-19-24(25)22(4)5/h17-19,21-22,32H,6-16,20H2,1-5H3,(H3,28,29,30,31,34) | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Acyl coenzyme A:cholesterol acyltransferase 1 inhibition in vitro measured in rabbit intestinal microsomes |
J Med Chem 39: 4382-95 (1996)
Article DOI: 10.1021/jm960404v
BindingDB Entry DOI: 10.7270/Q2571B3F |
More data for this
Ligand-Target Pair | |
Acyl-CoA:cholesterol acyltransferase
(Oryctolagus cuniculus) | BDBM50054305
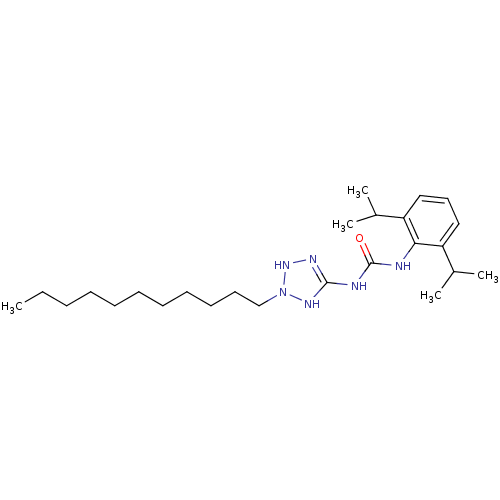
(1-(2,6-Diisopropyl-phenyl)-3-(2-undecyl-2,3-dihydr...)Show SMILES CCCCCCCCCCCN1NN=C(NC(=O)Nc2c(cccc2C(C)C)C(C)C)N1 |t:13| Show InChI InChI=1S/C25H44N6O/c1-6-7-8-9-10-11-12-13-14-18-31-29-24(28-30-31)27-25(32)26-23-21(19(2)3)16-15-17-22(23)20(4)5/h15-17,19-20,30H,6-14,18H2,1-5H3,(H3,26,27,28,29,32) | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Acyl coenzyme A:cholesterol acyltransferase 1 inhibition in vitro measured in rabbit intestinal microsomes |
J Med Chem 39: 4382-95 (1996)
Article DOI: 10.1021/jm960404v
BindingDB Entry DOI: 10.7270/Q2571B3F |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50011208
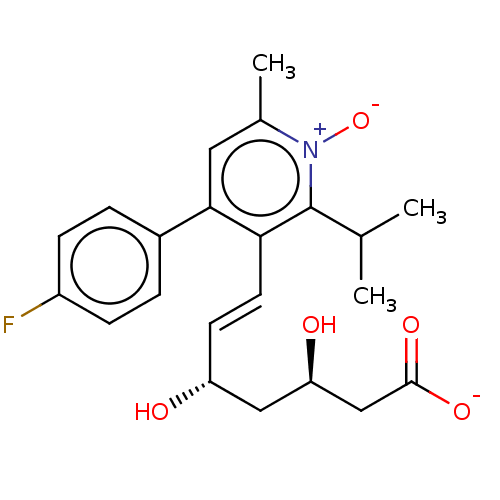
(7-[4-(4-Fluoro-phenyl)-2-isopropyl-6-methyl-1-oxy-...)Show SMILES [Na+].CC(C)c1c(\C=C\[C@@H](O)C[C@@H](O)CC([O-])=O)c(cc(C)[n+]1[O-])-c1ccc(F)cc1 Show InChI InChI=1S/C22H26FNO5.Na/c1-13(2)22-19(9-8-17(25)11-18(26)12-21(27)28)20(10-14(3)24(22)29)15-4-6-16(23)7-5-15;/h4-10,13,17-18,25-26H,11-12H2,1-3H3,(H,27,28);/q;+1/p-1/b9-8+;/t17-,18-;/m1./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Ability to inhibit microsomal preparation of HMG-CoA reductase in rat liver. |
J Med Chem 34: 463-6 (1991)
BindingDB Entry DOI: 10.7270/Q2MS3TC4 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50006410
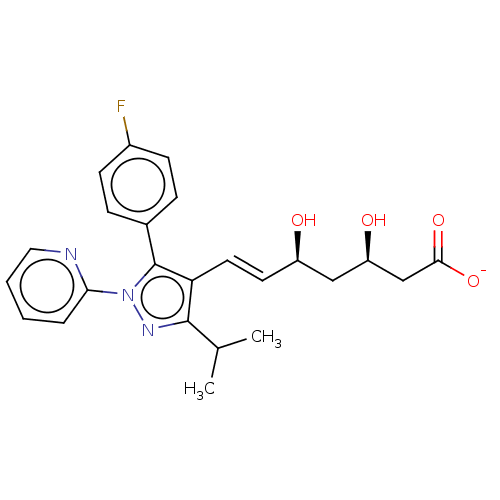
(CHEMBL2367477 | Sodium; 7-[5-(4-fluoro-phenyl)-3-i...)Show SMILES [Na+].CC(C)c1nn(c(c1\C=C\[C@@H](O)C[C@@H](O)CC([O-])=O)-c1ccc(F)cc1)-c1ccccn1 Show InChI InChI=1S/C24H26FN3O4.Na/c1-15(2)23-20(11-10-18(29)13-19(30)14-22(31)32)24(16-6-8-17(25)9-7-16)28(27-23)21-5-3-4-12-26-21;/h3-12,15,18-19,29-30H,13-14H2,1-2H3,(H,31,32);/q;+1/p-1/b11-10+;/t18-,19-;/m1./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Ability to inhibit microsomal preparation of HMG-CoA reductase in rat liver. |
J Med Chem 34: 463-6 (1991)
BindingDB Entry DOI: 10.7270/Q2MS3TC4 |
More data for this
Ligand-Target Pair | |
Acyl-CoA:cholesterol acyltransferase
(Oryctolagus cuniculus) | BDBM50054288
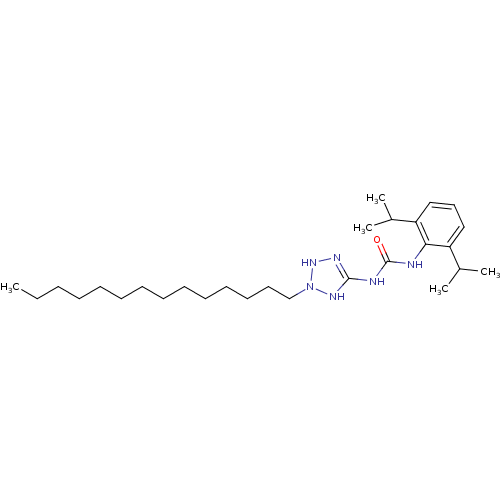
(1-(2,6-Diisopropyl-phenyl)-3-(2-tetradecyl-2,3-dih...)Show SMILES CCCCCCCCCCCCCCN1NN=C(NC(=O)Nc2c(cccc2C(C)C)C(C)C)N1 |t:16| Show InChI InChI=1S/C28H50N6O/c1-6-7-8-9-10-11-12-13-14-15-16-17-21-34-32-27(31-33-34)30-28(35)29-26-24(22(2)3)19-18-20-25(26)23(4)5/h18-20,22-23,33H,6-17,21H2,1-5H3,(H3,29,30,31,32,35) | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Acyl coenzyme A:cholesterol acyltransferase 1 inhibition in vitro measured in rabbit intestinal microsomes |
J Med Chem 39: 4382-95 (1996)
Article DOI: 10.1021/jm960404v
BindingDB Entry DOI: 10.7270/Q2571B3F |
More data for this
Ligand-Target Pair | |
Acyl-CoA:cholesterol acyltransferase
(Oryctolagus cuniculus) | BDBM50054279
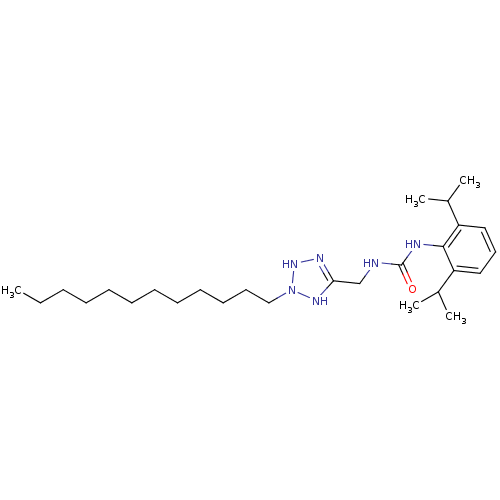
(1-(2,6-Diisopropyl-phenyl)-3-(2-dodecyl-2,3-dihydr...)Show SMILES CCCCCCCCCCCCN1NN=C(CNC(=O)Nc2c(cccc2C(C)C)C(C)C)N1 |t:14| Show InChI InChI=1S/C27H48N6O/c1-6-7-8-9-10-11-12-13-14-15-19-33-31-25(30-32-33)20-28-27(34)29-26-23(21(2)3)17-16-18-24(26)22(4)5/h16-18,21-22,32H,6-15,19-20H2,1-5H3,(H,30,31)(H2,28,29,34) | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Acyl coenzyme A:cholesterol acyltransferase 1 inhibition in vitro measured in rabbit intestinal microsomes |
J Med Chem 39: 4382-95 (1996)
Article DOI: 10.1021/jm960404v
BindingDB Entry DOI: 10.7270/Q2571B3F |
More data for this
Ligand-Target Pair | |
Acyl-CoA:cholesterol acyltransferase
(Oryctolagus cuniculus) | BDBM50054281
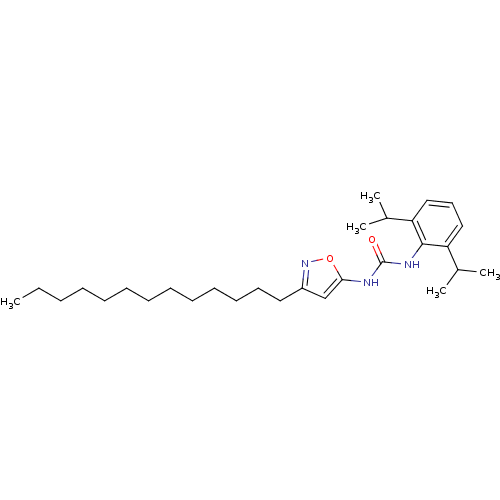
(1-(2,6-Diisopropyl-phenyl)-3-(3-tridecyl-isoxazol-...)Show SMILES CCCCCCCCCCCCCc1cc(NC(=O)Nc2c(cccc2C(C)C)C(C)C)on1 Show InChI InChI=1S/C29H47N3O2/c1-6-7-8-9-10-11-12-13-14-15-16-18-24-21-27(34-32-24)30-29(33)31-28-25(22(2)3)19-17-20-26(28)23(4)5/h17,19-23H,6-16,18H2,1-5H3,(H2,30,31,33) | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Acyl coenzyme A:cholesterol acyltransferase 1 inhibition in vitro measured in rabbit intestinal microsomes |
J Med Chem 39: 4382-95 (1996)
Article DOI: 10.1021/jm960404v
BindingDB Entry DOI: 10.7270/Q2571B3F |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50014345
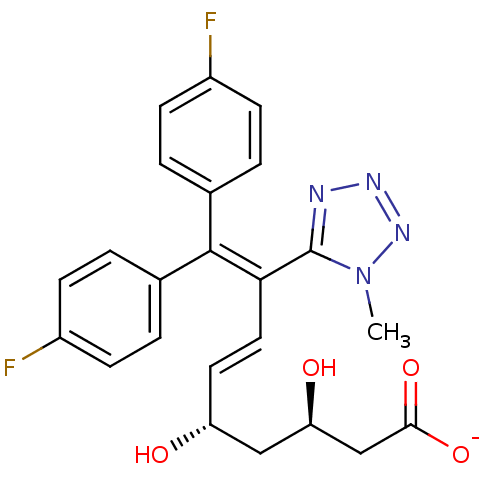
(CHEMBL11804 | Sodium; (E)-(3R,5S)-9,9-bis-(4-fluor...)Show SMILES [#6]-n1nnnc1\[#6](\[#6]=[#6]\[#6@@H](-[#8])-[#6]-[#6@@H](-[#8])-[#6]-[#6](-[#8-])=O)=[#6](\c1ccc(F)cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C23H22F2N4O4/c1-29-23(26-27-28-29)20(11-10-18(30)12-19(31)13-21(32)33)22(14-2-6-16(24)7-3-14)15-4-8-17(25)9-5-15/h2-11,18-19,30-31H,12-13H2,1H3,(H,32,33)/p-1/b11-10+/t18-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Ability to inhibit microsomal preparation of HMG-CoA reductase in rat liver. |
J Med Chem 34: 463-6 (1991)
BindingDB Entry DOI: 10.7270/Q2MS3TC4 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50011206
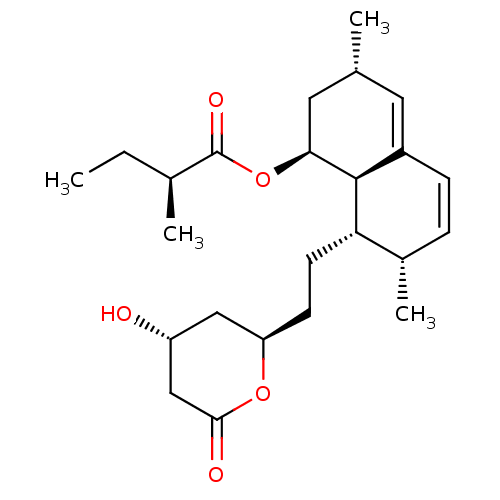
(2-Methyl-butyric acid 8-[2-(4-hydroxy-6-oxo-tetrah...)Show SMILES [H][C@]12[C@H](C[C@H](C)C=C1C=C[C@H](C)[C@@H]2CC[C@@H]1C[C@@H](O)CC(=O)O1)OC(=O)[C@@H](C)CC |c:6,9| Show InChI InChI=1S/C24H36O5/c1-5-15(3)24(27)29-21-11-14(2)10-17-7-6-16(4)20(23(17)21)9-8-19-12-18(25)13-22(26)28-19/h6-7,10,14-16,18-21,23,25H,5,8-9,11-13H2,1-4H3/t14-,15+,16+,18-,19?,20+,21+,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Ability to inhibit microsomal preparation of HMG-CoA reductase in rat liver. |
J Med Chem 34: 463-6 (1991)
BindingDB Entry DOI: 10.7270/Q2MS3TC4 |
More data for this
Ligand-Target Pair | |
Acyl-CoA:cholesterol acyltransferase
(Oryctolagus cuniculus) | BDBM50054302
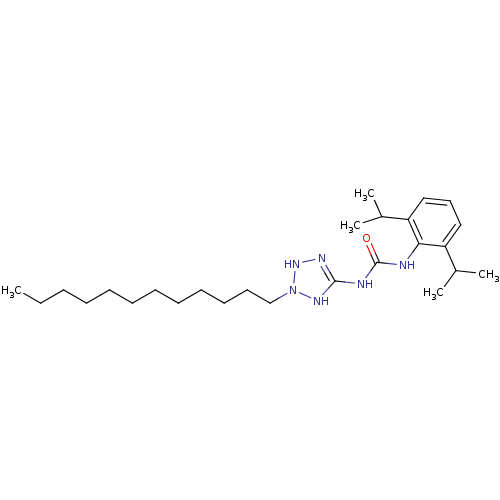
(1-(2,6-Diisopropyl-phenyl)-3-(2-dodecyl-2,3-dihydr...)Show SMILES CCCCCCCCCCCCN1NN=C(NC(=O)Nc2c(cccc2C(C)C)C(C)C)N1 |t:14| Show InChI InChI=1S/C26H46N6O/c1-6-7-8-9-10-11-12-13-14-15-19-32-30-25(29-31-32)28-26(33)27-24-22(20(2)3)17-16-18-23(24)21(4)5/h16-18,20-21,31H,6-15,19H2,1-5H3,(H3,27,28,29,30,33) | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Acyl coenzyme A:cholesterol acyltransferase 1 inhibition in vitro measured in rabbit intestinal microsomes |
J Med Chem 39: 4382-95 (1996)
Article DOI: 10.1021/jm960404v
BindingDB Entry DOI: 10.7270/Q2571B3F |
More data for this
Ligand-Target Pair | |
Acyl-CoA:cholesterol acyltransferase
(Oryctolagus cuniculus) | BDBM50054278
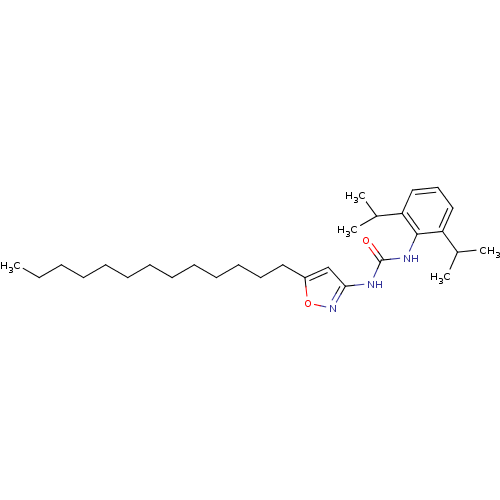
(1-(2,6-Diisopropyl-phenyl)-3-(5-tridecyl-isoxazol-...)Show SMILES CCCCCCCCCCCCCc1cc(NC(=O)Nc2c(cccc2C(C)C)C(C)C)no1 Show InChI InChI=1S/C29H47N3O2/c1-6-7-8-9-10-11-12-13-14-15-16-18-24-21-27(32-34-24)30-29(33)31-28-25(22(2)3)19-17-20-26(28)23(4)5/h17,19-23H,6-16,18H2,1-5H3,(H2,30,31,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Acyl coenzyme A:cholesterol acyltransferase 1 inhibition in vitro measured in rabbit intestinal microsomes |
J Med Chem 39: 4382-95 (1996)
Article DOI: 10.1021/jm960404v
BindingDB Entry DOI: 10.7270/Q2571B3F |
More data for this
Ligand-Target Pair | |
Acyl-CoA:cholesterol acyltransferase
(Oryctolagus cuniculus) | BDBM50054284

(1-(2-Decyl-2,3-dihydro-1H-tetrazol-5-yl)-3-(2,6-di...)Show SMILES CCCCCCCCCCN1NN=C(NC(=O)Nc2c(cccc2C(C)C)C(C)C)N1 |t:12| Show InChI InChI=1S/C24H42N6O/c1-6-7-8-9-10-11-12-13-17-30-28-23(27-29-30)26-24(31)25-22-20(18(2)3)15-14-16-21(22)19(4)5/h14-16,18-19,29H,6-13,17H2,1-5H3,(H3,25,26,27,28,31) | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Acyl coenzyme A:cholesterol acyltransferase 1 inhibition in vitro measured in rabbit intestinal microsomes |
J Med Chem 39: 4382-95 (1996)
Article DOI: 10.1021/jm960404v
BindingDB Entry DOI: 10.7270/Q2571B3F |
More data for this
Ligand-Target Pair | |
Acyl-CoA:cholesterol acyltransferase
(Oryctolagus cuniculus) | BDBM50054297
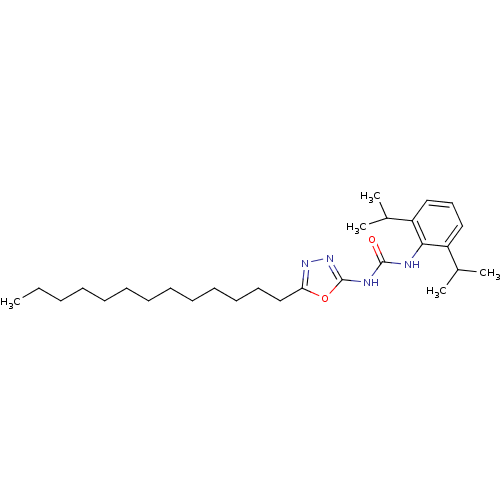
(1-(2,6-Diisopropyl-phenyl)-3-(5-tridecyl-[1,3,4]ox...)Show SMILES CCCCCCCCCCCCCc1nnc(NC(=O)Nc2c(cccc2C(C)C)C(C)C)o1 Show InChI InChI=1S/C28H46N4O2/c1-6-7-8-9-10-11-12-13-14-15-16-20-25-31-32-28(34-25)30-27(33)29-26-23(21(2)3)18-17-19-24(26)22(4)5/h17-19,21-22H,6-16,20H2,1-5H3,(H2,29,30,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Acyl coenzyme A:cholesterol acyltransferase 1 inhibition in vitro measured in rabbit intestinal microsomes |
J Med Chem 39: 4382-95 (1996)
Article DOI: 10.1021/jm960404v
BindingDB Entry DOI: 10.7270/Q2571B3F |
More data for this
Ligand-Target Pair | |
Acyl-CoA:cholesterol acyltransferase
(Oryctolagus cuniculus) | BDBM50054294

(1-(2,6-Diisopropyl-phenyl)-3-(3-dodecyl-isoxazol-5...)Show SMILES CCCCCCCCCCCCc1cc(NC(=O)Nc2c(cccc2C(C)C)C(C)C)on1 Show InChI InChI=1S/C28H45N3O2/c1-6-7-8-9-10-11-12-13-14-15-17-23-20-26(33-31-23)29-28(32)30-27-24(21(2)3)18-16-19-25(27)22(4)5/h16,18-22H,6-15,17H2,1-5H3,(H2,29,30,32) | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Acyl coenzyme A:cholesterol acyltransferase 1 inhibition in vitro measured in rabbit intestinal microsomes |
J Med Chem 39: 4382-95 (1996)
Article DOI: 10.1021/jm960404v
BindingDB Entry DOI: 10.7270/Q2571B3F |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50011032

(5-(4-Fluoro-phenyl)-1-[2-(4-hydroxy-6-oxo-tetrahyd...)Show SMILES CC(C)c1c(C(=O)Nc2ccccc2)c(c(-c2ccc(F)cc2)n1CC[C@@H]1C[C@@H](O)CC(=O)O1)-c1ccccc1 Show InChI InChI=1S/C33H33FN2O4/c1-21(2)31-30(33(39)35-25-11-7-4-8-12-25)29(22-9-5-3-6-10-22)32(23-13-15-24(34)16-14-23)36(31)18-17-27-19-26(37)20-28(38)40-27/h3-16,21,26-27,37H,17-20H2,1-2H3,(H,35,39)/t26-,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase activity in partially purified rat liver |
J Med Chem 34: 357-66 (1991)
BindingDB Entry DOI: 10.7270/Q2NV9H6F |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50011032

(5-(4-Fluoro-phenyl)-1-[2-(4-hydroxy-6-oxo-tetrahyd...)Show SMILES CC(C)c1c(C(=O)Nc2ccccc2)c(c(-c2ccc(F)cc2)n1CC[C@@H]1C[C@@H](O)CC(=O)O1)-c1ccccc1 Show InChI InChI=1S/C33H33FN2O4/c1-21(2)31-30(33(39)35-25-11-7-4-8-12-25)29(22-9-5-3-6-10-22)32(23-13-15-24(34)16-14-23)36(31)18-17-27-19-26(37)20-28(38)40-27/h3-16,21,26-27,37H,17-20H2,1-2H3,(H,35,39)/t26-,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase activity in partially purified rat liver |
J Med Chem 34: 357-66 (1991)
BindingDB Entry DOI: 10.7270/Q2NV9H6F |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50011203
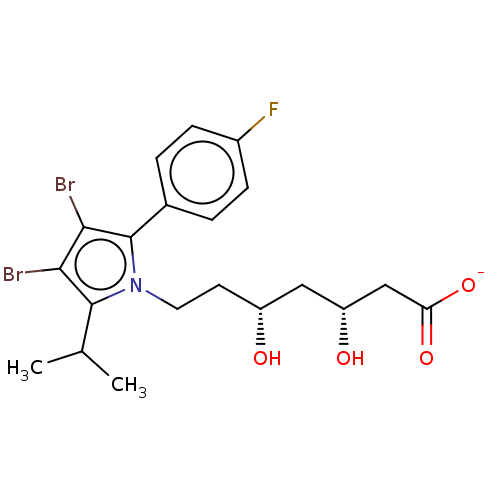
(CHEMBL3349880 | Sodium; 7-[3,4-dibromo-2-(4-fluoro...)Show SMILES [Na+].CC(C)c1c(Br)c(Br)c(-c2ccc(F)cc2)n1CC[C@@H](O)C[C@@H](O)CC([O-])=O Show InChI InChI=1S/C20H24Br2FNO4/c1-11(2)19-17(21)18(22)20(12-3-5-13(23)6-4-12)24(19)8-7-14(25)9-15(26)10-16(27)28/h3-6,11,14-15,25-26H,7-10H2,1-2H3,(H,27,28)/p-1/t14?,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Ability to inhibit microsomal preparation of HMG-CoA reductase in rat liver. |
J Med Chem 34: 463-6 (1991)
BindingDB Entry DOI: 10.7270/Q2MS3TC4 |
More data for this
Ligand-Target Pair | |
Acyl-CoA:cholesterol acyltransferase
(Oryctolagus cuniculus) | BDBM50054304
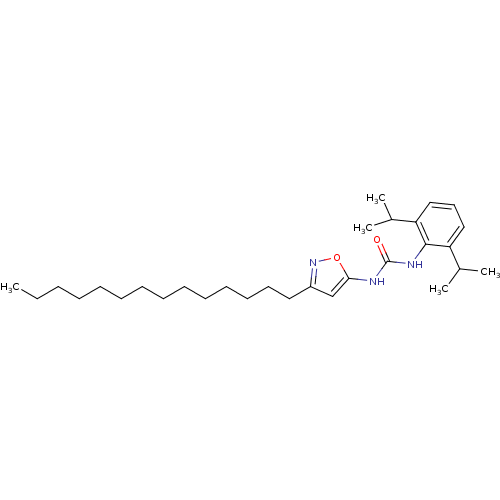
(1-(2,6-Diisopropyl-phenyl)-3-(3-tetradecyl-isoxazo...)Show SMILES CCCCCCCCCCCCCCc1cc(NC(=O)Nc2c(cccc2C(C)C)C(C)C)on1 Show InChI InChI=1S/C30H49N3O2/c1-6-7-8-9-10-11-12-13-14-15-16-17-19-25-22-28(35-33-25)31-30(34)32-29-26(23(2)3)20-18-21-27(29)24(4)5/h18,20-24H,6-17,19H2,1-5H3,(H2,31,32,34) | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Acyl coenzyme A:cholesterol acyltransferase 1 inhibition in vitro measured in rabbit intestinal microsomes |
J Med Chem 39: 4382-95 (1996)
Article DOI: 10.1021/jm960404v
BindingDB Entry DOI: 10.7270/Q2571B3F |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50011020
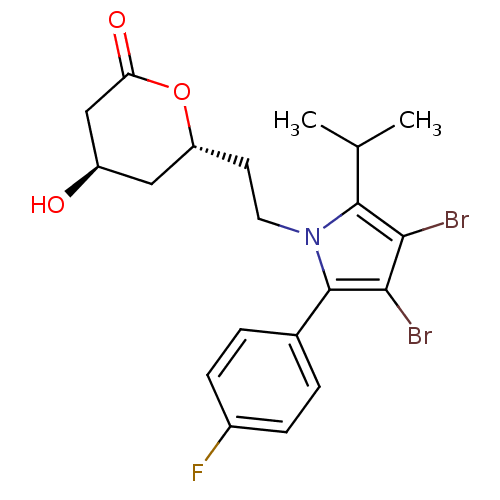
(6-{2-[3,4-Dibromo-2-(4-fluoro-phenyl)-5-isopropyl-...)Show SMILES CC(C)c1c(Br)c(Br)c(-c2ccc(F)cc2)n1CC[C@@H]1C[C@@H](O)CC(=O)O1 Show InChI InChI=1S/C20H22Br2FNO3/c1-11(2)19-17(21)18(22)20(12-3-5-13(23)6-4-12)24(19)8-7-15-9-14(25)10-16(26)27-15/h3-6,11,14-15,25H,7-10H2,1-2H3/t14-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase activity in partially purified rat liver |
J Med Chem 34: 357-66 (1991)
BindingDB Entry DOI: 10.7270/Q2NV9H6F |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50011018
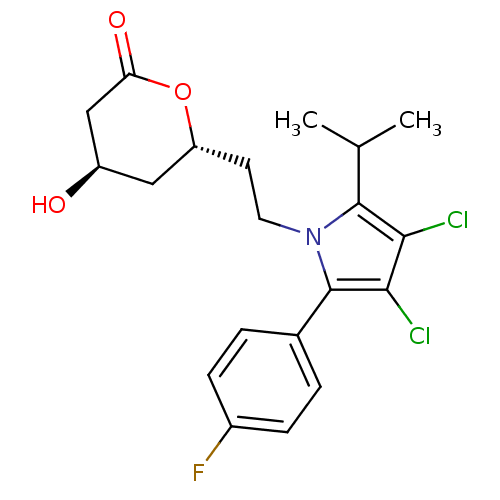
(6-{2-[3,4-Dichloro-2-(4-fluoro-phenyl)-5-isopropyl...)Show SMILES CC(C)c1c(Cl)c(Cl)c(-c2ccc(F)cc2)n1CC[C@@H]1C[C@@H](O)CC(=O)O1 Show InChI InChI=1S/C20H22Cl2FNO3/c1-11(2)19-17(21)18(22)20(12-3-5-13(23)6-4-12)24(19)8-7-15-9-14(25)10-16(26)27-15/h3-6,11,14-15,25H,7-10H2,1-2H3/t14-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase activity in partially purified rat liver |
J Med Chem 34: 357-66 (1991)
BindingDB Entry DOI: 10.7270/Q2NV9H6F |
More data for this
Ligand-Target Pair | |
Acyl-CoA:cholesterol acyltransferase
(Oryctolagus cuniculus) | BDBM50054277
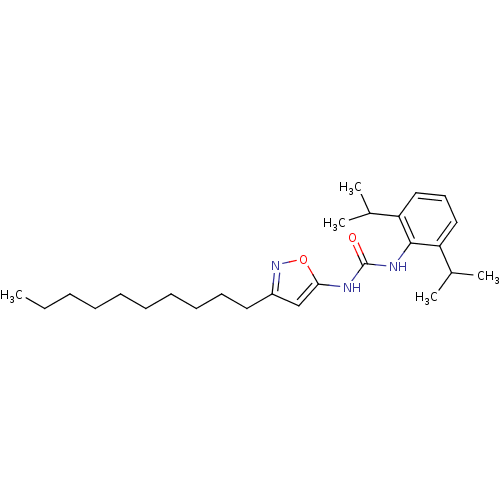
(1-(3-Decyl-isoxazol-5-yl)-3-(2,6-diisopropyl-pheny...)Show SMILES CCCCCCCCCCc1cc(NC(=O)Nc2c(cccc2C(C)C)C(C)C)on1 Show InChI InChI=1S/C26H41N3O2/c1-6-7-8-9-10-11-12-13-15-21-18-24(31-29-21)27-26(30)28-25-22(19(2)3)16-14-17-23(25)20(4)5/h14,16-20H,6-13,15H2,1-5H3,(H2,27,28,30) | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Acyl coenzyme A:cholesterol acyltransferase 1 inhibition in vitro measured in rabbit intestinal microsomes |
J Med Chem 39: 4382-95 (1996)
Article DOI: 10.1021/jm960404v
BindingDB Entry DOI: 10.7270/Q2571B3F |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50011036
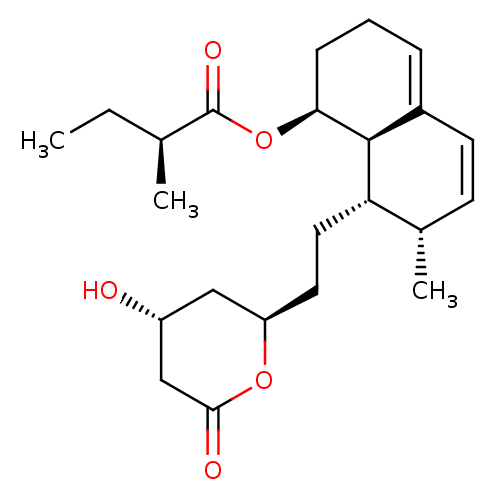
((S)-((1S,7S,8S,8aR)-8-(2-((2R,4R)-4-hydroxy-6-oxo-...)Show SMILES CC[C@H](C)C(=O)O[C@H]1CCC=C2C=C[C@H](C)[C@H](CC[C@@H]3C[C@@H](O)CC(=O)O3)[C@@H]12 |r,c:12,t:10| Show InChI InChI=1S/C23H34O5/c1-4-14(2)23(26)28-20-7-5-6-16-9-8-15(3)19(22(16)20)11-10-18-12-17(24)13-21(25)27-18/h6,8-9,14-15,17-20,22,24H,4-5,7,10-13H2,1-3H3/t14-,15-,17+,18+,19-,20-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase activity in partially purified rat liver |
J Med Chem 34: 357-66 (1991)
BindingDB Entry DOI: 10.7270/Q2NV9H6F |
More data for this
Ligand-Target Pair | |
Acyl-CoA:cholesterol acyltransferase
(Oryctolagus cuniculus) | BDBM50054312
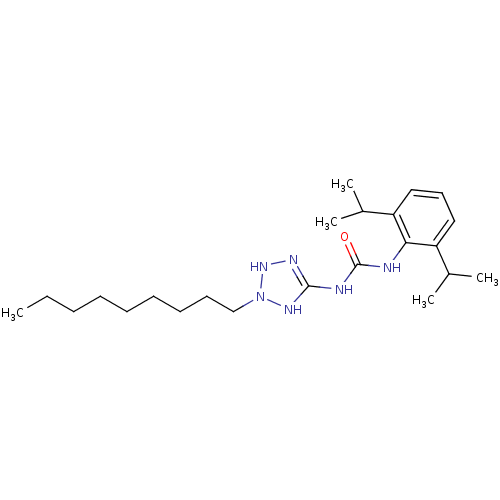
(1-(2,6-Diisopropyl-phenyl)-3-(2-nonyl-2,3-dihydro-...)Show SMILES CCCCCCCCCN1NN=C(NC(=O)Nc2c(cccc2C(C)C)C(C)C)N1 |t:11| Show InChI InChI=1S/C23H40N6O/c1-6-7-8-9-10-11-12-16-29-27-22(26-28-29)25-23(30)24-21-19(17(2)3)14-13-15-20(21)18(4)5/h13-15,17-18,28H,6-12,16H2,1-5H3,(H3,24,25,26,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Acyl coenzyme A:cholesterol acyltransferase 1 inhibition in vitro measured in rabbit intestinal microsomes |
J Med Chem 39: 4382-95 (1996)
Article DOI: 10.1021/jm960404v
BindingDB Entry DOI: 10.7270/Q2571B3F |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50368147
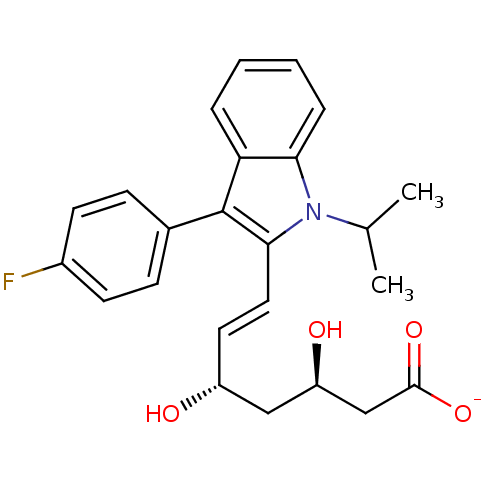
((+)-(3R,5S)-fluvastatin | (3R,5S)-fluvastatin | (3...)Show SMILES CC(C)n1c(\C=C\[C@@H](O)C[C@@H](O)CC([O-])=O)c(-c2ccc(F)cc2)c2ccccc12 |r| Show InChI InChI=1S/C24H26FNO4/c1-15(2)26-21-6-4-3-5-20(21)24(16-7-9-17(25)10-8-16)22(26)12-11-18(27)13-19(28)14-23(29)30/h3-12,15,18-19,27-28H,13-14H2,1-2H3,(H,29,30)/p-1/b12-11+/t18-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Ability to inhibit microsomal preparation of HMG-CoA reductase in rat liver. |
J Med Chem 34: 463-6 (1991)
BindingDB Entry DOI: 10.7270/Q2MS3TC4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acyl-CoA:cholesterol acyltransferase
(Oryctolagus cuniculus) | BDBM50054308
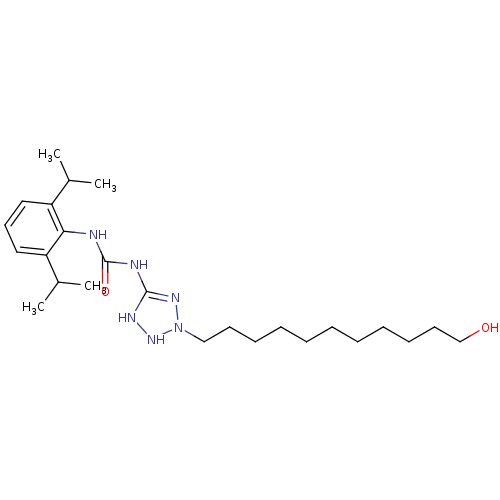
(1-(2,6-Diisopropyl-phenyl)-3-[2-(11-hydroxy-undecy...)Show SMILES CC(C)c1cccc(C(C)C)c1NC(=O)NC1=NN(CCCCCCCCCCCO)NN1 |t:17| Show InChI InChI=1S/C25H44N6O2/c1-19(2)21-15-14-16-22(20(3)4)23(21)26-25(33)27-24-28-30-31(29-24)17-12-10-8-6-5-7-9-11-13-18-32/h14-16,19-20,30,32H,5-13,17-18H2,1-4H3,(H3,26,27,28,29,33) | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Acyl coenzyme A:cholesterol acyltransferase 1 inhibition in vitro measured in rabbit intestinal microsomes |
J Med Chem 39: 4382-95 (1996)
Article DOI: 10.1021/jm960404v
BindingDB Entry DOI: 10.7270/Q2571B3F |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50282396
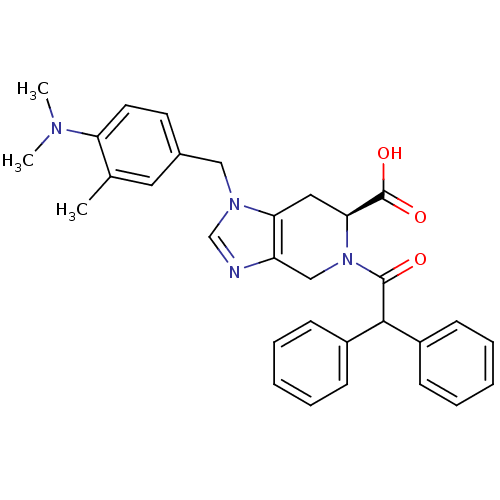
((S)-1-(4-Dimethylamino-3-methyl-benzyl)-5-diphenyl...)Show SMILES CN(C)c1ccc(Cn2cnc3CN([C@@H](Cc23)C(O)=O)C(=O)C(c2ccccc2)c2ccccc2)cc1C Show InChI InChI=1S/C31H32N4O3/c1-21-16-22(14-15-26(21)33(2)3)18-34-20-32-25-19-35(28(31(37)38)17-27(25)34)30(36)29(23-10-6-4-7-11-23)24-12-8-5-9-13-24/h4-16,20,28-29H,17-19H2,1-3H3,(H,37,38)/t28-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner Lambert Company
Curated by ChEMBL
| Assay Description
Binding affinity against Angiotensin II receptor, from rat adrenal gland |
J Med Chem 34: 3248-60 (1991)
BindingDB Entry DOI: 10.7270/Q24X5B06 |
More data for this
Ligand-Target Pair | |
Acyl-CoA:cholesterol acyltransferase
(Oryctolagus cuniculus) | BDBM50054289
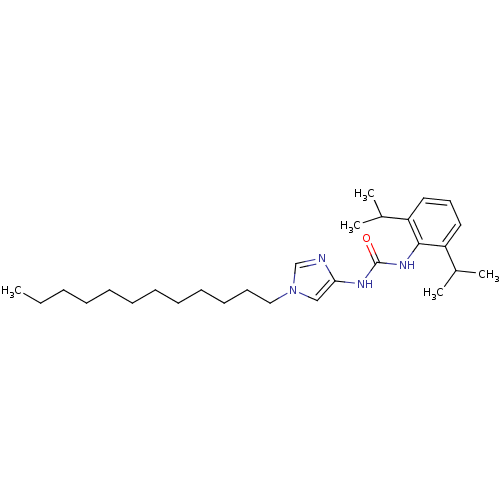
(1-(2,6-Diisopropyl-phenyl)-3-(1-dodecyl-1H-imidazo...)Show SMILES CCCCCCCCCCCCn1cnc(NC(=O)Nc2c(cccc2C(C)C)C(C)C)c1 Show InChI InChI=1S/C28H46N4O/c1-6-7-8-9-10-11-12-13-14-15-19-32-20-26(29-21-32)30-28(33)31-27-24(22(2)3)17-16-18-25(27)23(4)5/h16-18,20-23H,6-15,19H2,1-5H3,(H2,30,31,33) | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Acyl coenzyme A:cholesterol acyltransferase 1 inhibition in vitro measured in rabbit intestinal microsomes |
J Med Chem 39: 4382-95 (1996)
Article DOI: 10.1021/jm960404v
BindingDB Entry DOI: 10.7270/Q2571B3F |
More data for this
Ligand-Target Pair | |
Acyl-CoA:cholesterol acyltransferase
(Oryctolagus cuniculus) | BDBM50054298
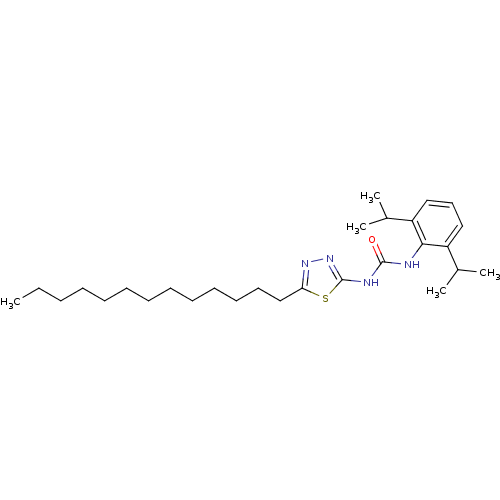
(1-(2,6-Diisopropyl-phenyl)-3-(5-tridecyl-[1,3,4]th...)Show SMILES CCCCCCCCCCCCCc1nnc(NC(=O)Nc2c(cccc2C(C)C)C(C)C)s1 Show InChI InChI=1S/C28H46N4OS/c1-6-7-8-9-10-11-12-13-14-15-16-20-25-31-32-28(34-25)30-27(33)29-26-23(21(2)3)18-17-19-24(26)22(4)5/h17-19,21-22H,6-16,20H2,1-5H3,(H2,29,30,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Acyl coenzyme A:cholesterol acyltransferase 1 inhibition in vitro measured in rabbit intestinal microsomes |
J Med Chem 39: 4382-95 (1996)
Article DOI: 10.1021/jm960404v
BindingDB Entry DOI: 10.7270/Q2571B3F |
More data for this
Ligand-Target Pair | |
Acyl-CoA:cholesterol acyltransferase
(Oryctolagus cuniculus) | BDBM50054313
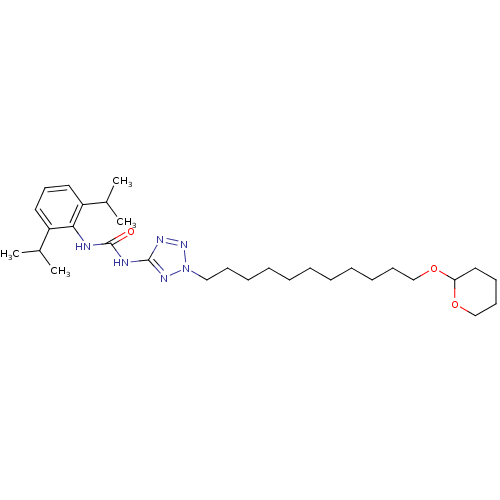
(1-(2,6-Diisopropyl-phenyl)-3-{2-[11-(tetrahydro-py...)Show SMILES CC(C)c1cccc(C(C)C)c1NC(=O)Nc1nnn(CCCCCCCCCCCOC2CCCCO2)n1 Show InChI InChI=1S/C30H50N6O3/c1-23(2)25-17-16-18-26(24(3)4)28(25)31-30(37)32-29-33-35-36(34-29)20-13-10-8-6-5-7-9-11-14-21-38-27-19-12-15-22-39-27/h16-18,23-24,27H,5-15,19-22H2,1-4H3,(H2,31,32,34,37) | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Acyl coenzyme A:cholesterol acyltransferase 1 inhibition in vitro measured in rabbit intestinal microsomes |
J Med Chem 39: 4382-95 (1996)
Article DOI: 10.1021/jm960404v
BindingDB Entry DOI: 10.7270/Q2571B3F |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50368146
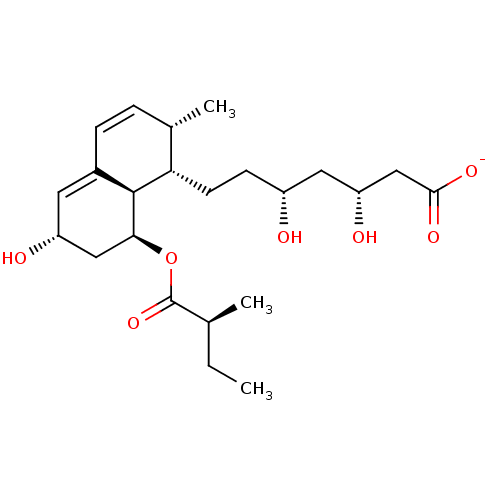
(CS-514 | Eptastatin Sodium | PRAVASTATIN SODIUM | ...)Show SMILES CC[C@H](C)C(=O)O[C@H]1C[C@H](O)C=C2C=C[C@H](C)[C@H](CC[C@@H](O)C[C@@H](O)CC([O-])=O)[C@@H]12 |r,c:13,t:11| Show InChI InChI=1S/C23H36O7/c1-4-13(2)23(29)30-20-11-17(25)9-15-6-5-14(3)19(22(15)20)8-7-16(24)10-18(26)12-21(27)28/h5-6,9,13-14,16-20,22,24-26H,4,7-8,10-12H2,1-3H3,(H,27,28)/p-1/t13-,14-,16+,17+,18+,19-,20-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Ability to inhibit microsomal preparation of HMG-CoA reductase in rat liver. |
J Med Chem 34: 463-6 (1991)
BindingDB Entry DOI: 10.7270/Q2MS3TC4 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50011035

(5-(4-Fluoro-phenyl)-1-[2-(4-hydroxy-6-oxo-tetrahyd...)Show SMILES CC(C)c1c(C(=O)OCc2ccccc2)c(c(-c2ccc(F)cc2)n1CC[C@@H]1C[C@@H](O)CC(=O)O1)-c1ccccc1 Show InChI InChI=1S/C34H34FNO5/c1-22(2)32-31(34(39)40-21-23-9-5-3-6-10-23)30(24-11-7-4-8-12-24)33(25-13-15-26(35)16-14-25)36(32)18-17-28-19-27(37)20-29(38)41-28/h3-16,22,27-28,37H,17-21H2,1-2H3/t27-,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase activity in partially purified rat liver |
J Med Chem 34: 357-66 (1991)
BindingDB Entry DOI: 10.7270/Q2NV9H6F |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50228885
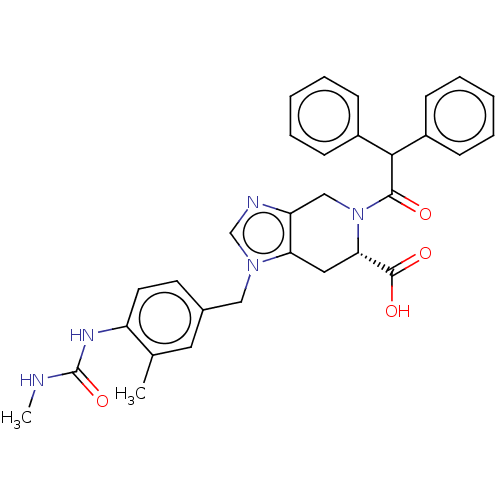
(CHEMBL109832)Show SMILES CNC(=O)Nc1ccc(Cn2cnc3CN([C@@H](Cc23)C(O)=O)C(=O)C(c2ccccc2)c2ccccc2)cc1C Show InChI InChI=1S/C31H31N5O4/c1-20-15-21(13-14-24(20)34-31(40)32-2)17-35-19-33-25-18-36(27(30(38)39)16-26(25)35)29(37)28(22-9-5-3-6-10-22)23-11-7-4-8-12-23/h3-15,19,27-28H,16-18H2,1-2H3,(H,38,39)(H2,32,34,40)/t27-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner Lambert Company
Curated by ChEMBL
| Assay Description
Binding affinity against Angiotensin II receptor, from rat adrenal gland |
J Med Chem 34: 3248-60 (1991)
BindingDB Entry DOI: 10.7270/Q24X5B06 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50011025
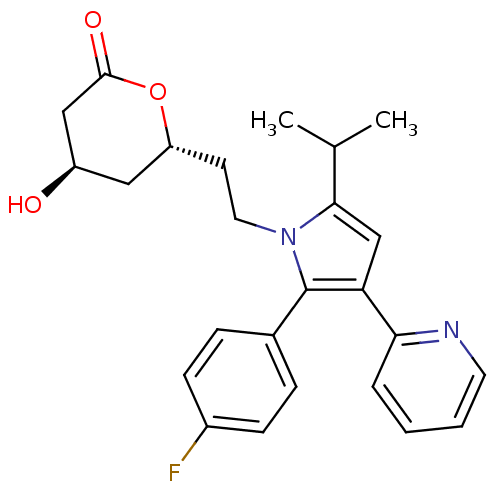
(6-{2-[2-(4-Fluoro-phenyl)-5-isopropyl-3-pyridin-2-...)Show SMILES CC(C)c1cc(c(-c2ccc(F)cc2)n1CC[C@@H]1C[C@@H](O)CC(=O)O1)-c1ccccn1 Show InChI InChI=1S/C25H27FN2O3/c1-16(2)23-15-21(22-5-3-4-11-27-22)25(17-6-8-18(26)9-7-17)28(23)12-10-20-13-19(29)14-24(30)31-20/h3-9,11,15-16,19-20,29H,10,12-14H2,1-2H3/t19-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase activity in partially purified rat liver |
J Med Chem 34: 357-66 (1991)
BindingDB Entry DOI: 10.7270/Q2NV9H6F |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data