

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lipopolysaccharide heptosyltransferase 1 (Escherichia coli (strain K12)) | BDBM34103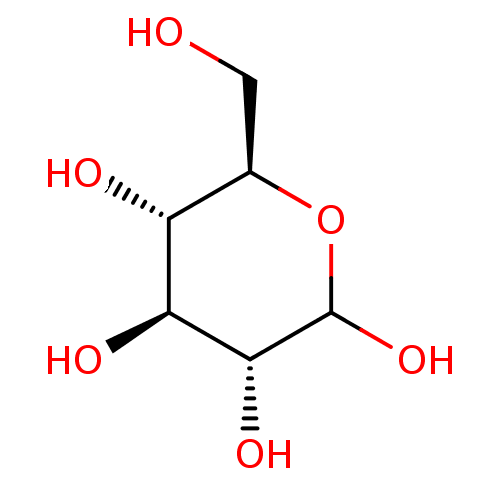 (D-glucose | dextrose | glucose) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wesleyan University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli Heptosyltransferase I assessed as reduction in ADP release using ODLA and ADP-heptose substrates in presence of phosph... | Bioorg Med Chem Lett 28: 594-600 (2018) Article DOI: 10.1016/j.bmcl.2018.01.040 BindingDB Entry DOI: 10.7270/Q2J38W63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipopolysaccharide heptosyltransferase 1 (Escherichia coli (strain K12)) | BDBM50454776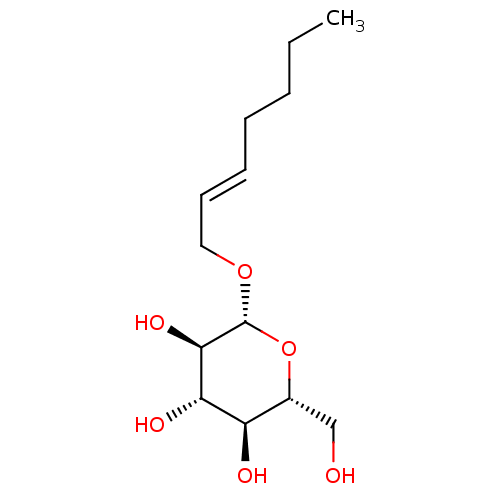 (CHEMBL4209246) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 3.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wesleyan University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli Heptosyltransferase I assessed as reduction in ADP release using ODLA and ADP-heptose substrates in presence of phosph... | Bioorg Med Chem Lett 28: 594-600 (2018) Article DOI: 10.1016/j.bmcl.2018.01.040 BindingDB Entry DOI: 10.7270/Q2J38W63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipopolysaccharide heptosyltransferase 1 (Escherichia coli (strain K12)) | BDBM20876 ((2R,3S,4S,5R,6S)-2-(hydroxymethyl)-6-methoxyoxane-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wesleyan University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli Heptosyltransferase I assessed as reduction in ADP release using ODLA and ADP-heptose substrates in presence of phosph... | Bioorg Med Chem Lett 28: 594-600 (2018) Article DOI: 10.1016/j.bmcl.2018.01.040 BindingDB Entry DOI: 10.7270/Q2J38W63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipopolysaccharide heptosyltransferase 1 (Escherichia coli (strain K12)) | BDBM228805 (Galactose) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wesleyan University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli Heptosyltransferase I assessed as reduction in ADP release using ODLA and ADP-heptose substrates in presence of phosph... | Bioorg Med Chem Lett 28: 594-600 (2018) Article DOI: 10.1016/j.bmcl.2018.01.040 BindingDB Entry DOI: 10.7270/Q2J38W63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipopolysaccharide heptosyltransferase 1 (Escherichia coli (strain K12)) | BDBM50454780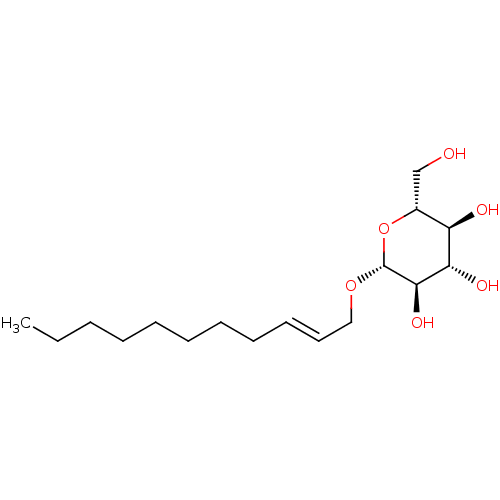 (CHEMBL4212177) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 5.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wesleyan University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli Heptosyltransferase I assessed as reduction in ADP release using ODLA and ADP-heptose substrates in presence of phosph... | Bioorg Med Chem Lett 28: 594-600 (2018) Article DOI: 10.1016/j.bmcl.2018.01.040 BindingDB Entry DOI: 10.7270/Q2J38W63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipopolysaccharide heptosyltransferase 1 (Escherichia coli (strain K12)) | BDBM50454777 (CHEMBL4205643) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 5.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wesleyan University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli Heptosyltransferase I assessed as reduction in ADP release using ODLA and ADP-heptose substrates in presence of phosph... | Bioorg Med Chem Lett 28: 594-600 (2018) Article DOI: 10.1016/j.bmcl.2018.01.040 BindingDB Entry DOI: 10.7270/Q2J38W63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipopolysaccharide heptosyltransferase 1 (Escherichia coli (strain K12)) | BDBM50454779 (CHEMBL4213641) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem | Article PubMed | 6.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wesleyan University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli Heptosyltransferase I assessed as reduction in ADP release using ODLA and ADP-heptose substrates in presence of phosph... | Bioorg Med Chem Lett 28: 594-600 (2018) Article DOI: 10.1016/j.bmcl.2018.01.040 BindingDB Entry DOI: 10.7270/Q2J38W63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipopolysaccharide heptosyltransferase 1 (Escherichia coli (strain K12)) | BDBM50454778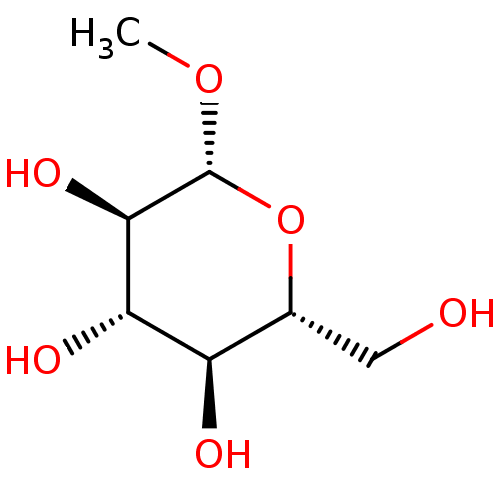 (CHEBI:320055 | CHEMBL132186) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem | Article PubMed | 6.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wesleyan University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli Heptosyltransferase I assessed as reduction in ADP release using ODLA and ADP-heptose substrates in presence of phosph... | Bioorg Med Chem Lett 28: 594-600 (2018) Article DOI: 10.1016/j.bmcl.2018.01.040 BindingDB Entry DOI: 10.7270/Q2J38W63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50555366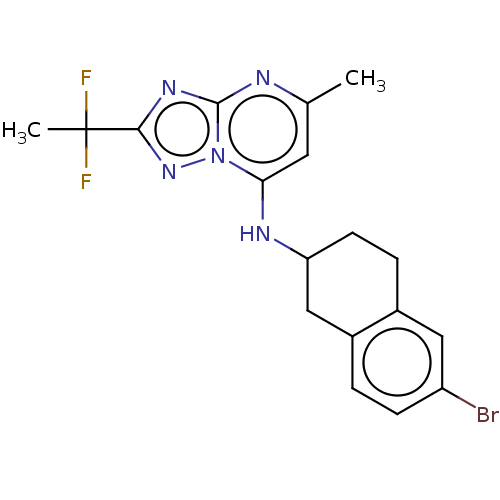 (CHEMBL4747214) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human ERG by patch clamp electrophysiology assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b00275 BindingDB Entry DOI: 10.7270/Q2R78JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50555369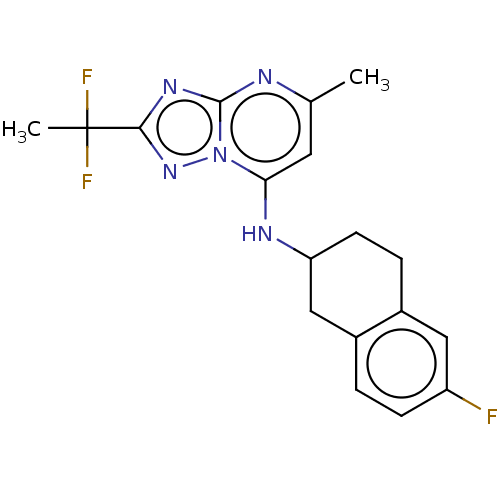 (CHEMBL4740960) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human ERG by patch clamp electrophysiology assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b00275 BindingDB Entry DOI: 10.7270/Q2R78JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50555367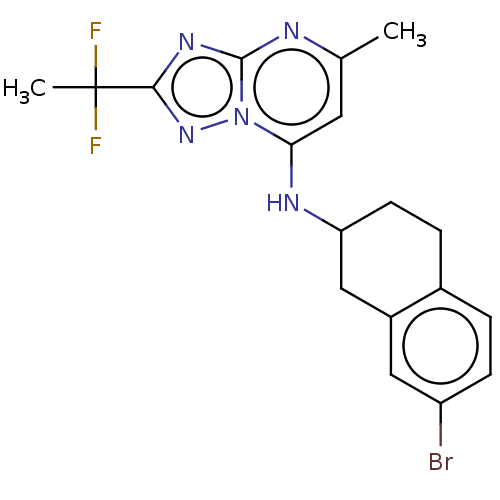 (CHEMBL4753862) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human ERG by patch clamp electrophysiology assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b00275 BindingDB Entry DOI: 10.7270/Q2R78JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50555364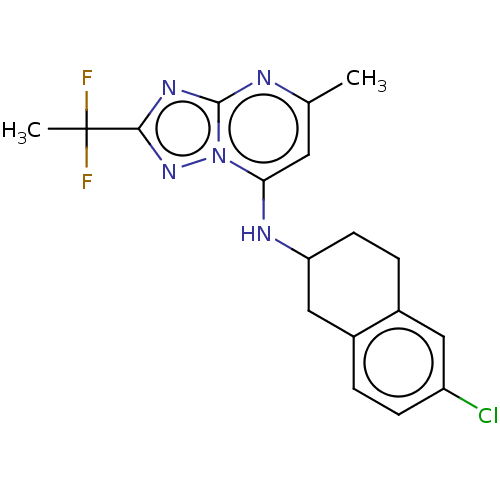 (CHEMBL4761666) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human ERG by patch clamp electrophysiology assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b00275 BindingDB Entry DOI: 10.7270/Q2R78JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50555365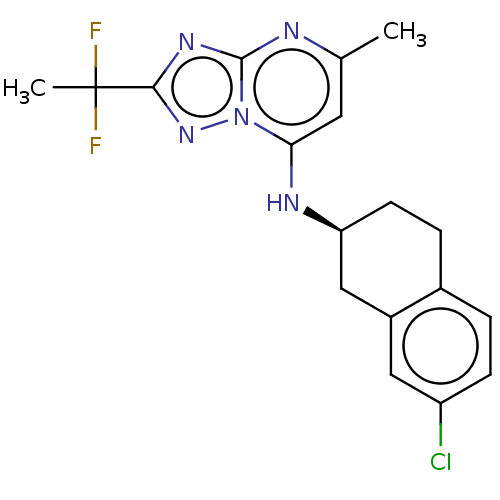 (CHEMBL4751920) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human ERG by patch clamp electrophysiology assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b00275 BindingDB Entry DOI: 10.7270/Q2R78JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50365230 (CHEMBL1956285 | US11903936, Compound DSM265 | US92...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human ERG by patch clamp electrophysiology assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b00275 BindingDB Entry DOI: 10.7270/Q2R78JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50555368 (CHEMBL4794107) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human ERG by patch clamp electrophysiology assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b00275 BindingDB Entry DOI: 10.7270/Q2R78JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50555372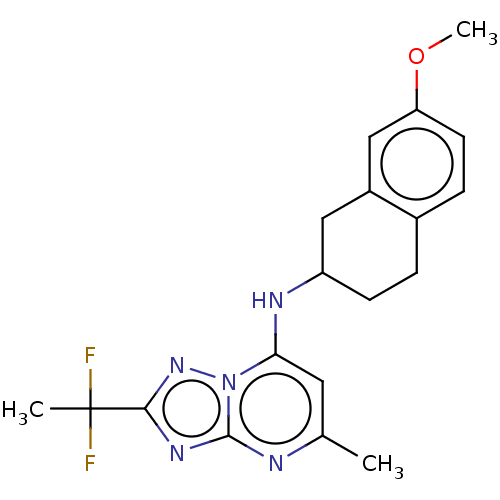 (CHEMBL4744317) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human ERG by patch clamp electrophysiology assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b00275 BindingDB Entry DOI: 10.7270/Q2R78JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50555365 (CHEMBL4751920) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP2C9 in human liver microsomes using isoform-specific probe substrates in presence of NADPH-generating system by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b00275 BindingDB Entry DOI: 10.7270/Q2R78JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Mus musculus) | BDBM50365230 (CHEMBL1956285 | US11903936, Compound DSM265 | US92...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of N-terminal His6-tagged mouse DHODH expressed in Escherichia coli BL21 using L-DHO as substrate and CoQ as co-substrate by DCIP dye base... | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b00275 BindingDB Entry DOI: 10.7270/Q2R78JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Rattus norvegicus (rat)) | BDBM50365230 (CHEMBL1956285 | US11903936, Compound DSM265 | US92...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of N-terminal His6-tagged rat DHODH expressed in Escherichia coli BL21 expressed in Escherichia coli BL21 using L-DHO as substrate and CoQ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b00275 BindingDB Entry DOI: 10.7270/Q2R78JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50555365 (CHEMBL4751920) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP2D6 in human liver microsomes using isoform-specific probe substrates in presence of NADPH-generating system by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b00275 BindingDB Entry DOI: 10.7270/Q2R78JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50555367 (CHEMBL4753862) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP2C9 in human liver microsomes using isoform-specific probe substrates in presence of NADPH-generating system by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b00275 BindingDB Entry DOI: 10.7270/Q2R78JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50555367 (CHEMBL4753862) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP2D6 in human liver microsomes using isoform-specific probe substrates in presence of NADPH-generating system by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b00275 BindingDB Entry DOI: 10.7270/Q2R78JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50555368 (CHEMBL4794107) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP2D6 in human liver microsomes using isoform-specific probe substrates in presence of NADPH-generating system by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b00275 BindingDB Entry DOI: 10.7270/Q2R78JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50555366 (CHEMBL4747214) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP2C9 in human liver microsomes using isoform-specific probe substrates in presence of NADPH-generating system by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b00275 BindingDB Entry DOI: 10.7270/Q2R78JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50555364 (CHEMBL4761666) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP2C9 in human liver microsomes using isoform-specific probe substrates in presence of NADPH-generating system by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b00275 BindingDB Entry DOI: 10.7270/Q2R78JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50555367 (CHEMBL4753862) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP2C8 in human liver microsomes using isoform-specific probe substrates in presence of NADPH-generating system by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b00275 BindingDB Entry DOI: 10.7270/Q2R78JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B6 (Homo sapiens (Human)) | BDBM50555366 (CHEMBL4747214) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP2B6 in human liver microsomes using isoform-specific probe substrates in presence of NADPH-generating system by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b00275 BindingDB Entry DOI: 10.7270/Q2R78JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B6 (Homo sapiens (Human)) | BDBM50555364 (CHEMBL4761666) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP2B6 in human liver microsomes using isoform-specific probe substrates in presence of NADPH-generating system by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b00275 BindingDB Entry DOI: 10.7270/Q2R78JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50555368 (CHEMBL4794107) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP2C9 in human liver microsomes using isoform-specific probe substrates in presence of NADPH-generating system by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b00275 BindingDB Entry DOI: 10.7270/Q2R78JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50555366 (CHEMBL4747214) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.87E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP2C8 in human liver microsomes using isoform-specific probe substrates in presence of NADPH-generating system by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b00275 BindingDB Entry DOI: 10.7270/Q2R78JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50555366 (CHEMBL4747214) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP2D6 in human liver microsomes using isoform-specific probe substrates in presence of NADPH-generating system by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b00275 BindingDB Entry DOI: 10.7270/Q2R78JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50555364 (CHEMBL4761666) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP2D6 in human liver microsomes using isoform-specific probe substrates in presence of NADPH-generating system by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b00275 BindingDB Entry DOI: 10.7270/Q2R78JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50555365 (CHEMBL4751920) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP2C8 in human liver microsomes using isoform-specific probe substrates in presence of NADPH-generating system by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b00275 BindingDB Entry DOI: 10.7270/Q2R78JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50555364 (CHEMBL4761666) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP2C8 in human liver microsomes using isoform-specific probe substrates in presence of NADPH-generating system by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b00275 BindingDB Entry DOI: 10.7270/Q2R78JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B6 (Homo sapiens (Human)) | BDBM50555368 (CHEMBL4794107) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP2B6 in human liver microsomes using isoform-specific probe substrates in presence of NADPH-generating system by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b00275 BindingDB Entry DOI: 10.7270/Q2R78JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B6 (Homo sapiens (Human)) | BDBM50555367 (CHEMBL4753862) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP2B6 in human liver microsomes using isoform-specific probe substrates in presence of NADPH-generating system by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b00275 BindingDB Entry DOI: 10.7270/Q2R78JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B6 (Homo sapiens (Human)) | BDBM50555365 (CHEMBL4751920) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP2B6 in human liver microsomes using isoform-specific probe substrates in presence of NADPH-generating system by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b00275 BindingDB Entry DOI: 10.7270/Q2R78JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50555368 (CHEMBL4794107) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP1A2 in human liver microsomes using isoform-specific probe substrate in presence of NADPH-generating system by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b00275 BindingDB Entry DOI: 10.7270/Q2R78JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50555367 (CHEMBL4753862) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP1A2 in human liver microsomes using isoform-specific probe substrate in presence of NADPH-generating system by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b00275 BindingDB Entry DOI: 10.7270/Q2R78JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50555366 (CHEMBL4747214) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP1A2 in human liver microsomes using isoform-specific probe substrate in presence of NADPH-generating system by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b00275 BindingDB Entry DOI: 10.7270/Q2R78JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50555365 (CHEMBL4751920) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP1A2 in human liver microsomes using isoform-specific probe substrate in presence of NADPH-generating system by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b00275 BindingDB Entry DOI: 10.7270/Q2R78JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50555364 (CHEMBL4761666) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP1A2 in human liver microsomes using isoform-specific probe substrate in presence of NADPH-generating system by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b00275 BindingDB Entry DOI: 10.7270/Q2R78JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50555364 (CHEMBL4761666) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP2C19 in human liver microsomes using isoform-specific probe substrates in presence of NADPH-generating system by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b00275 BindingDB Entry DOI: 10.7270/Q2R78JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50555368 (CHEMBL4794107) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP2C19 in human liver microsomes using isoform-specific probe substrates in presence of NADPH-generating system by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b00275 BindingDB Entry DOI: 10.7270/Q2R78JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50555365 (CHEMBL4751920) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP2C19 in human liver microsomes using isoform-specific probe substrates in presence of NADPH-generating system by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b00275 BindingDB Entry DOI: 10.7270/Q2R78JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50555366 (CHEMBL4747214) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP2C19 in human liver microsomes using isoform-specific probe substrates in presence of NADPH-generating system by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b00275 BindingDB Entry DOI: 10.7270/Q2R78JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50555367 (CHEMBL4753862) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP2C19 in human liver microsomes using isoform-specific probe substrates in presence of NADPH-generating system by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b00275 BindingDB Entry DOI: 10.7270/Q2R78JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50555368 (CHEMBL4794107) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP3A4 in human liver microsomes using isoform-specific probe substrates in presence of NADPH-generating system by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b00275 BindingDB Entry DOI: 10.7270/Q2R78JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50555364 (CHEMBL4761666) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP3A4 in human liver microsomes using isoform-specific probe substrates in presence of NADPH-generating system by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b00275 BindingDB Entry DOI: 10.7270/Q2R78JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50555365 (CHEMBL4751920) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP3A4 in human liver microsomes using isoform-specific probe substrates in presence of NADPH-generating system by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b00275 BindingDB Entry DOI: 10.7270/Q2R78JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 109 total ) | Next | Last >> |