

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50315314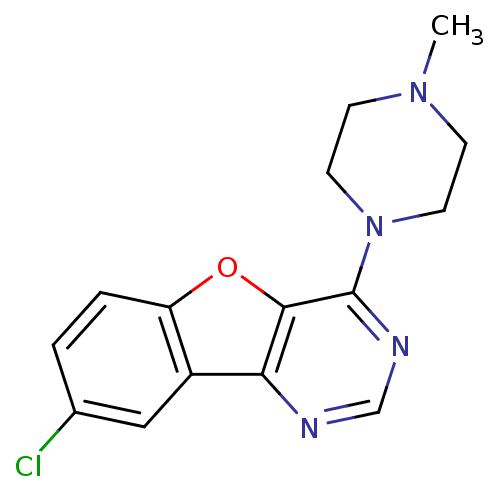 (8-chloro-4-(4-methylpiperazin-1-yl)benzofuro[3,2-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]histamine human recombinant histamine H4 receptor | Bioorg Med Chem Lett 20: 2516-9 (2010) Article DOI: 10.1016/j.bmcl.2010.02.097 BindingDB Entry DOI: 10.7270/Q2CN74V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM22914 (CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]histamine human recombinant histamine H4 receptor | Bioorg Med Chem Lett 20: 2516-9 (2010) Article DOI: 10.1016/j.bmcl.2010.02.097 BindingDB Entry DOI: 10.7270/Q2CN74V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM22566 (5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]histamine human recombinant histamine H4 receptor | Bioorg Med Chem Lett 20: 2516-9 (2010) Article DOI: 10.1016/j.bmcl.2010.02.097 BindingDB Entry DOI: 10.7270/Q2CN74V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50315348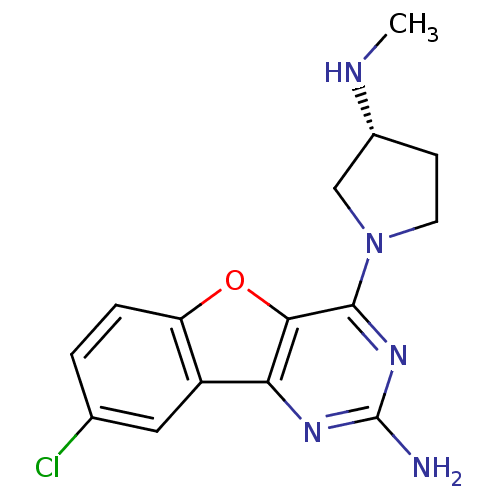 ((R)-8-chloro-4-(3-(methylamino)pyrrolidin-1-yl)ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]histamine dihydrochloride from human histamine H4 receptor after 2.5 hrs by scintillation proximity assay | Bioorg Med Chem Lett 20: 2516-9 (2010) Article DOI: 10.1016/j.bmcl.2010.02.097 BindingDB Entry DOI: 10.7270/Q2CN74V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50544399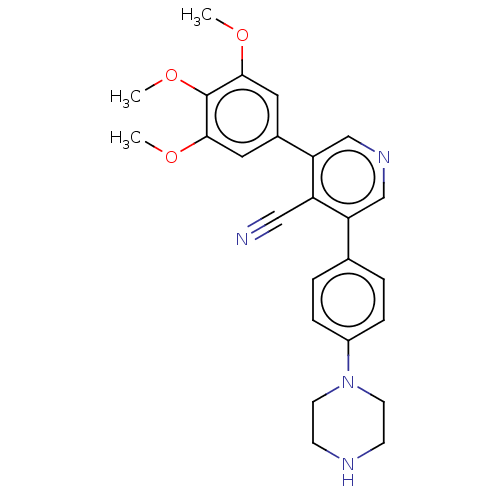 (CHEMBL4638273) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human ALK2 using casein as substrate in presence of [gamma-33P]-ATP by radiometric hotspot assay | J Med Chem 63: 10061-10085 (2020) Article DOI: 10.1021/acs.jmedchem.0c01199 BindingDB Entry DOI: 10.7270/Q2VM4GV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50315348 ((R)-8-chloro-4-(3-(methylamino)pyrrolidin-1-yl)ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Inverse agonist activity at human histamine H4 receptor assessed as inhibition of [35S]GTPgammaS binding after 15 mins by scintillation proximity ass... | Bioorg Med Chem Lett 20: 2516-9 (2010) Article DOI: 10.1016/j.bmcl.2010.02.097 BindingDB Entry DOI: 10.7270/Q2CN74V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50544408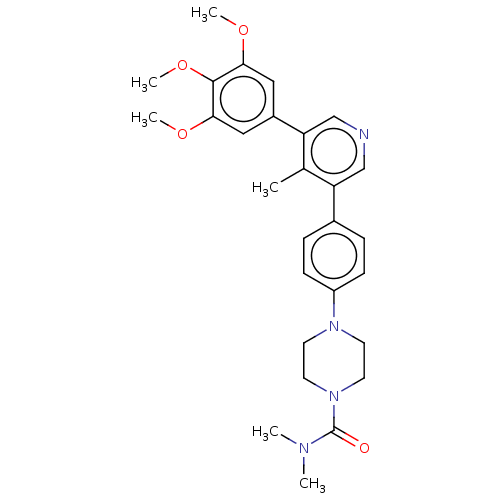 (CHEMBL4641207) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human ALK2 using casein as substrate in presence of [gamma-33P]-ATP by radiometric hotspot assay | J Med Chem 63: 10061-10085 (2020) Article DOI: 10.1021/acs.jmedchem.0c01199 BindingDB Entry DOI: 10.7270/Q2VM4GV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50315349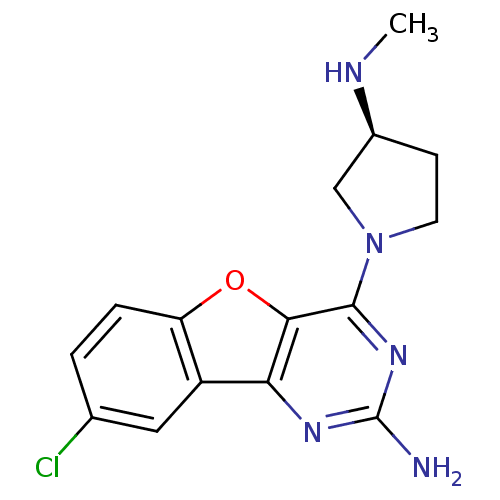 ((S)-8-chloro-4-(3-(methylamino)pyrrolidin-1-yl)ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Inverse agonist activity at human histamine H4 receptor assessed as inhibition of [35S]GTPgammaS binding after 15 mins by scintillation proximity ass... | Bioorg Med Chem Lett 20: 2516-9 (2010) Article DOI: 10.1016/j.bmcl.2010.02.097 BindingDB Entry DOI: 10.7270/Q2CN74V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50544414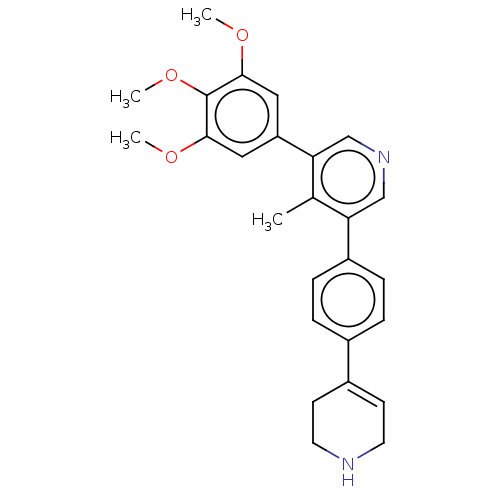 (CHEMBL4641530) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human ALK2 using casein as substrate in presence of [gamma-33P]-ATP by radiometric hotspot assay | J Med Chem 63: 10061-10085 (2020) Article DOI: 10.1021/acs.jmedchem.0c01199 BindingDB Entry DOI: 10.7270/Q2VM4GV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50544418 (CHEMBL4648102) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human ALK2 using casein as substrate in presence of [gamma-33P]-ATP by radiometric hotspot assay | J Med Chem 63: 10061-10085 (2020) Article DOI: 10.1021/acs.jmedchem.0c01199 BindingDB Entry DOI: 10.7270/Q2VM4GV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50528194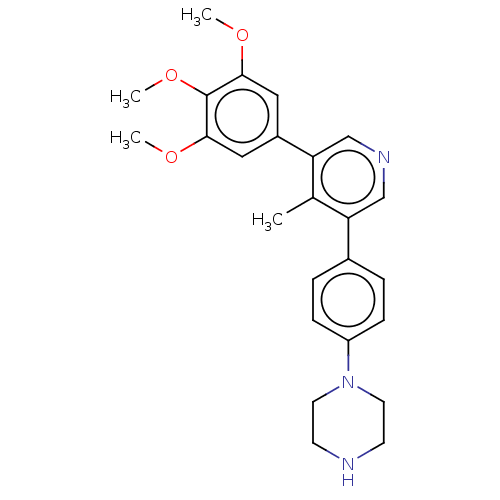 (CHEMBL4517408) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research Curated by ChEMBL | Assay Description Competitive displacement of PBI-6908 from nanoluciferase-fused ALK2 G328V mutant (unknown origin) expressed in HEK293 cells incubated for 2 hrs by Na... | J Med Chem 63: 10061-10085 (2020) Article DOI: 10.1021/acs.jmedchem.0c01199 BindingDB Entry DOI: 10.7270/Q2VM4GV3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50544419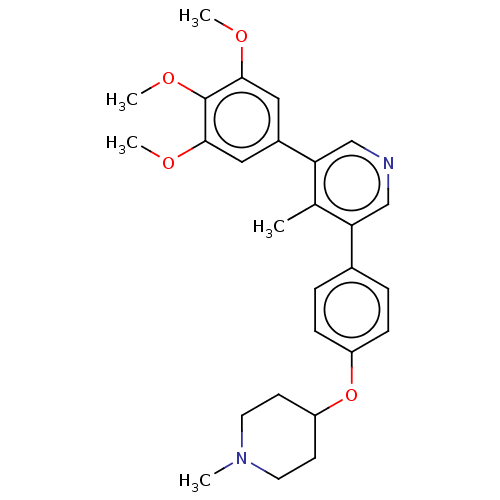 (CHEMBL4647724) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human ALK2 using casein as substrate in presence of [gamma-33P]-ATP by radiometric hotspot assay | J Med Chem 63: 10061-10085 (2020) Article DOI: 10.1021/acs.jmedchem.0c01199 BindingDB Entry DOI: 10.7270/Q2VM4GV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50528194 (CHEMBL4517408) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research Curated by ChEMBL | Assay Description Competitive displacement of PBI-6908 from nanoluciferase-fused ALK2 Q207D mutant (unknown origin) expressed in HEK293 cells incubated for 2 hrs by Na... | J Med Chem 63: 10061-10085 (2020) Article DOI: 10.1021/acs.jmedchem.0c01199 BindingDB Entry DOI: 10.7270/Q2VM4GV3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50544404 (CHEMBL4644571) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human ALK2 using casein as substrate in presence of [gamma-33P]-ATP by radiometric hotspot assay | J Med Chem 63: 10061-10085 (2020) Article DOI: 10.1021/acs.jmedchem.0c01199 BindingDB Entry DOI: 10.7270/Q2VM4GV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50544413 (CHEMBL4633241) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human ALK2 using casein as substrate in presence of [gamma-33P]-ATP by radiometric hotspot assay | J Med Chem 63: 10061-10085 (2020) Article DOI: 10.1021/acs.jmedchem.0c01199 BindingDB Entry DOI: 10.7270/Q2VM4GV3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50528194 (CHEMBL4517408) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of ALK2 G328V mutant (unknown origin) by radiometric assay | J Med Chem 63: 10061-10085 (2020) Article DOI: 10.1021/acs.jmedchem.0c01199 BindingDB Entry DOI: 10.7270/Q2VM4GV3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50544405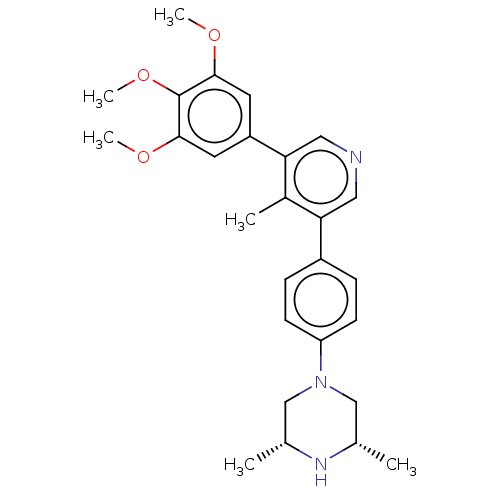 (CHEMBL4634857) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human ALK2 using casein as substrate in presence of [gamma-33P]-ATP by radiometric hotspot assay | J Med Chem 63: 10061-10085 (2020) Article DOI: 10.1021/acs.jmedchem.0c01199 BindingDB Entry DOI: 10.7270/Q2VM4GV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50200783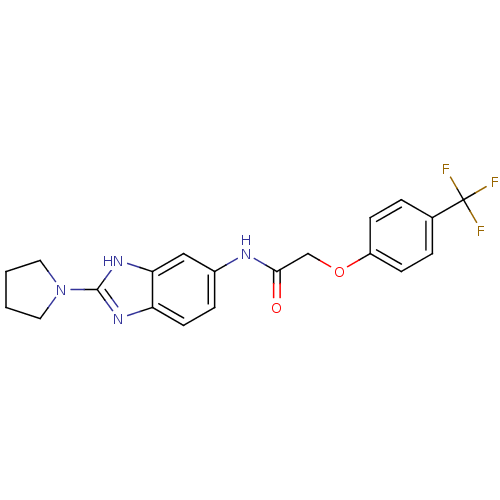 (ADS-103168 | CHEMBL424739 | N-(2-(pyrrolidin-1-yl)...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Limited Curated by ChEMBL | Assay Description Inhibition of MCH-mediated calcium influx into MCH-R1 expressing cells | Bioorg Med Chem Lett 17: 1403-7 (2007) Article DOI: 10.1016/j.bmcl.2006.11.092 BindingDB Entry DOI: 10.7270/Q2G73DCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50528194 (CHEMBL4517408) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human ALK2 R206H mutant using casein as substrate in presence of [gamma-33P]-ATP by radiometric hotspot assay | J Med Chem 63: 10061-10085 (2020) Article DOI: 10.1021/acs.jmedchem.0c01199 BindingDB Entry DOI: 10.7270/Q2VM4GV3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50544412 (CHEMBL4635321) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human ALK2 using casein as substrate in presence of [gamma-33P]-ATP by radiometric hotspot assay | J Med Chem 63: 10061-10085 (2020) Article DOI: 10.1021/acs.jmedchem.0c01199 BindingDB Entry DOI: 10.7270/Q2VM4GV3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50544403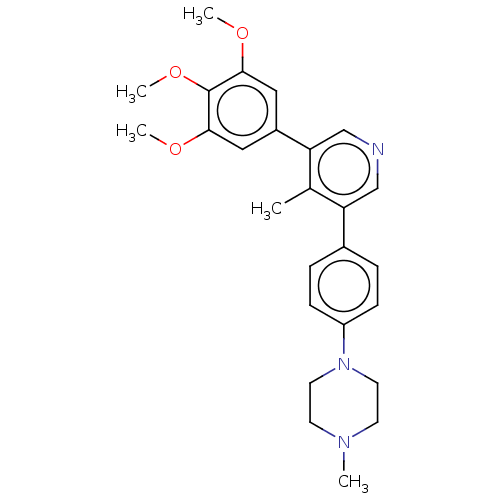 (CHEMBL4643265) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human ALK2 using casein as substrate in presence of [gamma-33P]-ATP by radiometric hotspot assay | J Med Chem 63: 10061-10085 (2020) Article DOI: 10.1021/acs.jmedchem.0c01199 BindingDB Entry DOI: 10.7270/Q2VM4GV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50200793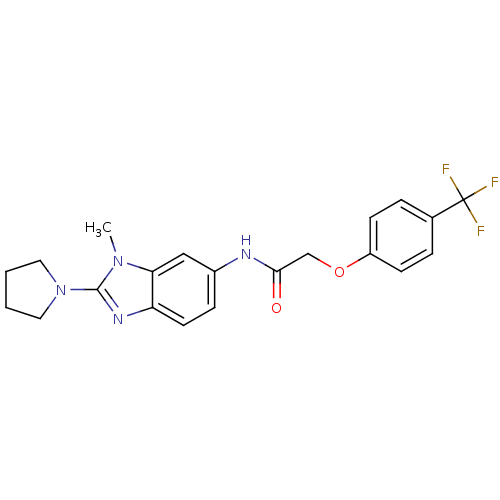 (ADS-103214 | CHEMBL216430 | N-(1-methyl-2-(pyrroli...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Limited Curated by ChEMBL | Assay Description Inhibition of MCH-mediated calcium influx into MCH-R1 expressing cells | Bioorg Med Chem Lett 17: 1403-7 (2007) Article DOI: 10.1016/j.bmcl.2006.11.092 BindingDB Entry DOI: 10.7270/Q2G73DCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50150059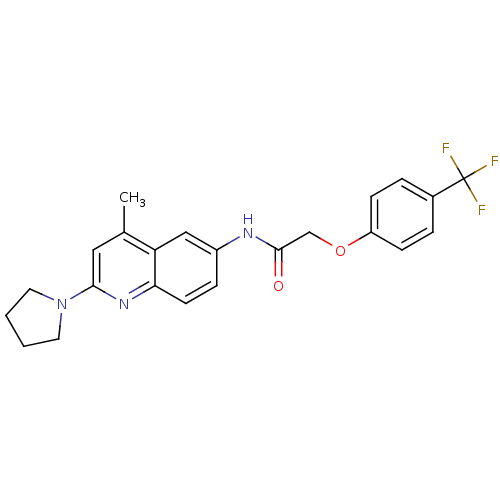 (CHEMBL183215 | N-(4-Methyl-2-pyrrolidin-1-yl-quino...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Limited Curated by ChEMBL | Assay Description Inhibition of MCH-mediated calcium influx into MCH-R1 expressing cells | Bioorg Med Chem Lett 17: 1403-7 (2007) Article DOI: 10.1016/j.bmcl.2006.11.092 BindingDB Entry DOI: 10.7270/Q2G73DCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50150059 (CHEMBL183215 | N-(4-Methyl-2-pyrrolidin-1-yl-quino...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Limited Curated by ChEMBL | Assay Description Tested for MCH-1 induced [Ca2+] release from CHO cells transfected with human MCH-1R | Bioorg Med Chem Lett 14: 4099-102 (2004) Article DOI: 10.1016/j.bmcl.2004.05.051 BindingDB Entry DOI: 10.7270/Q2WW7H49 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50544402 (CHEMBL4646278) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human ALK2 using casein as substrate in presence of [gamma-33P]-ATP by radiometric hotspot assay | J Med Chem 63: 10061-10085 (2020) Article DOI: 10.1021/acs.jmedchem.0c01199 BindingDB Entry DOI: 10.7270/Q2VM4GV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50528194 (CHEMBL4517408) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research Curated by ChEMBL | Assay Description Competitive displacement of PBI-6908 from nanoluciferase-fused ALK2 R206H mutant (unknown origin) expressed in HEK293 cells incubated for 2 hrs by Na... | J Med Chem 63: 10061-10085 (2020) Article DOI: 10.1021/acs.jmedchem.0c01199 BindingDB Entry DOI: 10.7270/Q2VM4GV3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50528194 (CHEMBL4517408) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human ALK2 using casein as substrate in presence of [gamma-33P]-ATP by radiometric hotspot assay | J Med Chem 63: 10061-10085 (2020) Article DOI: 10.1021/acs.jmedchem.0c01199 BindingDB Entry DOI: 10.7270/Q2VM4GV3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50544416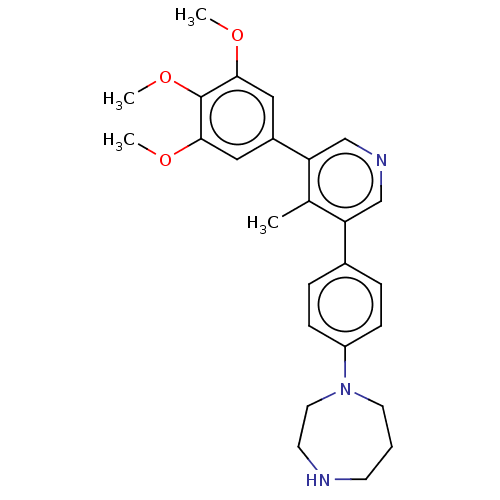 (CHEMBL4639700) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human ALK2 using casein as substrate in presence of [gamma-33P]-ATP by radiometric hotspot assay | J Med Chem 63: 10061-10085 (2020) Article DOI: 10.1021/acs.jmedchem.0c01199 BindingDB Entry DOI: 10.7270/Q2VM4GV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50315349 ((S)-8-chloro-4-(3-(methylamino)pyrrolidin-1-yl)ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]histamine dihydrochloride from human histamine H4 receptor after 2.5 hrs by scintillation proximity assay | Bioorg Med Chem Lett 20: 2516-9 (2010) Article DOI: 10.1016/j.bmcl.2010.02.097 BindingDB Entry DOI: 10.7270/Q2CN74V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50528194 (CHEMBL4517408) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of ALK2 R258G mutant (unknown origin) by radiometric hotspot assay | J Med Chem 63: 10061-10085 (2020) Article DOI: 10.1021/acs.jmedchem.0c01199 BindingDB Entry DOI: 10.7270/Q2VM4GV3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50544415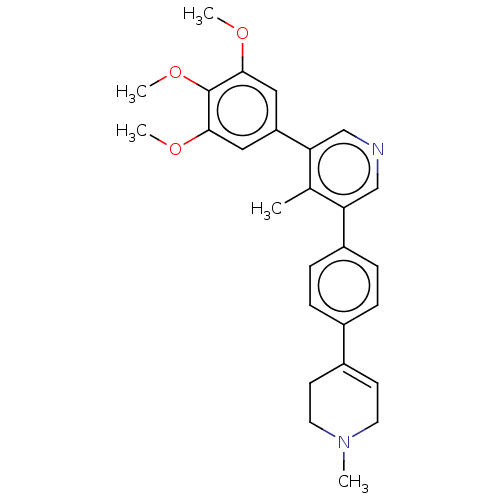 (CHEMBL4646444) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human ALK2 using casein as substrate in presence of [gamma-33P]-ATP by radiometric hotspot assay | J Med Chem 63: 10061-10085 (2020) Article DOI: 10.1021/acs.jmedchem.0c01199 BindingDB Entry DOI: 10.7270/Q2VM4GV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50544399 (CHEMBL4638273) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research Curated by ChEMBL | Assay Description Competitive displacement of PBI-6908 from nanoluciferase-fused ALK2 (unknown origin) expressed in HEK293 cells incubated for 2 hrs by NanoBRET NanoGl... | J Med Chem 63: 10061-10085 (2020) Article DOI: 10.1021/acs.jmedchem.0c01199 BindingDB Entry DOI: 10.7270/Q2VM4GV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50150059 (CHEMBL183215 | N-(4-Methyl-2-pyrrolidin-1-yl-quino...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Limited Curated by ChEMBL | Assay Description Displacement of 125I-[Phe13,Tyr19]-MCH from MCH-R1 expressing cell membranes | Bioorg Med Chem Lett 17: 1403-7 (2007) Article DOI: 10.1016/j.bmcl.2006.11.092 BindingDB Entry DOI: 10.7270/Q2G73DCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase receptor R3 (Homo sapiens (Human)) | BDBM50528194 (CHEMBL4517408) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human ALK1 using casein as substrate in presence of [gamma-33P]-ATP by radiometric hotspot assay | J Med Chem 63: 10061-10085 (2020) Article DOI: 10.1021/acs.jmedchem.0c01199 BindingDB Entry DOI: 10.7270/Q2VM4GV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50544396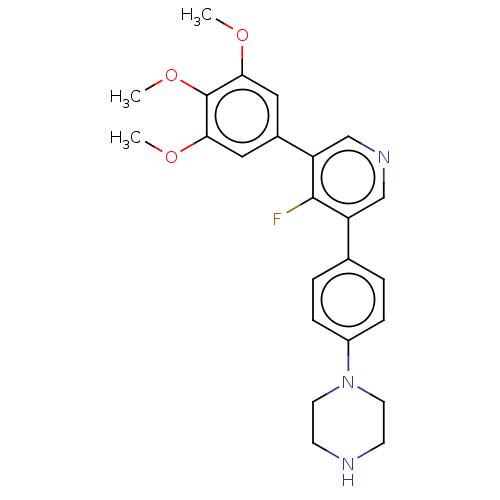 (CHEMBL4633121) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human ALK2 using casein as substrate in presence of [gamma-33P]-ATP by radiometric hotspot assay | J Med Chem 63: 10061-10085 (2020) Article DOI: 10.1021/acs.jmedchem.0c01199 BindingDB Entry DOI: 10.7270/Q2VM4GV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50528194 (CHEMBL4517408) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research Curated by ChEMBL | Assay Description Competitive displacement of PBI-6908 from nanoluciferase-fused ALK2 G356D mutant (unknown origin) expressed in HEK293 cells incubated for 2 hrs by Na... | J Med Chem 63: 10061-10085 (2020) Article DOI: 10.1021/acs.jmedchem.0c01199 BindingDB Entry DOI: 10.7270/Q2VM4GV3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50544411 (CHEMBL4648735) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human ALK2 using casein as substrate in presence of [gamma-33P]-ATP by radiometric hotspot assay | J Med Chem 63: 10061-10085 (2020) Article DOI: 10.1021/acs.jmedchem.0c01199 BindingDB Entry DOI: 10.7270/Q2VM4GV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50544397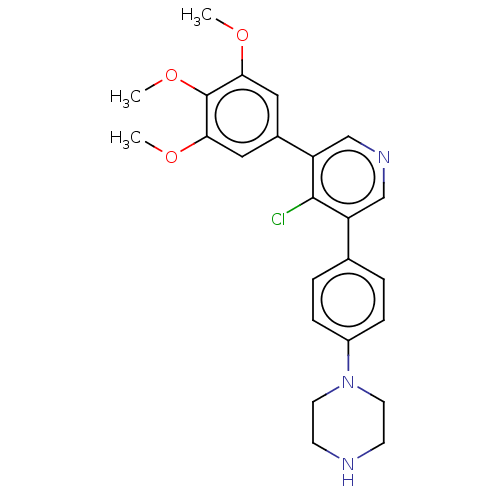 (CHEMBL4642165) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human ALK2 using casein as substrate in presence of [gamma-33P]-ATP by radiometric hotspot assay | J Med Chem 63: 10061-10085 (2020) Article DOI: 10.1021/acs.jmedchem.0c01199 BindingDB Entry DOI: 10.7270/Q2VM4GV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50544406 (CHEMBL4647991) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human ALK2 using casein as substrate in presence of [gamma-33P]-ATP by radiometric hotspot assay | J Med Chem 63: 10061-10085 (2020) Article DOI: 10.1021/acs.jmedchem.0c01199 BindingDB Entry DOI: 10.7270/Q2VM4GV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50315314 (8-chloro-4-(4-methylpiperazin-1-yl)benzofuro[3,2-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]histamine dihydrochloride from human histamine H4 receptor after 2.5 hrs by scintillation proximity assay | Bioorg Med Chem Lett 20: 2516-9 (2010) Article DOI: 10.1016/j.bmcl.2010.02.097 BindingDB Entry DOI: 10.7270/Q2CN74V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50544401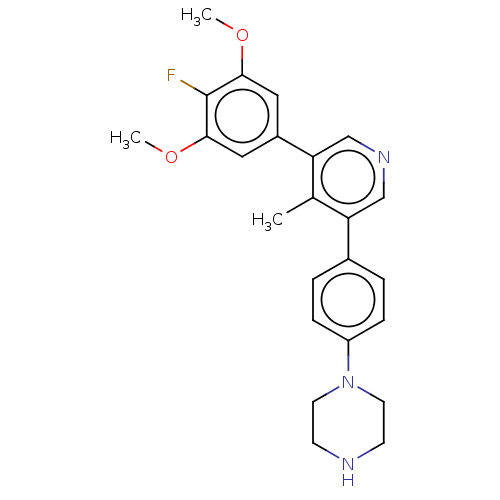 (CHEMBL4636120) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human ALK2 using casein as substrate in presence of [gamma-33P]-ATP by radiometric hotspot assay | J Med Chem 63: 10061-10085 (2020) Article DOI: 10.1021/acs.jmedchem.0c01199 BindingDB Entry DOI: 10.7270/Q2VM4GV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50544417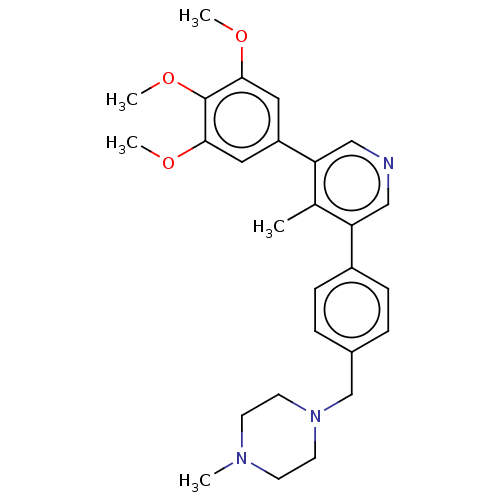 (CHEMBL4638893) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human ALK2 using casein as substrate in presence of [gamma-33P]-ATP by radiometric hotspot assay | J Med Chem 63: 10061-10085 (2020) Article DOI: 10.1021/acs.jmedchem.0c01199 BindingDB Entry DOI: 10.7270/Q2VM4GV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50544407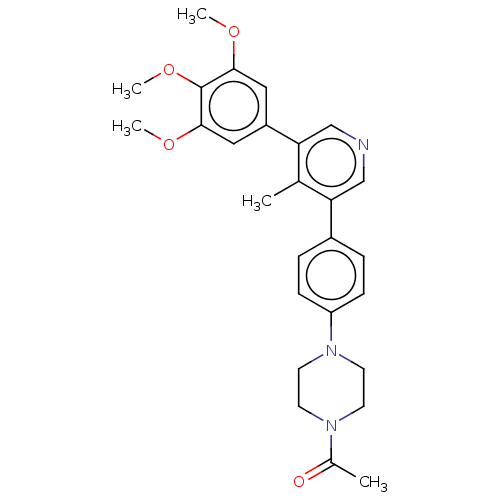 (CHEMBL4648707) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human ALK2 using casein as substrate in presence of [gamma-33P]-ATP by radiometric hotspot assay | J Med Chem 63: 10061-10085 (2020) Article DOI: 10.1021/acs.jmedchem.0c01199 BindingDB Entry DOI: 10.7270/Q2VM4GV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50241099 (ADS-103274 | CHEMBL276393 | N-(2-((2-(dimethylamin...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Limited Curated by ChEMBL | Assay Description Displacement of 125I-[Phe13,Tyr19]-MCH from MCH-R1 expressing cell membranes | Bioorg Med Chem Lett 17: 1403-7 (2007) Article DOI: 10.1016/j.bmcl.2006.11.092 BindingDB Entry DOI: 10.7270/Q2G73DCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50544410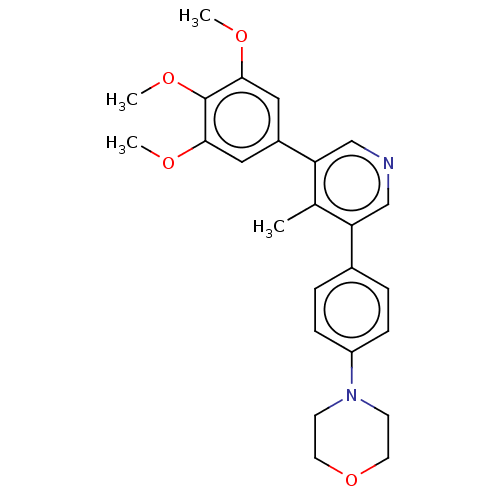 (CHEMBL4642622) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human ALK2 using casein as substrate in presence of [gamma-33P]-ATP by radiometric hotspot assay | J Med Chem 63: 10061-10085 (2020) Article DOI: 10.1021/acs.jmedchem.0c01199 BindingDB Entry DOI: 10.7270/Q2VM4GV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50241099 (ADS-103274 | CHEMBL276393 | N-(2-((2-(dimethylamin...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Limited Curated by ChEMBL | Assay Description Inhibition of MCH-mediated calcium influx into MCH-R1 expressing cells | Bioorg Med Chem Lett 17: 1403-7 (2007) Article DOI: 10.1016/j.bmcl.2006.11.092 BindingDB Entry DOI: 10.7270/Q2G73DCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50544409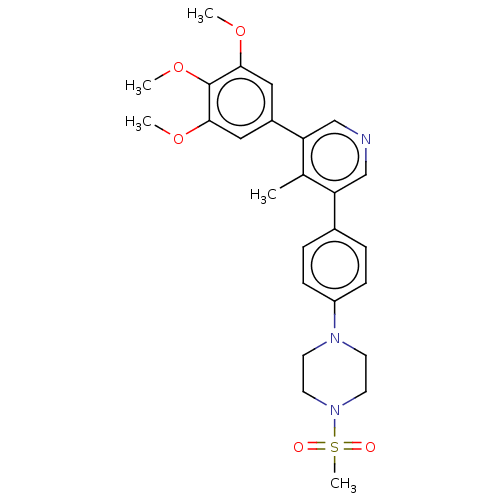 (CHEMBL4638544) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human ALK2 using casein as substrate in presence of [gamma-33P]-ATP by radiometric hotspot assay | J Med Chem 63: 10061-10085 (2020) Article DOI: 10.1021/acs.jmedchem.0c01199 BindingDB Entry DOI: 10.7270/Q2VM4GV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50544413 (CHEMBL4633241) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research Curated by ChEMBL | Assay Description Competitive displacement of PBI-6908 from nanoluciferase-fused ALK2 (unknown origin) expressed in HEK293 cells incubated for 2 hrs by NanoBRET NanoGl... | J Med Chem 63: 10061-10085 (2020) Article DOI: 10.1021/acs.jmedchem.0c01199 BindingDB Entry DOI: 10.7270/Q2VM4GV3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50315333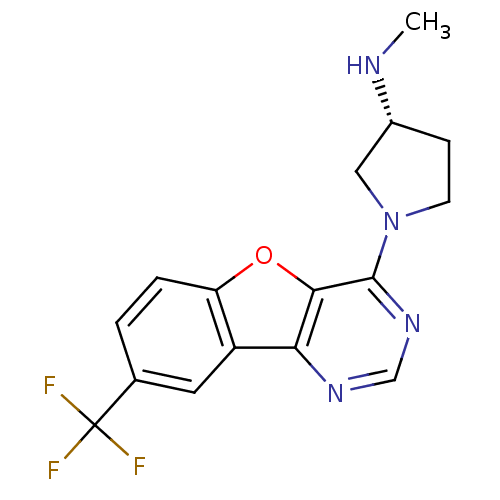 ((R)-N-methyl-1-(8-(trifluoromethyl)benzofuro[3,2-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]histamine dihydrochloride from human histamine H4 receptor after 2.5 hrs by scintillation proximity assay | Bioorg Med Chem Lett 20: 2516-9 (2010) Article DOI: 10.1016/j.bmcl.2010.02.097 BindingDB Entry DOI: 10.7270/Q2CN74V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50544418 (CHEMBL4648102) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research Curated by ChEMBL | Assay Description Competitive displacement of PBI-6908 from nanoluciferase-fused ALK2 (unknown origin) expressed in HEK293 cells incubated for 2 hrs by NanoBRET NanoGl... | J Med Chem 63: 10061-10085 (2020) Article DOI: 10.1021/acs.jmedchem.0c01199 BindingDB Entry DOI: 10.7270/Q2VM4GV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 258 total ) | Next | Last >> |