 Found 451 hits with Last Name = 'cockerill' and Initial = 'gs'
Found 451 hits with Last Name = 'cockerill' and Initial = 'gs' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Fusion glycoprotein F0
(Human respiratory syncytial virus A (strain A2)) | BDBM50572440

(CHEMBL4469438)Show SMILES CC(C)CCn1c(CN2C(=O)C3(CC3)c3ccccc23)nc2cc(CN)ccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Respiratory syncytial virus A2 fusion protein expressed in HEK293T cells harboring GAL4-NF-kappaB assessed as reduction in cell-cell fu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01882
BindingDB Entry DOI: 10.7270/Q2QF8XNB |
More data for this
Ligand-Target Pair | |
Fusion glycoprotein F0
(Human respiratory syncytial virus A (strain A2)) | BDBM50572444
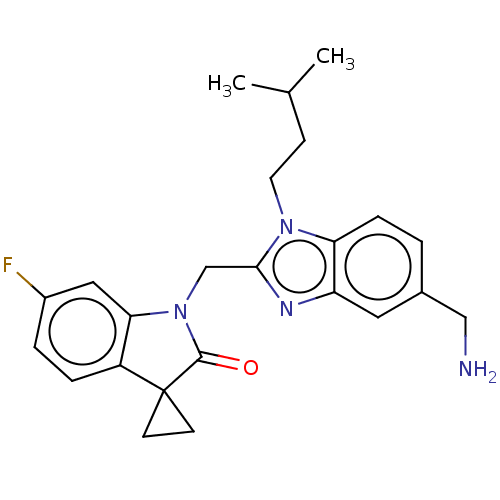
(CHEMBL4878302)Show SMILES CC(C)CCn1c(CN2C(=O)C3(CC3)c3ccc(F)cc23)nc2cc(CN)ccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Respiratory syncytial virus A2 fusion protein expressed in HEK293T cells harboring GAL4-NF-kappaB assessed as reduction in cell-cell fu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01882
BindingDB Entry DOI: 10.7270/Q2QF8XNB |
More data for this
Ligand-Target Pair | |
Fusion glycoprotein F0
(Human respiratory syncytial virus A (strain A2)) | BDBM50572466

(CHEMBL4876200)Show SMILES CC(C)CCn1c(CN2C(=O)C3(CCOCC3)c3ccccc23)nc2cc(CN)ccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Respiratory syncytial virus fusion protein |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01882
BindingDB Entry DOI: 10.7270/Q2QF8XNB |
More data for this
Ligand-Target Pair | |
Fusion glycoprotein F0
(Human respiratory syncytial virus A (strain A2)) | BDBM50572442

(CHEMBL4872095)Show SMILES CC(C)CCn1c(CN2C(=O)C3(CC3)c3cc(F)ccc23)nc2cc(CN)ccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Respiratory syncytial virus A2 fusion protein expressed in HEK293T cells harboring GAL4-NF-kappaB assessed as reduction in cell-cell fu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01882
BindingDB Entry DOI: 10.7270/Q2QF8XNB |
More data for this
Ligand-Target Pair | |
Fusion glycoprotein F0
(Human respiratory syncytial virus A (strain A2)) | BDBM50572464

(CHEMBL4858040)Show SMILES CC(C)CCn1c(CN2C(=O)C3(CC3)c3ccncc23)nc2cc(CN)ccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Respiratory syncytial virus fusion protein |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01882
BindingDB Entry DOI: 10.7270/Q2QF8XNB |
More data for this
Ligand-Target Pair | |
Fusion glycoprotein F0
(Human respiratory syncytial virus A (strain A2)) | BDBM50572463

(CHEMBL4871101)Show SMILES CC(C)CCn1c(CN2C(=O)C3(CC3)c3cccnc23)nc2cc(CN)ccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Respiratory syncytial virus fusion protein |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01882
BindingDB Entry DOI: 10.7270/Q2QF8XNB |
More data for this
Ligand-Target Pair | |
Fusion glycoprotein F0
(Human respiratory syncytial virus A (strain A2)) | BDBM50572450
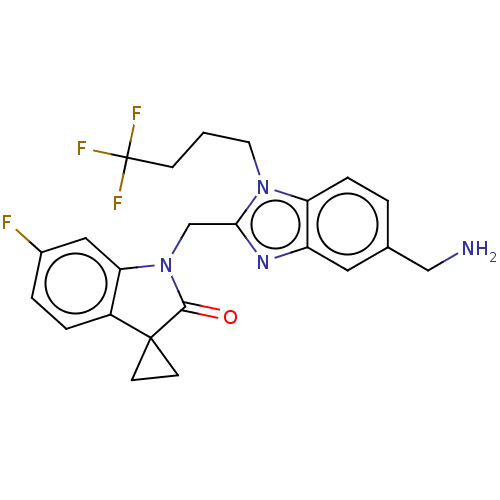
(Rv-521 | Rv521 | Sisunatovir)Show SMILES NCc1ccc2n(CCCC(F)(F)F)c(CN3C(=O)C4(CC4)c4ccc(F)cc34)nc2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Respiratory syncytial virus A2 fusion protein expressed in HEK293T cells harboring GAL4-NF-kappaB assessed as reduction in cell-cell fu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01882
BindingDB Entry DOI: 10.7270/Q2QF8XNB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Fusion glycoprotein F0
(Human respiratory syncytial virus A (strain A2)) | BDBM50572451
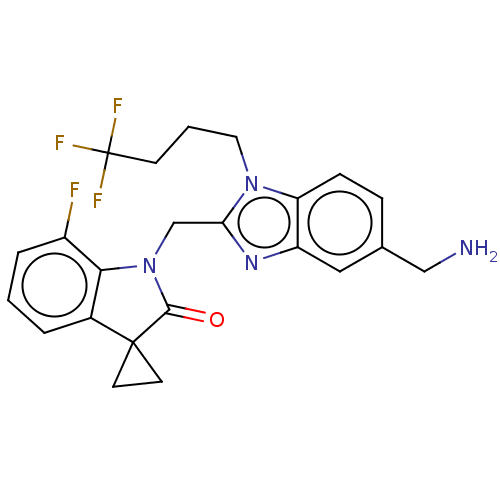
(CHEMBL4867108)Show SMILES NCc1ccc2n(CCCC(F)(F)F)c(CN3C(=O)C4(CC4)c4cccc(F)c34)nc2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Respiratory syncytial virus A2 fusion protein expressed in HEK293T cells harboring GAL4-NF-kappaB assessed as reduction in cell-cell fu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01882
BindingDB Entry DOI: 10.7270/Q2QF8XNB |
More data for this
Ligand-Target Pair | |
Fusion glycoprotein F0
(Human respiratory syncytial virus A (strain A2)) | BDBM50572465
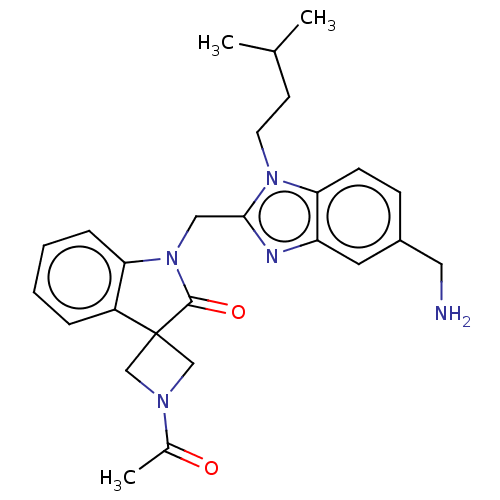
(CHEMBL4858949)Show SMILES CC(C)CCn1c(CN2C(=O)C3(CN(C3)C(C)=O)c3ccccc23)nc2cc(CN)ccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Respiratory syncytial virus fusion protein |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01882
BindingDB Entry DOI: 10.7270/Q2QF8XNB |
More data for this
Ligand-Target Pair | |
Fusion glycoprotein F0
(Human respiratory syncytial virus A (strain A2)) | BDBM50572445

(CHEMBL4865906)Show SMILES CC(C)CCn1c(CN2C(=O)C3(CC3)c3ccc(Cl)cc23)nc2cc(CN)ccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Respiratory syncytial virus A2 fusion protein expressed in HEK293T cells harboring GAL4-NF-kappaB assessed as reduction in cell-cell fu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01882
BindingDB Entry DOI: 10.7270/Q2QF8XNB |
More data for this
Ligand-Target Pair | |
Fusion glycoprotein F0
(Human respiratory syncytial virus A (strain A2)) | BDBM50572453

(CHEMBL4874709)Show SMILES NCc1ccc2n(C3CCOCC3)c(CN3C(=O)C4(CC4)c4ccc(F)cc34)nc2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Respiratory syncytial virus A2 fusion protein expressed in HEK293T cells harboring GAL4-NF-kappaB assessed as reduction in cell-cell fu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01882
BindingDB Entry DOI: 10.7270/Q2QF8XNB |
More data for this
Ligand-Target Pair | |
Fusion glycoprotein F0
(Human respiratory syncytial virus A (strain A2)) | BDBM50572447
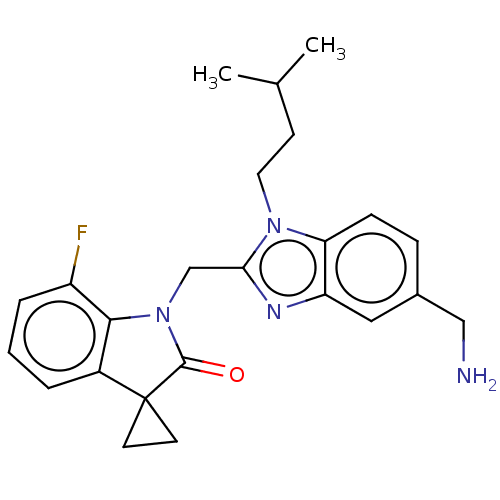
(CHEMBL4860271)Show SMILES CC(C)CCn1c(CN2C(=O)C3(CC3)c3cccc(F)c23)nc2cc(CN)ccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Respiratory syncytial virus A2 fusion protein expressed in HEK293T cells harboring GAL4-NF-kappaB assessed as reduction in cell-cell fu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01882
BindingDB Entry DOI: 10.7270/Q2QF8XNB |
More data for this
Ligand-Target Pair | |
Fusion glycoprotein F0
(Human respiratory syncytial virus A (strain A2)) | BDBM50572449

(CHEMBL4853602)Show SMILES NCc1ccc2n(CCCCO)c(CN3C(=O)C4(CC4)c4ccc(F)cc34)nc2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Respiratory syncytial virus A2 fusion protein expressed in HEK293T cells harboring GAL4-NF-kappaB assessed as reduction in cell-cell fu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01882
BindingDB Entry DOI: 10.7270/Q2QF8XNB |
More data for this
Ligand-Target Pair | |
Fusion glycoprotein F0
(Human respiratory syncytial virus A (strain A2)) | BDBM50572452
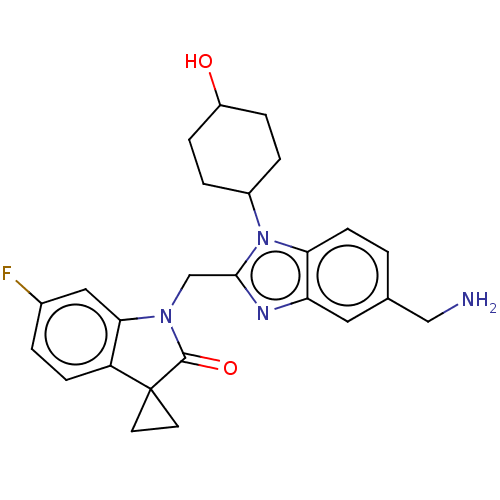
(CHEMBL4873528)Show SMILES NCc1ccc2n(C3CCC(O)CC3)c(CN3C(=O)C4(CC4)c4ccc(F)cc34)nc2c1 |(23.93,-12.4,;22.59,-11.64,;21.26,-12.41,;21.26,-13.96,;19.93,-14.74,;18.59,-13.97,;17.13,-14.45,;16.66,-15.91,;15.16,-16.23,;14.69,-17.69,;15.72,-18.84,;15.24,-20.3,;17.23,-18.52,;17.7,-17.05,;16.22,-13.21,;14.68,-13.21,;13.91,-11.88,;14.52,-10.48,;16.02,-10.15,;13.36,-9.45,;12.58,-8.11,;14.13,-8.11,;12.04,-10.23,;10.58,-9.76,;9.44,-10.79,;9.77,-12.3,;8.64,-13.34,;11.23,-12.76,;12.37,-11.73,;17.12,-11.96,;18.59,-12.43,;19.92,-11.65,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Respiratory syncytial virus A2 fusion protein expressed in HEK293T cells harboring GAL4-NF-kappaB assessed as reduction in cell-cell fu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01882
BindingDB Entry DOI: 10.7270/Q2QF8XNB |
More data for this
Ligand-Target Pair | |
Fusion glycoprotein F0
(Human respiratory syncytial virus A (strain A2)) | BDBM50572448
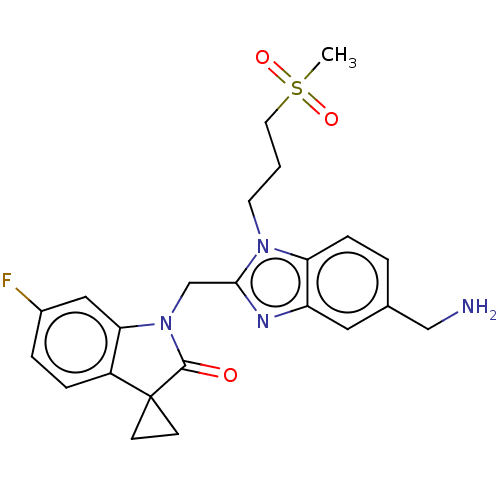
(CHEMBL4863172)Show SMILES CS(=O)(=O)CCCn1c(CN2C(=O)C3(CC3)c3ccc(F)cc23)nc2cc(CN)ccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Respiratory syncytial virus A2 fusion protein expressed in HEK293T cells harboring GAL4-NF-kappaB assessed as reduction in cell-cell fu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01882
BindingDB Entry DOI: 10.7270/Q2QF8XNB |
More data for this
Ligand-Target Pair | |
Fusion glycoprotein F0
(Human respiratory syncytial virus A (strain A2)) | BDBM50572454

(CHEMBL4855358)Show SMILES NC(=N)c1ccc2n(CCCC(F)(F)F)c(CN3C(=O)C4(CC4)c4ccc(F)cc34)nc2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Respiratory syncytial virus A2 fusion protein expressed in HEK293T cells harboring GAL4-NF-kappaB assessed as reduction in cell-cell fu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01882
BindingDB Entry DOI: 10.7270/Q2QF8XNB |
More data for this
Ligand-Target Pair | |
Fusion glycoprotein F0
(Human respiratory syncytial virus A (strain A2)) | BDBM50572443
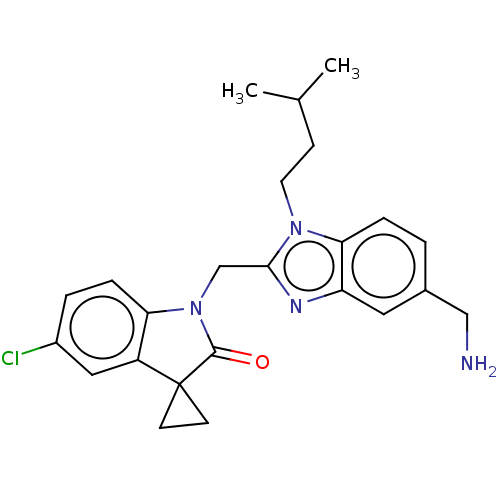
(CHEMBL4872263)Show SMILES CC(C)CCn1c(CN2C(=O)C3(CC3)c3cc(Cl)ccc23)nc2cc(CN)ccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Respiratory syncytial virus A2 fusion protein expressed in HEK293T cells harboring GAL4-NF-kappaB assessed as reduction in cell-cell fu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01882
BindingDB Entry DOI: 10.7270/Q2QF8XNB |
More data for this
Ligand-Target Pair | |
Fusion glycoprotein F0
(Human respiratory syncytial virus A (strain A2)) | BDBM50572462
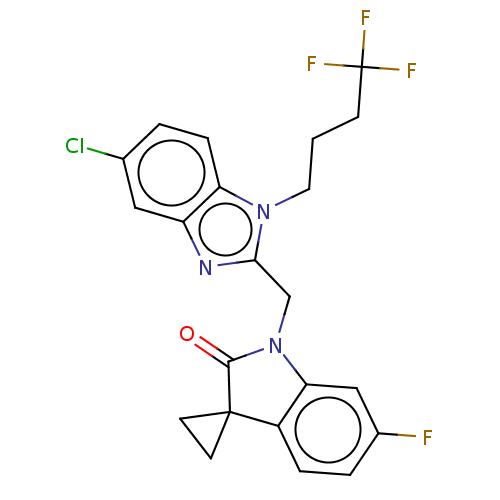
(CHEMBL4876843)Show SMILES Fc1ccc2c(c1)N(Cc1nc3cc(Cl)ccc3n1CCCC(F)(F)F)C(=O)C21CC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Respiratory syncytial virus A2 fusion protein expressed in HEK293T cells harboring GAL4-NF-kappaB assessed as reduction in cell-cell fu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01882
BindingDB Entry DOI: 10.7270/Q2QF8XNB |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM5445

(CHEMBL554 | GW572016 | LAPATINIB DITOSYLATE | Lapa...)Show SMILES CS(=O)(=O)CCNCc1ccc(o1)-c1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C29H26ClFN4O4S/c1-40(36,37)12-11-32-16-23-7-10-27(39-23)20-5-8-26-24(14-20)29(34-18-33-26)35-22-6-9-28(25(30)15-22)38-17-19-3-2-4-21(31)13-19/h2-10,13-15,18,32H,11-12,16-17H2,1H3,(H,33,34,35) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 |
Bioorg Med Chem Lett 16: 4686-91 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.090
BindingDB Entry DOI: 10.7270/Q2VM4D2Z |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM5459

(6-alkoxy-4-anilinoquinazoline 8e | N-{3-chloro-4-[...)Show SMILES CS(=O)(=O)CCNCCCCOc1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C28H30ClFN4O4S/c1-39(35,36)14-12-31-11-2-3-13-37-23-8-9-26-24(17-23)28(33-19-32-26)34-22-7-10-27(25(29)16-22)38-18-20-5-4-6-21(30)15-20/h4-10,15-17,19,31H,2-3,11-14,18H2,1H3,(H,32,33,34) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GlaxoSmithKline
| Assay Description
Enzymatic reactions were initiated by adding kinase to the reaction mixture containing ATP, [gamma-33P] ATP, peptide substrate and test inhibitor com... |
Bioorg Med Chem Lett 13: 637-40 (2003)
Article DOI: 10.1016/s0960-894x(02)01047-8
BindingDB Entry DOI: 10.7270/Q2D21VS2 |
More data for this
Ligand-Target Pair | |
Fusion glycoprotein F0
(Human respiratory syncytial virus A (strain A2)) | BDBM50572441

(CHEMBL4862291)Show SMILES CC(C)CCn1c(CN2C(=O)C3(CC3)c3c2cccc3Cl)nc2cc(CN)ccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Respiratory syncytial virus A2 fusion protein expressed in HEK293T cells harboring GAL4-NF-kappaB assessed as reduction in cell-cell fu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01882
BindingDB Entry DOI: 10.7270/Q2QF8XNB |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5456

(6-alkoxy-4-anilinoquinazoline 8b | N-(3-ethynylphe...)Show SMILES CS(=O)(=O)CCNCCCCOc1ccc2ncnc(Nc3cccc(c3)C#C)c2c1 Show InChI InChI=1S/C23H26N4O3S/c1-3-18-7-6-8-19(15-18)27-23-21-16-20(9-10-22(21)25-17-26-23)30-13-5-4-11-24-12-14-31(2,28)29/h1,6-10,15-17,24H,4-5,11-14H2,2H3,(H,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GlaxoSmithKline
| Assay Description
Enzymatic reactions were initiated by adding kinase to the reaction mixture containing ATP, [gamma-33P] ATP, peptide substrate and test inhibitor com... |
Bioorg Med Chem Lett 13: 637-40 (2003)
Article DOI: 10.1016/s0960-894x(02)01047-8
BindingDB Entry DOI: 10.7270/Q2D21VS2 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5445

(CHEMBL554 | GW572016 | LAPATINIB DITOSYLATE | Lapa...)Show SMILES CS(=O)(=O)CCNCc1ccc(o1)-c1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C29H26ClFN4O4S/c1-40(36,37)12-11-32-16-23-7-10-27(39-23)20-5-8-26-24(14-20)29(34-18-33-26)35-22-6-9-28(25(30)15-22)38-17-19-3-2-4-21(31)13-19/h2-10,13-15,18,32H,11-12,16-17H2,1H3,(H,33,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of ErbB1 |
Bioorg Med Chem Lett 16: 4686-91 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.090
BindingDB Entry DOI: 10.7270/Q2VM4D2Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50189123

((4-benzyloxy-3-chloro-phenyl)-{6-[5-(1-oxo-1lambda...)Show SMILES Clc1cc(Nc2ncnc3ccc(cc23)-c2ccc(CN3CCS(=O)CC3)o2)ccc1OCc1ccccc1 Show InChI InChI=1S/C30H27ClN4O3S/c31-26-17-23(7-10-29(26)37-19-21-4-2-1-3-5-21)34-30-25-16-22(6-9-27(25)32-20-33-30)28-11-8-24(38-28)18-35-12-14-39(36)15-13-35/h1-11,16-17,20H,12-15,18-19H2,(H,32,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of ErbB1 |
Bioorg Med Chem Lett 16: 4686-91 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.090
BindingDB Entry DOI: 10.7270/Q2VM4D2Z |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50189113
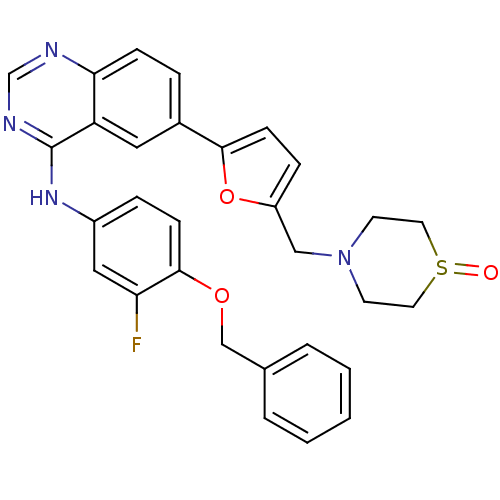
((4-benzyloxy-3-fluoro-phenyl)-{6-[5-(1-oxo-1lambda...)Show SMILES Fc1cc(Nc2ncnc3ccc(cc23)-c2ccc(CN3CCS(=O)CC3)o2)ccc1OCc1ccccc1 Show InChI InChI=1S/C30H27FN4O3S/c31-26-17-23(7-10-29(26)37-19-21-4-2-1-3-5-21)34-30-25-16-22(6-9-27(25)32-20-33-30)28-11-8-24(38-28)18-35-12-14-39(36)15-13-35/h1-11,16-17,20H,12-15,18-19H2,(H,32,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of ErbB1 |
Bioorg Med Chem Lett 16: 4686-91 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.090
BindingDB Entry DOI: 10.7270/Q2VM4D2Z |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50189107

(CHEMBL215171 | N-(4-(3-fluorobenzyloxy)-3-chloroph...)Show SMILES CS(=O)(=O)CCOCc1ccc(o1)-c1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C29H25ClFN3O5S/c1-40(35,36)12-11-37-17-23-7-10-27(39-23)20-5-8-26-24(14-20)29(33-18-32-26)34-22-6-9-28(25(30)15-22)38-16-19-3-2-4-21(31)13-19/h2-10,13-15,18H,11-12,16-17H2,1H3,(H,32,33,34) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 |
Bioorg Med Chem Lett 16: 4686-91 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.090
BindingDB Entry DOI: 10.7270/Q2VM4D2Z |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50189114

(CHEMBL265367 | N-(4-(3-fluorobenzyloxy)-3-(trifluo...)Show SMILES Cn1ccnc1S(=O)(=O)CCNCc1ccc(o1)-c1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(c3)C(F)(F)F)c2c1 Show InChI InChI=1S/C33H28F4N6O4S/c1-43-13-11-39-32(43)48(44,45)14-12-38-18-25-7-10-29(47-25)22-5-8-28-26(16-22)31(41-20-40-28)42-24-6-9-30(27(17-24)33(35,36)37)46-19-21-3-2-4-23(34)15-21/h2-11,13,15-17,20,38H,12,14,18-19H2,1H3,(H,40,41,42) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of ErbB1 |
Bioorg Med Chem Lett 16: 4686-91 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.090
BindingDB Entry DOI: 10.7270/Q2VM4D2Z |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50189109

(CHEMBL212954 | [3-chloro-4-(3-fluoro-benzyloxy)-ph...)Show SMILES Fc1cccc(COc2ccc(Nc3ncnc4ccc(cc34)-c3ccc(CN4CCS(=O)CC4)o3)cc2Cl)c1 Show InChI InChI=1S/C30H26ClFN4O3S/c31-26-16-23(5-8-29(26)38-18-20-2-1-3-22(32)14-20)35-30-25-15-21(4-7-27(25)33-19-34-30)28-9-6-24(39-28)17-36-10-12-40(37)13-11-36/h1-9,14-16,19H,10-13,17-18H2,(H,33,34,35) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 |
Bioorg Med Chem Lett 16: 4686-91 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.090
BindingDB Entry DOI: 10.7270/Q2VM4D2Z |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50189108

(CHEMBL380247 | GW574783B | N-(4-(benzyloxy)-3-chlo...)Show SMILES CS(=O)(=O)CCNCc1ccc(o1)-c1ccc2ncnc(Nc3ccc(OCc4ccccc4)c(Cl)c3)c2c1 Show InChI InChI=1S/C29H27ClN4O4S/c1-39(35,36)14-13-31-17-23-9-12-27(38-23)21-7-10-26-24(15-21)29(33-19-32-26)34-22-8-11-28(25(30)16-22)37-18-20-5-3-2-4-6-20/h2-12,15-16,19,31H,13-14,17-18H2,1H3,(H,32,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of ErbB1 |
Bioorg Med Chem Lett 16: 4686-91 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.090
BindingDB Entry DOI: 10.7270/Q2VM4D2Z |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50189126

(CHEMBL214487 | N-(4-(3-fluorobenzyloxy)-3-chloroph...)Show SMILES CC(C)S(=O)(=O)CCNCc1ccc(o1)-c1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C31H30ClFN4O4S/c1-20(2)42(38,39)13-12-34-17-25-8-11-29(41-25)22-6-9-28-26(15-22)31(36-19-35-28)37-24-7-10-30(27(32)16-24)40-18-21-4-3-5-23(33)14-21/h3-11,14-16,19-20,34H,12-13,17-18H2,1-2H3,(H,35,36,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of ErbB1 |
Bioorg Med Chem Lett 16: 4686-91 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.090
BindingDB Entry DOI: 10.7270/Q2VM4D2Z |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50189121
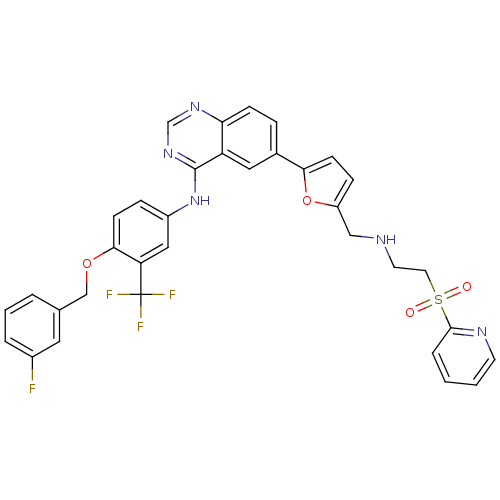
(CHEMBL214023 | N-(4-(3-fluorobenzyloxy)-3-(trifluo...)Show SMILES Fc1cccc(COc2ccc(Nc3ncnc4ccc(cc34)-c3ccc(CNCCS(=O)(=O)c4ccccn4)o3)cc2C(F)(F)F)c1 Show InChI InChI=1S/C34H27F4N5O4S/c35-24-5-3-4-22(16-24)20-46-31-11-8-25(18-28(31)34(36,37)38)43-33-27-17-23(7-10-29(27)41-21-42-33)30-12-9-26(47-30)19-39-14-15-48(44,45)32-6-1-2-13-40-32/h1-13,16-18,21,39H,14-15,19-20H2,(H,41,42,43) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of ErbB1 |
Bioorg Med Chem Lett 16: 4686-91 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.090
BindingDB Entry DOI: 10.7270/Q2VM4D2Z |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50370758

(CHEMBL1794063)Show SMILES Fc1cccc(COc2ccc(Nc3ncnc4ccc(cc34)-c3ccc(CNCCS(=O)(=O)c4ccccc4)o3)cc2Cl)c1 Show InChI InChI=1S/C34H28ClFN4O4S/c35-30-19-26(10-13-33(30)43-21-23-5-4-6-25(36)17-23)40-34-29-18-24(9-12-31(29)38-22-39-34)32-14-11-27(44-32)20-37-15-16-45(41,42)28-7-2-1-3-8-28/h1-14,17-19,22,37H,15-16,20-21H2,(H,38,39,40) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of ErbB1 |
Bioorg Med Chem Lett 16: 4686-91 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.090
BindingDB Entry DOI: 10.7270/Q2VM4D2Z |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM5460
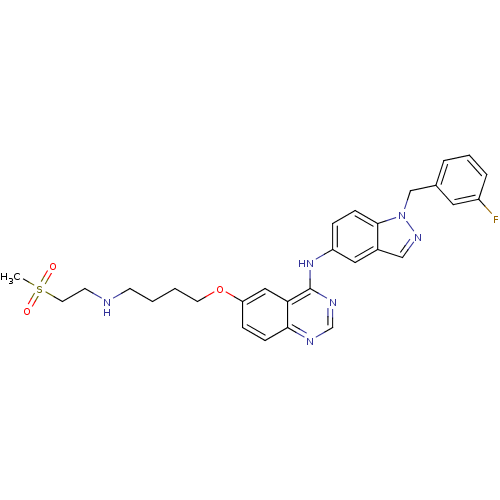
(6-alkoxy-4-anilinoquinazoline 8f | N-{1-[(3-fluoro...)Show SMILES CS(=O)(=O)CCNCCCCOc1ccc2ncnc(Nc3ccc4n(Cc5cccc(F)c5)ncc4c3)c2c1 Show InChI InChI=1S/C29H31FN6O3S/c1-40(37,38)14-12-31-11-2-3-13-39-25-8-9-27-26(17-25)29(33-20-32-27)35-24-7-10-28-22(16-24)18-34-36(28)19-21-5-4-6-23(30)15-21/h4-10,15-18,20,31H,2-3,11-14,19H2,1H3,(H,32,33,35) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GlaxoSmithKline
| Assay Description
Enzymatic reactions were initiated by adding kinase to the reaction mixture containing ATP, [gamma-33P] ATP, peptide substrate and test inhibitor com... |
Bioorg Med Chem Lett 13: 637-40 (2003)
Article DOI: 10.1016/s0960-894x(02)01047-8
BindingDB Entry DOI: 10.7270/Q2D21VS2 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50189127
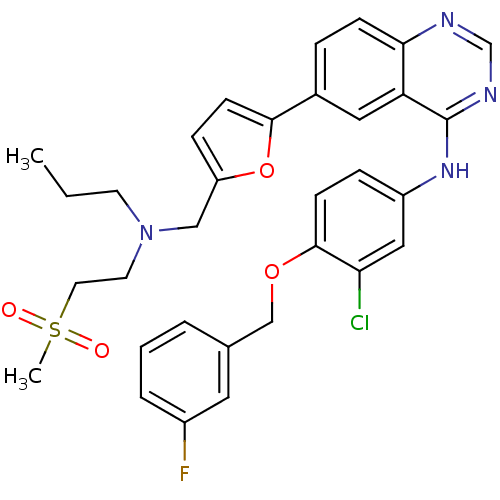
(CHEMBL212251 | N-(4-(3-fluorobenzyloxy)-3-chloroph...)Show SMILES CCCN(CCS(C)(=O)=O)Cc1ccc(o1)-c1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C32H32ClFN4O4S/c1-3-13-38(14-15-43(2,39)40)19-26-9-12-30(42-26)23-7-10-29-27(17-23)32(36-21-35-29)37-25-8-11-31(28(33)18-25)41-20-22-5-4-6-24(34)16-22/h4-12,16-18,21H,3,13-15,19-20H2,1-2H3,(H,35,36,37) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 |
Bioorg Med Chem Lett 16: 4686-91 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.090
BindingDB Entry DOI: 10.7270/Q2VM4D2Z |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5457
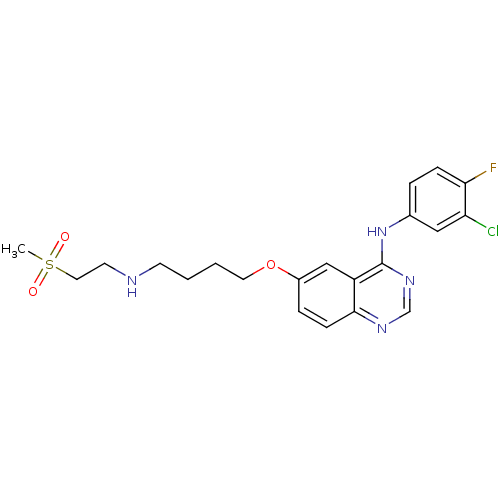
(6-alkoxy-4-anilinoquinazoline 8c | N-(3-chloro-4-f...)Show SMILES CS(=O)(=O)CCNCCCCOc1ccc2ncnc(Nc3ccc(F)c(Cl)c3)c2c1 Show InChI InChI=1S/C21H24ClFN4O3S/c1-31(28,29)11-9-24-8-2-3-10-30-16-5-7-20-17(13-16)21(26-14-25-20)27-15-4-6-19(23)18(22)12-15/h4-7,12-14,24H,2-3,8-11H2,1H3,(H,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GlaxoSmithKline
| Assay Description
Enzymatic reactions were initiated by adding kinase to the reaction mixture containing ATP, [gamma-33P] ATP, peptide substrate and test inhibitor com... |
Bioorg Med Chem Lett 13: 637-40 (2003)
Article DOI: 10.1016/s0960-894x(02)01047-8
BindingDB Entry DOI: 10.7270/Q2D21VS2 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50189112
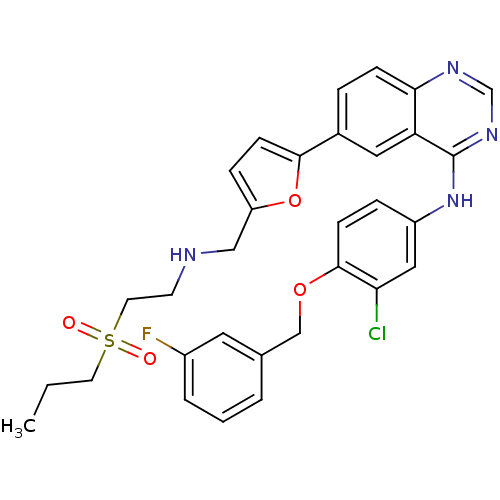
(CHEMBL383965 | N-(4-(3-fluorobenzyloxy)-3-chloroph...)Show SMILES CCCS(=O)(=O)CCNCc1ccc(o1)-c1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C31H30ClFN4O4S/c1-2-13-42(38,39)14-12-34-18-25-8-11-29(41-25)22-6-9-28-26(16-22)31(36-20-35-28)37-24-7-10-30(27(32)17-24)40-19-21-4-3-5-23(33)15-21/h3-11,15-17,20,34H,2,12-14,18-19H2,1H3,(H,35,36,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of ErbB1 |
Bioorg Med Chem Lett 16: 4686-91 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.090
BindingDB Entry DOI: 10.7270/Q2VM4D2Z |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50189108

(CHEMBL380247 | GW574783B | N-(4-(benzyloxy)-3-chlo...)Show SMILES CS(=O)(=O)CCNCc1ccc(o1)-c1ccc2ncnc(Nc3ccc(OCc4ccccc4)c(Cl)c3)c2c1 Show InChI InChI=1S/C29H27ClN4O4S/c1-39(35,36)14-13-31-17-23-9-12-27(38-23)21-7-10-26-24(15-21)29(33-19-32-26)34-22-8-11-28(25(30)16-22)37-18-20-5-3-2-4-6-20/h2-12,15-16,19,31H,13-14,17-18H2,1H3,(H,32,33,34) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 |
Bioorg Med Chem Lett 16: 4686-91 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.090
BindingDB Entry DOI: 10.7270/Q2VM4D2Z |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50189117

(2-(((5-(4-(4-(3-fluorobenzyloxy)-3-chlorophenylami...)Show SMILES CS(=O)(=O)CCN(CC#N)Cc1ccc(o1)-c1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C31H27ClFN5O4S/c1-43(39,40)14-13-38(12-11-34)18-25-7-10-29(42-25)22-5-8-28-26(16-22)31(36-20-35-28)37-24-6-9-30(27(32)17-24)41-19-21-3-2-4-23(33)15-21/h2-10,15-17,20H,12-14,18-19H2,1H3,(H,35,36,37) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 |
Bioorg Med Chem Lett 16: 4686-91 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.090
BindingDB Entry DOI: 10.7270/Q2VM4D2Z |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50189123

((4-benzyloxy-3-chloro-phenyl)-{6-[5-(1-oxo-1lambda...)Show SMILES Clc1cc(Nc2ncnc3ccc(cc23)-c2ccc(CN3CCS(=O)CC3)o2)ccc1OCc1ccccc1 Show InChI InChI=1S/C30H27ClN4O3S/c31-26-17-23(7-10-29(26)37-19-21-4-2-1-3-5-21)34-30-25-16-22(6-9-27(25)32-20-33-30)28-11-8-24(38-28)18-35-12-14-39(36)15-13-35/h1-11,16-17,20H,12-15,18-19H2,(H,32,33,34) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 |
Bioorg Med Chem Lett 16: 4686-91 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.090
BindingDB Entry DOI: 10.7270/Q2VM4D2Z |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50189124

(CHEMBL213818 | N-(4-(3-fluorobenzyloxy)phenyl)-6-(...)Show SMILES CS(=O)(=O)CCNCc1ccc(o1)-c1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)cc3)c2c1 Show InChI InChI=1S/C29H27FN4O4S/c1-39(35,36)14-13-31-17-25-10-12-28(38-25)21-5-11-27-26(16-21)29(33-19-32-27)34-23-6-8-24(9-7-23)37-18-20-3-2-4-22(30)15-20/h2-12,15-16,19,31H,13-14,17-18H2,1H3,(H,32,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of ErbB1 |
Bioorg Med Chem Lett 16: 4686-91 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.090
BindingDB Entry DOI: 10.7270/Q2VM4D2Z |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50189126

(CHEMBL214487 | N-(4-(3-fluorobenzyloxy)-3-chloroph...)Show SMILES CC(C)S(=O)(=O)CCNCc1ccc(o1)-c1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C31H30ClFN4O4S/c1-20(2)42(38,39)13-12-34-17-25-8-11-29(41-25)22-6-9-28-26(15-22)31(36-19-35-28)37-24-7-10-30(27(32)16-24)40-18-21-4-3-5-23(33)14-21/h3-11,14-16,19-20,34H,12-13,17-18H2,1-2H3,(H,35,36,37) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 |
Bioorg Med Chem Lett 16: 4686-91 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.090
BindingDB Entry DOI: 10.7270/Q2VM4D2Z |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5460

(6-alkoxy-4-anilinoquinazoline 8f | N-{1-[(3-fluoro...)Show SMILES CS(=O)(=O)CCNCCCCOc1ccc2ncnc(Nc3ccc4n(Cc5cccc(F)c5)ncc4c3)c2c1 Show InChI InChI=1S/C29H31FN6O3S/c1-40(37,38)14-12-31-11-2-3-13-39-25-8-9-27-26(17-25)29(33-20-32-27)35-24-7-10-28-22(16-24)18-34-36(28)19-21-5-4-6-23(30)15-21/h4-10,15-18,20,31H,2-3,11-14,19H2,1H3,(H,32,33,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GlaxoSmithKline
| Assay Description
Enzymatic reactions were initiated by adding kinase to the reaction mixture containing ATP, [gamma-33P] ATP, peptide substrate and test inhibitor com... |
Bioorg Med Chem Lett 13: 637-40 (2003)
Article DOI: 10.1016/s0960-894x(02)01047-8
BindingDB Entry DOI: 10.7270/Q2D21VS2 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50189105

(CHEMBL214689 | N-(4-(benzyloxy)-3-bromophenyl)-6-(...)Show SMILES CS(=O)(=O)CCNCc1ccc(o1)-c1ccc2ncnc(Nc3ccc(OCc4ccccc4)c(Br)c3)c2c1 Show InChI InChI=1S/C29H27BrN4O4S/c1-39(35,36)14-13-31-17-23-9-12-27(38-23)21-7-10-26-24(15-21)29(33-19-32-26)34-22-8-11-28(25(30)16-22)37-18-20-5-3-2-4-6-20/h2-12,15-16,19,31H,13-14,17-18H2,1H3,(H,32,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of ErbB1 |
Bioorg Med Chem Lett 16: 4686-91 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.090
BindingDB Entry DOI: 10.7270/Q2VM4D2Z |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50189128

(CHEMBL212250 | N-(4-(3-fluorobenzyloxy)-3-chloroph...)Show SMILES Fc1cccc(COc2ccc(Nc3ncnc4ccc(cc34)-c3ccc(COCCS(=O)(=O)c4ccccc4)o3)cc2Cl)c1 Show InChI InChI=1S/C34H27ClFN3O5S/c35-30-19-26(10-13-33(30)43-20-23-5-4-6-25(36)17-23)39-34-29-18-24(9-12-31(29)37-22-38-34)32-14-11-27(44-32)21-42-15-16-45(40,41)28-7-2-1-3-8-28/h1-14,17-19,22H,15-16,20-21H2,(H,37,38,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of ErbB1 |
Bioorg Med Chem Lett 16: 4686-91 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.090
BindingDB Entry DOI: 10.7270/Q2VM4D2Z |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50189105

(CHEMBL214689 | N-(4-(benzyloxy)-3-bromophenyl)-6-(...)Show SMILES CS(=O)(=O)CCNCc1ccc(o1)-c1ccc2ncnc(Nc3ccc(OCc4ccccc4)c(Br)c3)c2c1 Show InChI InChI=1S/C29H27BrN4O4S/c1-39(35,36)14-13-31-17-23-9-12-27(38-23)21-7-10-26-24(15-21)29(33-19-32-26)34-22-8-11-28(25(30)16-22)37-18-20-5-3-2-4-6-20/h2-12,15-16,19,31H,13-14,17-18H2,1H3,(H,32,33,34) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 |
Bioorg Med Chem Lett 16: 4686-91 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.090
BindingDB Entry DOI: 10.7270/Q2VM4D2Z |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50189114

(CHEMBL265367 | N-(4-(3-fluorobenzyloxy)-3-(trifluo...)Show SMILES Cn1ccnc1S(=O)(=O)CCNCc1ccc(o1)-c1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(c3)C(F)(F)F)c2c1 Show InChI InChI=1S/C33H28F4N6O4S/c1-43-13-11-39-32(43)48(44,45)14-12-38-18-25-7-10-29(47-25)22-5-8-28-26(16-22)31(41-20-40-28)42-24-6-9-30(27(17-24)33(35,36)37)46-19-21-3-2-4-23(34)15-21/h2-11,13,15-17,20,38H,12,14,18-19H2,1H3,(H,40,41,42) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 |
Bioorg Med Chem Lett 16: 4686-91 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.090
BindingDB Entry DOI: 10.7270/Q2VM4D2Z |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50189116

(CHEMBL213342 | N-(4-(benzyloxy)phenyl)-6-(5-((2-(m...)Show SMILES CS(=O)(=O)CCNCc1ccc(o1)-c1ccc2ncnc(Nc3ccc(OCc4ccccc4)cc3)c2c1 Show InChI InChI=1S/C29H28N4O4S/c1-38(34,35)16-15-30-18-25-12-14-28(37-25)22-7-13-27-26(17-22)29(32-20-31-27)33-23-8-10-24(11-9-23)36-19-21-5-3-2-4-6-21/h2-14,17,20,30H,15-16,18-19H2,1H3,(H,31,32,33) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 |
Bioorg Med Chem Lett 16: 4686-91 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.090
BindingDB Entry DOI: 10.7270/Q2VM4D2Z |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50189107

(CHEMBL215171 | N-(4-(3-fluorobenzyloxy)-3-chloroph...)Show SMILES CS(=O)(=O)CCOCc1ccc(o1)-c1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C29H25ClFN3O5S/c1-40(35,36)12-11-37-17-23-7-10-27(39-23)20-5-8-26-24(14-20)29(33-18-32-26)34-22-6-9-28(25(30)15-22)38-16-19-3-2-4-21(31)13-19/h2-10,13-15,18H,11-12,16-17H2,1H3,(H,32,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of ErbB1 |
Bioorg Med Chem Lett 16: 4686-91 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.090
BindingDB Entry DOI: 10.7270/Q2VM4D2Z |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50189112

(CHEMBL383965 | N-(4-(3-fluorobenzyloxy)-3-chloroph...)Show SMILES CCCS(=O)(=O)CCNCc1ccc(o1)-c1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C31H30ClFN4O4S/c1-2-13-42(38,39)14-12-34-18-25-8-11-29(41-25)22-6-9-28-26(16-22)31(36-20-35-28)37-24-7-10-30(27(32)17-24)40-19-21-4-3-5-23(33)15-21/h3-11,15-17,20,34H,2,12-14,18-19H2,1H3,(H,35,36,37) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 |
Bioorg Med Chem Lett 16: 4686-91 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.090
BindingDB Entry DOI: 10.7270/Q2VM4D2Z |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5459

(6-alkoxy-4-anilinoquinazoline 8e | N-{3-chloro-4-[...)Show SMILES CS(=O)(=O)CCNCCCCOc1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C28H30ClFN4O4S/c1-39(35,36)14-12-31-11-2-3-13-37-23-8-9-26-24(17-23)28(33-19-32-26)34-22-7-10-27(25(29)16-22)38-18-20-5-4-6-21(30)15-20/h4-10,15-17,19,31H,2-3,11-14,18H2,1H3,(H,32,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GlaxoSmithKline
| Assay Description
Enzymatic reactions were initiated by adding kinase to the reaction mixture containing ATP, [gamma-33P] ATP, peptide substrate and test inhibitor com... |
Bioorg Med Chem Lett 13: 637-40 (2003)
Article DOI: 10.1016/s0960-894x(02)01047-8
BindingDB Entry DOI: 10.7270/Q2D21VS2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data