 Found 49 hits with Last Name = 'chung' and Initial = 'by'
Found 49 hits with Last Name = 'chung' and Initial = 'by' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Voltage-dependent T-type calcium channel subunit alpha-1G
(Homo sapiens (Human)) | BDBM50148172
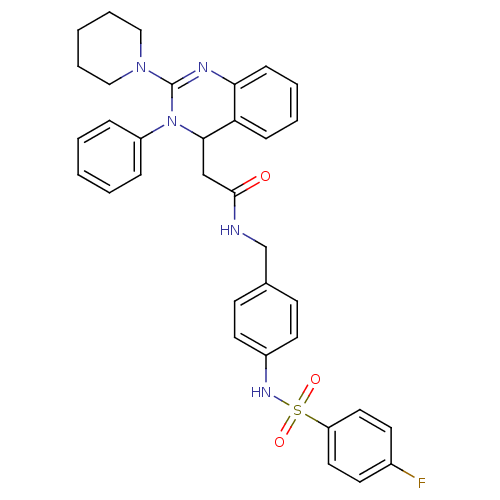
(CHEMBL251949 | KYS-05042 | N-(4-(4-fluorophenylsul...)Show SMILES Fc1ccc(cc1)S(=O)(=O)Nc1ccc(CNC(=O)CC2N(C(=Nc3ccccc23)N2CCCCC2)c2ccccc2)cc1 |c:23| Show InChI InChI=1S/C34H34FN5O3S/c35-26-15-19-29(20-16-26)44(42,43)38-27-17-13-25(14-18-27)24-36-33(41)23-32-30-11-5-6-12-31(30)37-34(39-21-7-2-8-22-39)40(32)28-9-3-1-4-10-28/h1,3-6,9-20,32,38H,2,7-8,21-24H2,(H,36,41) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science& Technology
Curated by ChEMBL
| Assay Description
Inhibition of T-type [Ca2+] channel (alpha1G) expressed in HEK293 cells |
Bioorg Med Chem Lett 15: 283-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.078
BindingDB Entry DOI: 10.7270/Q2KS6SB3 |
More data for this
Ligand-Target Pair | |
Voltage-dependent T-type calcium channel subunit alpha-1G
(Homo sapiens (Human)) | BDBM50157857
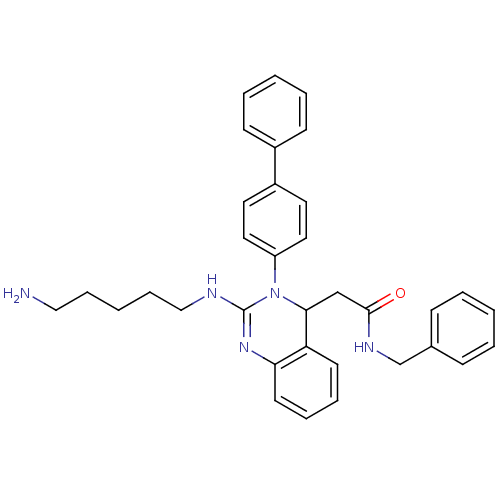
(2-(2-(5-aminopentylamino)-3-(biphenyl-4-yl)-3,4-di...)Show SMILES NCCCCCNC1=Nc2ccccc2C(CC(=O)NCc2ccccc2)N1c1ccc(cc1)-c1ccccc1 |t:7| Show InChI InChI=1S/C34H37N5O/c35-22-10-3-11-23-36-34-38-31-17-9-8-16-30(31)32(24-33(40)37-25-26-12-4-1-5-13-26)39(34)29-20-18-28(19-21-29)27-14-6-2-7-15-27/h1-2,4-9,12-21,32H,3,10-11,22-25,35H2,(H,36,38)(H,37,40) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science& Technology
Curated by ChEMBL
| Assay Description
Inhibition of T-type [Ca2+] channel (alpha1G) expressed in HEK293 cells |
Bioorg Med Chem Lett 15: 283-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.078
BindingDB Entry DOI: 10.7270/Q2KS6SB3 |
More data for this
Ligand-Target Pair | |
Voltage-dependent T-type calcium channel subunit alpha-1G
(Homo sapiens (Human)) | BDBM50157862
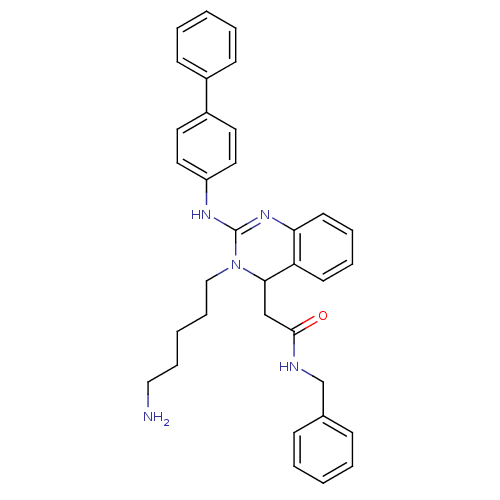
(2-(3-(5-aminopentyl)-2-(biphenyl-4-ylamino)-3,4-di...)Show SMILES NCCCCCN1C(CC(=O)NCc2ccccc2)c2ccccc2N=C1Nc1ccc(cc1)-c1ccccc1 |c:27| Show InChI InChI=1S/C34H37N5O/c35-22-10-3-11-23-39-32(24-33(40)36-25-26-12-4-1-5-13-26)30-16-8-9-17-31(30)38-34(39)37-29-20-18-28(19-21-29)27-14-6-2-7-15-27/h1-2,4-9,12-21,32H,3,10-11,22-25,35H2,(H,36,40)(H,37,38) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science& Technology
Curated by ChEMBL
| Assay Description
Inhibition of T-type [Ca2+] channel (alpha1G) expressed in HEK293 cells |
Bioorg Med Chem Lett 15: 283-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.078
BindingDB Entry DOI: 10.7270/Q2KS6SB3 |
More data for this
Ligand-Target Pair | |
Voltage-dependent T-type calcium channel subunit alpha-1G
(Homo sapiens (Human)) | BDBM50157864
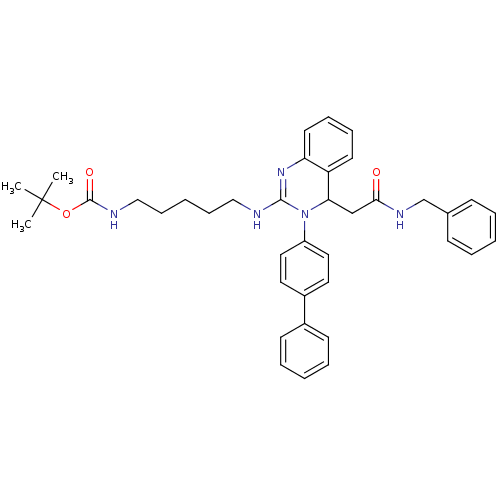
(CHEMBL51435 | tert-butyl 5-(4-(2-(benzylamino)-2-o...)Show SMILES CC(C)(C)OC(=O)NCCCCCNC1=Nc2ccccc2C(CC(=O)NCc2ccccc2)N1c1ccc(cc1)-c1ccccc1 |t:14| Show InChI InChI=1S/C39H45N5O3/c1-39(2,3)47-38(46)41-26-14-6-13-25-40-37-43-34-20-12-11-19-33(34)35(27-36(45)42-28-29-15-7-4-8-16-29)44(37)32-23-21-31(22-24-32)30-17-9-5-10-18-30/h4-5,7-12,15-24,35H,6,13-14,25-28H2,1-3H3,(H,40,43)(H,41,46)(H,42,45) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science& Technology
Curated by ChEMBL
| Assay Description
Inhibition of T-type [Ca2+] channel (alpha1G) expressed in HEK293 cells |
Bioorg Med Chem Lett 15: 283-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.078
BindingDB Entry DOI: 10.7270/Q2KS6SB3 |
More data for this
Ligand-Target Pair | |
Voltage-dependent T-type calcium channel subunit alpha-1G
(Homo sapiens (Human)) | BDBM50157861
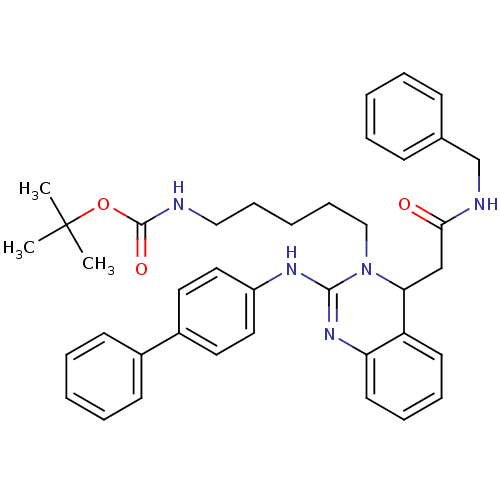
(CHEMBL362667 | tert-butyl 5-(4-(2-(benzylamino)-2-...)Show SMILES CC(C)(C)OC(=O)NCCCCCN1C(CC(=O)NCc2ccccc2)c2ccccc2N=C1Nc1ccc(cc1)-c1ccccc1 |c:34| Show InChI InChI=1S/C39H45N5O3/c1-39(2,3)47-38(46)40-25-13-6-14-26-44-35(27-36(45)41-28-29-15-7-4-8-16-29)33-19-11-12-20-34(33)43-37(44)42-32-23-21-31(22-24-32)30-17-9-5-10-18-30/h4-5,7-12,15-24,35H,6,13-14,25-28H2,1-3H3,(H,40,46)(H,41,45)(H,42,43) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science& Technology
Curated by ChEMBL
| Assay Description
Inhibition of T-type [Ca2+] channel (alpha1G) expressed in HEK293 cells |
Bioorg Med Chem Lett 15: 283-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.078
BindingDB Entry DOI: 10.7270/Q2KS6SB3 |
More data for this
Ligand-Target Pair | |
Voltage-dependent T-type calcium channel subunit alpha-1G
(Homo sapiens (Human)) | BDBM50148180
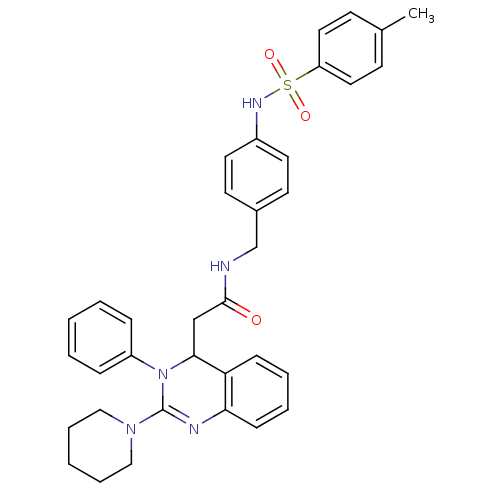
(2-(3-Phenyl-2-piperidin-1-yl-3,4-dihydro-quinazoli...)Show SMILES Cc1ccc(cc1)S(=O)(=O)Nc1ccc(CNC(=O)CC2N(C(=Nc3ccccc23)N2CCCCC2)c2ccccc2)cc1 |c:23| Show InChI InChI=1S/C35H37N5O3S/c1-26-14-20-30(21-15-26)44(42,43)38-28-18-16-27(17-19-28)25-36-34(41)24-33-31-12-6-7-13-32(31)37-35(39-22-8-3-9-23-39)40(33)29-10-4-2-5-11-29/h2,4-7,10-21,33,38H,3,8-9,22-25H2,1H3,(H,36,41) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science& Technology
Curated by ChEMBL
| Assay Description
Inhibition of T-type [Ca2+] channel (alpha1G) expressed in HEK293 cells |
Bioorg Med Chem Lett 15: 283-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.078
BindingDB Entry DOI: 10.7270/Q2KS6SB3 |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50073850
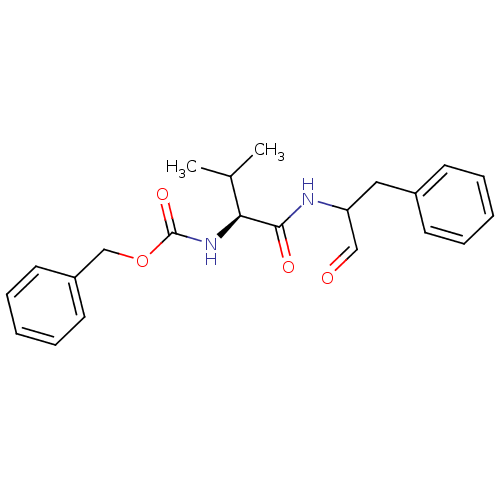
((S)-2-((S)-2-Benzyloxycarbonylamino-3-methyl-butyr...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)NC(Cc1ccccc1)C=O |r| Show InChI InChI=1S/C22H26N2O4/c1-16(2)20(24-22(27)28-15-18-11-7-4-8-12-18)21(26)23-19(14-25)13-17-9-5-3-6-10-17/h3-12,14,16,19-20H,13,15H2,1-2H3,(H,23,26)(H,24,27)/t19?,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science & Technology
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human plasma mu-calpain |
Bioorg Med Chem Lett 15: 2857-60 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.095
BindingDB Entry DOI: 10.7270/Q2Z31Z5J |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50167706
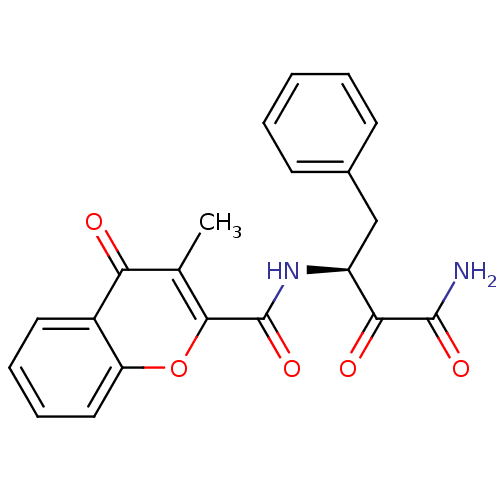
((S)-N-(4-amino-3,4-dioxo-1-phenylbutan-2-yl)-3-met...)Show SMILES Cc1c(oc2ccccc2c1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)C(N)=O |r| Show InChI InChI=1S/C21H18N2O5/c1-12-17(24)14-9-5-6-10-16(14)28-19(12)21(27)23-15(18(25)20(22)26)11-13-7-3-2-4-8-13/h2-10,15H,11H2,1H3,(H2,22,26)(H,23,27)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science & Technology
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human plasma mu-calpain |
Bioorg Med Chem Lett 15: 2857-60 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.095
BindingDB Entry DOI: 10.7270/Q2Z31Z5J |
More data for this
Ligand-Target Pair | |
Voltage-dependent T-type calcium channel subunit alpha-1G
(Homo sapiens (Human)) | BDBM50157856
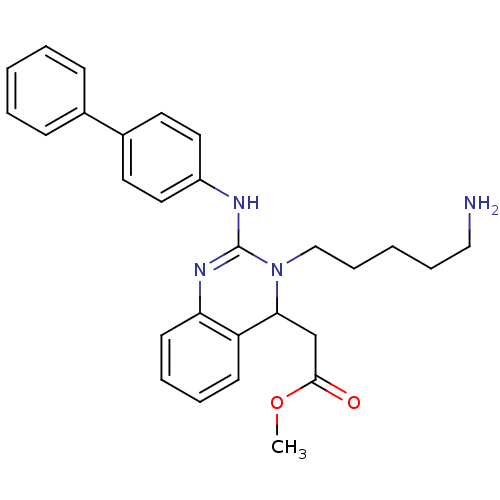
(CHEMBL182332 | [3-(5-Amino-pentyl)-2-(biphenyl-4-y...)Show SMILES COC(=O)CC1N(CCCCCN)C(Nc2ccc(cc2)-c2ccccc2)=Nc2ccccc12 |c:28| Show InChI InChI=1S/C28H32N4O2/c1-34-27(33)20-26-24-12-6-7-13-25(24)31-28(32(26)19-9-3-8-18-29)30-23-16-14-22(15-17-23)21-10-4-2-5-11-21/h2,4-7,10-17,26H,3,8-9,18-20,29H2,1H3,(H,30,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science& Technology
Curated by ChEMBL
| Assay Description
Inhibition of T-type [Ca2+] channel (alpha1G) expressed in HEK293 cells |
Bioorg Med Chem Lett 15: 283-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.078
BindingDB Entry DOI: 10.7270/Q2KS6SB3 |
More data for this
Ligand-Target Pair | |
Voltage-dependent T-type calcium channel subunit alpha-1G
(Homo sapiens (Human)) | BDBM50157860
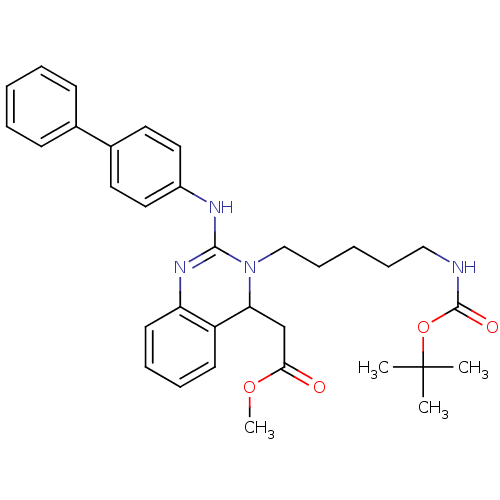
(CHEMBL182518 | [2-(Biphenyl-4-ylamino)-3-(5-tert-b...)Show SMILES COC(=O)CC1N(CCCCCNC(=O)OC(C)(C)C)C(Nc2ccc(cc2)-c2ccccc2)=Nc2ccccc12 |c:35| Show InChI InChI=1S/C33H40N4O4/c1-33(2,3)41-32(39)34-21-11-6-12-22-37-29(23-30(38)40-4)27-15-9-10-16-28(27)36-31(37)35-26-19-17-25(18-20-26)24-13-7-5-8-14-24/h5,7-10,13-20,29H,6,11-12,21-23H2,1-4H3,(H,34,39)(H,35,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science& Technology
Curated by ChEMBL
| Assay Description
Inhibition of T-type [Ca2+] channel (alpha1G) expressed in HEK293 cells |
Bioorg Med Chem Lett 15: 283-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.078
BindingDB Entry DOI: 10.7270/Q2KS6SB3 |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50167708
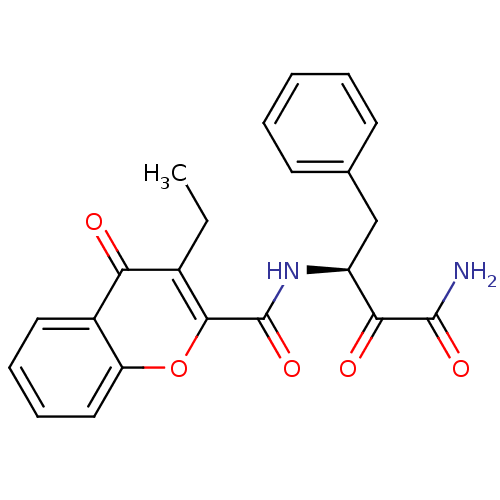
(3-Ethyl-4-oxo-4H-chromene-2-carboxylic acid ((S)-1...)Show SMILES CCc1c(oc2ccccc2c1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)C(N)=O Show InChI InChI=1S/C22H20N2O5/c1-2-14-18(25)15-10-6-7-11-17(15)29-20(14)22(28)24-16(19(26)21(23)27)12-13-8-4-3-5-9-13/h3-11,16H,2,12H2,1H3,(H2,23,27)(H,24,28)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science & Technology
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human plasma mu-calpain |
Bioorg Med Chem Lett 15: 2857-60 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.095
BindingDB Entry DOI: 10.7270/Q2Z31Z5J |
More data for this
Ligand-Target Pair | |
Voltage-dependent T-type calcium channel subunit alpha-1G
(Homo sapiens (Human)) | BDBM50192093
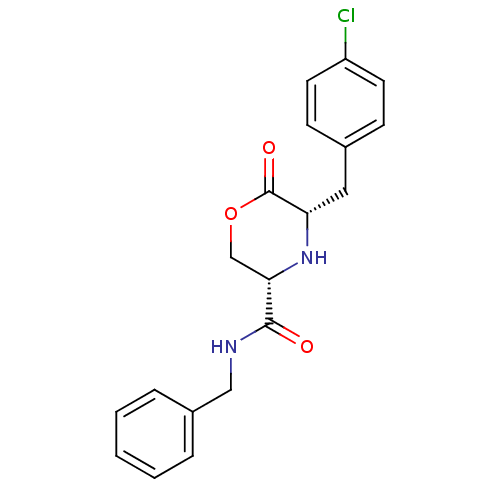
(CHEMBL386255 | cis-5-(4-chlorobenzyl)-N-benzyl-6-o...)Show SMILES Clc1ccc(C[C@@H]2N[C@@H](COC2=O)C(=O)NCc2ccccc2)cc1 Show InChI InChI=1S/C19H19ClN2O3/c20-15-8-6-13(7-9-15)10-16-19(24)25-12-17(22-16)18(23)21-11-14-4-2-1-3-5-14/h1-9,16-17,22H,10-12H2,(H,21,23)/t16-,17-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Ability to block calcium channel T type 3.1v expressed in HEK293 cells by whole cell patch clamp method |
Bioorg Med Chem Lett 16: 5244-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.031
BindingDB Entry DOI: 10.7270/Q2N58KZV |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50167707

(3-Methyl-4-oxo-4H-chromene-2-carboxylic acid ((S)-...)Show SMILES Cc1c(oc2ccccc2c1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C29H26N2O5/c1-19-25(32)22-14-8-9-15-24(22)36-27(19)29(35)31-23(18-21-12-6-3-7-13-21)26(33)28(34)30-17-16-20-10-4-2-5-11-20/h2-15,23H,16-18H2,1H3,(H,30,34)(H,31,35)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science & Technology
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human plasma mu-calpain |
Bioorg Med Chem Lett 15: 2857-60 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.095
BindingDB Entry DOI: 10.7270/Q2Z31Z5J |
More data for this
Ligand-Target Pair | |
Voltage-dependent T-type calcium channel subunit alpha-1G
(Homo sapiens (Human)) | BDBM50157859
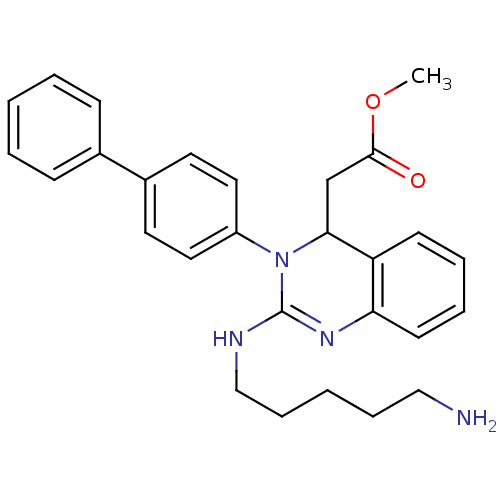
(CHEMBL360359 | [2-(5-amino-pentylamino)-3-biphenyl...)Show SMILES COC(=O)CC1N(C(NCCCCCN)=Nc2ccccc12)c1ccc(cc1)-c1ccccc1 |c:14| Show InChI InChI=1S/C28H32N4O2/c1-34-27(33)20-26-24-12-6-7-13-25(24)31-28(30-19-9-3-8-18-29)32(26)23-16-14-22(15-17-23)21-10-4-2-5-11-21/h2,4-7,10-17,26H,3,8-9,18-20,29H2,1H3,(H,30,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science& Technology
Curated by ChEMBL
| Assay Description
Inhibition of T-type [Ca2+] channel (alpha1G) expressed in HEK293 cells |
Bioorg Med Chem Lett 15: 283-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.078
BindingDB Entry DOI: 10.7270/Q2KS6SB3 |
More data for this
Ligand-Target Pair | |
Pancreatic triacylglycerol lipase
(Sus scrofa (Pig)) | BDBM24567

((2S)-1-[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]tridecan-...)Show SMILES CCCCCCCCCCC[C@@H](C[C@@H]1OC(=O)[C@H]1CCCCCC)OC(=O)[C@H](CC(C)C)NC=O |r| Show InChI InChI=1S/C29H53NO5/c1-5-7-9-11-12-13-14-15-16-18-24(34-29(33)26(30-22-31)20-23(3)4)21-27-25(28(32)35-27)19-17-10-8-6-2/h22-27H,5-21H2,1-4H3,(H,30,31)/t24-,25-,26-,27-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Atomic Energy Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of pig pancreatic lipase assessed as hydrolysis of p-nitrophenylbutyrate to p-nitrophenol |
Bioorg Med Chem Lett 23: 1099-103 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.003
BindingDB Entry DOI: 10.7270/Q2P55PTG |
More data for this
Ligand-Target Pair | |
Pancreatic triacylglycerol lipase
(Sus scrofa (Pig)) | BDBM24567

((2S)-1-[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]tridecan-...)Show SMILES CCCCCCCCCCC[C@@H](C[C@@H]1OC(=O)[C@H]1CCCCCC)OC(=O)[C@H](CC(C)C)NC=O |r| Show InChI InChI=1S/C29H53NO5/c1-5-7-9-11-12-13-14-15-16-18-24(34-29(33)26(30-22-31)20-23(3)4)21-27-25(28(32)35-27)19-17-10-8-6-2/h22-27H,5-21H2,1-4H3,(H,30,31)/t24-,25-,26-,27-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| US Patent
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Korea Atomic Energy Research Institute
US Patent
| Assay Description
Particularly, pancreatic lipase inhibiting activity was measured by the conventional method known to those in the art (Kim, J. H.; Kim, H. J.; Park, ... |
US Patent US9328123 (2016)
BindingDB Entry DOI: 10.7270/Q2Q81BXH |
More data for this
Ligand-Target Pair | |
Voltage-dependent T-type calcium channel subunit alpha-1G
(Homo sapiens (Human)) | BDBM50157863
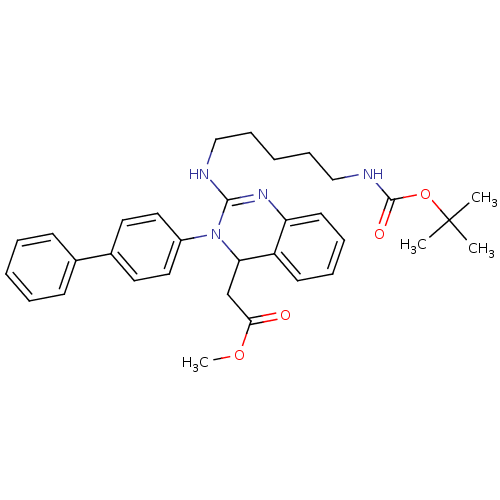
(CHEMBL182968 | [3-Biphenyl-4-yl-2-(5-tert-butoxyca...)Show SMILES COC(=O)CC1N(C(NCCCCCNC(=O)OC(C)(C)C)=Nc2ccccc12)c1ccc(cc1)-c1ccccc1 |c:21| Show InChI InChI=1S/C33H40N4O4/c1-33(2,3)41-32(39)35-22-12-6-11-21-34-31-36-28-16-10-9-15-27(28)29(23-30(38)40-4)37(31)26-19-17-25(18-20-26)24-13-7-5-8-14-24/h5,7-10,13-20,29H,6,11-12,21-23H2,1-4H3,(H,34,36)(H,35,39) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science& Technology
Curated by ChEMBL
| Assay Description
Inhibition of T-type [Ca2+] channel (alpha1G) expressed in HEK293 cells |
Bioorg Med Chem Lett 15: 283-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.078
BindingDB Entry DOI: 10.7270/Q2KS6SB3 |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50167705

(3-Methyl-4-oxo-4H-chromene-2-carboxylic acid ((S)-...)Show SMILES Cc1c(oc2ccccc2c1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)C(=O)NCc1ccccc1 Show InChI InChI=1S/C28H24N2O5/c1-18-24(31)21-14-8-9-15-23(21)35-26(18)28(34)30-22(16-19-10-4-2-5-11-19)25(32)27(33)29-17-20-12-6-3-7-13-20/h2-15,22H,16-17H2,1H3,(H,29,33)(H,30,34)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science & Technology
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human plasma mu-calpain |
Bioorg Med Chem Lett 15: 2857-60 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.095
BindingDB Entry DOI: 10.7270/Q2Z31Z5J |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50167711

(7-Methyl-8-oxo-2,3-dihydro-8H-1,4,5-trioxa-anthrac...)Show SMILES Cc1c(oc2cc3OCCOc3cc2c1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)C(N)=O Show InChI InChI=1S/C23H20N2O7/c1-12-19(26)14-10-17-18(31-8-7-30-17)11-16(14)32-21(12)23(29)25-15(20(27)22(24)28)9-13-5-3-2-4-6-13/h2-6,10-11,15H,7-9H2,1H3,(H2,24,28)(H,25,29)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science & Technology
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human plasma mu-calpain |
Bioorg Med Chem Lett 15: 2857-60 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.095
BindingDB Entry DOI: 10.7270/Q2Z31Z5J |
More data for this
Ligand-Target Pair | |
Voltage-dependent T-type calcium channel subunit alpha-1G
(Homo sapiens (Human)) | BDBM50192090
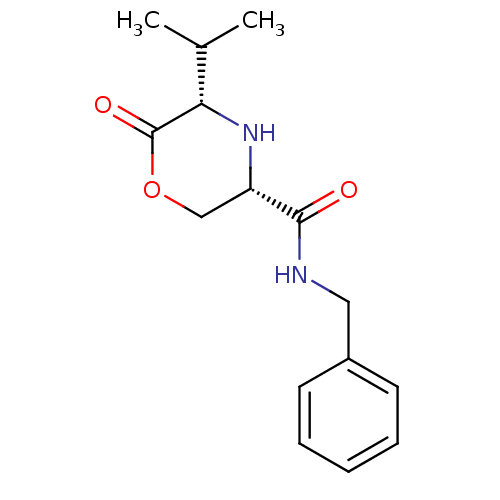
(CHEMBL424892 | cis-5-isopropyl-6-oxo-morpholine-3-...)Show InChI InChI=1S/C15H20N2O3/c1-10(2)13-15(19)20-9-12(17-13)14(18)16-8-11-6-4-3-5-7-11/h3-7,10,12-13,17H,8-9H2,1-2H3,(H,16,18)/t12-,13-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 990 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Ability to block calcium channel T type 3.1v expressed in HEK293 cells by whole cell patch clamp method |
Bioorg Med Chem Lett 16: 5244-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.031
BindingDB Entry DOI: 10.7270/Q2N58KZV |
More data for this
Ligand-Target Pair | |
Voltage-dependent T-type calcium channel subunit alpha-1G
(Homo sapiens (Human)) | BDBM50192085
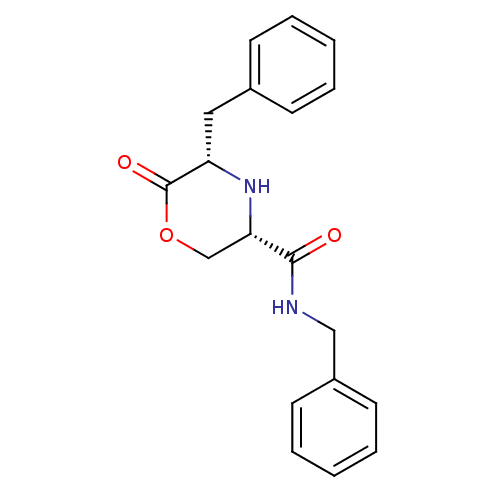
(CHEMBL215688 | cis-N,5-dibenzyl-6-oxomorpholine-3-...)Show SMILES O=C(NCc1ccccc1)[C@@H]1COC(=O)[C@H](Cc2ccccc2)N1 Show InChI InChI=1S/C19H20N2O3/c22-18(20-12-15-9-5-2-6-10-15)17-13-24-19(23)16(21-17)11-14-7-3-1-4-8-14/h1-10,16-17,21H,11-13H2,(H,20,22)/t16-,17-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Ability to block calcium channel T type 3.1v expressed in HEK293 cells by whole cell patch clamp method |
Bioorg Med Chem Lett 16: 5244-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.031
BindingDB Entry DOI: 10.7270/Q2N58KZV |
More data for this
Ligand-Target Pair | |
Voltage-dependent T-type calcium channel subunit alpha-1G
(Homo sapiens (Human)) | BDBM50192088
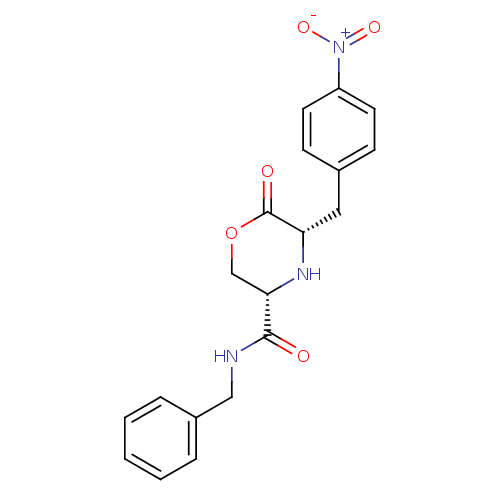
(CHEMBL438287 | cis-5-(4-nitrobenzyl)-N-benzyl-6-ox...)Show SMILES [O-][N+](=O)c1ccc(C[C@@H]2N[C@@H](COC2=O)C(=O)NCc2ccccc2)cc1 Show InChI InChI=1S/C19H19N3O5/c23-18(20-11-14-4-2-1-3-5-14)17-12-27-19(24)16(21-17)10-13-6-8-15(9-7-13)22(25)26/h1-9,16-17,21H,10-12H2,(H,20,23)/t16-,17-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Ability to block calcium channel T type 3.1v expressed in HEK293 cells by whole cell patch clamp method |
Bioorg Med Chem Lett 16: 5244-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.031
BindingDB Entry DOI: 10.7270/Q2N58KZV |
More data for this
Ligand-Target Pair | |
Voltage-dependent T-type calcium channel subunit alpha-1G
(Homo sapiens (Human)) | BDBM50148175
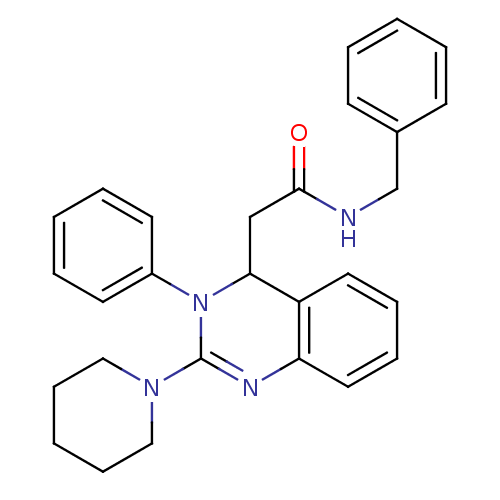
(CHEMBL114695 | KYS-05001 | N-Benzyl-2-(3-phenyl-2-...)Show SMILES O=C(CC1N(C(=Nc2ccccc12)N1CCCCC1)c1ccccc1)NCc1ccccc1 |c:5| Show InChI InChI=1S/C28H30N4O/c33-27(29-21-22-12-4-1-5-13-22)20-26-24-16-8-9-17-25(24)30-28(31-18-10-3-11-19-31)32(26)23-14-6-2-7-15-23/h1-2,4-9,12-17,26H,3,10-11,18-21H2,(H,29,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science& Technology
Curated by ChEMBL
| Assay Description
Inhibition of T-type [Ca2+] channel (alpha1G) expressed in HEK293 cells |
Bioorg Med Chem Lett 15: 283-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.078
BindingDB Entry DOI: 10.7270/Q2KS6SB3 |
More data for this
Ligand-Target Pair | |
Voltage-dependent T-type calcium channel subunit alpha-1G
(Homo sapiens (Human)) | BDBM50192092
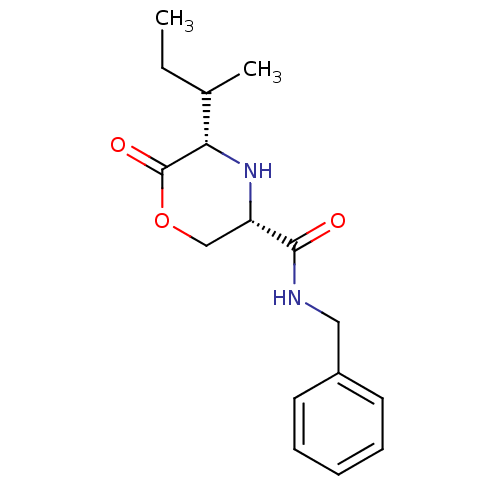
(CHEMBL384620 | cis-N-benzyl-5-((R)-sec-butyl)-6-ox...)Show SMILES CCC(C)[C@@H]1N[C@@H](COC1=O)C(=O)NCc1ccccc1 Show InChI InChI=1S/C16H22N2O3/c1-3-11(2)14-16(20)21-10-13(18-14)15(19)17-9-12-7-5-4-6-8-12/h4-8,11,13-14,18H,3,9-10H2,1-2H3,(H,17,19)/t11?,13-,14-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Ability to block calcium channel T type 3.1v expressed in HEK293 cells by whole cell patch clamp method |
Bioorg Med Chem Lett 16: 5244-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.031
BindingDB Entry DOI: 10.7270/Q2N58KZV |
More data for this
Ligand-Target Pair | |
Voltage-dependent T-type calcium channel subunit alpha-1G
(Homo sapiens (Human)) | BDBM50192089
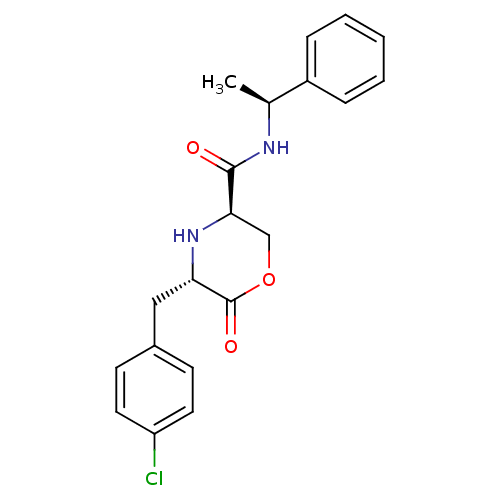
(CHEMBL384456 | trans-5-(4-chlorobenzyl)-6-oxo-N-((...)Show SMILES C[C@H](NC(=O)[C@H]1COC(=O)[C@H](Cc2ccc(Cl)cc2)N1)c1ccccc1 Show InChI InChI=1S/C20H21ClN2O3/c1-13(15-5-3-2-4-6-15)22-19(24)18-12-26-20(25)17(23-18)11-14-7-9-16(21)10-8-14/h2-10,13,17-18,23H,11-12H2,1H3,(H,22,24)/t13-,17-,18+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Ability to block calcium channel T type 3.1v expressed in HEK293 cells by whole cell patch clamp method |
Bioorg Med Chem Lett 16: 5244-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.031
BindingDB Entry DOI: 10.7270/Q2N58KZV |
More data for this
Ligand-Target Pair | |
Voltage-dependent T-type calcium channel subunit alpha-1G
(Homo sapiens (Human)) | BDBM50117922
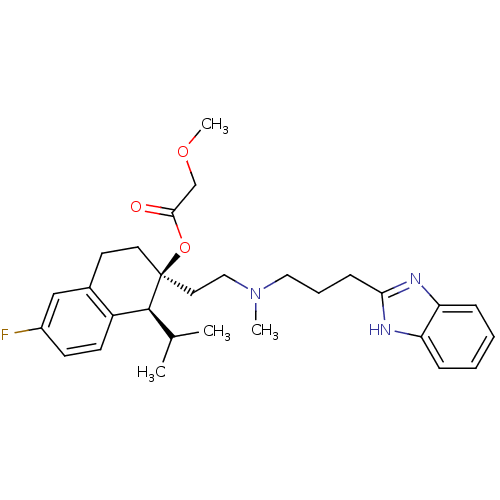
((1S,2S)-2-(2-((3-(1H-benzo[d]imidazol-2-yl)propyl)...)Show SMILES COCC(=O)O[C@]1(CCN(C)CCCc2nc3ccccc3[nH]2)CCc2cc(F)ccc2[C@@H]1C(C)C |r| Show InChI InChI=1S/C29H38FN3O3/c1-20(2)28-23-12-11-22(30)18-21(23)13-14-29(28,36-27(34)19-35-4)15-17-33(3)16-7-10-26-31-24-8-5-6-9-25(24)32-26/h5-6,8-9,11-12,18,20,28H,7,10,13-17,19H2,1-4H3,(H,31,32)/t28-,29-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Ability to block calcium channel T type 3.1v expressed in HEK293 cells by whole cell patch clamp method |
Bioorg Med Chem Lett 16: 5244-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.031
BindingDB Entry DOI: 10.7270/Q2N58KZV |
More data for this
Ligand-Target Pair | |
Voltage-dependent T-type calcium channel subunit alpha-1G
(Homo sapiens (Human)) | BDBM50117922

((1S,2S)-2-(2-((3-(1H-benzo[d]imidazol-2-yl)propyl)...)Show SMILES COCC(=O)O[C@]1(CCN(C)CCCc2nc3ccccc3[nH]2)CCc2cc(F)ccc2[C@@H]1C(C)C |r| Show InChI InChI=1S/C29H38FN3O3/c1-20(2)28-23-12-11-22(30)18-21(23)13-14-29(28,36-27(34)19-35-4)15-17-33(3)16-7-10-26-31-24-8-5-6-9-25(24)32-26/h5-6,8-9,11-12,18,20,28H,7,10,13-17,19H2,1-4H3,(H,31,32)/t28-,29-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science& Technology
Curated by ChEMBL
| Assay Description
Inhibition of T-type [Ca2+] channel (alpha1G) expressed in HEK293 cells |
Bioorg Med Chem Lett 15: 283-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.078
BindingDB Entry DOI: 10.7270/Q2KS6SB3 |
More data for this
Ligand-Target Pair | |
Voltage-dependent T-type calcium channel subunit alpha-1G
(Homo sapiens (Human)) | BDBM50192086
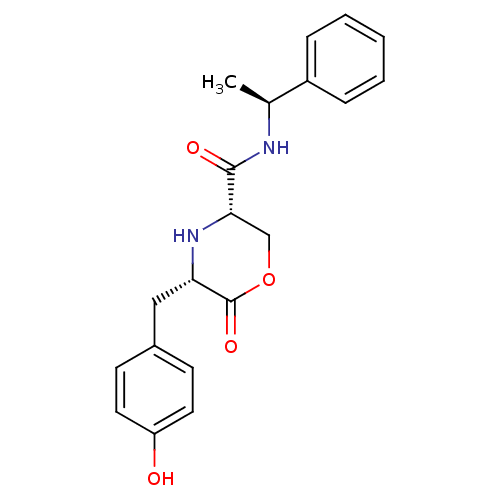
(CHEMBL385050 | cis-5-(4-hydroxybenzyl)-6-oxo-N-((S...)Show SMILES C[C@H](NC(=O)[C@@H]1COC(=O)[C@H](Cc2ccc(O)cc2)N1)c1ccccc1 Show InChI InChI=1S/C20H22N2O4/c1-13(15-5-3-2-4-6-15)21-19(24)18-12-26-20(25)17(22-18)11-14-7-9-16(23)10-8-14/h2-10,13,17-18,22-23H,11-12H2,1H3,(H,21,24)/t13-,17-,18-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Ability to block calcium channel T type 3.1v expressed in HEK293 cells by whole cell patch clamp method |
Bioorg Med Chem Lett 16: 5244-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.031
BindingDB Entry DOI: 10.7270/Q2N58KZV |
More data for this
Ligand-Target Pair | |
Voltage-dependent T-type calcium channel subunit alpha-1G
(Homo sapiens (Human)) | BDBM50192087
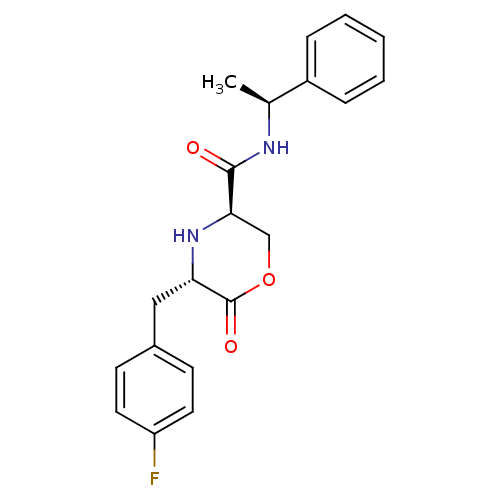
(CHEMBL215629 | trans-5-(4-fluorobenzyl)-6-oxo-N-((...)Show SMILES C[C@H](NC(=O)[C@H]1COC(=O)[C@H](Cc2ccc(F)cc2)N1)c1ccccc1 Show InChI InChI=1S/C20H21FN2O3/c1-13(15-5-3-2-4-6-15)22-19(24)18-12-26-20(25)17(23-18)11-14-7-9-16(21)10-8-14/h2-10,13,17-18,23H,11-12H2,1H3,(H,22,24)/t13-,17-,18+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Ability to block calcium channel T type 3.1v expressed in HEK293 cells by whole cell patch clamp method |
Bioorg Med Chem Lett 16: 5244-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.031
BindingDB Entry DOI: 10.7270/Q2N58KZV |
More data for this
Ligand-Target Pair | |
Voltage-dependent T-type calcium channel subunit alpha-1G
(Homo sapiens (Human)) | BDBM50192097
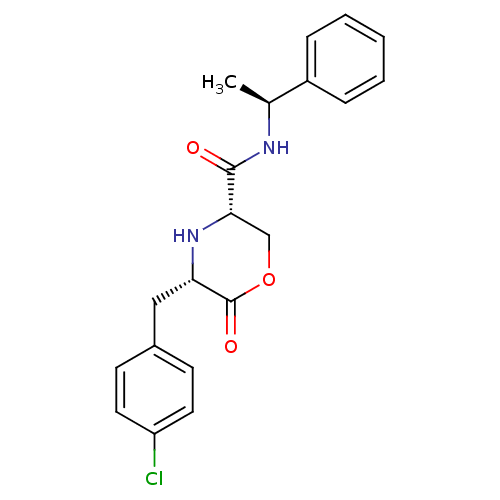
(CHEMBL215982 | cis-5-(4-chlorobenzyl)-6-oxo-N-((S)...)Show SMILES C[C@H](NC(=O)[C@@H]1COC(=O)[C@H](Cc2ccc(Cl)cc2)N1)c1ccccc1 Show InChI InChI=1S/C20H21ClN2O3/c1-13(15-5-3-2-4-6-15)22-19(24)18-12-26-20(25)17(23-18)11-14-7-9-16(21)10-8-14/h2-10,13,17-18,23H,11-12H2,1H3,(H,22,24)/t13-,17-,18-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Ability to block calcium channel T type 3.1v expressed in HEK293 cells by whole cell patch clamp method |
Bioorg Med Chem Lett 16: 5244-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.031
BindingDB Entry DOI: 10.7270/Q2N58KZV |
More data for this
Ligand-Target Pair | |
Voltage-dependent T-type calcium channel subunit alpha-1G
(Homo sapiens (Human)) | BDBM50192096
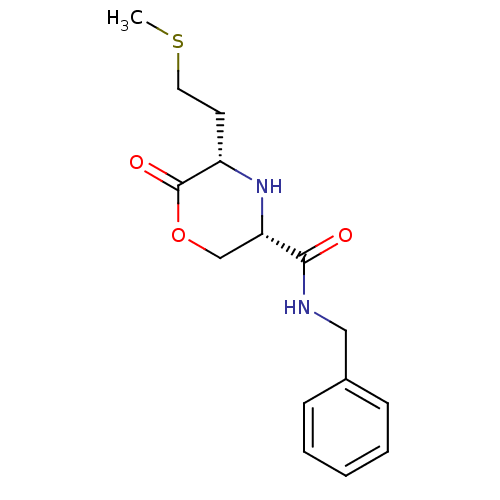
(CHEMBL215092 | cis-N-benzyl-5-(2-(methylthio)ethyl...)Show InChI InChI=1S/C15H20N2O3S/c1-21-8-7-12-15(19)20-10-13(17-12)14(18)16-9-11-5-3-2-4-6-11/h2-6,12-13,17H,7-10H2,1H3,(H,16,18)/t12-,13-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Ability to block calcium channel T type 3.1v expressed in HEK293 cells by whole cell patch clamp method |
Bioorg Med Chem Lett 16: 5244-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.031
BindingDB Entry DOI: 10.7270/Q2N58KZV |
More data for this
Ligand-Target Pair | |
Voltage-dependent T-type calcium channel subunit alpha-1G
(Homo sapiens (Human)) | BDBM50192094
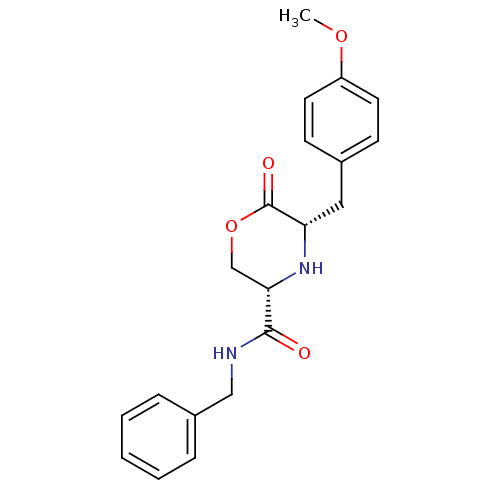
(CHEMBL214709 | cis-5-(4-methoxybenzyl)-N-benzyl-6-...)Show SMILES COc1ccc(C[C@@H]2N[C@@H](COC2=O)C(=O)NCc2ccccc2)cc1 Show InChI InChI=1S/C20H22N2O4/c1-25-16-9-7-14(8-10-16)11-17-20(24)26-13-18(22-17)19(23)21-12-15-5-3-2-4-6-15/h2-10,17-18,22H,11-13H2,1H3,(H,21,23)/t17-,18-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Ability to block calcium channel T type 3.1v expressed in HEK293 cells by whole cell patch clamp method |
Bioorg Med Chem Lett 16: 5244-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.031
BindingDB Entry DOI: 10.7270/Q2N58KZV |
More data for this
Ligand-Target Pair | |
Voltage-dependent T-type calcium channel subunit alpha-1G
(Homo sapiens (Human)) | BDBM50192095
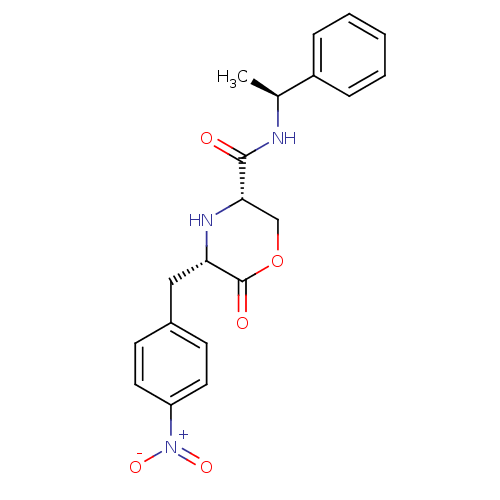
(CHEMBL215930 | cis-5-(4-nitrobenzyl)-6-oxo-N-((S)-...)Show SMILES C[C@H](NC(=O)[C@@H]1COC(=O)[C@H](Cc2ccc(cc2)[N+]([O-])=O)N1)c1ccccc1 Show InChI InChI=1S/C20H21N3O5/c1-13(15-5-3-2-4-6-15)21-19(24)18-12-28-20(25)17(22-18)11-14-7-9-16(10-8-14)23(26)27/h2-10,13,17-18,22H,11-12H2,1H3,(H,21,24)/t13-,17-,18-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Ability to block calcium channel T type 3.1v expressed in HEK293 cells by whole cell patch clamp method |
Bioorg Med Chem Lett 16: 5244-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.031
BindingDB Entry DOI: 10.7270/Q2N58KZV |
More data for this
Ligand-Target Pair | |
Voltage-dependent T-type calcium channel subunit alpha-1G
(Homo sapiens (Human)) | BDBM50192091
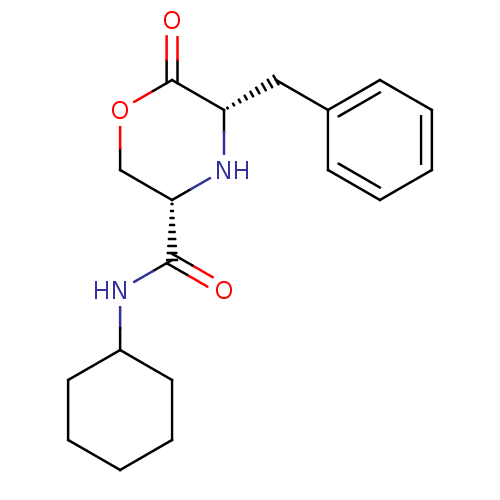
(CHEMBL215215 | cis-5-benzyl-N-cyclohexyl-6-oxomorp...)Show SMILES O=C(NC1CCCCC1)[C@@H]1COC(=O)[C@H](Cc2ccccc2)N1 Show InChI InChI=1S/C18H24N2O3/c21-17(19-14-9-5-2-6-10-14)16-12-23-18(22)15(20-16)11-13-7-3-1-4-8-13/h1,3-4,7-8,14-16,20H,2,5-6,9-12H2,(H,19,21)/t15-,16-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Ability to block calcium channel T type 3.1v expressed in HEK293 cells by whole cell patch clamp method |
Bioorg Med Chem Lett 16: 5244-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.031
BindingDB Entry DOI: 10.7270/Q2N58KZV |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50167715
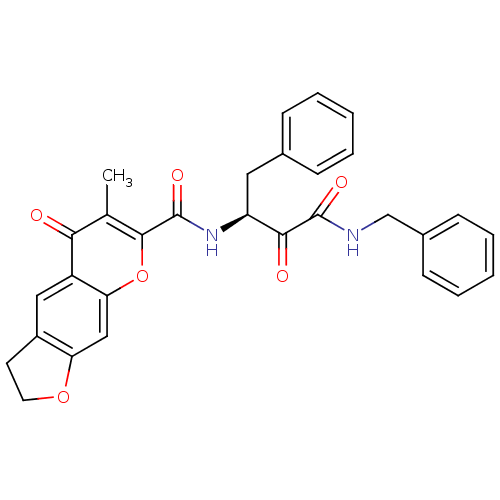
(6-Methyl-5-oxo-2,3-dihydro-5H-furo[3,2-g]chromene-...)Show SMILES Cc1c(oc2cc3OCCc3cc2c1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)C(=O)NCc1ccccc1 Show InChI InChI=1S/C30H26N2O6/c1-18-26(33)22-15-21-12-13-37-24(21)16-25(22)38-28(18)30(36)32-23(14-19-8-4-2-5-9-19)27(34)29(35)31-17-20-10-6-3-7-11-20/h2-11,15-16,23H,12-14,17H2,1H3,(H,31,35)(H,32,36)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science & Technology
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human plasma mu-calpain |
Bioorg Med Chem Lett 15: 2857-60 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.095
BindingDB Entry DOI: 10.7270/Q2Z31Z5J |
More data for this
Ligand-Target Pair | |
Voltage-dependent T-type calcium channel subunit alpha-1G
(Homo sapiens (Human)) | BDBM50192084
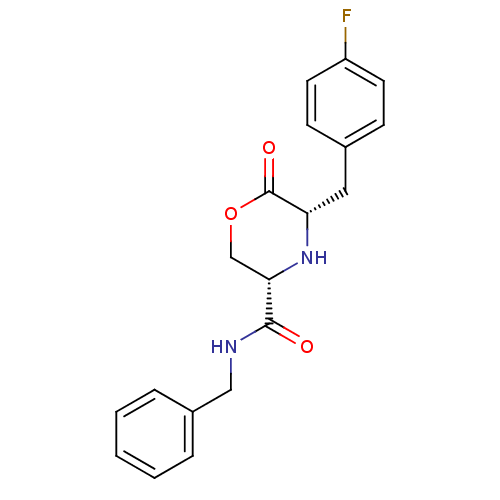
(CHEMBL215095 | cis-5-(4-fluorobenzyl)-N-benzyl-6-o...)Show SMILES Fc1ccc(C[C@@H]2N[C@@H](COC2=O)C(=O)NCc2ccccc2)cc1 Show InChI InChI=1S/C19H19FN2O3/c20-15-8-6-13(7-9-15)10-16-19(24)25-12-17(22-16)18(23)21-11-14-4-2-1-3-5-14/h1-9,16-17,22H,10-12H2,(H,21,23)/t16-,17-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Ability to block calcium channel T type 3.1v expressed in HEK293 cells by whole cell patch clamp method |
Bioorg Med Chem Lett 16: 5244-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.031
BindingDB Entry DOI: 10.7270/Q2N58KZV |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50167709
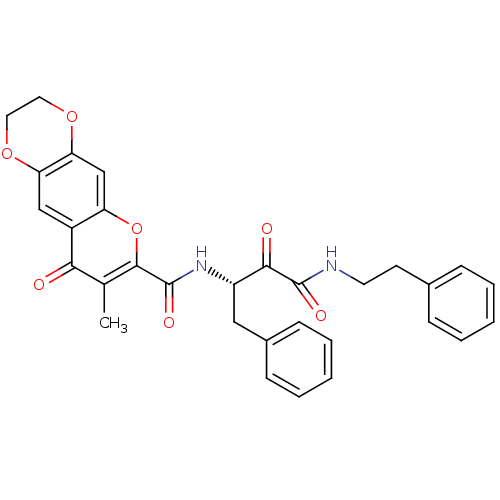
(7-Methyl-8-oxo-2,3-dihydro-8H-1,4,5-trioxa-anthrac...)Show SMILES Cc1c(oc2cc3OCCOc3cc2c1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C31H28N2O7/c1-19-27(34)22-17-25-26(39-15-14-38-25)18-24(22)40-29(19)31(37)33-23(16-21-10-6-3-7-11-21)28(35)30(36)32-13-12-20-8-4-2-5-9-20/h2-11,17-18,23H,12-16H2,1H3,(H,32,36)(H,33,37)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science & Technology
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human plasma mu-calpain |
Bioorg Med Chem Lett 15: 2857-60 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.095
BindingDB Entry DOI: 10.7270/Q2Z31Z5J |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50167704

(3-Methyl-4-oxo-4H-chromene-2-carboxylic acid ((S)-...)Show SMILES CC(C)NC(=O)C(=O)[C@H](Cc1ccccc1)NC(=O)c1oc2ccccc2c(=O)c1C Show InChI InChI=1S/C24H24N2O5/c1-14(2)25-23(29)21(28)18(13-16-9-5-4-6-10-16)26-24(30)22-15(3)20(27)17-11-7-8-12-19(17)31-22/h4-12,14,18H,13H2,1-3H3,(H,25,29)(H,26,30)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science & Technology
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human plasma mu-calpain |
Bioorg Med Chem Lett 15: 2857-60 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.095
BindingDB Entry DOI: 10.7270/Q2Z31Z5J |
More data for this
Ligand-Target Pair | |
Pancreatic triacylglycerol lipase
(Sus scrofa (Pig)) | BDBM224806
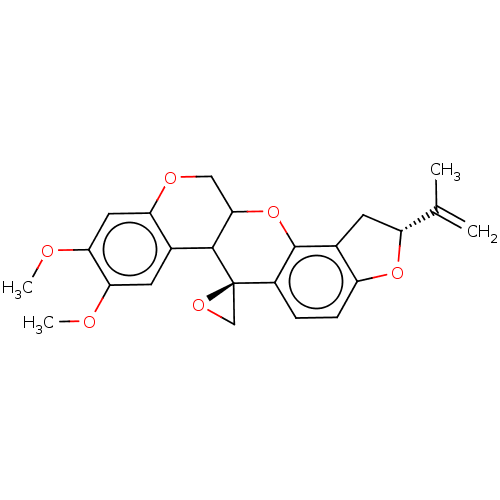
(US9328123, Rotenone A)Show SMILES COc1cc2OCC3Oc4c5C[C@@H](Oc5ccc4[C@]4(CO4)C3c2cc1OC)C(C)=C |r| Show InChI InChI=1S/C24H24O6/c1-12(2)17-8-14-16(29-17)6-5-15-23(14)30-21-10-27-18-9-20(26-4)19(25-3)7-13(18)22(21)24(15)11-28-24/h5-7,9,17,21-22H,1,8,10-11H2,2-4H3/t17-,21?,22?,24-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Korea Atomic Energy Research Institute
US Patent
| Assay Description
Particularly, pancreatic lipase inhibiting activity was measured by the conventional method known to those in the art (Kim, J. H.; Kim, H. J.; Park, ... |
US Patent US9328123 (2016)
BindingDB Entry DOI: 10.7270/Q2Q81BXH |
More data for this
Ligand-Target Pair | |
Pancreatic triacylglycerol lipase
(Sus scrofa (Pig)) | BDBM50429043
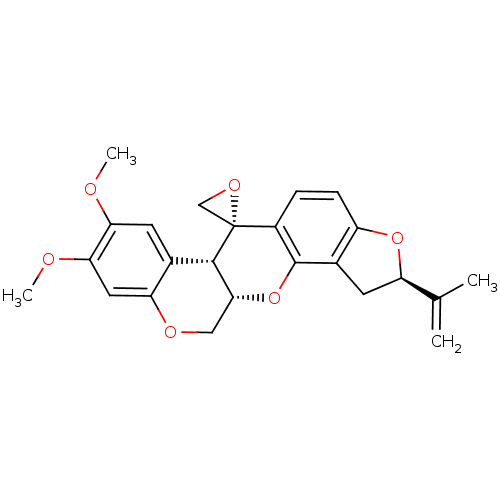
(CHEMBL2334103 | Rotenoisin A)Show SMILES COc1cc2OC[C@H]3Oc4c5C[C@@H](Oc5ccc4[C@]4(CO4)[C@H]3c2cc1OC)C(C)=C |r| Show InChI InChI=1S/C24H24O6/c1-12(2)17-8-14-16(29-17)6-5-15-23(14)30-21-10-27-18-9-20(26-4)19(25-3)7-13(18)22(21)24(15)11-28-24/h5-7,9,17,21-22H,1,8,10-11H2,2-4H3/t17-,21-,22+,24-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Atomic Energy Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of pig pancreatic lipase assessed as hydrolysis of p-nitrophenylbutyrate to p-nitrophenol |
Bioorg Med Chem Lett 23: 1099-103 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.003
BindingDB Entry DOI: 10.7270/Q2P55PTG |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50167710

(3-Methyl-4-oxo-4H-chromene-2-carboxylic acid [(S)-...)Show SMILES Cc1c(oc2ccccc2c1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)C(=O)NCCN1CCOCC1 Show InChI InChI=1S/C27H29N3O6/c1-18-23(31)20-9-5-6-10-22(20)36-25(18)27(34)29-21(17-19-7-3-2-4-8-19)24(32)26(33)28-11-12-30-13-15-35-16-14-30/h2-10,21H,11-17H2,1H3,(H,28,33)(H,29,34)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science & Technology
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human plasma mu-calpain |
Bioorg Med Chem Lett 15: 2857-60 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.095
BindingDB Entry DOI: 10.7270/Q2Z31Z5J |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50167712
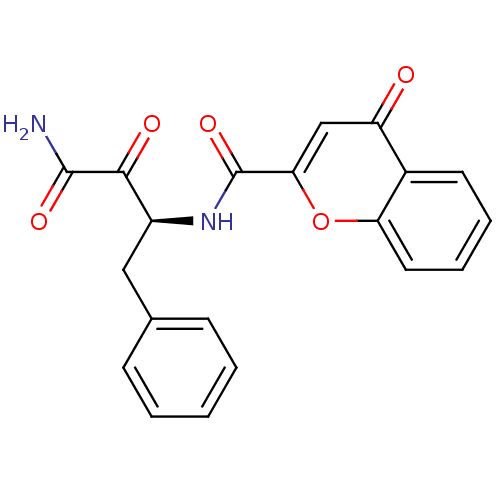
(4-Oxo-4H-chromene-2-carboxylic acid ((S)-1-benzyl-...)Show SMILES NC(=O)C(=O)[C@H](Cc1ccccc1)NC(=O)c1cc(=O)c2ccccc2o1 Show InChI InChI=1S/C20H16N2O5/c21-19(25)18(24)14(10-12-6-2-1-3-7-12)22-20(26)17-11-15(23)13-8-4-5-9-16(13)27-17/h1-9,11,14H,10H2,(H2,21,25)(H,22,26)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science & Technology
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human plasma mu-calpain |
Bioorg Med Chem Lett 15: 2857-60 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.095
BindingDB Entry DOI: 10.7270/Q2Z31Z5J |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50167713

(7-Methyl-8-oxo-2,3-dihydro-8H-1,4,5-trioxa-anthrac...)Show SMILES CC(C)NC(=O)C(=O)[C@H](Cc1ccccc1)NC(=O)c1oc2cc3OCCOc3cc2c(=O)c1C Show InChI InChI=1S/C26H26N2O7/c1-14(2)27-25(31)23(30)18(11-16-7-5-4-6-8-16)28-26(32)24-15(3)22(29)17-12-20-21(13-19(17)35-24)34-10-9-33-20/h4-8,12-14,18H,9-11H2,1-3H3,(H,27,31)(H,28,32)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science & Technology
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human plasma mu-calpain |
Bioorg Med Chem Lett 15: 2857-60 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.095
BindingDB Entry DOI: 10.7270/Q2Z31Z5J |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50167714
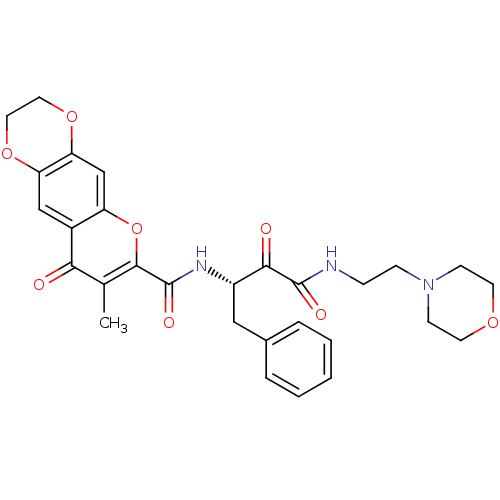
(7-Methyl-8-oxo-2,3-dihydro-8H-1,4,5-trioxa-anthrac...)Show SMILES Cc1c(oc2cc3OCCOc3cc2c1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)C(=O)NCCN1CCOCC1 Show InChI InChI=1S/C29H31N3O8/c1-18-25(33)20-16-23-24(39-14-13-38-23)17-22(20)40-27(18)29(36)31-21(15-19-5-3-2-4-6-19)26(34)28(35)30-7-8-32-9-11-37-12-10-32/h2-6,16-17,21H,7-15H2,1H3,(H,30,35)(H,31,36)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science & Technology
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human plasma mu-calpain |
Bioorg Med Chem Lett 15: 2857-60 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.095
BindingDB Entry DOI: 10.7270/Q2Z31Z5J |
More data for this
Ligand-Target Pair | |
Pancreatic triacylglycerol lipase
(Sus scrofa (Pig)) | BDBM224807
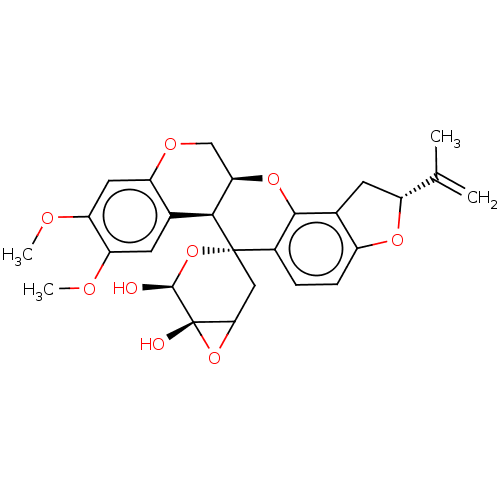
(Gamma-irradiated rotenone | US9328123, Rotenone B)Show SMILES COc1cc2OC[C@H]3Oc4c5C[C@@H](Oc5ccc4[C@@]4(CC5O[C@]5(O)[C@@H](O)O4)[C@H]3c2cc1OC)C(C)=C |r| Show InChI InChI=1S/C27H28O9/c1-12(2)17-8-14-16(33-17)6-5-15-24(14)34-21-11-32-18-9-20(31-4)19(30-3)7-13(18)23(21)26(15)10-22-27(29,35-22)25(28)36-26/h5-7,9,17,21-23,25,28-29H,1,8,10-11H2,2-4H3/t17-,21-,22?,23+,25+,26+,27+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.84E+4 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Korea Atomic Energy Research Institute
US Patent
| Assay Description
Particularly, pancreatic lipase inhibiting activity was measured by the conventional method known to those in the art (Kim, J. H.; Kim, H. J.; Park, ... |
US Patent US9328123 (2016)
BindingDB Entry DOI: 10.7270/Q2Q81BXH |
More data for this
Ligand-Target Pair | |
Pancreatic triacylglycerol lipase
(Sus scrofa (Pig)) | BDBM50429042
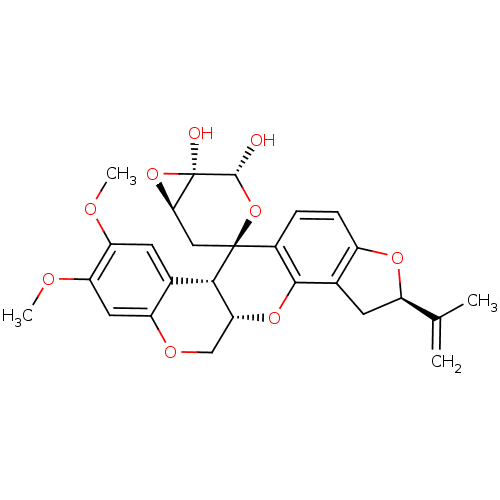
(CHEMBL2334102 | Rotenoisin B)Show SMILES COc1cc2OC[C@H]3Oc4c5C[C@@H](Oc5ccc4[C@@]4(C[C@H]5O[C@@]5(O)[C@H](O)O4)[C@H]3c2cc1OC)C(C)=C |r| Show InChI InChI=1S/C27H28O9/c1-12(2)17-8-14-16(33-17)6-5-15-24(14)34-21-11-32-18-9-20(31-4)19(30-3)7-13(18)23(21)26(15)10-22-27(29,35-22)25(28)36-26/h5-7,9,17,21-23,25,28-29H,1,8,10-11H2,2-4H3/t17-,21-,22-,23+,25-,26+,27-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Atomic Energy Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of pig pancreatic lipase assessed as hydrolysis of p-nitrophenylbutyrate to p-nitrophenol |
Bioorg Med Chem Lett 23: 1099-103 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.003
BindingDB Entry DOI: 10.7270/Q2P55PTG |
More data for this
Ligand-Target Pair | |
Pancreatic triacylglycerol lipase
(Sus scrofa (Pig)) | BDBM224807

(Gamma-irradiated rotenone | US9328123, Rotenone B)Show SMILES COc1cc2OC[C@H]3Oc4c5C[C@@H](Oc5ccc4[C@@]4(CC5O[C@]5(O)[C@@H](O)O4)[C@H]3c2cc1OC)C(C)=C |r| Show InChI InChI=1S/C27H28O9/c1-12(2)17-8-14-16(33-17)6-5-15-24(14)34-21-11-32-18-9-20(31-4)19(30-3)7-13(18)23(21)26(15)10-22-27(29,35-22)25(28)36-26/h5-7,9,17,21-23,25,28-29H,1,8,10-11H2,2-4H3/t17-,21-,22?,23+,25+,26+,27+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.12E+4 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Korea Atomic Energy Research Institute
US Patent
| Assay Description
Particularly, pancreatic lipase inhibiting activity was measured by the conventional method known to those in the art (Kim, J. H.; Kim, H. J.; Park, ... |
US Patent US9328123 (2016)
BindingDB Entry DOI: 10.7270/Q2Q81BXH |
More data for this
Ligand-Target Pair | |
Pancreatic triacylglycerol lipase
(Sus scrofa (Pig)) | BDBM50135527
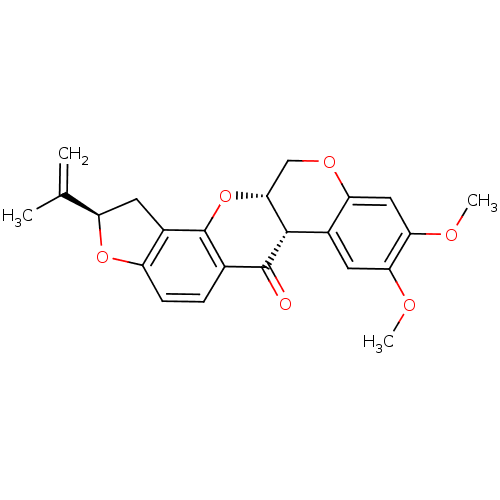
((-)-cis-rotenone | (-)-rotenone | (2R,6aS,12aS)-8,...)Show SMILES COc1cc2OC[C@H]3Oc4c5C[C@@H](Oc5ccc4C(=O)[C@H]3c2cc1OC)C(C)=C |r| Show InChI InChI=1S/C23H22O6/c1-11(2)16-8-14-15(28-16)6-5-12-22(24)21-13-7-18(25-3)19(26-4)9-17(13)27-10-20(21)29-23(12)14/h5-7,9,16,20-21H,1,8,10H2,2-4H3/t16-,20-,21+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Atomic Energy Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of pig pancreatic lipase assessed as hydrolysis of p-nitrophenylbutyrate to p-nitrophenol |
Bioorg Med Chem Lett 23: 1099-103 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.003
BindingDB Entry DOI: 10.7270/Q2P55PTG |
More data for this
Ligand-Target Pair | |
Pancreatic triacylglycerol lipase
(Sus scrofa (Pig)) | BDBM224805
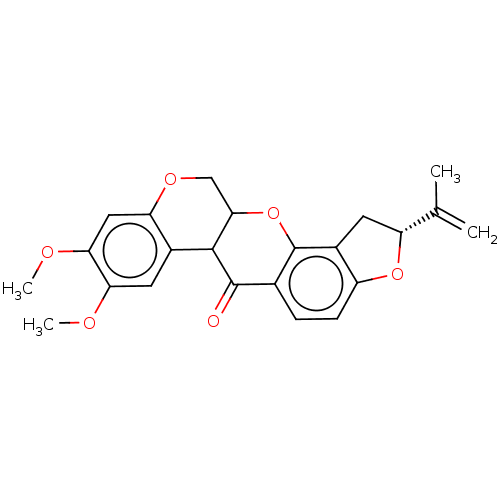
(US9328123, Rotenone)Show SMILES COc1cc2OCC3Oc4c5C[C@@H](Oc5ccc4C(=O)C3c2cc1OC)C(C)=C |r| Show InChI InChI=1S/C23H22O6/c1-11(2)16-8-14-15(28-16)6-5-12-22(24)21-13-7-18(25-3)19(26-4)9-17(13)27-10-20(21)29-23(12)14/h5-7,9,16,20-21H,1,8,10H2,2-4H3/t16-,20?,21?/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Korea Atomic Energy Research Institute
US Patent
| Assay Description
Particularly, pancreatic lipase inhibiting activity was measured by the conventional method known to those in the art (Kim, J. H.; Kim, H. J.; Park, ... |
US Patent US9328123 (2016)
BindingDB Entry DOI: 10.7270/Q2Q81BXH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data