

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sigma intracellular receptor 2 (Rattus norvegicus (Rat)) | BDBM82353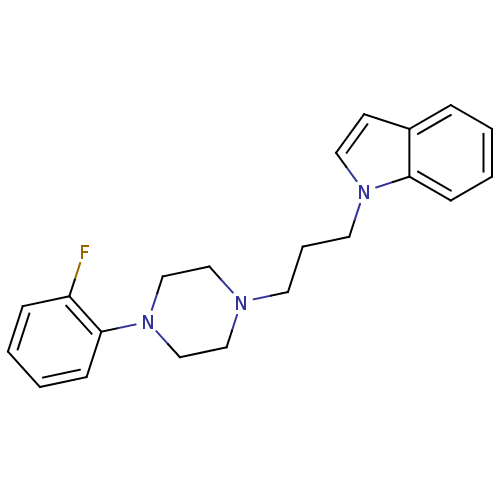 (1-{3-[4-(substitutedphenyl)piperazin1-yl]propyl}-1...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mazandaran University of Medical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]-di-o-tolylguanidine from sigma-2 receptor in rat liver membranes after 180 mins by scintillation counting method | Eur J Med Chem 150: 9-29 (2018) Article DOI: 10.1016/j.ejmech.2018.02.065 BindingDB Entry DOI: 10.7270/Q2X92DXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM82351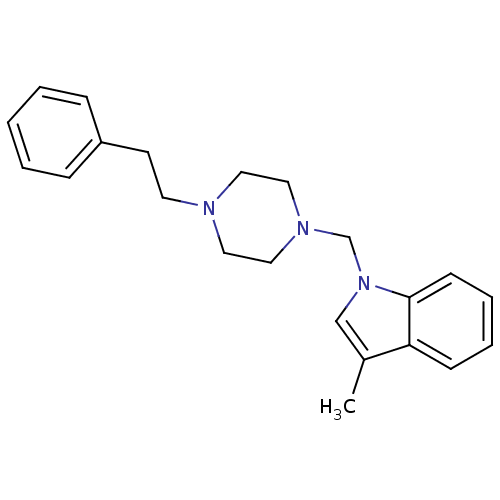 (1-{[4-(substitutedphenyl/phenylethyl)piperazin1-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mazandaran University of Medical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-pentazocine from sigma-1 receptor in guinea pig brain membranes after 180 mins by scintillation counting method | Eur J Med Chem 150: 9-29 (2018) Article DOI: 10.1016/j.ejmech.2018.02.065 BindingDB Entry DOI: 10.7270/Q2X92DXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Rattus norvegicus (Rat)) | BDBM82351 (1-{[4-(substitutedphenyl/phenylethyl)piperazin1-yl...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mazandaran University of Medical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]-di-o-tolylguanidine from sigma-2 receptor in rat liver membranes after 180 mins by scintillation counting method | Eur J Med Chem 150: 9-29 (2018) Article DOI: 10.1016/j.ejmech.2018.02.065 BindingDB Entry DOI: 10.7270/Q2X92DXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50397360 (CHEMBL2170177 | US10188756, Compound CN110) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Mazandaran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of HDAC8 (unknown origin) preincubated for 15 mins followed by acetyl-Gly-Ala-(N-acetyl-Lys)-amino-4-methylcoumarin substrate addition and... | Eur J Med Chem 150: 9-29 (2018) Article DOI: 10.1016/j.ejmech.2018.02.065 BindingDB Entry DOI: 10.7270/Q2X92DXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (HDAC1 and HDAC2) (Homo sapiens (Human)) | BDBM50220811 (CHEMBL56157) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Mazandaran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of HDAC1/2 in human K562 nuclear extract using (QSY-7)-RGGRGLGK(Ac)-GGARRHRK(TAMRA)NH2 as substrate incubated for 30 mins by fluorescence ... | Eur J Med Chem 150: 9-29 (2018) Article DOI: 10.1016/j.ejmech.2018.02.065 BindingDB Entry DOI: 10.7270/Q2X92DXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Homo sapiens (Human)) | BDBM19428 ((2E)-N-hydroxy-3-(4-{[(2-hydroxyethyl)[2-(1H-indol...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Mazandaran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of human H1299 cells derived HDAC using biotin-labeled SGRGKGGKGLGKGGAKRHRKVLRD peracetylated with [3H]acetate at lysine residue as substr... | Eur J Med Chem 150: 9-29 (2018) Article DOI: 10.1016/j.ejmech.2018.02.065 BindingDB Entry DOI: 10.7270/Q2X92DXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50458326 (CHEMBL4209288) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Mazandaran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) | Eur J Med Chem 150: 9-29 (2018) Article DOI: 10.1016/j.ejmech.2018.02.065 BindingDB Entry DOI: 10.7270/Q2X92DXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Homo sapiens (Human)) | BDBM50134232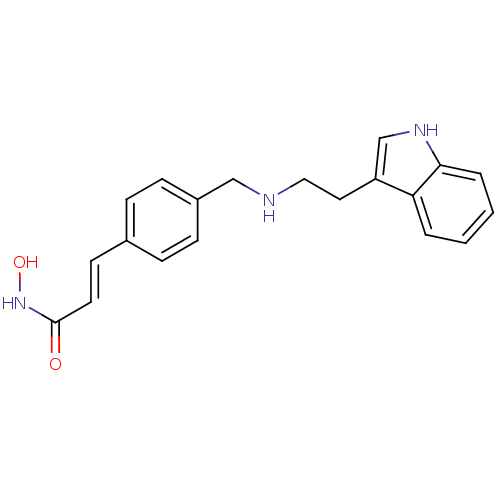 ((E)-N-Hydroxy-3-(4-{[2-(1H-indol-3-yl)-ethylamino]...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Mazandaran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of human H1299 cells derived HDAC using biotin-labeled SGRGKGGKGLGKGGAKRHRKVLRD peracetylated with [3H]acetate at lysine residue as substr... | Eur J Med Chem 150: 9-29 (2018) Article DOI: 10.1016/j.ejmech.2018.02.065 BindingDB Entry DOI: 10.7270/Q2X92DXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50458322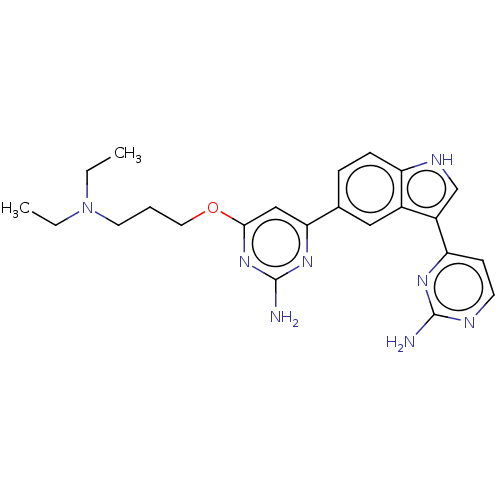 (CHEMBL4206921) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Mazandaran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) | Eur J Med Chem 150: 9-29 (2018) Article DOI: 10.1016/j.ejmech.2018.02.065 BindingDB Entry DOI: 10.7270/Q2X92DXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Homo sapiens (Human)) | BDBM50458324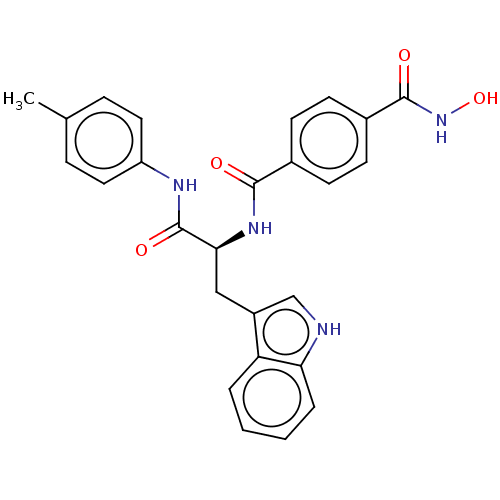 (CHEMBL4202863) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Mazandaran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of HDAC in human HeLa nuclear extract preincubated for 10 mins followed by Boc-Lys(acetyl)-AMC substrate addition measured after 30 mins b... | Eur J Med Chem 150: 9-29 (2018) Article DOI: 10.1016/j.ejmech.2018.02.065 BindingDB Entry DOI: 10.7270/Q2X92DXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50458323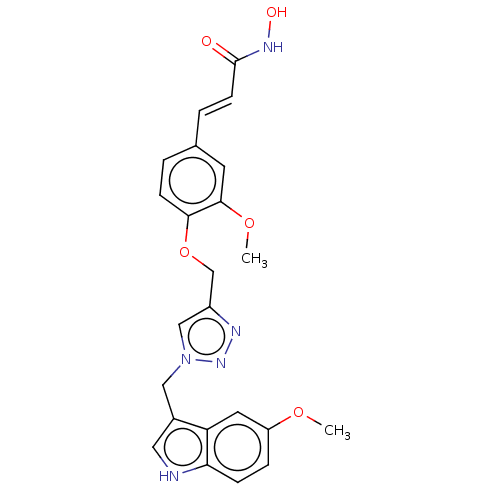 (CHEMBL4207448) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Mazandaran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of HDAC1 (unknown origin) | Eur J Med Chem 150: 9-29 (2018) Article DOI: 10.1016/j.ejmech.2018.02.065 BindingDB Entry DOI: 10.7270/Q2X92DXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM152804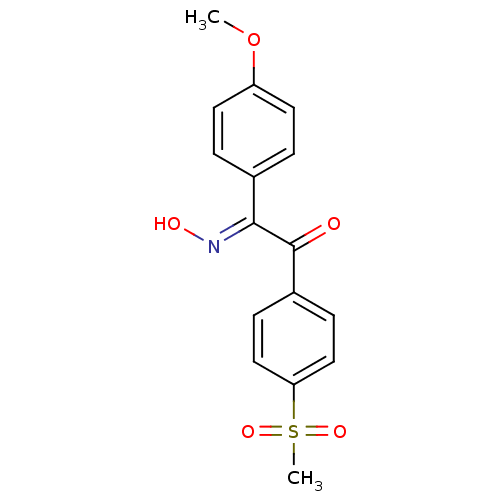 ((2E)-2-(N-hydroxyimino)-1-(4-methanesulfonylphenyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Mazandaran University of Medical Sciences | Assay Description Reaction mixtures were prepared in 100 mM Tris澦Cl buffer, pH 8.0 containing 1 然 heme and COX-1 or COX-2 and preincubated for 10 min in a waterbath ... | Chem Biol Drug Des 85: 494-503 (2015) Article DOI: 10.1111/cbdd.12435 BindingDB Entry DOI: 10.7270/Q21C1VK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Homo sapiens (Human)) | BDBM50458325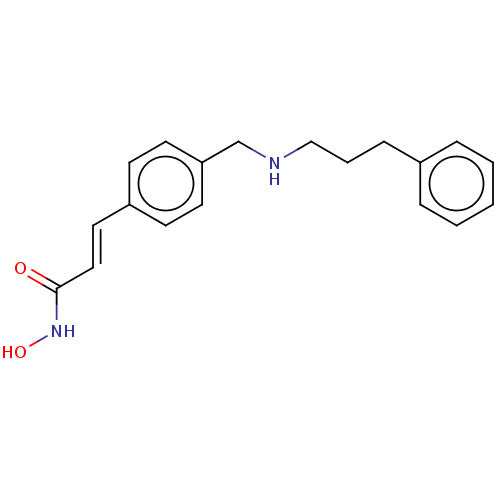 (CHEMBL342474) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Mazandaran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of human H1299 cells derived HDAC using biotin-labeled SGRGKGGKGLGKGGAKRHRKVLRD peracetylated with [3H]acetate at lysine residue as substr... | Eur J Med Chem 150: 9-29 (2018) Article DOI: 10.1016/j.ejmech.2018.02.065 BindingDB Entry DOI: 10.7270/Q2X92DXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Mazandaran University of Medical Sciences | Assay Description Reaction mixtures were prepared in 100 mM Tris澦Cl buffer, pH 8.0 containing 1 然 heme and COX-1 or COX-2 and preincubated for 10 min in a waterbath ... | Chem Biol Drug Des 85: 494-503 (2015) Article DOI: 10.1111/cbdd.12435 BindingDB Entry DOI: 10.7270/Q21C1VK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM152806 ((2E)-2-(4-fluorophenyl)-2-(N-hydroxyimino)-1-(4-me...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Mazandaran University of Medical Sciences | Assay Description Reaction mixtures were prepared in 100 mM Tris澦Cl buffer, pH 8.0 containing 1 然 heme and COX-1 or COX-2 and preincubated for 10 min in a waterbath ... | Chem Biol Drug Des 85: 494-503 (2015) Article DOI: 10.1111/cbdd.12435 BindingDB Entry DOI: 10.7270/Q21C1VK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50029593 (CHEMBL7162 | N-(2-(cyclohexyloxy)-4-nitrophenyl)me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Mazandaran University of Medical Sciences | Assay Description Reaction mixtures were prepared in 100 mM Tris澦Cl buffer, pH 8.0 containing 1 然 heme and COX-1 or COX-2 and preincubated for 10 min in a waterbath ... | Chem Biol Drug Des 85: 494-503 (2015) Article DOI: 10.1111/cbdd.12435 BindingDB Entry DOI: 10.7270/Q21C1VK2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 1.85E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Mazandaran University of Medical Sciences | Assay Description Reaction mixtures were prepared in 100 mM Tris澦Cl buffer, pH 8.0 containing 1 然 heme and COX-1 or COX-2 and preincubated for 10 min in a waterbath ... | Chem Biol Drug Des 85: 494-503 (2015) Article DOI: 10.1111/cbdd.12435 BindingDB Entry DOI: 10.7270/Q21C1VK2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM152805 ((2E)-2-(4-chlorophenyl)-2-(N-hydroxyimino)-1-(4-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Mazandaran University of Medical Sciences | Assay Description Reaction mixtures were prepared in 100 mM Tris澦Cl buffer, pH 8.0 containing 1 然 heme and COX-1 or COX-2 and preincubated for 10 min in a waterbath ... | Chem Biol Drug Des 85: 494-503 (2015) Article DOI: 10.1111/cbdd.12435 BindingDB Entry DOI: 10.7270/Q21C1VK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM152804 ((2E)-2-(N-hydroxyimino)-1-(4-methanesulfonylphenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Mazandaran University of Medical Sciences | Assay Description Reaction mixtures were prepared in 100 mM Tris澦Cl buffer, pH 8.0 containing 1 然 heme and COX-1 or COX-2 and preincubated for 10 min in a waterbath ... | Chem Biol Drug Des 85: 494-503 (2015) Article DOI: 10.1111/cbdd.12435 BindingDB Entry DOI: 10.7270/Q21C1VK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM152803 ((2E)-2-(N-hydroxyimino)-1-(4-methanesulfonylphenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Mazandaran University of Medical Sciences | Assay Description Reaction mixtures were prepared in 100 mM Tris澦Cl buffer, pH 8.0 containing 1 然 heme and COX-1 or COX-2 and preincubated for 10 min in a waterbath ... | Chem Biol Drug Des 85: 494-503 (2015) Article DOI: 10.1111/cbdd.12435 BindingDB Entry DOI: 10.7270/Q21C1VK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM152802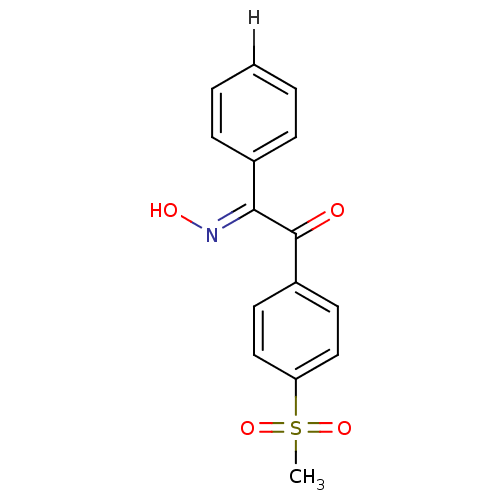 ((2E)-2-(N-hydroxyimino)-1-(4-methanesulfonylphenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Mazandaran University of Medical Sciences | Assay Description Reaction mixtures were prepared in 100 mM Tris澦Cl buffer, pH 8.0 containing 1 然 heme and COX-1 or COX-2 and preincubated for 10 min in a waterbath ... | Chem Biol Drug Des 85: 494-503 (2015) Article DOI: 10.1111/cbdd.12435 BindingDB Entry DOI: 10.7270/Q21C1VK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM152806 ((2E)-2-(4-fluorophenyl)-2-(N-hydroxyimino)-1-(4-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Mazandaran University of Medical Sciences | Assay Description Reaction mixtures were prepared in 100 mM Tris澦Cl buffer, pH 8.0 containing 1 然 heme and COX-1 or COX-2 and preincubated for 10 min in a waterbath ... | Chem Biol Drug Des 85: 494-503 (2015) Article DOI: 10.1111/cbdd.12435 BindingDB Entry DOI: 10.7270/Q21C1VK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50458324 (CHEMBL4202863) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Mazandaran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of HDAC4 (unknown origin) | Eur J Med Chem 150: 9-29 (2018) Article DOI: 10.1016/j.ejmech.2018.02.065 BindingDB Entry DOI: 10.7270/Q2X92DXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM152805 ((2E)-2-(4-chlorophenyl)-2-(N-hydroxyimino)-1-(4-me...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Mazandaran University of Medical Sciences | Assay Description Reaction mixtures were prepared in 100 mM Tris澦Cl buffer, pH 8.0 containing 1 然 heme and COX-1 or COX-2 and preincubated for 10 min in a waterbath ... | Chem Biol Drug Des 85: 494-503 (2015) Article DOI: 10.1111/cbdd.12435 BindingDB Entry DOI: 10.7270/Q21C1VK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM152803 ((2E)-2-(N-hydroxyimino)-1-(4-methanesulfonylphenyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Mazandaran University of Medical Sciences | Assay Description Reaction mixtures were prepared in 100 mM Tris澦Cl buffer, pH 8.0 containing 1 然 heme and COX-1 or COX-2 and preincubated for 10 min in a waterbath ... | Chem Biol Drug Des 85: 494-503 (2015) Article DOI: 10.1111/cbdd.12435 BindingDB Entry DOI: 10.7270/Q21C1VK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM152802 ((2E)-2-(N-hydroxyimino)-1-(4-methanesulfonylphenyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Mazandaran University of Medical Sciences | Assay Description Reaction mixtures were prepared in 100 mM Tris澦Cl buffer, pH 8.0 containing 1 然 heme and COX-1 or COX-2 and preincubated for 10 min in a waterbath ... | Chem Biol Drug Des 85: 494-503 (2015) Article DOI: 10.1111/cbdd.12435 BindingDB Entry DOI: 10.7270/Q21C1VK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50029593 (CHEMBL7162 | N-(2-(cyclohexyloxy)-4-nitrophenyl)me...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.13E+5 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Mazandaran University of Medical Sciences | Assay Description Reaction mixtures were prepared in 100 mM Tris澦Cl buffer, pH 8.0 containing 1 然 heme and COX-1 or COX-2 and preincubated for 10 min in a waterbath ... | Chem Biol Drug Des 85: 494-503 (2015) Article DOI: 10.1111/cbdd.12435 BindingDB Entry DOI: 10.7270/Q21C1VK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||