 Found 42 hits with Last Name = 'da nascimento' and Initial = 's'
Found 42 hits with Last Name = 'da nascimento' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50070420
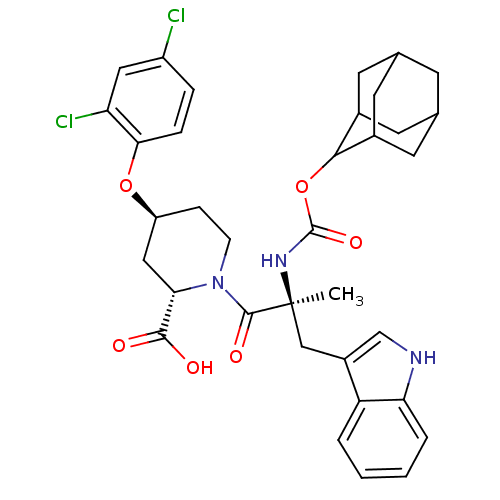
((2S,4S)-1-[(S)-2-(Adamantan-2-yloxycarbonylamino)-...)Show SMILES C[C@@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)N1CC[C@@H](C[C@H]1C(O)=O)Oc1ccc(Cl)cc1Cl |wU:33.39,1.0,wD:1.13,31.42,TLB:22:17:25:21.23.20,22:21:16.17.18:25,THB:20:19:16:21.22.23,20:21:16:19.18.25,15:16:19.18.25:21.22.23,15:16:25:21.23.20,(10.79,-4.05,;10.79,-5.59,;10.82,-7.13,;10.02,-8.45,;8.48,-8.58,;8.16,-10.09,;9.48,-10.86,;9.76,-12.36,;11.21,-12.88,;12.36,-11.85,;12.07,-10.37,;10.63,-9.86,;9.44,-4.82,;8.13,-5.63,;8.16,-7.17,;6.78,-4.89,;5.47,-5.69,;3.96,-5.24,;3.96,-3.67,;2.93,-2.42,;1.52,-3.03,;1.55,-4.53,;2.55,-5.82,;2.93,-4.89,;4.25,-4.41,;4.28,-2.93,;12.11,-4.79,;12.07,-3.25,;13.65,-4.79,;14.42,-3.48,;15.96,-3.48,;16.73,-4.79,;15.96,-6.14,;14.42,-6.14,;14.42,-7.68,;15.73,-8.45,;13.07,-8.42,;18.27,-4.82,;19.81,-4.82,;20.58,-6.14,;22.08,-6.14,;22.85,-4.82,;24.39,-4.82,;22.08,-3.48,;20.54,-3.48,;19.77,-2.16,)| Show InChI InChI=1S/C35H39Cl2N3O6/c1-35(17-23-18-38-28-5-3-2-4-26(23)28,39-34(44)46-31-21-11-19-10-20(13-21)14-22(31)12-19)33(43)40-9-8-25(16-29(40)32(41)42)45-30-7-6-24(36)15-27(30)37/h2-7,15,18-22,25,29,31,38H,8-14,16-17H2,1H3,(H,39,44)(H,41,42)/t19?,20?,21?,22?,25-,29-,31?,35-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Capacity to inhibit [3H]-p CCK 8 binding to membrane preparations of CHO cells transfected with the rat CCK-B receptor |
Bioorg Med Chem Lett 8: 1419-24 (1999)
BindingDB Entry DOI: 10.7270/Q2NZ86S0 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50070414
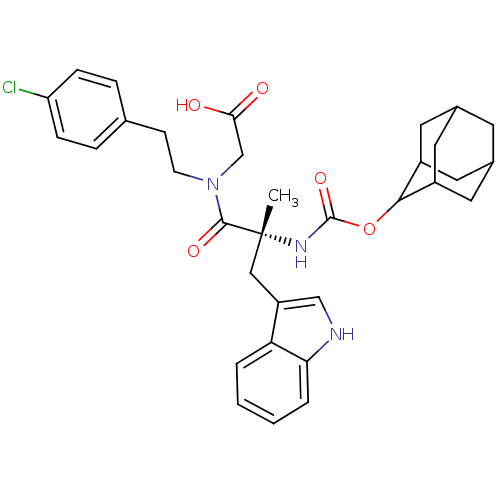
(CHEMBL286247 | {[(R)-2-(Adamantan-2-yloxycarbonyla...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)N(CCc1ccc(Cl)cc1)CC(O)=O |wU:1.13,wD:1.0,TLB:15:16:19.18.25:21.23.22,THB:23:24:18:21.22.20,23:21:18:16.24.25,20:19:16:21.23.22,20:21:16:19.18.25,15:16:18:21.22.20,(7.91,-7.33,;7.94,-8.88,;7.94,-10.43,;7.17,-11.75,;5.62,-11.9,;5.27,-13.39,;6.62,-14.19,;6.91,-15.68,;8.36,-16.2,;9.52,-15.17,;9.23,-13.68,;7.78,-13.17,;6.59,-8.13,;5.23,-8.91,;5.27,-10.46,;3.91,-8.17,;2.56,-8.97,;1.07,-8.54,;-.34,-9.1,;-1.38,-7.84,;-1.38,-6.3,;.01,-5.72,;1.07,-6.94,;1.37,-6.2,;1.37,-7.69,;.04,-8.17,;9.27,-8.07,;9.23,-6.52,;10.81,-8.07,;11.55,-6.74,;13.09,-6.72,;13.84,-5.38,;13.06,-4.07,;13.8,-2.75,;15.35,-2.72,;16.1,-1.4,;16.12,-4.04,;15.36,-5.38,;11.55,-9.42,;13.1,-9.39,;13.9,-10.72,;13.87,-8.04,)| Show InChI InChI=1S/C33H38ClN3O5/c1-33(17-25-18-35-28-5-3-2-4-27(25)28,31(40)37(19-29(38)39)11-10-20-6-8-26(34)9-7-20)36-32(41)42-30-23-13-21-12-22(15-23)16-24(30)14-21/h2-9,18,21-24,30,35H,10-17,19H2,1H3,(H,36,41)(H,38,39)/t21?,22?,23?,24?,30?,33-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Capacity to inhibit [3H]-p CCK 8 binding to membrane preparations of CHO cells transfected with the rat CCK-B receptor |
Bioorg Med Chem Lett 8: 1419-24 (1999)
BindingDB Entry DOI: 10.7270/Q2NZ86S0 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50070412
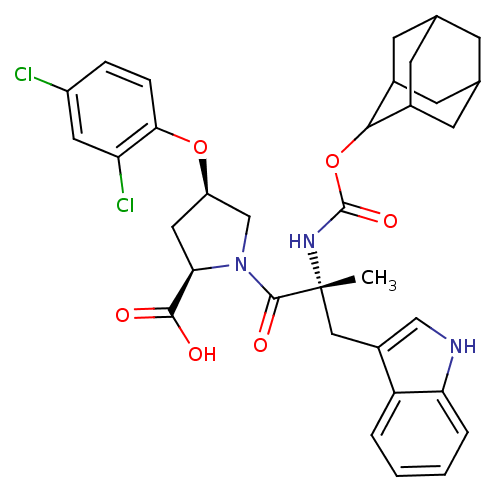
((2R,4R)-1-[(R)-2-(Adamantan-2-yloxycarbonylamino)-...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)N1C[C@@H](C[C@@H]1C(O)=O)Oc1ccc(Cl)cc1Cl |wU:1.13,wD:32.38,30.41,1.0,TLB:22:17:25:21.23.20,22:21:16.17.18:25,THB:20:19:16:21.22.23,20:21:16:19.18.25,15:16:19.18.25:21.22.23,15:16:25:21.23.20,(22.06,-1.42,;22.08,-2.96,;22.09,-4.51,;21.3,-5.84,;19.77,-5.96,;19.42,-7.48,;20.76,-8.25,;21.05,-9.76,;22.49,-10.27,;23.65,-9.25,;23.36,-7.76,;21.92,-7.25,;20.72,-2.2,;19.38,-3,;19.42,-4.55,;18.05,-2.26,;16.71,-3.06,;15.22,-2.62,;15.23,-1.04,;14.17,.19,;12.79,-.39,;12.79,-1.91,;13.82,-3.2,;14.2,-2.26,;15.52,-1.77,;15.52,-.29,;23.4,-2.17,;23.37,-.62,;24.94,-2.17,;25.84,-.91,;27.3,-1.38,;27.3,-2.91,;25.85,-3.39,;25.37,-4.87,;26.42,-6,;23.88,-5.19,;28.56,-.46,;29.96,-1.09,;30.13,-2.62,;31.54,-3.25,;32.79,-2.33,;34.19,-2.96,;32.61,-.78,;31.19,-.17,;31.03,1.35,)| Show InChI InChI=1S/C34H37Cl2N3O6/c1-34(15-22-16-37-27-5-3-2-4-25(22)27,38-33(43)45-30-20-9-18-8-19(11-20)12-21(30)10-18)32(42)39-17-24(14-28(39)31(40)41)44-29-7-6-23(35)13-26(29)36/h2-7,13,16,18-21,24,28,30,37H,8-12,14-15,17H2,1H3,(H,38,43)(H,40,41)/t18?,19?,20?,21?,24-,28-,30?,34-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Capacity to inhibit [3H]-p CCK 8 binding to membrane preparations of CHO cells transfected with the rat CCK-B receptor |
Bioorg Med Chem Lett 8: 1419-24 (1999)
BindingDB Entry DOI: 10.7270/Q2NZ86S0 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50006878

((R)-1-(1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)Nc2ccc(C)cc2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C24H22N4O2/c1-16-12-14-18(15-13-16)25-24(30)27-22-23(29)28(2)20-11-7-6-10-19(20)21(26-22)17-8-4-3-5-9-17/h3-15,22H,1-2H3,(H2,25,27,30)/t22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Capacity to inhibit [3H]-p CCK 8 binding to membrane preparations of CHO cells transfected with the rat CCK-B receptor |
Bioorg Med Chem Lett 8: 1419-24 (1999)
BindingDB Entry DOI: 10.7270/Q2NZ86S0 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50070415

((2R,4R)-1-[(R)-2-(Adamantan-2-yloxycarbonylamino)-...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)N1C[C@@H](C[C@@H]1C(O)=O)Oc1ccc(F)cc1F |wU:1.13,wD:32.38,30.41,1.0,TLB:22:17:25:21.23.20,22:21:16.17.18:25,THB:20:19:16:21.22.23,20:21:16:19.18.25,15:16:19.18.25:21.22.23,15:16:25:21.23.20,(18.68,-9.94,;18.7,-11.49,;18.71,-13.03,;17.93,-14.36,;16.39,-14.49,;16.04,-16,;17.38,-16.78,;17.67,-18.29,;19.12,-18.79,;20.27,-17.77,;19.98,-16.28,;18.54,-15.78,;17.35,-10.73,;16,-11.53,;16.04,-13.08,;14.67,-10.79,;13.33,-11.59,;11.84,-11.15,;11.85,-9.57,;10.79,-8.33,;9.41,-8.91,;9.41,-10.44,;10.44,-11.72,;10.82,-10.79,;12.14,-10.3,;12.14,-8.82,;20.02,-10.7,;20,-9.15,;21.56,-10.7,;22.46,-9.44,;23.92,-9.91,;23.92,-11.44,;22.47,-11.92,;21.99,-13.4,;23.04,-14.52,;20.5,-13.72,;25.18,-8.99,;26.58,-9.62,;26.75,-11.15,;28.16,-11.78,;29.41,-10.86,;30.81,-11.49,;29.23,-9.31,;27.82,-8.7,;27.65,-7.18,)| Show InChI InChI=1S/C34H37F2N3O6/c1-34(15-22-16-37-27-5-3-2-4-25(22)27,38-33(43)45-30-20-9-18-8-19(11-20)12-21(30)10-18)32(42)39-17-24(14-28(39)31(40)41)44-29-7-6-23(35)13-26(29)36/h2-7,13,16,18-21,24,28,30,37H,8-12,14-15,17H2,1H3,(H,38,43)(H,40,41)/t18?,19?,20?,21?,24-,28-,30?,34-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibitory activity against CCK-B receptor |
Bioorg Med Chem Lett 8: 1419-24 (1999)
BindingDB Entry DOI: 10.7270/Q2NZ86S0 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50070413
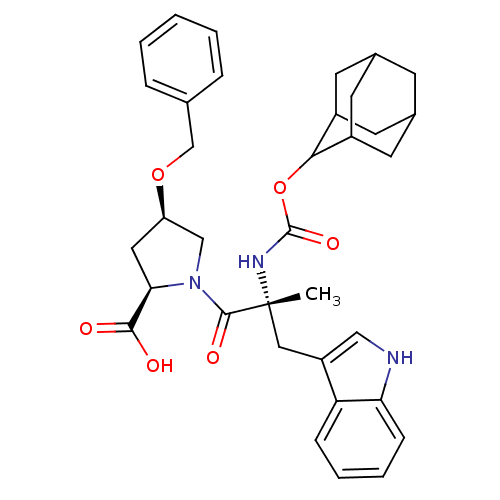
((2R,4R)-1-[(R)-2-(Adamantan-2-yloxycarbonylamino)-...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)N1C[C@@H](C[C@@H]1C(O)=O)OCc1ccccc1 |wU:1.13,wD:32.38,30.41,1.0,TLB:25:24:22:19.18.20,15:16:19.18.25:21.23.22,THB:25:19:16.24.23:22,20:21:16:19.18.25,20:19:16:21.23.22,15:16:22:19.18.20,(18.68,-9.94,;18.7,-11.49,;18.71,-13.03,;17.93,-14.36,;16.39,-14.49,;16.04,-16,;17.38,-16.78,;17.67,-18.29,;19.12,-18.79,;20.27,-17.77,;19.98,-16.28,;18.54,-15.78,;17.35,-10.73,;16,-11.53,;16.04,-13.08,;14.67,-10.79,;13.33,-11.59,;12.14,-10.3,;10.82,-10.79,;9.41,-10.44,;9.41,-8.91,;10.79,-8.33,;12.14,-8.82,;11.85,-9.57,;11.84,-11.15,;10.44,-11.72,;20.02,-10.7,;20,-9.15,;21.56,-10.7,;22.46,-9.44,;23.92,-9.91,;23.92,-11.44,;22.47,-11.92,;21.99,-13.4,;23.04,-14.52,;20.5,-13.72,;25.18,-8.99,;26.58,-9.62,;27.82,-8.7,;27.65,-7.18,;28.9,-6.25,;30.31,-6.89,;30.49,-8.41,;29.23,-9.33,)| Show InChI InChI=1S/C35H41N3O6/c1-35(17-26-18-36-29-10-6-5-9-28(26)29,37-34(42)44-31-24-12-22-11-23(14-24)15-25(31)13-22)33(41)38-19-27(16-30(38)32(39)40)43-20-21-7-3-2-4-8-21/h2-10,18,22-25,27,30-31,36H,11-17,19-20H2,1H3,(H,37,42)(H,39,40)/t22?,23?,24?,25?,27-,30-,31?,35-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Capacity to inhibit [3H]-p CCK 8 binding to membrane preparations of CHO cells transfected with the rat CCK-B receptor |
Bioorg Med Chem Lett 8: 1419-24 (1999)
BindingDB Entry DOI: 10.7270/Q2NZ86S0 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50070419
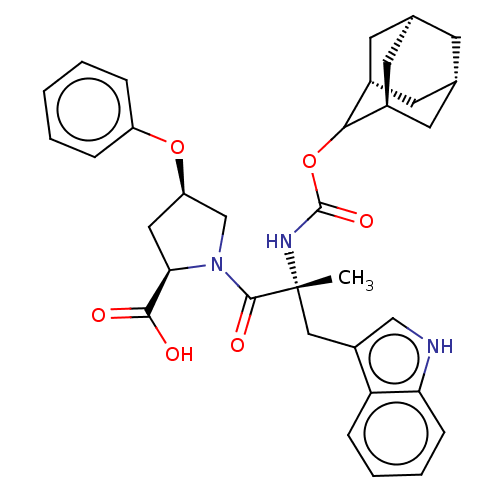
((2R,4R)-1-[(R)-2-(Adamantan-2-yloxycarbonylamino)-...)Show SMILES [H][C@@]12C[C@@]3([H])C[C@@]([H])(C1)C(OC(=O)N[C@](C)(Cc1c[nH]c4ccccc14)C(=O)N1C[C@@H](C[C@@H]1C(O)=O)Oc1ccccc1)[C@@]([H])(C2)C3 |wU:14.28,43.48,3.3,wD:32.36,30.39,6.6,1.0,TLB:5:3:45:9.6.8,10:9:45:3.46.2,THB:5:6:45:3.46.2,2:3:9:1.45.8,2:1:9:3.5.46,(5.34,-8.16,;6.78,-8.71,;5.58,-10,;7.08,-9.56,;6.98,-11.1,;8.47,-10.13,;9.49,-8.85,;10.92,-9.37,;8.08,-9.21,;9.5,-7.34,;10.82,-6.56,;12.14,-7.3,;12.56,-5.72,;12.17,-8.85,;13.69,-8.85,;13.69,-7.3,;13.69,-10.37,;12.82,-11.81,;13.47,-13.37,;12.21,-14.47,;10.78,-13.56,;9.18,-14.05,;7.95,-12.89,;8.34,-11.26,;9.95,-10.79,;11.17,-11.95,;15.23,-8.85,;15.98,-10.18,;15.98,-7.52,;15.37,-6.11,;16.5,-5.1,;17.84,-5.85,;17.52,-7.36,;18.53,-8.5,;20.04,-8.17,;18.07,-9.95,;16.34,-3.56,;17.6,-2.66,;17.43,-1.14,;18.65,-.24,;20.07,-.87,;20.23,-2.4,;18.99,-3.29,;8.11,-6.76,;8.14,-5.24,;6.76,-7.24,;7.07,-8,)| Show InChI InChI=1S/C34H39N3O6/c1-34(17-24-18-35-28-10-6-5-9-27(24)28,36-33(41)43-30-22-12-20-11-21(14-22)15-23(30)13-20)32(40)37-19-26(16-29(37)31(38)39)42-25-7-3-2-4-8-25/h2-10,18,20-23,26,29-30,35H,11-17,19H2,1H3,(H,36,41)(H,38,39)/t20-,21+,22-,23+,26-,29-,30?,34-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Capacity to inhibit [3H]-p CCK 8 binding to membrane preparations of CHO cells transfected with the rat CCK-B receptor |
Bioorg Med Chem Lett 8: 1419-24 (1999)
BindingDB Entry DOI: 10.7270/Q2NZ86S0 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50070416
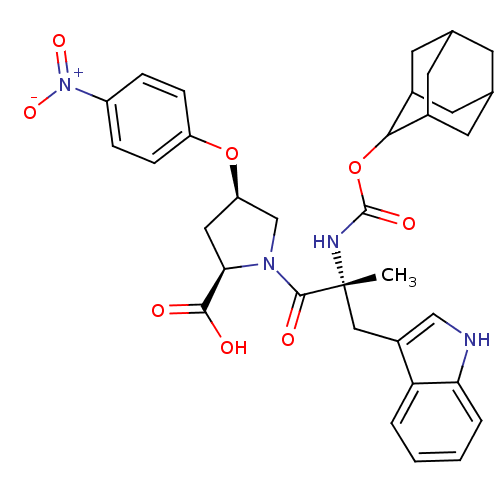
((2R,4R)-1-[(R)-2-(Adamantan-2-yloxycarbonylamino)-...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)N1C[C@@H](C[C@@H]1C(O)=O)Oc1ccc(cc1)[N+]([O-])=O |wU:1.13,wD:32.38,30.41,1.0,TLB:15:16:19.18.25:21.23.22,15:16:25:21.23.20,22:17:25:21.23.20,22:21:16.17.18:25,THB:20:19:16:21.23.22,20:21:16:19.18.25,(18.67,-9.94,;18.69,-11.49,;18.7,-13.03,;17.92,-14.36,;16.39,-14.49,;16.03,-16,;17.37,-16.77,;17.66,-18.28,;19.11,-18.79,;20.27,-17.77,;19.98,-16.28,;18.53,-15.78,;17.34,-10.72,;16,-11.53,;16.03,-13.07,;14.67,-10.79,;13.33,-11.58,;12.14,-10.3,;10.82,-10.79,;9.41,-10.43,;9.41,-8.91,;10.79,-8.33,;12.14,-8.82,;11.85,-9.57,;11.84,-11.14,;10.43,-11.72,;20.01,-10.69,;19.99,-9.15,;21.56,-10.69,;22.46,-9.44,;23.91,-9.91,;23.91,-11.43,;22.47,-11.91,;21.98,-13.39,;23.04,-14.52,;20.49,-13.72,;25.17,-8.99,;26.57,-9.62,;27.81,-8.7,;29.22,-9.31,;29.41,-10.85,;28.15,-11.78,;26.75,-11.14,;30.82,-11.49,;30.99,-13.01,;32.05,-10.56,)| Show InChI InChI=1S/C34H38N4O8/c1-34(16-23-17-35-28-5-3-2-4-27(23)28,36-33(42)46-30-21-11-19-10-20(13-21)14-22(30)12-19)32(41)37-18-26(15-29(37)31(39)40)45-25-8-6-24(7-9-25)38(43)44/h2-9,17,19-22,26,29-30,35H,10-16,18H2,1H3,(H,36,42)(H,39,40)/t19?,20?,21?,22?,26-,29-,30?,34-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Capacity to inhibit [3H]-p CCK 8 binding to membrane preparations of CHO cells transfected with the rat CCK-B receptor |
Bioorg Med Chem Lett 8: 1419-24 (1999)
BindingDB Entry DOI: 10.7270/Q2NZ86S0 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50070411
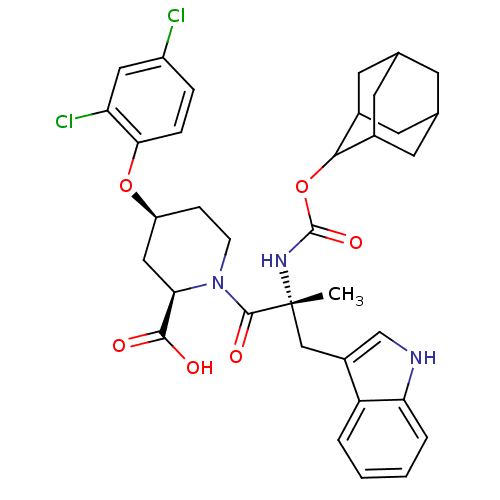
((2R,4S)-1-[(R)-2-(Adamantan-2-yloxycarbonylamino)-...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)N1CC[C@@H](C[C@@H]1C(O)=O)Oc1ccc(Cl)cc1Cl |wU:1.13,wD:33.39,31.42,1.0,TLB:22:17:25:21.23.20,22:21:16.17.18:25,THB:20:19:16:21.22.23,20:21:16:19.18.25,15:16:19.18.25:21.22.23,15:16:25:21.23.20,(10.82,-4.07,;10.82,-5.61,;10.86,-7.16,;10.05,-8.5,;8.51,-8.62,;8.18,-10.14,;9.5,-10.91,;9.79,-12.42,;11.24,-12.92,;12.4,-11.91,;12.11,-10.41,;10.66,-9.91,;9.47,-4.86,;8.15,-5.66,;8.18,-7.21,;6.8,-4.92,;5.48,-5.71,;3.97,-5.28,;3.97,-3.7,;2.94,-2.45,;1.52,-3.04,;1.56,-4.57,;2.55,-5.86,;2.94,-4.92,;4.26,-4.42,;4.29,-2.94,;12.14,-4.83,;12.11,-3.28,;13.69,-4.83,;14.46,-3.48,;16,-3.48,;16.78,-4.83,;16,-6.16,;14.46,-6.16,;14.46,-7.7,;15.78,-8.5,;13.11,-8.46,;18.32,-4.83,;19.87,-4.83,;20.64,-6.16,;22.15,-6.18,;22.92,-4.83,;24.47,-4.83,;22.15,-3.51,;20.61,-3.51,;19.83,-2.17,)| Show InChI InChI=1S/C35H39Cl2N3O6/c1-35(17-23-18-38-28-5-3-2-4-26(23)28,39-34(44)46-31-21-11-19-10-20(13-21)14-22(31)12-19)33(43)40-9-8-25(16-29(40)32(41)42)45-30-7-6-24(36)15-27(30)37/h2-7,15,18-22,25,29,31,38H,8-14,16-17H2,1H3,(H,39,44)(H,41,42)/t19?,20?,21?,22?,25-,29+,31?,35+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 175 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Capacity to inhibit [3H]-p CCK 8 binding to membrane preparations of CHO cells transfected with the rat CCK-B receptor |
Bioorg Med Chem Lett 8: 1419-24 (1999)
BindingDB Entry DOI: 10.7270/Q2NZ86S0 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50070424
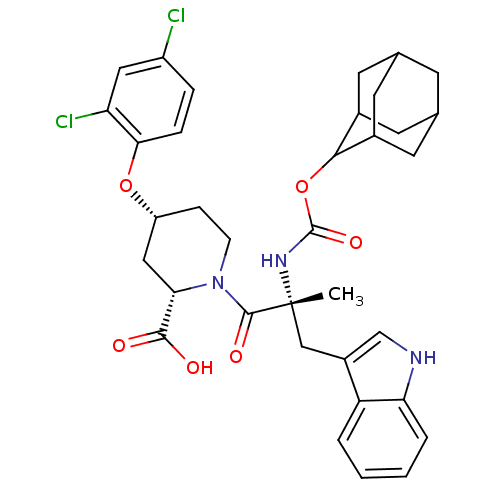
((2S,4R)-1-[(R)-2-(Adamantan-2-yloxycarbonylamino)-...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)N1CC[C@H](C[C@H]1C(O)=O)Oc1ccc(Cl)cc1Cl |wU:1.13,33.39,31.42,wD:1.0,TLB:22:17:25:21.23.20,22:21:16.17.18:25,THB:20:19:16:21.22.23,20:21:16:19.18.25,15:16:19.18.25:21.22.23,15:16:25:21.23.20,(10.82,-4.07,;10.82,-5.61,;10.86,-7.16,;10.05,-8.5,;8.51,-8.62,;8.18,-10.14,;9.5,-10.91,;9.79,-12.42,;11.24,-12.92,;12.4,-11.91,;12.11,-10.41,;10.66,-9.91,;9.47,-4.86,;8.15,-5.66,;8.18,-7.21,;6.8,-4.92,;5.48,-5.71,;3.97,-5.28,;3.97,-3.7,;2.94,-2.45,;1.52,-3.04,;1.56,-4.57,;2.55,-5.86,;2.94,-4.92,;4.26,-4.42,;4.29,-2.94,;12.14,-4.83,;12.11,-3.28,;13.69,-4.83,;14.46,-3.48,;16,-3.48,;16.78,-4.83,;16,-6.16,;14.46,-6.16,;14.46,-7.7,;15.78,-8.5,;13.11,-8.46,;18.32,-4.83,;19.87,-4.83,;20.64,-6.16,;22.15,-6.18,;22.92,-4.83,;24.47,-4.83,;22.15,-3.51,;20.61,-3.51,;19.83,-2.17,)| Show InChI InChI=1S/C35H39Cl2N3O6/c1-35(17-23-18-38-28-5-3-2-4-26(23)28,39-34(44)46-31-21-11-19-10-20(13-21)14-22(31)12-19)33(43)40-9-8-25(16-29(40)32(41)42)45-30-7-6-24(36)15-27(30)37/h2-7,15,18-22,25,29,31,38H,8-14,16-17H2,1H3,(H,39,44)(H,41,42)/t19?,20?,21?,22?,25-,29+,31?,35-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 543 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Capacity to inhibit [3H]-p CCK 8 binding to membrane preparations of CHO cells transfected with the rat CCK-B receptor |
Bioorg Med Chem Lett 8: 1419-24 (1999)
BindingDB Entry DOI: 10.7270/Q2NZ86S0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Cavia porcellus) | BDBM50070412

((2R,4R)-1-[(R)-2-(Adamantan-2-yloxycarbonylamino)-...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)N1C[C@@H](C[C@@H]1C(O)=O)Oc1ccc(Cl)cc1Cl |wU:1.13,wD:32.38,30.41,1.0,TLB:22:17:25:21.23.20,22:21:16.17.18:25,THB:20:19:16:21.22.23,20:21:16:19.18.25,15:16:19.18.25:21.22.23,15:16:25:21.23.20,(22.06,-1.42,;22.08,-2.96,;22.09,-4.51,;21.3,-5.84,;19.77,-5.96,;19.42,-7.48,;20.76,-8.25,;21.05,-9.76,;22.49,-10.27,;23.65,-9.25,;23.36,-7.76,;21.92,-7.25,;20.72,-2.2,;19.38,-3,;19.42,-4.55,;18.05,-2.26,;16.71,-3.06,;15.22,-2.62,;15.23,-1.04,;14.17,.19,;12.79,-.39,;12.79,-1.91,;13.82,-3.2,;14.2,-2.26,;15.52,-1.77,;15.52,-.29,;23.4,-2.17,;23.37,-.62,;24.94,-2.17,;25.84,-.91,;27.3,-1.38,;27.3,-2.91,;25.85,-3.39,;25.37,-4.87,;26.42,-6,;23.88,-5.19,;28.56,-.46,;29.96,-1.09,;30.13,-2.62,;31.54,-3.25,;32.79,-2.33,;34.19,-2.96,;32.61,-.78,;31.19,-.17,;31.03,1.35,)| Show InChI InChI=1S/C34H37Cl2N3O6/c1-34(15-22-16-37-27-5-3-2-4-25(22)27,38-33(43)45-30-20-9-18-8-19(11-20)12-21(30)10-18)32(42)39-17-24(14-28(39)31(40)41)44-29-7-6-23(35)13-26(29)36/h2-7,13,16,18-21,24,28,30,37H,8-12,14-15,17H2,1H3,(H,38,43)(H,40,41)/t18?,19?,20?,21?,24-,28-,30?,34-/m1/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 751 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Binding affinity against CCK-A receptor in guinea pig pancreatic membranes |
Bioorg Med Chem Lett 8: 1419-24 (1999)
BindingDB Entry DOI: 10.7270/Q2NZ86S0 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50070417
((2R,4R)-1-[(R)-2-(Adamantan-2-yloxycarbonylamino)-...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)N1CC[C@H](C[C@@H]1C(O)=O)Oc1ccc(Cl)cc1Cl |wU:1.13,31.42,wD:33.39,1.0,TLB:15:16:19.18.25:21.23.22,25:24:22:19.18.20,THB:20:21:16:19.18.25,20:19:16:21.23.22,15:16:22:19.18.20,25:19:16.24.23:22,(14.46,-7.69,;14.47,-9.23,;14.49,-10.78,;13.7,-12.11,;12.16,-12.25,;11.82,-13.75,;13.15,-14.53,;13.44,-16.03,;14.89,-16.53,;16.04,-15.52,;15.76,-14.02,;14.31,-13.53,;13.12,-8.49,;11.79,-9.29,;11.82,-10.83,;10.45,-8.55,;9.13,-9.33,;7.92,-8.04,;6.6,-8.55,;5.21,-8.2,;5.18,-6.66,;6.59,-6.08,;7.94,-6.57,;7.63,-7.33,;7.63,-8.91,;6.21,-9.48,;15.79,-8.45,;15.76,-6.91,;17.33,-8.45,;18.07,-7.11,;19.61,-7.11,;20.38,-8.43,;19.61,-9.77,;18.09,-9.78,;17.33,-11.1,;18.1,-12.45,;15.79,-11.1,;21.92,-8.43,;22.7,-9.78,;21.92,-11.1,;22.7,-12.45,;24.24,-12.45,;25.01,-13.78,;25.01,-11.1,;24.24,-9.77,;25,-8.43,)| Show InChI InChI=1S/C35H39Cl2N3O6/c1-35(17-23-18-38-28-5-3-2-4-26(23)28,39-34(44)46-31-21-11-19-10-20(13-21)14-22(31)12-19)33(43)40-9-8-25(16-29(40)32(41)42)45-30-7-6-24(36)15-27(30)37/h2-7,15,18-22,25,29,31,38H,8-14,16-17H2,1H3,(H,39,44)(H,41,42)/t19?,20?,21?,22?,25-,29-,31?,35-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 789 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Capacity to inhibit [3H]-p CCK 8 binding to membrane preparations of CHO cells transfected with the rat CCK-B receptor |
Bioorg Med Chem Lett 8: 1419-24 (1999)
BindingDB Entry DOI: 10.7270/Q2NZ86S0 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50070423
((2S,4S)-1-[(R)-2-(Adamantan-2-yloxycarbonylamino)-...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)N1CC[C@@H](C[C@H]1C(O)=O)Oc1ccc(Cl)cc1Cl |wU:1.13,33.39,wD:31.42,1.0,TLB:22:17:25:21.23.20,22:21:16.17.18:25,THB:20:19:16:21.22.23,20:21:16:19.18.25,15:16:19.18.25:21.22.23,15:16:25:21.23.20,(10.79,-4.05,;10.79,-5.59,;10.82,-7.13,;10.02,-8.45,;8.48,-8.58,;8.16,-10.09,;9.48,-10.86,;9.76,-12.36,;11.21,-12.88,;12.36,-11.85,;12.07,-10.37,;10.63,-9.86,;9.44,-4.82,;8.13,-5.63,;8.16,-7.17,;6.78,-4.89,;5.47,-5.69,;3.96,-5.24,;3.96,-3.67,;2.93,-2.42,;1.52,-3.03,;1.55,-4.53,;2.55,-5.82,;2.93,-4.89,;4.25,-4.41,;4.28,-2.93,;12.11,-4.79,;12.07,-3.25,;13.65,-4.79,;14.42,-3.48,;15.96,-3.48,;16.73,-4.79,;15.96,-6.14,;14.42,-6.14,;14.42,-7.68,;15.73,-8.45,;13.07,-8.42,;18.27,-4.82,;19.81,-4.82,;20.58,-6.14,;22.08,-6.14,;22.85,-4.82,;24.39,-4.82,;22.08,-3.48,;20.54,-3.48,;19.77,-2.16,)| Show InChI InChI=1S/C35H39Cl2N3O6/c1-35(17-23-18-38-28-5-3-2-4-26(23)28,39-34(44)46-31-21-11-19-10-20(13-21)14-22(31)12-19)33(43)40-9-8-25(16-29(40)32(41)42)45-30-7-6-24(36)15-27(30)37/h2-7,15,18-22,25,29,31,38H,8-14,16-17H2,1H3,(H,39,44)(H,41,42)/t19?,20?,21?,22?,25-,29-,31?,35+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 791 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Capacity to inhibit [3H]-p CCK 8 binding to membrane preparations of CHO cells transfected with the rat CCK-B receptor |
Bioorg Med Chem Lett 8: 1419-24 (1999)
BindingDB Entry DOI: 10.7270/Q2NZ86S0 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50070418

((2R,4S)-1-[(S)-2-(Adamantan-2-yloxycarbonylamino)-...)Show SMILES C[C@@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)N1CC[C@@H](C[C@@H]1C(O)=O)Oc1ccc(Cl)cc1Cl |wU:1.0,wD:1.13,33.39,31.42,TLB:22:17:25:21.23.20,22:21:16.17.18:25,THB:20:19:16:21.22.23,20:21:16:19.18.25,15:16:19.18.25:21.22.23,15:16:25:21.23.20,(10.82,-4.07,;10.82,-5.61,;10.86,-7.16,;10.05,-8.5,;8.51,-8.62,;8.18,-10.14,;9.5,-10.91,;9.79,-12.42,;11.24,-12.92,;12.4,-11.91,;12.11,-10.41,;10.66,-9.91,;9.47,-4.86,;8.15,-5.66,;8.18,-7.21,;6.8,-4.92,;5.48,-5.71,;3.97,-5.28,;3.97,-3.7,;2.94,-2.45,;1.52,-3.04,;1.56,-4.57,;2.55,-5.86,;2.94,-4.92,;4.26,-4.42,;4.29,-2.94,;12.14,-4.83,;12.11,-3.28,;13.69,-4.83,;14.46,-3.48,;16,-3.48,;16.78,-4.83,;16,-6.16,;14.46,-6.16,;14.46,-7.7,;15.78,-8.5,;13.11,-8.46,;18.32,-4.83,;19.87,-4.83,;20.64,-6.16,;22.15,-6.18,;22.92,-4.83,;24.47,-4.83,;22.15,-3.51,;20.61,-3.51,;19.83,-2.17,)| Show InChI InChI=1S/C35H39Cl2N3O6/c1-35(17-23-18-38-28-5-3-2-4-26(23)28,39-34(44)46-31-21-11-19-10-20(13-21)14-22(31)12-19)33(43)40-9-8-25(16-29(40)32(41)42)45-30-7-6-24(36)15-27(30)37/h2-7,15,18-22,25,29,31,38H,8-14,16-17H2,1H3,(H,39,44)(H,41,42)/t19?,20?,21?,22?,25-,29+,31?,35-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 812 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Capacity to inhibit [3H]-p CCK 8 binding to membrane preparations of CHO cells transfected with the rat CCK-B receptor |
Bioorg Med Chem Lett 8: 1419-24 (1999)
BindingDB Entry DOI: 10.7270/Q2NZ86S0 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50070421
((2S,4R)-1-[(S)-2-(Adamantan-2-yloxycarbonylamino)-...)Show SMILES C[C@@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)N1CC[C@H](C[C@H]1C(O)=O)Oc1ccc(Cl)cc1Cl |wU:33.39,31.42,1.0,wD:1.13,TLB:22:17:25:21.23.20,22:21:16.17.18:25,THB:20:19:16:21.22.23,20:21:16:19.18.25,15:16:19.18.25:21.22.23,15:16:25:21.23.20,(10.79,-4.05,;10.79,-5.59,;10.82,-7.13,;10.02,-8.45,;8.48,-8.58,;8.16,-10.09,;9.48,-10.86,;9.76,-12.36,;11.21,-12.88,;12.36,-11.85,;12.07,-10.37,;10.63,-9.86,;9.44,-4.82,;8.13,-5.63,;8.16,-7.17,;6.78,-4.89,;5.47,-5.69,;3.96,-5.24,;3.96,-3.67,;2.93,-2.42,;1.52,-3.03,;1.55,-4.53,;2.55,-5.82,;2.93,-4.89,;4.25,-4.41,;4.28,-2.93,;12.11,-4.79,;12.07,-3.25,;13.65,-4.79,;14.42,-3.48,;15.96,-3.48,;16.73,-4.79,;15.96,-6.14,;14.42,-6.14,;14.42,-7.68,;15.73,-8.45,;13.07,-8.42,;18.27,-4.82,;19.81,-4.82,;20.58,-6.14,;22.08,-6.14,;22.85,-4.82,;24.39,-4.82,;22.08,-3.48,;20.54,-3.48,;19.77,-2.16,)| Show InChI InChI=1S/C35H39Cl2N3O6/c1-35(17-23-18-38-28-5-3-2-4-26(23)28,39-34(44)46-31-21-11-19-10-20(13-21)14-22(31)12-19)33(43)40-9-8-25(16-29(40)32(41)42)45-30-7-6-24(36)15-27(30)37/h2-7,15,18-22,25,29,31,38H,8-14,16-17H2,1H3,(H,39,44)(H,41,42)/t19?,20?,21?,22?,25-,29+,31?,35+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 896 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Capacity to inhibit [3H]-p CCK 8 binding to membrane preparations of CHO cells transfected with the rat CCK-B receptor |
Bioorg Med Chem Lett 8: 1419-24 (1999)
BindingDB Entry DOI: 10.7270/Q2NZ86S0 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50450641
(CHEMBL2114379)Show SMILES C[C@@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)N1CC[C@H](C[C@@H]1C(O)=O)Oc1ccc(Cl)cc1Cl |wU:31.42,1.0,wD:33.39,TLB:15:16:19.18.25:21.23.22,25:24:22:19.18.20,THB:20:21:16:19.18.25,20:19:16:21.23.22,15:16:22:19.18.20,25:19:16.24.23:22,(14.46,-7.69,;14.47,-9.23,;14.49,-10.78,;13.7,-12.11,;12.16,-12.25,;11.82,-13.75,;13.15,-14.53,;13.44,-16.03,;14.89,-16.53,;16.04,-15.52,;15.76,-14.02,;14.31,-13.53,;13.12,-8.49,;11.79,-9.29,;11.82,-10.83,;10.45,-8.55,;9.13,-9.33,;7.92,-8.04,;6.6,-8.55,;5.21,-8.2,;5.18,-6.66,;6.59,-6.08,;7.94,-6.57,;7.63,-7.33,;7.63,-8.91,;6.21,-9.48,;15.79,-8.45,;15.76,-6.91,;17.33,-8.45,;18.07,-7.11,;19.61,-7.11,;20.38,-8.43,;19.61,-9.77,;18.09,-9.78,;17.33,-11.1,;18.1,-12.45,;15.79,-11.1,;21.92,-8.43,;22.7,-9.78,;21.92,-11.1,;22.7,-12.45,;24.24,-12.45,;25.01,-13.78,;25.01,-11.1,;24.24,-9.77,;25,-8.43,)| Show InChI InChI=1S/C35H39Cl2N3O6/c1-35(17-23-18-38-28-5-3-2-4-26(23)28,39-34(44)46-31-21-11-19-10-20(13-21)14-22(31)12-19)33(43)40-9-8-25(16-29(40)32(41)42)45-30-7-6-24(36)15-27(30)37/h2-7,15,18-22,25,29,31,38H,8-14,16-17H2,1H3,(H,39,44)(H,41,42)/t19?,20?,21?,22?,25-,29-,31?,35+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Capacity to inhibit [3H]-p CCK 8 binding to membrane preparations of CHO cells transfected with the rat CCK-B receptor |
Bioorg Med Chem Lett 8: 1419-24 (1999)
BindingDB Entry DOI: 10.7270/Q2NZ86S0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Cavia porcellus) | BDBM50070420

((2S,4S)-1-[(S)-2-(Adamantan-2-yloxycarbonylamino)-...)Show SMILES C[C@@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)N1CC[C@@H](C[C@H]1C(O)=O)Oc1ccc(Cl)cc1Cl |wU:33.39,1.0,wD:1.13,31.42,TLB:22:17:25:21.23.20,22:21:16.17.18:25,THB:20:19:16:21.22.23,20:21:16:19.18.25,15:16:19.18.25:21.22.23,15:16:25:21.23.20,(10.79,-4.05,;10.79,-5.59,;10.82,-7.13,;10.02,-8.45,;8.48,-8.58,;8.16,-10.09,;9.48,-10.86,;9.76,-12.36,;11.21,-12.88,;12.36,-11.85,;12.07,-10.37,;10.63,-9.86,;9.44,-4.82,;8.13,-5.63,;8.16,-7.17,;6.78,-4.89,;5.47,-5.69,;3.96,-5.24,;3.96,-3.67,;2.93,-2.42,;1.52,-3.03,;1.55,-4.53,;2.55,-5.82,;2.93,-4.89,;4.25,-4.41,;4.28,-2.93,;12.11,-4.79,;12.07,-3.25,;13.65,-4.79,;14.42,-3.48,;15.96,-3.48,;16.73,-4.79,;15.96,-6.14,;14.42,-6.14,;14.42,-7.68,;15.73,-8.45,;13.07,-8.42,;18.27,-4.82,;19.81,-4.82,;20.58,-6.14,;22.08,-6.14,;22.85,-4.82,;24.39,-4.82,;22.08,-3.48,;20.54,-3.48,;19.77,-2.16,)| Show InChI InChI=1S/C35H39Cl2N3O6/c1-35(17-23-18-38-28-5-3-2-4-26(23)28,39-34(44)46-31-21-11-19-10-20(13-21)14-22(31)12-19)33(43)40-9-8-25(16-29(40)32(41)42)45-30-7-6-24(36)15-27(30)37/h2-7,15,18-22,25,29,31,38H,8-14,16-17H2,1H3,(H,39,44)(H,41,42)/t19?,20?,21?,22?,25-,29-,31?,35-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Binding affinity against CCK-A receptor in guinea pig pancreatic membranes |
Bioorg Med Chem Lett 8: 1419-24 (1999)
BindingDB Entry DOI: 10.7270/Q2NZ86S0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Cavia porcellus) | BDBM50070414

(CHEMBL286247 | {[(R)-2-(Adamantan-2-yloxycarbonyla...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)N(CCc1ccc(Cl)cc1)CC(O)=O |wU:1.13,wD:1.0,TLB:15:16:19.18.25:21.23.22,THB:23:24:18:21.22.20,23:21:18:16.24.25,20:19:16:21.23.22,20:21:16:19.18.25,15:16:18:21.22.20,(7.91,-7.33,;7.94,-8.88,;7.94,-10.43,;7.17,-11.75,;5.62,-11.9,;5.27,-13.39,;6.62,-14.19,;6.91,-15.68,;8.36,-16.2,;9.52,-15.17,;9.23,-13.68,;7.78,-13.17,;6.59,-8.13,;5.23,-8.91,;5.27,-10.46,;3.91,-8.17,;2.56,-8.97,;1.07,-8.54,;-.34,-9.1,;-1.38,-7.84,;-1.38,-6.3,;.01,-5.72,;1.07,-6.94,;1.37,-6.2,;1.37,-7.69,;.04,-8.17,;9.27,-8.07,;9.23,-6.52,;10.81,-8.07,;11.55,-6.74,;13.09,-6.72,;13.84,-5.38,;13.06,-4.07,;13.8,-2.75,;15.35,-2.72,;16.1,-1.4,;16.12,-4.04,;15.36,-5.38,;11.55,-9.42,;13.1,-9.39,;13.9,-10.72,;13.87,-8.04,)| Show InChI InChI=1S/C33H38ClN3O5/c1-33(17-25-18-35-28-5-3-2-4-27(25)28,31(40)37(19-29(38)39)11-10-20-6-8-26(34)9-7-20)36-32(41)42-30-23-13-21-12-22(15-23)16-24(30)14-21/h2-9,18,21-24,30,35H,10-17,19H2,1H3,(H,36,41)(H,38,39)/t21?,22?,23?,24?,30?,33-/m1/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Binding affinity against CCK-A receptor in guinea pig pancreatic membranes |
Bioorg Med Chem Lett 8: 1419-24 (1999)
BindingDB Entry DOI: 10.7270/Q2NZ86S0 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50070422
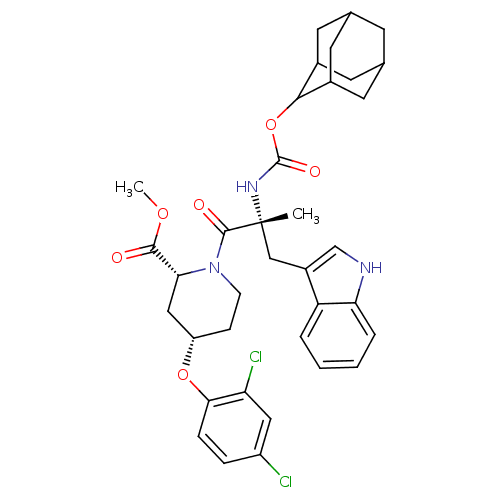
((2R,4S)-1-[(R)-2-(Adamantan-2-yloxycarbonylamino)-...)Show SMILES COC(=O)[C@H]1C[C@H](CCN1C(=O)[C@@](C)(Cc1c[nH]c2ccccc12)NC(=O)OC1C2CC3CC(C2)CC1C3)Oc1ccc(Cl)cc1Cl |wU:12.26,wD:4.3,6.43,12.13,TLB:34:29:37:33.35.32,34:33:28.29.30:37,THB:32:31:28:33.34.35,32:33:28:31.30.37,27:28:31.30.37:33.34.35,27:28:37:33.35.32,(15.75,-10.02,;15.78,-8.5,;14.46,-7.7,;13.11,-8.46,;14.46,-6.16,;16,-6.16,;16.78,-4.83,;16,-3.48,;14.46,-3.48,;13.69,-4.83,;12.14,-4.83,;12.11,-3.28,;10.82,-5.61,;10.82,-4.07,;10.86,-7.16,;10.05,-8.5,;8.51,-8.62,;8.18,-10.14,;9.5,-10.91,;9.79,-12.42,;11.24,-12.92,;12.4,-11.91,;12.11,-10.41,;10.66,-9.91,;9.47,-4.86,;8.15,-5.66,;8.18,-7.21,;6.8,-4.92,;5.48,-5.71,;3.97,-5.28,;3.97,-3.7,;2.94,-2.45,;1.52,-3.04,;1.56,-4.57,;2.55,-5.86,;2.94,-4.92,;4.26,-4.42,;4.29,-2.94,;18.32,-4.83,;19.87,-4.83,;20.64,-6.16,;22.15,-6.18,;22.92,-4.83,;24.47,-4.83,;22.15,-3.51,;20.61,-3.51,;19.83,-2.17,)| Show InChI InChI=1S/C36H41Cl2N3O6/c1-36(18-24-19-39-29-6-4-3-5-27(24)29,40-35(44)47-32-22-12-20-11-21(14-22)15-23(32)13-20)34(43)41-10-9-26(17-30(41)33(42)45-2)46-31-8-7-25(37)16-28(31)38/h3-8,16,19-23,26,30,32,39H,9-15,17-18H2,1-2H3,(H,40,44)/t20?,21?,22?,23?,26-,30+,32?,36+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Capacity to inhibit [3H]-p CCK 8 binding to membrane preparations of CHO cells transfected with the rat CCK-B receptor |
Bioorg Med Chem Lett 8: 1419-24 (1999)
BindingDB Entry DOI: 10.7270/Q2NZ86S0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Cavia porcellus) | BDBM50070410

(CHEMBL105618 | [2-(4-Benzoyl-piperidin-1-yl)-1-(1H...)Show SMILES [H][C@@]12C[C@@]3([H])C[C@@]([H])(C1)C(OC(=O)NC(C)(Cc1c[nH]c4ccccc14)C(=O)N1CCC(CC1)C(=O)c1ccccc1)[C@@]([H])(C2)C3 |wU:3.3,42.47,wD:1.0,6.6,TLB:10:9:5:1.8.2,THB:44:42:5:1.8.2,44:1:5:9.42.45,2:3:9:1.44.8,2:1:9:3.5.45,10:9:1.44.8:3.5.45,(.37,-4.95,;.95,-6.36,;-.8,-6.4,;.55,-7.17,;-.61,-8.19,;1.12,-8.59,;2.75,-8.43,;3.39,-9.82,;1.53,-7.65,;3.85,-7.36,;5.2,-8.11,;6.53,-7.31,;6.6,-5.77,;7.81,-8.15,;9.19,-7.46,;8.83,-8.96,;9.28,-5.92,;10.32,-4.79,;11.85,-4.96,;12.49,-3.57,;11.36,-2.53,;11.39,-1,;10.07,-.2,;8.74,-.97,;8.72,-2.5,;10.03,-3.27,;10.48,-8.3,;10.4,-9.84,;11.86,-7.6,;12.91,-6.47,;14.35,-7.45,;15.68,-7.32,;14.63,-8.47,;13.1,-7.47,;16.81,-8.39,;16.44,-9.9,;18.28,-7.95,;18.63,-6.46,;20.11,-6.01,;21.24,-7.08,;20.87,-8.58,;19.39,-9.01,;3.3,-5.96,;4.42,-4.92,;2.02,-5.33,;1.68,-6.07,)| Show InChI InChI=1S/C35H41N3O4/c1-35(20-28-21-36-30-10-6-5-9-29(28)30,37-34(41)42-32-26-16-22-15-23(18-26)19-27(32)17-22)33(40)38-13-11-25(12-14-38)31(39)24-7-3-2-4-8-24/h2-10,21-23,25-27,32,36H,11-20H2,1H3,(H,37,41)/t22-,23+,26-,27+,32?,35? | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Binding affinity against CCK-A receptor in guinea pig pancreatic membranes |
Bioorg Med Chem Lett 8: 1419-24 (1999)
BindingDB Entry DOI: 10.7270/Q2NZ86S0 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50070410

(CHEMBL105618 | [2-(4-Benzoyl-piperidin-1-yl)-1-(1H...)Show SMILES [H][C@@]12C[C@@]3([H])C[C@@]([H])(C1)C(OC(=O)NC(C)(Cc1c[nH]c4ccccc14)C(=O)N1CCC(CC1)C(=O)c1ccccc1)[C@@]([H])(C2)C3 |wU:3.3,42.47,wD:1.0,6.6,TLB:10:9:5:1.8.2,THB:44:42:5:1.8.2,44:1:5:9.42.45,2:3:9:1.44.8,2:1:9:3.5.45,10:9:1.44.8:3.5.45,(.37,-4.95,;.95,-6.36,;-.8,-6.4,;.55,-7.17,;-.61,-8.19,;1.12,-8.59,;2.75,-8.43,;3.39,-9.82,;1.53,-7.65,;3.85,-7.36,;5.2,-8.11,;6.53,-7.31,;6.6,-5.77,;7.81,-8.15,;9.19,-7.46,;8.83,-8.96,;9.28,-5.92,;10.32,-4.79,;11.85,-4.96,;12.49,-3.57,;11.36,-2.53,;11.39,-1,;10.07,-.2,;8.74,-.97,;8.72,-2.5,;10.03,-3.27,;10.48,-8.3,;10.4,-9.84,;11.86,-7.6,;12.91,-6.47,;14.35,-7.45,;15.68,-7.32,;14.63,-8.47,;13.1,-7.47,;16.81,-8.39,;16.44,-9.9,;18.28,-7.95,;18.63,-6.46,;20.11,-6.01,;21.24,-7.08,;20.87,-8.58,;19.39,-9.01,;3.3,-5.96,;4.42,-4.92,;2.02,-5.33,;1.68,-6.07,)| Show InChI InChI=1S/C35H41N3O4/c1-35(20-28-21-36-30-10-6-5-9-29(28)30,37-34(41)42-32-26-16-22-15-23(18-26)19-27(32)17-22)33(40)38-13-11-25(12-14-38)31(39)24-7-3-2-4-8-24/h2-10,21-23,25-27,32,36H,11-20H2,1H3,(H,37,41)/t22-,23+,26-,27+,32?,35? | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Capacity to inhibit [3H]-p CCK 8 binding to membrane preparations of CHO cells transfected with the rat CCK-B receptor |
Bioorg Med Chem Lett 8: 1419-24 (1999)
BindingDB Entry DOI: 10.7270/Q2NZ86S0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Cavia porcellus) | BDBM50070411

((2R,4S)-1-[(R)-2-(Adamantan-2-yloxycarbonylamino)-...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)N1CC[C@@H](C[C@@H]1C(O)=O)Oc1ccc(Cl)cc1Cl |wU:1.13,wD:33.39,31.42,1.0,TLB:22:17:25:21.23.20,22:21:16.17.18:25,THB:20:19:16:21.22.23,20:21:16:19.18.25,15:16:19.18.25:21.22.23,15:16:25:21.23.20,(10.82,-4.07,;10.82,-5.61,;10.86,-7.16,;10.05,-8.5,;8.51,-8.62,;8.18,-10.14,;9.5,-10.91,;9.79,-12.42,;11.24,-12.92,;12.4,-11.91,;12.11,-10.41,;10.66,-9.91,;9.47,-4.86,;8.15,-5.66,;8.18,-7.21,;6.8,-4.92,;5.48,-5.71,;3.97,-5.28,;3.97,-3.7,;2.94,-2.45,;1.52,-3.04,;1.56,-4.57,;2.55,-5.86,;2.94,-4.92,;4.26,-4.42,;4.29,-2.94,;12.14,-4.83,;12.11,-3.28,;13.69,-4.83,;14.46,-3.48,;16,-3.48,;16.78,-4.83,;16,-6.16,;14.46,-6.16,;14.46,-7.7,;15.78,-8.5,;13.11,-8.46,;18.32,-4.83,;19.87,-4.83,;20.64,-6.16,;22.15,-6.18,;22.92,-4.83,;24.47,-4.83,;22.15,-3.51,;20.61,-3.51,;19.83,-2.17,)| Show InChI InChI=1S/C35H39Cl2N3O6/c1-35(17-23-18-38-28-5-3-2-4-26(23)28,39-34(44)46-31-21-11-19-10-20(13-21)14-22(31)12-19)33(43)40-9-8-25(16-29(40)32(41)42)45-30-7-6-24(36)15-27(30)37/h2-7,15,18-22,25,29,31,38H,8-14,16-17H2,1H3,(H,39,44)(H,41,42)/t19?,20?,21?,22?,25-,29+,31?,35+/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Binding affinity against CCK-A receptor in guinea pig pancreatic membranes |
Bioorg Med Chem Lett 8: 1419-24 (1999)
BindingDB Entry DOI: 10.7270/Q2NZ86S0 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50070412

((2R,4R)-1-[(R)-2-(Adamantan-2-yloxycarbonylamino)-...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)N1C[C@@H](C[C@@H]1C(O)=O)Oc1ccc(Cl)cc1Cl |wU:1.13,wD:32.38,30.41,1.0,TLB:22:17:25:21.23.20,22:21:16.17.18:25,THB:20:19:16:21.22.23,20:21:16:19.18.25,15:16:19.18.25:21.22.23,15:16:25:21.23.20,(22.06,-1.42,;22.08,-2.96,;22.09,-4.51,;21.3,-5.84,;19.77,-5.96,;19.42,-7.48,;20.76,-8.25,;21.05,-9.76,;22.49,-10.27,;23.65,-9.25,;23.36,-7.76,;21.92,-7.25,;20.72,-2.2,;19.38,-3,;19.42,-4.55,;18.05,-2.26,;16.71,-3.06,;15.22,-2.62,;15.23,-1.04,;14.17,.19,;12.79,-.39,;12.79,-1.91,;13.82,-3.2,;14.2,-2.26,;15.52,-1.77,;15.52,-.29,;23.4,-2.17,;23.37,-.62,;24.94,-2.17,;25.84,-.91,;27.3,-1.38,;27.3,-2.91,;25.85,-3.39,;25.37,-4.87,;26.42,-6,;23.88,-5.19,;28.56,-.46,;29.96,-1.09,;30.13,-2.62,;31.54,-3.25,;32.79,-2.33,;34.19,-2.96,;32.61,-.78,;31.19,-.17,;31.03,1.35,)| Show InChI InChI=1S/C34H37Cl2N3O6/c1-34(15-22-16-37-27-5-3-2-4-25(22)27,38-33(43)45-30-20-9-18-8-19(11-20)12-21(30)10-18)32(42)39-17-24(14-28(39)31(40)41)44-29-7-6-23(35)13-26(29)36/h2-7,13,16,18-21,24,28,30,37H,8-12,14-15,17H2,1H3,(H,38,43)(H,40,41)/t18?,19?,20?,21?,24-,28-,30?,34-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibitory activity against CCK-B receptor |
Bioorg Med Chem Lett 8: 1419-24 (1999)
BindingDB Entry DOI: 10.7270/Q2NZ86S0 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50006878

((R)-1-(1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)Nc2ccc(C)cc2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C24H22N4O2/c1-16-12-14-18(15-13-16)25-24(30)27-22-23(29)28(2)20-11-7-6-10-19(20)21(26-22)17-8-4-3-5-9-17/h3-15,22H,1-2H3,(H2,25,27,30)/t22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibitory activity against CCK-B receptor |
Bioorg Med Chem Lett 8: 1419-24 (1999)
BindingDB Entry DOI: 10.7270/Q2NZ86S0 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50070420

((2S,4S)-1-[(S)-2-(Adamantan-2-yloxycarbonylamino)-...)Show SMILES C[C@@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)N1CC[C@@H](C[C@H]1C(O)=O)Oc1ccc(Cl)cc1Cl |wU:33.39,1.0,wD:1.13,31.42,TLB:22:17:25:21.23.20,22:21:16.17.18:25,THB:20:19:16:21.22.23,20:21:16:19.18.25,15:16:19.18.25:21.22.23,15:16:25:21.23.20,(10.79,-4.05,;10.79,-5.59,;10.82,-7.13,;10.02,-8.45,;8.48,-8.58,;8.16,-10.09,;9.48,-10.86,;9.76,-12.36,;11.21,-12.88,;12.36,-11.85,;12.07,-10.37,;10.63,-9.86,;9.44,-4.82,;8.13,-5.63,;8.16,-7.17,;6.78,-4.89,;5.47,-5.69,;3.96,-5.24,;3.96,-3.67,;2.93,-2.42,;1.52,-3.03,;1.55,-4.53,;2.55,-5.82,;2.93,-4.89,;4.25,-4.41,;4.28,-2.93,;12.11,-4.79,;12.07,-3.25,;13.65,-4.79,;14.42,-3.48,;15.96,-3.48,;16.73,-4.79,;15.96,-6.14,;14.42,-6.14,;14.42,-7.68,;15.73,-8.45,;13.07,-8.42,;18.27,-4.82,;19.81,-4.82,;20.58,-6.14,;22.08,-6.14,;22.85,-4.82,;24.39,-4.82,;22.08,-3.48,;20.54,-3.48,;19.77,-2.16,)| Show InChI InChI=1S/C35H39Cl2N3O6/c1-35(17-23-18-38-28-5-3-2-4-26(23)28,39-34(44)46-31-21-11-19-10-20(13-21)14-22(31)12-19)33(43)40-9-8-25(16-29(40)32(41)42)45-30-7-6-24(36)15-27(30)37/h2-7,15,18-22,25,29,31,38H,8-14,16-17H2,1H3,(H,39,44)(H,41,42)/t19?,20?,21?,22?,25-,29-,31?,35-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 217 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibitory activity against CCK-B receptor |
Bioorg Med Chem Lett 8: 1419-24 (1999)
BindingDB Entry DOI: 10.7270/Q2NZ86S0 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50070414

(CHEMBL286247 | {[(R)-2-(Adamantan-2-yloxycarbonyla...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)N(CCc1ccc(Cl)cc1)CC(O)=O |wU:1.13,wD:1.0,TLB:15:16:19.18.25:21.23.22,THB:23:24:18:21.22.20,23:21:18:16.24.25,20:19:16:21.23.22,20:21:16:19.18.25,15:16:18:21.22.20,(7.91,-7.33,;7.94,-8.88,;7.94,-10.43,;7.17,-11.75,;5.62,-11.9,;5.27,-13.39,;6.62,-14.19,;6.91,-15.68,;8.36,-16.2,;9.52,-15.17,;9.23,-13.68,;7.78,-13.17,;6.59,-8.13,;5.23,-8.91,;5.27,-10.46,;3.91,-8.17,;2.56,-8.97,;1.07,-8.54,;-.34,-9.1,;-1.38,-7.84,;-1.38,-6.3,;.01,-5.72,;1.07,-6.94,;1.37,-6.2,;1.37,-7.69,;.04,-8.17,;9.27,-8.07,;9.23,-6.52,;10.81,-8.07,;11.55,-6.74,;13.09,-6.72,;13.84,-5.38,;13.06,-4.07,;13.8,-2.75,;15.35,-2.72,;16.1,-1.4,;16.12,-4.04,;15.36,-5.38,;11.55,-9.42,;13.1,-9.39,;13.9,-10.72,;13.87,-8.04,)| Show InChI InChI=1S/C33H38ClN3O5/c1-33(17-25-18-35-28-5-3-2-4-27(25)28,31(40)37(19-29(38)39)11-10-20-6-8-26(34)9-7-20)36-32(41)42-30-23-13-21-12-22(15-23)16-24(30)14-21/h2-9,18,21-24,30,35H,10-17,19H2,1H3,(H,36,41)(H,38,39)/t21?,22?,23?,24?,30?,33-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 217 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Capacity to inhibit [3H]-p CCK 8 binding to membrane preparations of CHO cells transfected with the rat CCK-B receptor |
Bioorg Med Chem Lett 8: 1419-24 (1999)
BindingDB Entry DOI: 10.7270/Q2NZ86S0 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50070415

((2R,4R)-1-[(R)-2-(Adamantan-2-yloxycarbonylamino)-...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)N1C[C@@H](C[C@@H]1C(O)=O)Oc1ccc(F)cc1F |wU:1.13,wD:32.38,30.41,1.0,TLB:22:17:25:21.23.20,22:21:16.17.18:25,THB:20:19:16:21.22.23,20:21:16:19.18.25,15:16:19.18.25:21.22.23,15:16:25:21.23.20,(18.68,-9.94,;18.7,-11.49,;18.71,-13.03,;17.93,-14.36,;16.39,-14.49,;16.04,-16,;17.38,-16.78,;17.67,-18.29,;19.12,-18.79,;20.27,-17.77,;19.98,-16.28,;18.54,-15.78,;17.35,-10.73,;16,-11.53,;16.04,-13.08,;14.67,-10.79,;13.33,-11.59,;11.84,-11.15,;11.85,-9.57,;10.79,-8.33,;9.41,-8.91,;9.41,-10.44,;10.44,-11.72,;10.82,-10.79,;12.14,-10.3,;12.14,-8.82,;20.02,-10.7,;20,-9.15,;21.56,-10.7,;22.46,-9.44,;23.92,-9.91,;23.92,-11.44,;22.47,-11.92,;21.99,-13.4,;23.04,-14.52,;20.5,-13.72,;25.18,-8.99,;26.58,-9.62,;26.75,-11.15,;28.16,-11.78,;29.41,-10.86,;30.81,-11.49,;29.23,-9.31,;27.82,-8.7,;27.65,-7.18,)| Show InChI InChI=1S/C34H37F2N3O6/c1-34(15-22-16-37-27-5-3-2-4-25(22)27,38-33(43)45-30-20-9-18-8-19(11-20)12-21(30)10-18)32(42)39-17-24(14-28(39)31(40)41)44-29-7-6-23(35)13-26(29)36/h2-7,13,16,18-21,24,28,30,37H,8-12,14-15,17H2,1H3,(H,38,43)(H,40,41)/t18?,19?,20?,21?,24-,28-,30?,34-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 389 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibitory activity against CCK-B receptor |
Bioorg Med Chem Lett 8: 1419-24 (1999)
BindingDB Entry DOI: 10.7270/Q2NZ86S0 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50070416

((2R,4R)-1-[(R)-2-(Adamantan-2-yloxycarbonylamino)-...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)N1C[C@@H](C[C@@H]1C(O)=O)Oc1ccc(cc1)[N+]([O-])=O |wU:1.13,wD:32.38,30.41,1.0,TLB:15:16:19.18.25:21.23.22,15:16:25:21.23.20,22:17:25:21.23.20,22:21:16.17.18:25,THB:20:19:16:21.23.22,20:21:16:19.18.25,(18.67,-9.94,;18.69,-11.49,;18.7,-13.03,;17.92,-14.36,;16.39,-14.49,;16.03,-16,;17.37,-16.77,;17.66,-18.28,;19.11,-18.79,;20.27,-17.77,;19.98,-16.28,;18.53,-15.78,;17.34,-10.72,;16,-11.53,;16.03,-13.07,;14.67,-10.79,;13.33,-11.58,;12.14,-10.3,;10.82,-10.79,;9.41,-10.43,;9.41,-8.91,;10.79,-8.33,;12.14,-8.82,;11.85,-9.57,;11.84,-11.14,;10.43,-11.72,;20.01,-10.69,;19.99,-9.15,;21.56,-10.69,;22.46,-9.44,;23.91,-9.91,;23.91,-11.43,;22.47,-11.91,;21.98,-13.39,;23.04,-14.52,;20.49,-13.72,;25.17,-8.99,;26.57,-9.62,;27.81,-8.7,;29.22,-9.31,;29.41,-10.85,;28.15,-11.78,;26.75,-11.14,;30.82,-11.49,;30.99,-13.01,;32.05,-10.56,)| Show InChI InChI=1S/C34H38N4O8/c1-34(16-23-17-35-28-5-3-2-4-27(23)28,36-33(42)46-30-21-11-19-10-20(13-21)14-22(30)12-19)32(41)37-18-26(15-29(37)31(39)40)45-25-8-6-24(7-9-25)38(43)44/h2-9,17,19-22,26,29-30,35H,10-16,18H2,1H3,(H,36,42)(H,39,40)/t19?,20?,21?,22?,26-,29-,30?,34-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 507 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibitory activity against CCK-B receptor |
Bioorg Med Chem Lett 8: 1419-24 (1999)
BindingDB Entry DOI: 10.7270/Q2NZ86S0 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50240798
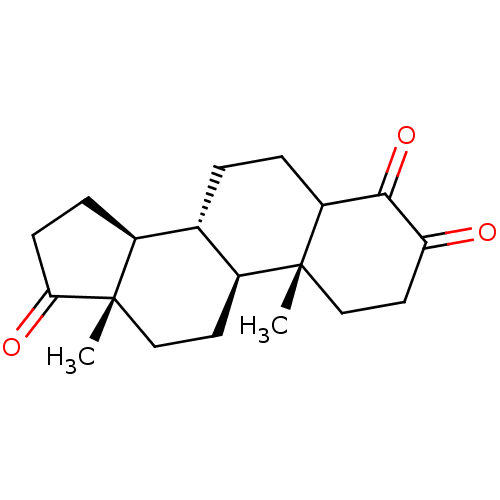
((8R,9S,10R,13S,14S)-4-Hydroxy-10,13-dimethyl-1,6,7...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCC4C(=O)C(=O)CC[C@]34C)[C@@H]1CCC2=O |r| Show InChI InChI=1S/C19H26O3/c1-18-10-8-15(20)17(22)14(18)4-3-11-12-5-6-16(21)19(12,2)9-7-13(11)18/h11-14H,3-10H2,1-2H3/t11-,12-,13-,14?,18+,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul (UFRGS)
Curated by ChEMBL
| Assay Description
Inhibition of aromatase in human placental microsomes assessed as tritiated water release after 15 mins using [1beta, 3H]androstenedione as substrate... |
Eur J Med Chem 43: 1865-77 (2008)
Article DOI: 10.1016/j.ejmech.2007.11.021
BindingDB Entry DOI: 10.7270/Q2ZW1KPN |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM7458

(5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...)Show InChI InChI=1S/C15H10O5/c16-9-3-1-8(2-4-9)13-7-12(19)15-11(18)5-10(17)6-14(15)20-13/h1-7,16-18H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul (UFRGS)
Curated by ChEMBL
| Assay Description
Inhibition of aromatase in human placental microsomes assessed as tritiated water release after 15 mins using [1beta, 3H]androstenedione as substrate... |
Eur J Med Chem 43: 1865-77 (2008)
Article DOI: 10.1016/j.ejmech.2007.11.021
BindingDB Entry DOI: 10.7270/Q2ZW1KPN |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50148911

((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...)Show SMILES C[C@@H]1CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2[C@H]1C)C(O)=O |r,c:9| Show InChI InChI=1S/C30H48O3/c1-18-10-15-30(25(32)33)17-16-28(6)20(24(30)19(18)2)8-9-22-27(5)13-12-23(31)26(3,4)21(27)11-14-29(22,28)7/h8,18-19,21-24,31H,9-17H2,1-7H3,(H,32,33)/t18-,19+,21+,22-,23+,24+,27+,28-,29-,30+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul (UFRGS)
Curated by ChEMBL
| Assay Description
Inhibition of aromatase in human placental microsomes assessed as tritiated water release after 15 mins using [1beta, 3H]androstenedione as substrate... |
Eur J Med Chem 43: 1865-77 (2008)
Article DOI: 10.1016/j.ejmech.2007.11.021
BindingDB Entry DOI: 10.7270/Q2ZW1KPN |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50245648
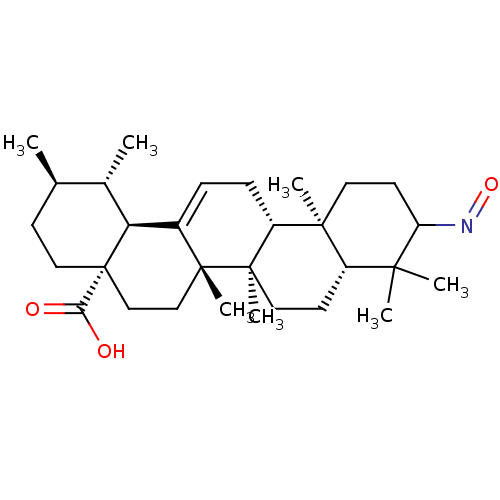
((E )-3-Oximeurs-12-en-28-oic acid | CHEMBL487888)Show SMILES C[C@@H]1CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CCC(N=O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2[C@H]1C)C(O)=O |r,c:9| Show InChI InChI=1S/C30H47NO3/c1-18-10-15-30(25(32)33)17-16-28(6)20(24(30)19(18)2)8-9-22-27(5)13-12-23(31-34)26(3,4)21(27)11-14-29(22,28)7/h8,18-19,21-24H,9-17H2,1-7H3,(H,32,33)/t18-,19+,21+,22-,23?,24+,27+,28-,29-,30+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul (UFRGS)
Curated by ChEMBL
| Assay Description
Inhibition of aromatase in human placental microsomes assessed as tritiated water release after 15 mins using [1beta, 3H]androstenedione as substrate... |
Eur J Med Chem 43: 1865-77 (2008)
Article DOI: 10.1016/j.ejmech.2007.11.021
BindingDB Entry DOI: 10.7270/Q2ZW1KPN |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50245649
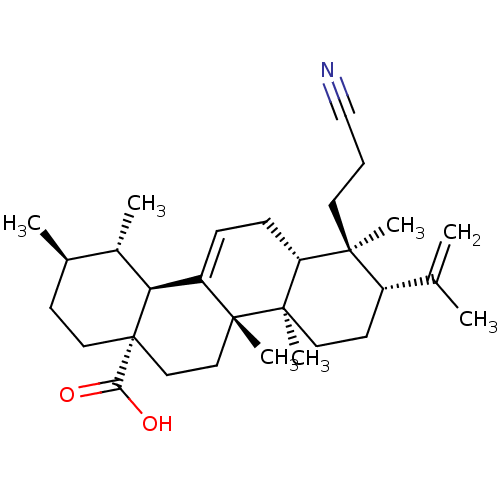
(2-Cyano-2,3-seco-4-yliden-olean-12-enoic acid | CH...)Show SMILES C[C@@H]1CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@](C)(CCC#N)[C@@H](CC[C@@]34C)C(C)=C)[C@@H]2[C@H]1C)C(O)=O |r,c:9| Show InChI InChI=1S/C30H45NO2/c1-19(2)22-12-14-29(7)24(27(22,5)13-8-18-31)10-9-23-25-21(4)20(3)11-15-30(25,26(32)33)17-16-28(23,29)6/h9,20-22,24-25H,1,8,10-17H2,2-7H3,(H,32,33)/t20-,21+,22+,24-,25+,27+,28-,29-,30+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul (UFRGS)
Curated by ChEMBL
| Assay Description
Inhibition of aromatase in human placental microsomes assessed as tritiated water release after 15 mins using [1beta, 3H]androstenedione as substrate... |
Eur J Med Chem 43: 1865-77 (2008)
Article DOI: 10.1016/j.ejmech.2007.11.021
BindingDB Entry DOI: 10.7270/Q2ZW1KPN |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50225900
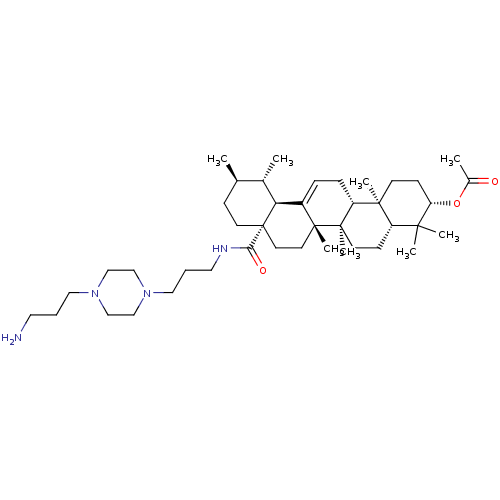
(CHEMBL270215 | N-{3-[4-(3-aminopropyl)piperazinyl]...)Show SMILES C[C@@H]1CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](OC(C)=O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2[C@H]1C)C(=O)NCCCN1CCN(CCCN)CC1 |r,c:9| Show InChI InChI=1S/C42H72N4O3/c1-29-13-18-42(37(48)44-22-10-24-46-27-25-45(26-28-46)23-9-21-43)20-19-40(7)32(36(42)30(29)2)11-12-34-39(6)16-15-35(49-31(3)47)38(4,5)33(39)14-17-41(34,40)8/h11,29-30,33-36H,9-10,12-28,43H2,1-8H3,(H,44,48)/t29-,30+,33+,34-,35+,36+,39+,40-,41-,42+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul (UFRGS)
Curated by ChEMBL
| Assay Description
Inhibition of aromatase in human placental microsomes assessed as tritiated water release after 15 mins using [1beta, 3H]androstenedione as substrate... |
Eur J Med Chem 43: 1865-77 (2008)
Article DOI: 10.1016/j.ejmech.2007.11.021
BindingDB Entry DOI: 10.7270/Q2ZW1KPN |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50245704
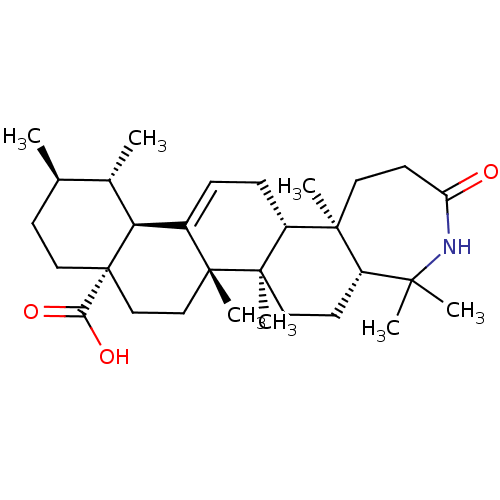
(4-aza-A-homo-3-oxo-ursolic acid | CHEMBL454421)Show SMILES C[C@@H]1CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CCC(=O)NC(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2[C@H]1C)C(O)=O |r,c:9| Show InChI InChI=1S/C30H47NO3/c1-18-10-15-30(25(33)34)17-16-28(6)20(24(30)19(18)2)8-9-22-27(5)13-12-23(32)31-26(3,4)21(27)11-14-29(22,28)7/h8,18-19,21-22,24H,9-17H2,1-7H3,(H,31,32)(H,33,34)/t18-,19+,21+,22-,24+,27+,28-,29-,30+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul (UFRGS)
Curated by ChEMBL
| Assay Description
Inhibition of aromatase in human placental microsomes assessed as tritiated water release after 15 mins using [1beta, 3H]androstenedione as substrate... |
Eur J Med Chem 43: 1865-77 (2008)
Article DOI: 10.1016/j.ejmech.2007.11.021
BindingDB Entry DOI: 10.7270/Q2ZW1KPN |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50225913
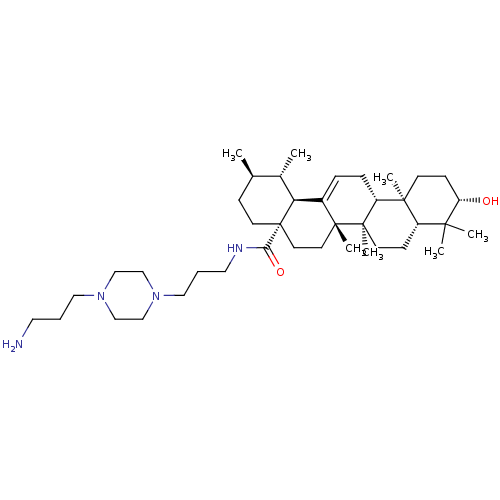
(CHEMBL264924 | N-{3-[4-(3-aminopropyl)piperazinyl]...)Show SMILES C[C@@H]1CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2[C@H]1C)C(=O)NCCCN1CCN(CCCN)CC1 |r,c:9| Show InChI InChI=1S/C40H70N4O2/c1-28-12-17-40(35(46)42-21-9-23-44-26-24-43(25-27-44)22-8-20-41)19-18-38(6)30(34(40)29(28)2)10-11-32-37(5)15-14-33(45)36(3,4)31(37)13-16-39(32,38)7/h10,28-29,31-34,45H,8-9,11-27,41H2,1-7H3,(H,42,46)/t28-,29+,31+,32-,33+,34+,37+,38-,39-,40+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul (UFRGS)
Curated by ChEMBL
| Assay Description
Inhibition of aromatase in human placental microsomes assessed as tritiated water release after 15 mins using [1beta, 3H]androstenedione as substrate... |
Eur J Med Chem 43: 1865-77 (2008)
Article DOI: 10.1016/j.ejmech.2007.11.021
BindingDB Entry DOI: 10.7270/Q2ZW1KPN |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50245647
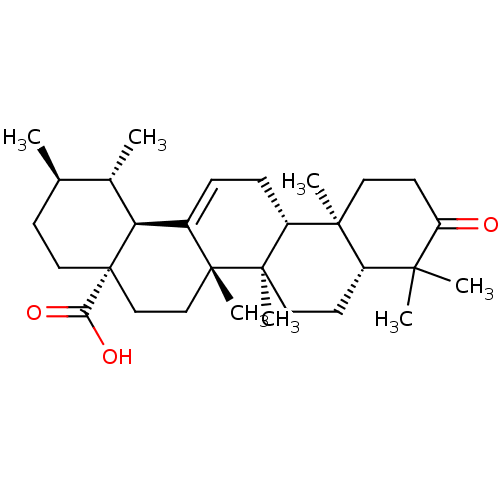
((1S,2R,4aS,6aS,6bR,8aR,12aR,12bR,14bS)-1,2,6a,6b,9...)Show SMILES C[C@@H]1CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CCC(=O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2[C@H]1C)C(O)=O |r,c:9| Show InChI InChI=1S/C30H46O3/c1-18-10-15-30(25(32)33)17-16-28(6)20(24(30)19(18)2)8-9-22-27(5)13-12-23(31)26(3,4)21(27)11-14-29(22,28)7/h8,18-19,21-22,24H,9-17H2,1-7H3,(H,32,33)/t18-,19+,21+,22-,24+,27+,28-,29-,30+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul (UFRGS)
Curated by ChEMBL
| Assay Description
Inhibition of aromatase in human placental microsomes assessed as tritiated water release after 15 mins using [1beta, 3H]androstenedione as substrate... |
Eur J Med Chem 43: 1865-77 (2008)
Article DOI: 10.1016/j.ejmech.2007.11.021
BindingDB Entry DOI: 10.7270/Q2ZW1KPN |
More data for this
Ligand-Target Pair | |
Histidine-rich protein PFHRP-II
(Plasmodium falciparum) | BDBM50411865
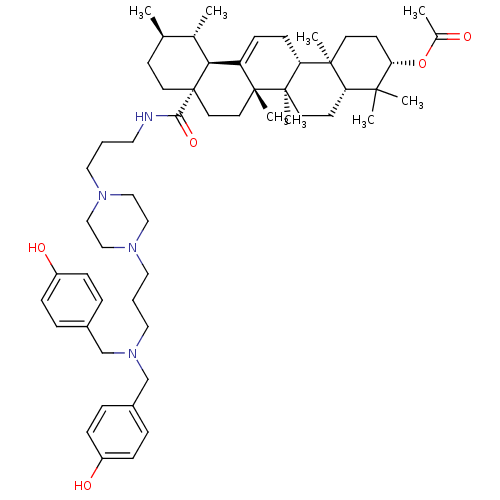
(CHEMBL261832)Show SMILES C[C@@H]1CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](OC(C)=O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2[C@H]1C)C(=O)NCCCN1CCN(CCCN(Cc2ccc(O)cc2)Cc2ccc(O)cc2)CC1 |c:9| Show InChI InChI=1S/C56H84N4O5/c1-39-21-26-56(28-27-54(7)46(50(56)40(39)2)19-20-48-53(6)24-23-49(65-41(3)61)52(4,5)47(53)22-25-55(48,54)8)51(64)57-29-9-30-58-33-35-59(36-34-58)31-10-32-60(37-42-11-15-44(62)16-12-42)38-43-13-17-45(63)18-14-43/h11-19,39-40,47-50,62-63H,9-10,20-38H2,1-8H3,(H,57,64)/t39-,40+,47+,48-,49+,50+,53+,54-,55-,56+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.25E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul (UFRGS)
Curated by ChEMBL
| Assay Description
Inhibition of beta-hematin formation |
Bioorg Med Chem 16: 771-82 (2008)
Article DOI: 10.1016/j.bmc.2007.10.031
BindingDB Entry DOI: 10.7270/Q23N24MK |
More data for this
Ligand-Target Pair | |
Histidine-rich protein PFHRP-II
(Plasmodium falciparum) | BDBM22985
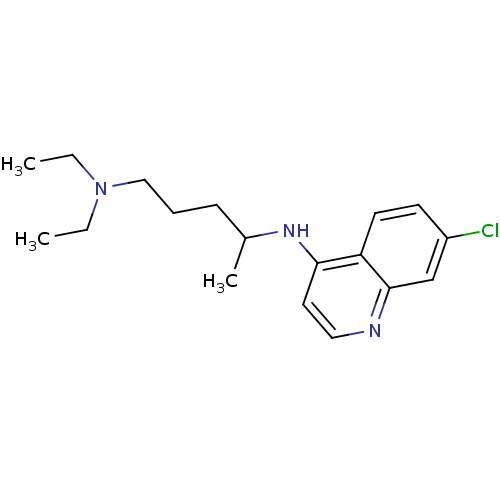
(Aralen | CHEMBL76 | CHLOROQUINE PHOSPHATE | Chloro...)Show InChI InChI=1S/C18H26ClN3/c1-4-22(5-2)12-6-7-14(3)21-17-10-11-20-18-13-15(19)8-9-16(17)18/h8-11,13-14H,4-7,12H2,1-3H3,(H,20,21) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.90E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul (UFRGS)
Curated by ChEMBL
| Assay Description
Inhibition of beta-hematin formation |
Bioorg Med Chem 16: 771-82 (2008)
Article DOI: 10.1016/j.bmc.2007.10.031
BindingDB Entry DOI: 10.7270/Q23N24MK |
More data for this
Ligand-Target Pair | |
Histidine-rich protein PFHRP-II
(Plasmodium falciparum) | BDBM50148911

((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...)Show SMILES C[C@@H]1CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2[C@H]1C)C(O)=O |r,c:9| Show InChI InChI=1S/C30H48O3/c1-18-10-15-30(25(32)33)17-16-28(6)20(24(30)19(18)2)8-9-22-27(5)13-12-23(31)26(3,4)21(27)11-14-29(22,28)7/h8,18-19,21-24,31H,9-17H2,1-7H3,(H,32,33)/t18-,19+,21+,22-,23+,24+,27+,28-,29-,30+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul (UFRGS)
Curated by ChEMBL
| Assay Description
Inhibition of beta-hematin formation |
Bioorg Med Chem 16: 771-82 (2008)
Article DOI: 10.1016/j.bmc.2007.10.031
BindingDB Entry DOI: 10.7270/Q23N24MK |
More data for this
Ligand-Target Pair | |
Histidine-rich protein PFHRP-II
(Plasmodium falciparum) | BDBM50411864
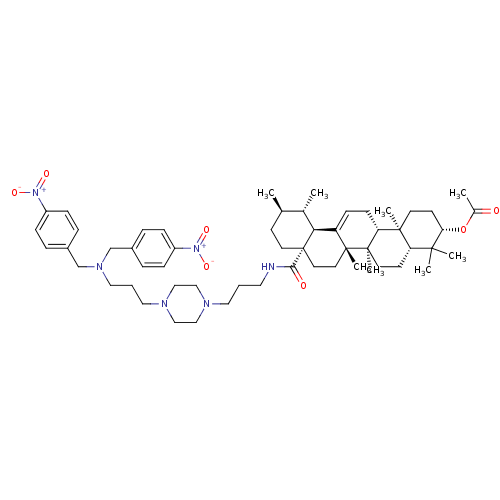
(CHEMBL436662)Show SMILES C[C@@H]1CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](OC(C)=O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2[C@H]1C)C(=O)NCCCN1CCN(CCCN(Cc2ccc(cc2)[N+]([O-])=O)Cc2ccc(cc2)[N+]([O-])=O)CC1 |c:9| Show InChI InChI=1S/C56H82N6O7/c1-39-21-26-56(28-27-54(7)46(50(56)40(39)2)19-20-48-53(6)24-23-49(69-41(3)63)52(4,5)47(53)22-25-55(48,54)8)51(64)57-29-9-30-58-33-35-59(36-34-58)31-10-32-60(37-42-11-15-44(16-12-42)61(65)66)38-43-13-17-45(18-14-43)62(67)68/h11-19,39-40,47-50H,9-10,20-38H2,1-8H3,(H,57,64)/t39-,40+,47+,48-,49+,50+,53+,54-,55-,56+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul (UFRGS)
Curated by ChEMBL
| Assay Description
Inhibition of beta-hematin formation |
Bioorg Med Chem 16: 771-82 (2008)
Article DOI: 10.1016/j.bmc.2007.10.031
BindingDB Entry DOI: 10.7270/Q23N24MK |
More data for this
Ligand-Target Pair | |
Histidine-rich protein PFHRP-II
(Plasmodium falciparum) | BDBM50225900

(CHEMBL270215 | N-{3-[4-(3-aminopropyl)piperazinyl]...)Show SMILES C[C@@H]1CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](OC(C)=O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2[C@H]1C)C(=O)NCCCN1CCN(CCCN)CC1 |r,c:9| Show InChI InChI=1S/C42H72N4O3/c1-29-13-18-42(37(48)44-22-10-24-46-27-25-45(26-28-46)23-9-21-43)20-19-40(7)32(36(42)30(29)2)11-12-34-39(6)16-15-35(49-31(3)47)38(4,5)33(39)14-17-41(34,40)8/h11,29-30,33-36H,9-10,12-28,43H2,1-8H3,(H,44,48)/t29-,30+,33+,34-,35+,36+,39+,40-,41-,42+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul (UFRGS)
Curated by ChEMBL
| Assay Description
Inhibition of beta-hematin formation |
Bioorg Med Chem 16: 771-82 (2008)
Article DOI: 10.1016/j.bmc.2007.10.031
BindingDB Entry DOI: 10.7270/Q23N24MK |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data









































