 Found 662 hits with Last Name = 'davies' and Initial = 'nl'
Found 662 hits with Last Name = 'davies' and Initial = 'nl' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50508937

(CHEMBL4448046)Show SMILES Cn1nc2CSCc3nn(C)c(Cl)c3-c3c(Cl)ccc4c(CCCOc5cc(SCc1c2)cc1ccccc51)c(C(O)=O)n(C)c34 Show InChI InChI=1S/C34H31Cl2N5O3S2/c1-39-31-25-10-11-26(35)29(31)30-27(38-41(3)33(30)36)18-45-16-20-14-21(40(2)37-20)17-46-22-13-19-7-4-5-8-23(19)28(15-22)44-12-6-9-24(25)32(39)34(42)43/h4-5,7-8,10-11,13-15H,6,9,12,16-18H2,1-3H3,(H,42,43) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Mcl1 (unknown origin) by TR-FRET assay |
J Med Chem 62: 9418-9437 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00716
BindingDB Entry DOI: 10.7270/Q2PV6PNH |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50508939

(CHEMBL4443085)Show SMILES Cc1cc(CSCc2nn(C)c(C)c2-c2cccc3c(CCCOc4cccc5ccccc45)c([nH]c23)C(O)=O)nn1C |(21.19,-26.33,;22.68,-25.93,;23.37,-24.56,;24.9,-24.79,;25.99,-23.71,;27.47,-24.11,;28.56,-23.02,;30.05,-23.42,;30.52,-24.88,;32.06,-24.88,;32.84,-26.21,;32.54,-23.42,;34.03,-23.02,;31.29,-22.51,;31.29,-20.97,;29.96,-20.21,;29.96,-18.67,;31.29,-17.89,;32.62,-18.66,;34.09,-18.19,;34.49,-16.7,;35.97,-16.3,;36.37,-14.82,;37.86,-14.42,;38.26,-12.93,;37.17,-11.83,;37.57,-10.35,;39.06,-9.95,;40.14,-11.03,;41.62,-10.63,;42.72,-11.71,;42.34,-13.2,;40.85,-13.61,;39.75,-12.52,;34.99,-19.43,;34.09,-20.68,;32.62,-20.2,;36.53,-19.43,;37.3,-20.76,;37.3,-18.1,;25.13,-26.32,;23.76,-27.02,;23.36,-28.5,)| Show InChI InChI=1S/C34H35N5O3S/c1-21-18-24(36-38(21)3)19-43-20-29-31(22(2)39(4)37-29)28-14-8-13-26-27(33(34(40)41)35-32(26)28)15-9-17-42-30-16-7-11-23-10-5-6-12-25(23)30/h5-8,10-14,16,18,35H,9,15,17,19-20H2,1-4H3,(H,40,41) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Mcl1 (unknown origin) by TR-FRET assay |
J Med Chem 62: 9418-9437 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00716
BindingDB Entry DOI: 10.7270/Q2PV6PNH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50508950

(CHEMBL4472439)Show SMILES C[C@H]1CCN(C)C(=O)C[C@@H](Cc2ccc(Cl)c(Cl)c2)NC(=O)CN2c3ccccc3CC[C@H](NC(=O)C[C@H](Cc3ccc(Cl)cc3)N1)C2=O |r| Show InChI InChI=1S/C37H42Cl3N5O4/c1-23-15-16-44(2)36(48)21-29(18-25-9-13-30(39)31(40)19-25)42-35(47)22-45-33-6-4-3-5-26(33)10-14-32(37(45)49)43-34(46)20-28(41-23)17-24-7-11-27(38)12-8-24/h3-9,11-13,19,23,28-29,32,41H,10,14-18,20-22H2,1-2H3,(H,42,47)(H,43,46)/t23-,28-,29+,32-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Mcl1 (unknown origin) by TR-FRET assay |
J Med Chem 62: 9418-9437 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00716
BindingDB Entry DOI: 10.7270/Q2PV6PNH |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50508947
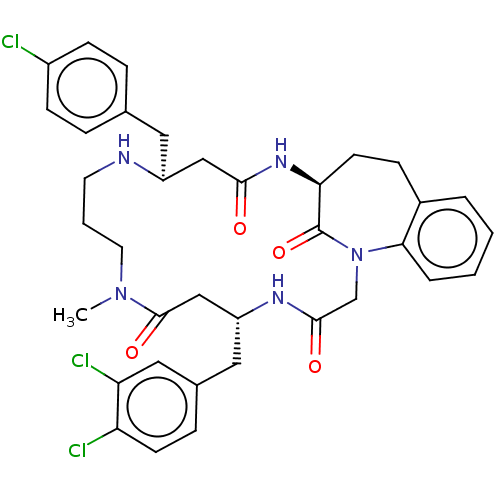
(CHEMBL4460550)Show SMILES CN1CCCN[C@@H](Cc2ccc(Cl)cc2)CC(=O)N[C@H]2CCc3ccccc3N(CC(=O)N[C@H](Cc3ccc(Cl)c(Cl)c3)CC1=O)C2=O |r| Show InChI InChI=1S/C36H40Cl3N5O4/c1-43-16-4-15-40-27(17-23-7-11-26(37)12-8-23)20-33(45)42-31-14-10-25-5-2-3-6-32(25)44(36(31)48)22-34(46)41-28(21-35(43)47)18-24-9-13-29(38)30(39)19-24/h2-3,5-9,11-13,19,27-28,31,40H,4,10,14-18,20-22H2,1H3,(H,41,46)(H,42,45)/t27-,28+,31-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Mcl1 (unknown origin) by TR-FRET assay |
J Med Chem 62: 9418-9437 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00716
BindingDB Entry DOI: 10.7270/Q2PV6PNH |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50508938

(CHEMBL1984039)Show SMILES Cc1nn(C)c(C)c1-c1cccc2c(CCCOc3cccc4ccccc34)c([nH]c12)C(O)=O |(-6.44,-15.46,;-4.9,-15.46,;-3.99,-16.71,;-2.53,-16.23,;-1.28,-17.14,;-2.53,-14.69,;-1.28,-13.79,;-3.99,-14.22,;-4.47,-12.75,;-5.97,-12.43,;-6.45,-10.97,;-5.42,-9.83,;-3.91,-10.15,;-2.67,-9.24,;-2.67,-7.7,;-1.33,-6.93,;-1.33,-5.39,;,-4.62,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;2.67,-3.08,;1.33,-2.31,;-1.42,-10.15,;-1.9,-11.61,;-3.44,-11.61,;.04,-9.67,;1.19,-10.7,;.36,-8.16,)| Show InChI InChI=1S/C28H27N3O3/c1-17-25(18(2)31(3)30-17)23-13-7-12-21-22(27(28(32)33)29-26(21)23)14-8-16-34-24-15-6-10-19-9-4-5-11-20(19)24/h4-7,9-13,15,29H,8,14,16H2,1-3H3,(H,32,33) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Mcl1 (unknown origin) by TR-FRET assay |
J Med Chem 62: 9418-9437 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00716
BindingDB Entry DOI: 10.7270/Q2PV6PNH |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50508940
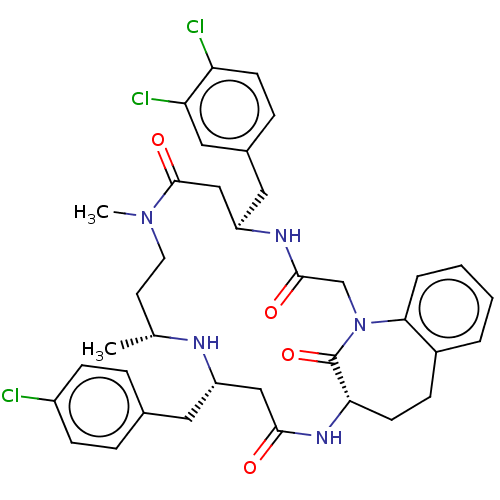
(CHEMBL4582512)Show SMILES C[C@@H]1CCN(C)C(=O)C[C@@H](Cc2ccc(Cl)c(Cl)c2)NC(=O)CN2c3ccccc3CC[C@H](NC(=O)C[C@H](Cc3ccc(Cl)cc3)N1)C2=O |r| Show InChI InChI=1S/C37H42Cl3N5O4/c1-23-15-16-44(2)36(48)21-29(18-25-9-13-30(39)31(40)19-25)42-35(47)22-45-33-6-4-3-5-26(33)10-14-32(37(45)49)43-34(46)20-28(41-23)17-24-7-11-27(38)12-8-24/h3-9,11-13,19,23,28-29,32,41H,10,14-18,20-22H2,1-2H3,(H,42,47)(H,43,46)/t23-,28+,29-,32+/m1/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 739 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Mcl1 (unknown origin) by TR-FRET assay |
J Med Chem 62: 9418-9437 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00716
BindingDB Entry DOI: 10.7270/Q2PV6PNH |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50508942
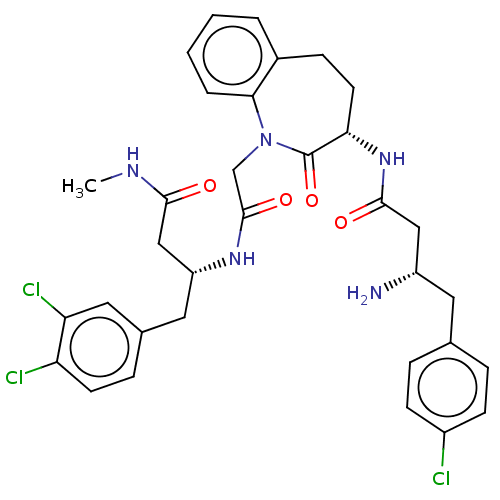
(CHEMBL4449849)Show SMILES CNC(=O)C[C@@H](Cc1ccc(Cl)c(Cl)c1)NC(=O)CN1c2ccccc2CC[C@H](NC(=O)C[C@@H](N)Cc2ccc(Cl)cc2)C1=O |r| Show InChI InChI=1S/C33H36Cl3N5O4/c1-38-30(42)18-25(15-21-8-12-26(35)27(36)16-21)39-32(44)19-41-29-5-3-2-4-22(29)9-13-28(33(41)45)40-31(43)17-24(37)14-20-6-10-23(34)11-7-20/h2-8,10-12,16,24-25,28H,9,13-15,17-19,37H2,1H3,(H,38,42)(H,39,44)(H,40,43)/t24-,25+,28-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Mcl1 (unknown origin) by TR-FRET assay |
J Med Chem 62: 9418-9437 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00716
BindingDB Entry DOI: 10.7270/Q2PV6PNH |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50508941

(CHEMBL4442625)Show SMILES CC1CCN[C@@H](Cc2ccc(Cl)cc2)CC(=O)N[C@H]2CCc3ccccc3N(CC(=O)N[C@H](Cc3ccc(Cl)c(Cl)c3)CC(=O)N1C)C2=O |r| Show InChI InChI=1S/C37H42Cl3N5O4/c1-23-15-16-41-28(17-24-7-11-27(38)12-8-24)20-34(46)43-32-14-10-26-5-3-4-6-33(26)45(37(32)49)22-35(47)42-29(21-36(48)44(23)2)18-25-9-13-30(39)31(40)19-25/h3-9,11-13,19,23,28-29,32,41H,10,14-18,20-22H2,1-2H3,(H,42,47)(H,43,46)/t23?,28-,29+,32-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Mcl1 (unknown origin) by TR-FRET assay |
J Med Chem 62: 9418-9437 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00716
BindingDB Entry DOI: 10.7270/Q2PV6PNH |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50508941

(CHEMBL4442625)Show SMILES CC1CCN[C@@H](Cc2ccc(Cl)cc2)CC(=O)N[C@H]2CCc3ccccc3N(CC(=O)N[C@H](Cc3ccc(Cl)c(Cl)c3)CC(=O)N1C)C2=O |r| Show InChI InChI=1S/C37H42Cl3N5O4/c1-23-15-16-41-28(17-24-7-11-27(38)12-8-24)20-34(46)43-32-14-10-26-5-3-4-6-33(26)45(37(32)49)22-35(47)42-29(21-36(48)44(23)2)18-25-9-13-30(39)31(40)19-25/h3-9,11-13,19,23,28-29,32,41H,10,14-18,20-22H2,1-2H3,(H,42,47)(H,43,46)/t23?,28-,29+,32-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Mcl1 (unknown origin) by TR-FRET assay |
J Med Chem 62: 9418-9437 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00716
BindingDB Entry DOI: 10.7270/Q2PV6PNH |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50508936
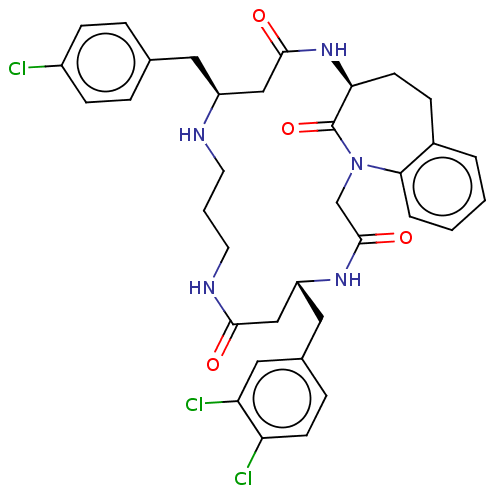
(CHEMBL4590345)Show SMILES Clc1ccc(C[C@H]2CC(=O)N[C@H]3CCc4ccccc4N(CC(=O)N[C@H](Cc4ccc(Cl)c(Cl)c4)CC(=O)NCCCN2)C3=O)cc1 |r| Show InChI InChI=1S/C35H38Cl3N5O4/c36-25-10-6-22(7-11-25)16-26-19-33(45)42-30-13-9-24-4-1-2-5-31(24)43(35(30)47)21-34(46)41-27(20-32(44)40-15-3-14-39-26)17-23-8-12-28(37)29(38)18-23/h1-2,4-8,10-12,18,26-27,30,39H,3,9,13-17,19-21H2,(H,40,44)(H,41,46)(H,42,45)/t26-,27+,30-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Mcl1 (unknown origin) by TR-FRET assay |
J Med Chem 62: 9418-9437 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00716
BindingDB Entry DOI: 10.7270/Q2PV6PNH |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM245474

(US9428503, 1)Show SMILES CN(C)CCCOc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n(C4CCOCC4)c3c2c1 Show InChI InChI=1S/C26H31N5O3/c1-29(2)11-4-12-34-24-8-6-19(16-28-24)18-5-7-22-21(15-18)25-23(17-27-22)30(3)26(32)31(25)20-9-13-33-14-10-20/h5-8,15-17,20H,4,9-14H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATM (unknown origin) using p53 as substrate preincubated for 30 mins followed by substrate addition and measured after 2 hrs by HTRF as... |
J Med Chem 61: 3823-3841 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01896
BindingDB Entry DOI: 10.7270/Q2RB775P |
More data for this
Ligand-Target Pair | |
Oxidized purine nucleoside triphosphate hydrolase
(Homo sapiens (Human)) | BDBM50548115

(CHEMBL4778773)Show SMILES CNC(=O)c1cnc2cc(OC)c(OCN3CCN(C)CC3)cc2c1Nc1ccccc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate preincubated for 15 mins followed by substrate addition and measured after 20 mins ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.5b01760
BindingDB Entry DOI: 10.7270/Q2TB1BHR |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM245500
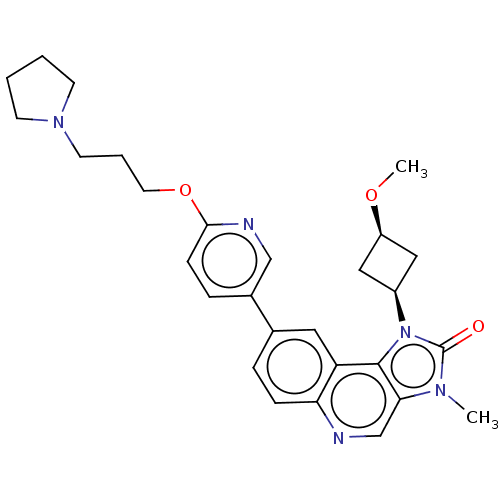
(US9428503, 28)Show SMILES CO[C@H]1C[C@H](C1)n1c2c(cnc3ccc(cc23)-c2ccc(OCCCN3CCCC3)nc2)n(C)c1=O |r,wD:4.6,2.1,(3.62,4.9,;3.22,3.41,;4.31,2.32,;4.31,.78,;5.85,.78,;5.85,2.32,;6.93,-.31,;6.61,-1.82,;7.95,-2.59,;7.95,-4.13,;6.61,-4.9,;5.28,-4.13,;3.95,-4.9,;2.61,-4.13,;2.61,-2.59,;3.95,-1.82,;5.28,-2.59,;1.28,-1.82,;1.28,-.28,;-.05,.49,;-1.39,-.28,;-2.72,.49,;-4.06,-.28,;-5.39,.49,;-6.72,-.28,;-8.06,.49,;-8.06,2.03,;-9.52,2.51,;-10.43,1.26,;-9.52,.02,;-1.39,-1.82,;-.05,-2.59,;9.09,-1.56,;10.43,-2.33,;8.47,-.15,;9.24,1.19,)| Show InChI InChI=1S/C28H33N5O3/c1-31-25-18-29-24-8-6-19(14-23(24)27(25)33(28(31)34)21-15-22(16-21)35-2)20-7-9-26(30-17-20)36-13-5-12-32-10-3-4-11-32/h6-9,14,17-18,21-22H,3-5,10-13,15-16H2,1-2H3/t21-,22+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATM autophosphorylation at Ser1981 in human HT29 cells preincubated for 1 hr followed by X ray irradiation and measured after 1 hr by H... |
J Med Chem 61: 3823-3841 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01896
BindingDB Entry DOI: 10.7270/Q2RB775P |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM245505

(US9428503, 33)Show SMILES CO[C@H]1C[C@H](C1)n1c2c(cnc3ccc(cc23)-c2ccc(OCCCN3CCCCC3)nc2)n(C)c1=O |r,wD:4.6,2.1,(3.76,4.9,;3.37,3.41,;4.45,2.32,;4.45,.78,;5.99,.78,;5.99,2.32,;7.08,-.31,;6.76,-1.82,;8.1,-2.59,;8.1,-4.13,;6.76,-4.9,;5.43,-4.13,;4.1,-4.9,;2.76,-4.13,;2.76,-2.59,;4.1,-1.82,;5.43,-2.59,;1.43,-1.82,;1.43,-.28,;.09,.49,;-1.24,-.28,;-2.57,.49,;-3.91,-.28,;-5.24,.49,;-6.57,-.28,;-7.91,.49,;-7.91,2.03,;-9.24,2.8,;-10.57,2.03,;-10.57,.49,;-9.24,-.28,;-1.24,-1.82,;.09,-2.59,;9.24,-1.56,;10.57,-2.33,;8.61,-.15,;9.38,1.19,)| Show InChI InChI=1S/C29H35N5O3/c1-32-26-19-30-25-9-7-20(15-24(25)28(26)34(29(32)35)22-16-23(17-22)36-2)21-8-10-27(31-18-21)37-14-6-13-33-11-4-3-5-12-33/h7-10,15,18-19,22-23H,3-6,11-14,16-17H2,1-2H3/t22-,23+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATM autophosphorylation at Ser1981 in human HT29 cells preincubated for 1 hr followed by X ray irradiation and measured after 1 hr by H... |
J Med Chem 61: 3823-3841 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01896
BindingDB Entry DOI: 10.7270/Q2RB775P |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM245478
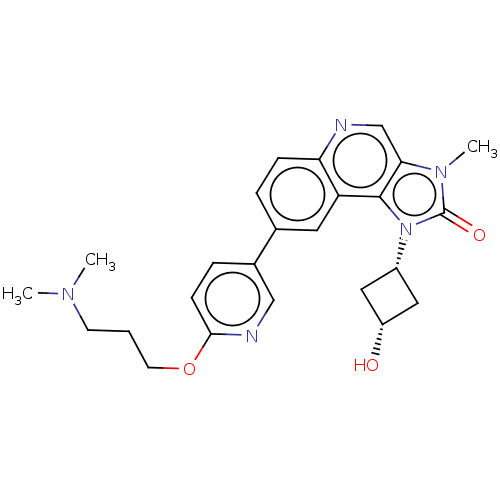
(US9428503, 5)Show SMILES CN(C)CCCOc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n([C@@H]4C[C@H](O)C4)c3c2c1 |r,wD:25.25,27.28,(-9.98,.47,;-8.65,1.24,;-8.65,2.78,;-7.32,.47,;-5.98,1.24,;-4.65,.47,;-3.32,1.24,;-1.98,.47,;-.65,1.24,;.68,.47,;.68,-1.07,;-.65,-1.84,;-1.98,-1.07,;2.02,-1.84,;2.02,-3.38,;3.35,-4.15,;4.69,-3.38,;6.02,-4.15,;7.35,-3.38,;7.35,-1.84,;8.5,-.81,;9.98,-1.21,;7.87,.6,;8.64,1.93,;6.34,.43,;5.25,1.52,;3.71,1.52,;3.71,3.06,;2.62,4.15,;5.25,3.06,;6.02,-1.07,;4.69,-1.84,;3.35,-1.07,)| Show InChI InChI=1S/C25H29N5O3/c1-28(2)9-4-10-33-23-8-6-17(14-27-23)16-5-7-21-20(11-16)24-22(15-26-21)29(3)25(32)30(24)18-12-19(31)13-18/h5-8,11,14-15,18-19,31H,4,9-10,12-13H2,1-3H3/t18-,19+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATM autophosphorylation at Ser1981 in human HT29 cells preincubated for 1 hr followed by X ray irradiation and measured after 1 hr by H... |
J Med Chem 61: 3823-3841 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01896
BindingDB Entry DOI: 10.7270/Q2RB775P |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM245475

(US9428503, 2)Show SMILES CO[C@H]1C[C@H](C1)n1c2c(cnc3ccc(cc23)-c2ccc(OCCCN(C)C)nc2)n(C)c1=O |r,wD:4.6,2.1,(3.02,4.9,;2.62,3.41,;3.71,2.32,;3.71,.78,;5.25,.78,;5.25,2.32,;6.34,-.31,;6.02,-1.82,;7.35,-2.59,;7.35,-4.13,;6.02,-4.9,;4.69,-4.13,;3.35,-4.9,;2.02,-4.13,;2.02,-2.59,;3.35,-1.82,;4.69,-2.59,;.68,-1.82,;.68,-.28,;-.65,.49,;-1.98,-.28,;-3.32,.49,;-4.65,-.28,;-5.98,.49,;-7.32,-.28,;-8.65,.49,;-9.99,-.28,;-8.65,2.03,;-1.98,-1.82,;-.65,-2.59,;8.5,-1.56,;9.99,-1.95,;7.87,-.15,;8.64,1.19,)| Show InChI InChI=1S/C26H31N5O3/c1-29(2)10-5-11-34-24-9-7-18(15-28-24)17-6-8-22-21(12-17)25-23(16-27-22)30(3)26(32)31(25)19-13-20(14-19)33-4/h6-9,12,15-16,19-20H,5,10-11,13-14H2,1-4H3/t19-,20+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATM autophosphorylation at Ser1981 in human HT29 cells preincubated for 1 hr followed by X ray irradiation and measured after 1 hr by H... |
J Med Chem 61: 3823-3841 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01896
BindingDB Entry DOI: 10.7270/Q2RB775P |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM245514
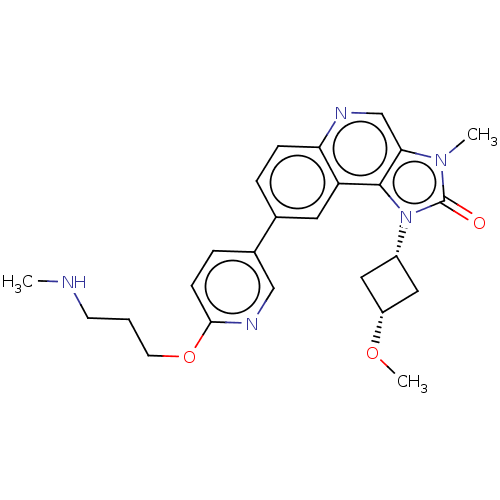
(US9428503, 42)Show SMILES CNCCCOc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n([C@@H]4C[C@@H](C4)OC)c3c2c1 |r,wD:24.24,26.29,(-9.91,.47,;-8.57,1.24,;-7.24,.47,;-5.91,1.24,;-4.57,.47,;-3.24,1.24,;-1.91,.47,;-.57,1.24,;.76,.47,;.76,-1.07,;-.57,-1.84,;-1.91,-1.07,;2.1,-1.84,;2.1,-3.38,;3.43,-4.15,;4.76,-3.38,;6.1,-4.15,;7.43,-3.38,;7.43,-1.84,;8.57,-.81,;9.91,-1.58,;7.95,.6,;8.72,1.93,;6.42,.43,;5.33,1.52,;3.79,1.52,;3.79,3.06,;5.33,3.06,;2.7,4.15,;1.21,3.75,;6.1,-1.07,;4.76,-1.84,;3.43,-1.07,)| Show InChI InChI=1S/C25H29N5O3/c1-26-9-4-10-33-23-8-6-17(14-28-23)16-5-7-21-20(11-16)24-22(15-27-21)29(2)25(31)30(24)18-12-19(13-18)32-3/h5-8,11,14-15,18-19,26H,4,9-10,12-13H2,1-3H3/t18-,19+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATM autophosphorylation at Ser1981 in human HT29 cells preincubated for 1 hr followed by X ray irradiation and measured after 1 hr by H... |
J Med Chem 61: 3823-3841 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01896
BindingDB Entry DOI: 10.7270/Q2RB775P |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM245510
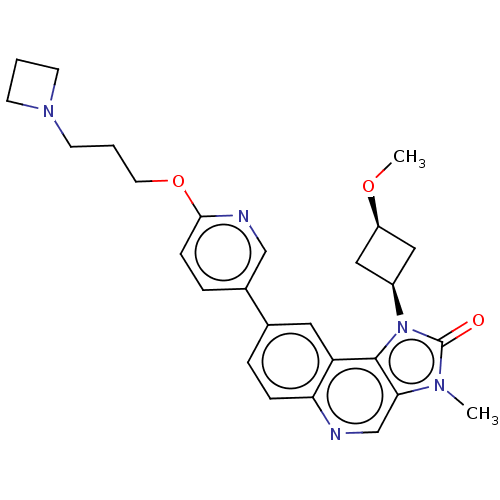
(US9428503, 38)Show SMILES CO[C@H]1C[C@H](C1)n1c2c(cnc3ccc(cc23)-c2ccc(OCCCN3CCC3)nc2)n(C)c1=O |r,wD:4.6,2.1,(3.37,4.9,;2.97,3.41,;4.06,2.32,;4.06,.78,;5.6,.78,;5.6,2.32,;6.69,-.31,;6.37,-1.82,;7.71,-2.59,;7.71,-4.13,;6.37,-4.9,;5.04,-4.13,;3.7,-4.9,;2.37,-4.13,;2.37,-2.59,;3.7,-1.82,;5.04,-2.59,;1.04,-1.82,;1.04,-.28,;-.3,.49,;-1.63,-.28,;-2.96,.49,;-4.3,-.28,;-5.63,.49,;-6.96,-.28,;-8.3,.49,;-8.7,1.98,;-10.18,1.58,;-9.79,.1,;-1.63,-1.82,;-.3,-2.59,;8.85,-1.56,;10.18,-2.33,;8.22,-.15,;8.99,1.19,)| Show InChI InChI=1S/C27H31N5O3/c1-30-24-17-28-23-7-5-18(13-22(23)26(24)32(27(30)33)20-14-21(15-20)34-2)19-6-8-25(29-16-19)35-12-4-11-31-9-3-10-31/h5-8,13,16-17,20-21H,3-4,9-12,14-15H2,1-2H3/t20-,21+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATM autophosphorylation at Ser1981 in human HT29 cells preincubated for 1 hr followed by X ray irradiation and measured after 1 hr by H... |
J Med Chem 61: 3823-3841 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01896
BindingDB Entry DOI: 10.7270/Q2RB775P |
More data for this
Ligand-Target Pair | |
Oxidized purine nucleoside triphosphate hydrolase
(Homo sapiens (Human)) | BDBM50548110

(CHEMBL4747532) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate preincubated for 15 mins followed by substrate addition and measured after 20 mins ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.5b01760
BindingDB Entry DOI: 10.7270/Q2TB1BHR |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM245490
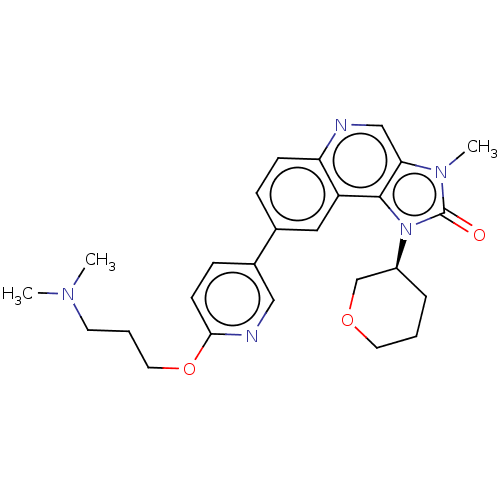
(US9428503, 17)Show SMILES CN(C)CCCOc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n([C@H]4CCCOC4)c3c2c1 |r| Show InChI InChI=1S/C26H31N5O3/c1-29(2)11-5-13-34-24-10-8-19(15-28-24)18-7-9-22-21(14-18)25-23(16-27-22)30(3)26(32)31(25)20-6-4-12-33-17-20/h7-10,14-16,20H,4-6,11-13,17H2,1-3H3/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATM autophosphorylation at Ser1981 in human HT29 cells preincubated for 1 hr followed by X ray irradiation and measured after 1 hr by H... |
J Med Chem 61: 3823-3841 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01896
BindingDB Entry DOI: 10.7270/Q2RB775P |
More data for this
Ligand-Target Pair | |
Oxidized purine nucleoside triphosphate hydrolase
(Homo sapiens (Human)) | BDBM50162075
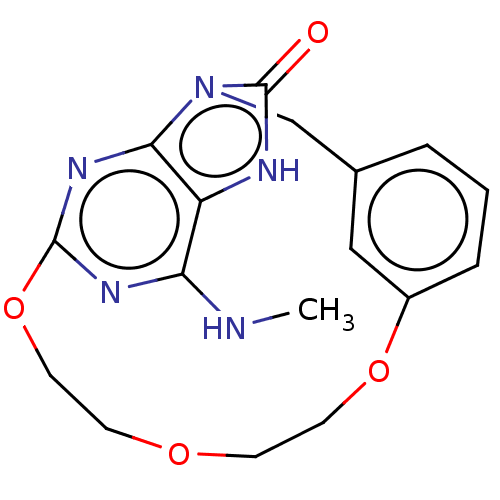
(CHEMBL3794167)Show InChI InChI=1S/C17H19N5O4/c1-18-14-13-15-21-16(20-14)26-8-6-24-5-7-25-12-4-2-3-11(9-12)10-22(15)17(23)19-13/h2-4,9H,5-8,10H2,1H3,(H,19,23)(H,18,20,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate preincubated for 15 mins followed by substrate addition and measured after 20 mins ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.5b01760
BindingDB Entry DOI: 10.7270/Q2TB1BHR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Oxidized purine nucleoside triphosphate hydrolase
(Homo sapiens (Human)) | BDBM50162074

(CHEMBL3792684)Show InChI InChI=1S/C19H19N3O3/c1-20-19(23)14-11-21-15-10-17(25-3)16(24-2)9-13(15)18(14)22-12-7-5-4-6-8-12/h4-11H,1-3H3,(H,20,23)(H,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate preincubated for 15 mins followed by substrate addition and measured after 20 mins ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.5b01760
BindingDB Entry DOI: 10.7270/Q2TB1BHR |
More data for this
Ligand-Target Pair | |
Oxidized purine nucleoside triphosphate hydrolase
(Homo sapiens (Human)) | BDBM50548117
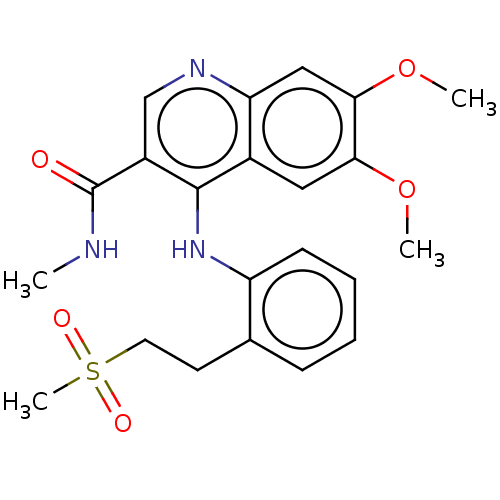
(CHEMBL4776026)Show SMILES CNC(=O)c1cnc2cc(OC)c(OC)cc2c1Nc1ccccc1CCS(C)(=O)=O | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate preincubated for 15 mins followed by substrate addition and measured after 20 mins ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.5b01760
BindingDB Entry DOI: 10.7270/Q2TB1BHR |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM245474

(US9428503, 1)Show SMILES CN(C)CCCOc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n(C4CCOCC4)c3c2c1 Show InChI InChI=1S/C26H31N5O3/c1-29(2)11-4-12-34-24-8-6-19(16-28-24)18-5-7-22-21(15-18)25-23(17-27-22)30(3)26(32)31(25)20-9-13-33-14-10-20/h5-8,15-17,20H,4,9-14H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATM autophosphorylation at Ser1981 in human HT29 cells preincubated for 1 hr followed by X ray irradiation and measured after 1 hr by H... |
J Med Chem 61: 3823-3841 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01896
BindingDB Entry DOI: 10.7270/Q2RB775P |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593696
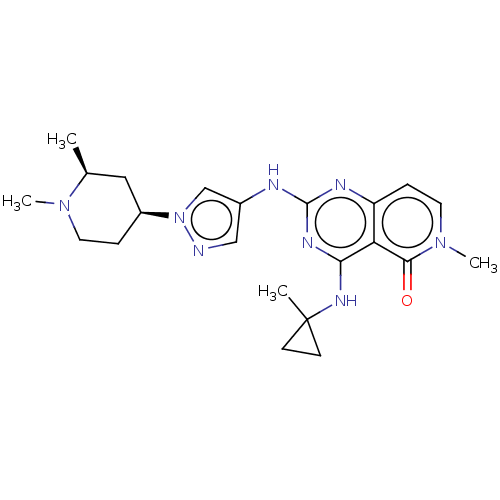
(CHEMBL5200601)Show SMILES C[C@H]1C[C@H](CCN1C)n1cc(Nc2nc(NC3(C)CC3)c3c(ccn(C)c3=O)n2)cn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593694
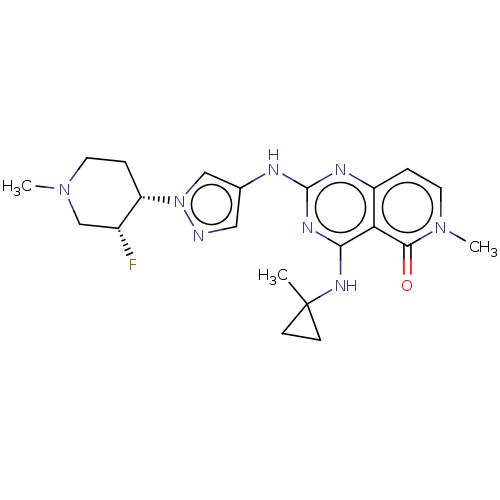
(CHEMBL5193253)Show SMILES CN1CC[C@@H]([C@H](F)C1)n1cc(Nc2nc(NC3(C)CC3)c3c(ccn(C)c3=O)n2)cn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM245491
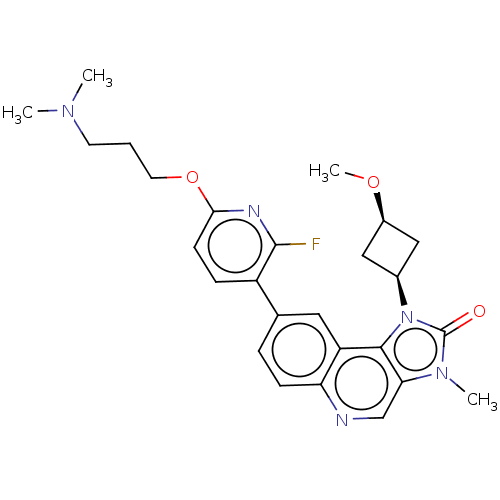
(US9428503, 18)Show SMILES CO[C@H]1C[C@H](C1)n1c2c(cnc3ccc(cc23)-c2ccc(OCCCN(C)C)nc2F)n(C)c1=O |r,wD:4.6,2.1,(3.02,4.9,;2.62,3.41,;3.71,2.32,;3.71,.78,;5.25,.78,;5.25,2.32,;6.34,-.31,;6.02,-1.82,;7.35,-2.59,;7.35,-4.13,;6.02,-4.9,;4.69,-4.13,;3.35,-4.9,;2.02,-4.13,;2.02,-2.59,;3.35,-1.82,;4.69,-2.59,;.68,-1.82,;.68,-.28,;-.65,.49,;-1.98,-.28,;-3.32,.49,;-4.65,-.28,;-5.98,.49,;-7.32,-.28,;-8.65,.49,;-9.98,-.28,;-8.65,2.03,;-1.98,-1.82,;-.65,-2.59,;-.65,-4.13,;8.5,-1.56,;9.98,-1.95,;7.87,-.15,;8.64,1.19,)| Show InChI InChI=1S/C26H30FN5O3/c1-30(2)10-5-11-35-23-9-7-19(25(27)29-23)16-6-8-21-20(12-16)24-22(15-28-21)31(3)26(33)32(24)17-13-18(14-17)34-4/h6-9,12,15,17-18H,5,10-11,13-14H2,1-4H3/t17-,18+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATM autophosphorylation at Ser1981 in human HT29 cells preincubated for 1 hr followed by X ray irradiation and measured after 1 hr by H... |
J Med Chem 61: 3823-3841 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01896
BindingDB Entry DOI: 10.7270/Q2RB775P |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM245481

(US9428503, 8)Show SMILES CN(C)CCCOc1ccc(cn1)-c1cc2c3n(C4CCOCC4)c(=O)n(C)c3cnc2cc1F Show InChI InChI=1S/C26H30FN5O3/c1-30(2)9-4-10-35-24-6-5-17(15-29-24)19-13-20-22(14-21(19)27)28-16-23-25(20)32(26(33)31(23)3)18-7-11-34-12-8-18/h5-6,13-16,18H,4,7-12H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATM autophosphorylation at Ser1981 in human HT29 cells preincubated for 1 hr followed by X ray irradiation and measured after 1 hr by H... |
J Med Chem 61: 3823-3841 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01896
BindingDB Entry DOI: 10.7270/Q2RB775P |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593693

(CHEMBL5201376)Show SMILES CN(C)C(=O)CN1CCC(CC1)n1cc(Nc2nc(NC3(C)CC3)c3c(ccn(C)c3=O)n2)cn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Oxidized purine nucleoside triphosphate hydrolase
(Homo sapiens (Human)) | BDBM50548124

(CHEMBL4784602) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate preincubated for 15 mins followed by substrate addition and measured after 20 mins ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.5b01760
BindingDB Entry DOI: 10.7270/Q2TB1BHR |
More data for this
Ligand-Target Pair | |
Oxidized purine nucleoside triphosphate hydrolase
(Homo sapiens (Human)) | BDBM50548109
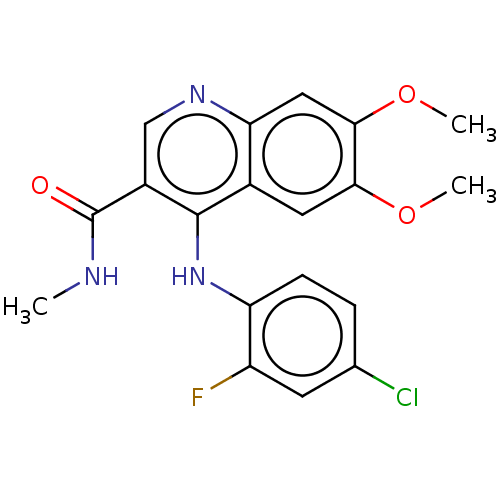
(CHEMBL4783492)Show SMILES CNC(=O)c1cnc2cc(OC)c(OC)cc2c1Nc1ccc(Cl)cc1F | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate preincubated for 15 mins followed by substrate addition and measured after 20 mins ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.5b01760
BindingDB Entry DOI: 10.7270/Q2TB1BHR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM245489

(US9428503, 16)Show SMILES CN(C)CCCOc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n([C@@H]4CCCOC4)c3c2c1 |r| Show InChI InChI=1S/C26H31N5O3/c1-29(2)11-5-13-34-24-10-8-19(15-28-24)18-7-9-22-21(14-18)25-23(16-27-22)30(3)26(32)31(25)20-6-4-12-33-17-20/h7-10,14-16,20H,4-6,11-13,17H2,1-3H3/t20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATM autophosphorylation at Ser1981 in human HT29 cells preincubated for 1 hr followed by X ray irradiation and measured after 1 hr by H... |
J Med Chem 61: 3823-3841 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01896
BindingDB Entry DOI: 10.7270/Q2RB775P |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593710
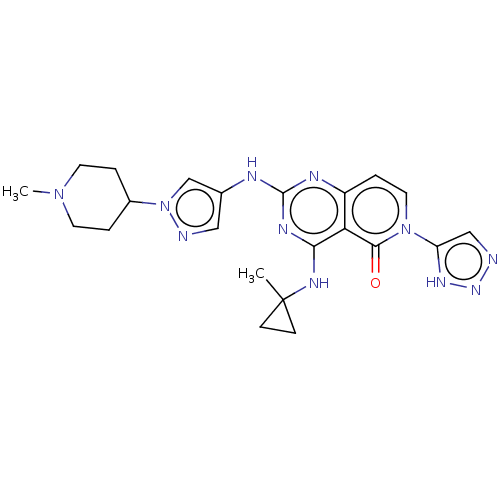
(CHEMBL5193053)Show SMILES CN1CCC(CC1)n1cc(Nc2nc(NC3(C)CC3)c3c(ccn(-c4cnn[nH]4)c3=O)n2)cn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593700

(CHEMBL5193024)Show SMILES CN1CC2(CC(C2)n2cc(Nc3nc(NC4(C)CC4)c4c(ccn(C)c4=O)n3)cn2)C1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593695
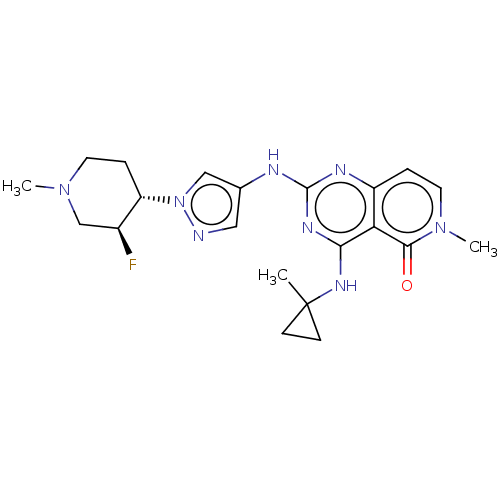
(CHEMBL5209502)Show SMILES CN1CC[C@@H]([C@@H](F)C1)n1cc(Nc2nc(NC3(C)CC3)c3c(ccn(C)c3=O)n2)cn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593689
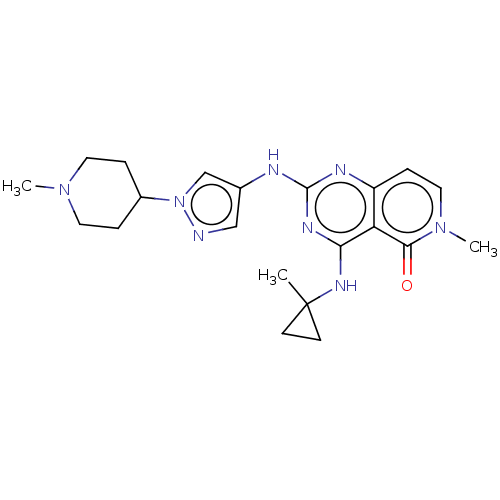
(CHEMBL5174529)Show SMILES CN1CCC(CC1)n1cc(Nc2nc(NC3(C)CC3)c3c(ccn(C)c3=O)n2)cn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593692

(CHEMBL5200432)Show SMILES CN(C)CC(=O)N1CCC(CC1)n1cc(Nc2nc(NC3(C)CC3)c3c(ccn(C)c3=O)n2)cn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593697

(CHEMBL5196755)Show SMILES C[C@@H]1C[C@H](CCN1C)n1cc(Nc2nc(NC3(C)CC3)c3c(ccn(C)c3=O)n2)cn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM245519

(US9428503, 47)Show SMILES Cn1c2cnc3cc(F)c(cc3c2n([C@H]2CCCOC2)c1=O)-c1ccc(OCCCN2CCCCC2)nc1 |r| Show InChI InChI=1S/C29H34FN5O3/c1-33-26-18-31-25-16-24(30)22(15-23(25)28(26)35(29(33)36)21-7-5-13-37-19-21)20-8-9-27(32-17-20)38-14-6-12-34-10-3-2-4-11-34/h8-9,15-18,21H,2-7,10-14,19H2,1H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATM autophosphorylation at Ser1981 in human HT29 cells preincubated for 1 hr followed by X ray irradiation and measured after 1 hr by H... |
J Med Chem 61: 3823-3841 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01896
BindingDB Entry DOI: 10.7270/Q2RB775P |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM245479
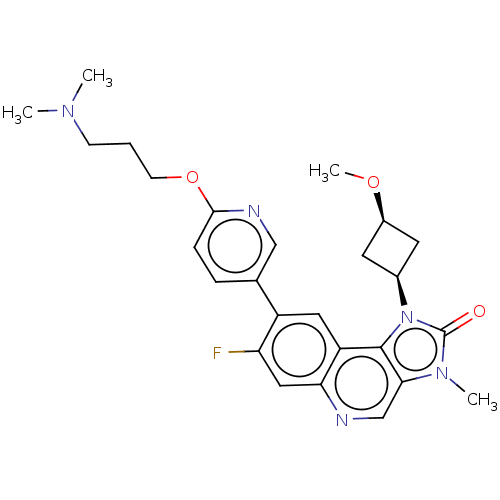
(US9428503, 6)Show SMILES CO[C@H]1C[C@H](C1)n1c2c(cnc3cc(F)c(cc23)-c2ccc(OCCCN(C)C)nc2)n(C)c1=O |r,wD:4.6,2.1,(3.02,4.9,;2.62,3.41,;3.71,2.32,;3.71,.78,;5.25,.78,;5.25,2.32,;6.34,-.31,;6.02,-1.82,;7.35,-2.59,;7.35,-4.13,;6.02,-4.9,;4.69,-4.13,;3.35,-4.9,;2.02,-4.13,;.68,-4.9,;2.02,-2.59,;3.35,-1.82,;4.69,-2.59,;.68,-1.82,;.68,-.28,;-.65,.49,;-1.98,-.28,;-3.32,.49,;-4.65,-.28,;-5.98,.49,;-7.32,-.28,;-8.65,.49,;-9.98,-.28,;-8.65,2.03,;-1.98,-1.82,;-.65,-2.59,;8.5,-1.56,;9.98,-1.95,;7.87,-.15,;8.64,1.19,)| Show InChI InChI=1S/C26H30FN5O3/c1-30(2)8-5-9-35-24-7-6-16(14-29-24)19-12-20-22(13-21(19)27)28-15-23-25(20)32(26(33)31(23)3)17-10-18(11-17)34-4/h6-7,12-15,17-18H,5,8-11H2,1-4H3/t17-,18+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATM autophosphorylation at Ser1981 in human HT29 cells preincubated for 1 hr followed by X ray irradiation and measured after 1 hr by H... |
J Med Chem 61: 3823-3841 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01896
BindingDB Entry DOI: 10.7270/Q2RB775P |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM245525
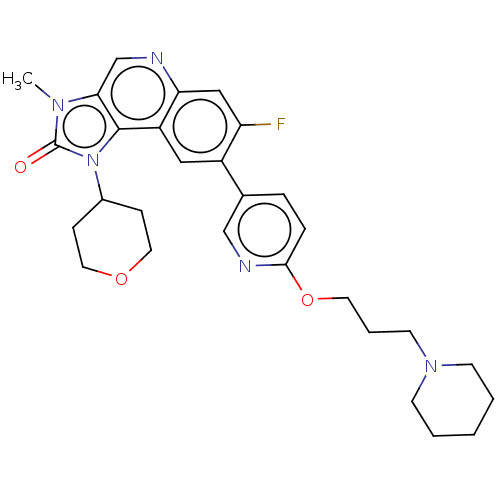
(US9428503, 53)Show SMILES Cn1c2cnc3cc(F)c(cc3c2n(C2CCOCC2)c1=O)-c1ccc(OCCCN2CCCCC2)nc1 Show InChI InChI=1S/C29H34FN5O3/c1-33-26-19-31-25-17-24(30)22(16-23(25)28(26)35(29(33)36)21-8-14-37-15-9-21)20-6-7-27(32-18-20)38-13-5-12-34-10-3-2-4-11-34/h6-7,16-19,21H,2-5,8-15H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATM autophosphorylation at Ser1981 in human HT29 cells preincubated for 1 hr followed by X ray irradiation and measured after 1 hr by H... |
J Med Chem 61: 3823-3841 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01896
BindingDB Entry DOI: 10.7270/Q2RB775P |
More data for this
Ligand-Target Pair | |
Oxidized purine nucleoside triphosphate hydrolase
(Homo sapiens (Human)) | BDBM50548121
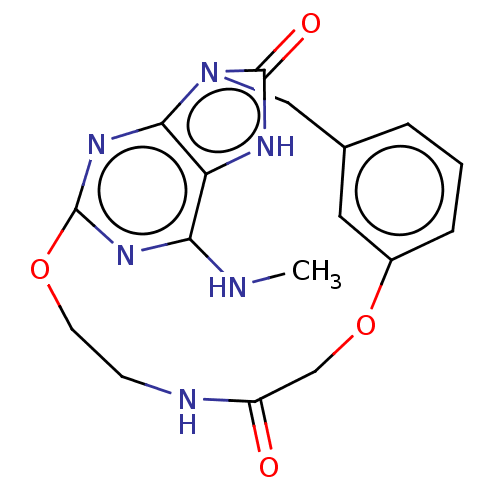
(CHEMBL4754128)Show SMILES CNc1nc2OCCNC(=O)COc3cccc(Cn4c(n2)c1[nH]c4=O)c3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate preincubated for 15 mins followed by substrate addition and measured after 20 mins ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.5b01760
BindingDB Entry DOI: 10.7270/Q2TB1BHR |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM245477
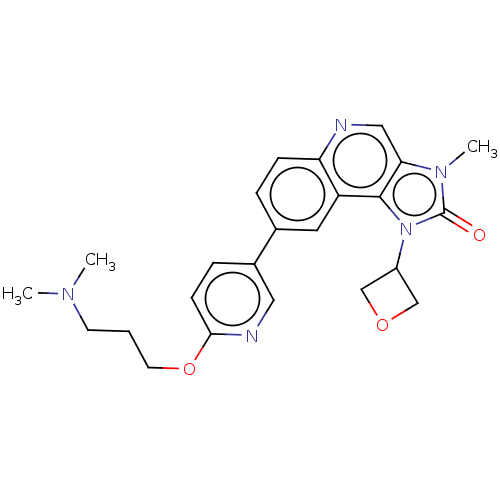
(US9428503, 4)Show SMILES CN(C)CCCOc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n(C4COC4)c3c2c1 Show InChI InChI=1S/C24H27N5O3/c1-27(2)9-4-10-32-22-8-6-17(12-26-22)16-5-7-20-19(11-16)23-21(13-25-20)28(3)24(30)29(23)18-14-31-15-18/h5-8,11-13,18H,4,9-10,14-15H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATM autophosphorylation at Ser1981 in human HT29 cells preincubated for 1 hr followed by X ray irradiation and measured after 1 hr by H... |
J Med Chem 61: 3823-3841 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01896
BindingDB Entry DOI: 10.7270/Q2RB775P |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50573136

(CHEMBL4875486)Show SMILES CN(Cc1ccc2nccn2c1)c1nnc(o1)-c1ccc(cc1C)-c1c(C)nn(C)c1C#N | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MER (unknown origin) by LC-MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00920
BindingDB Entry DOI: 10.7270/Q2S18686 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM50378199
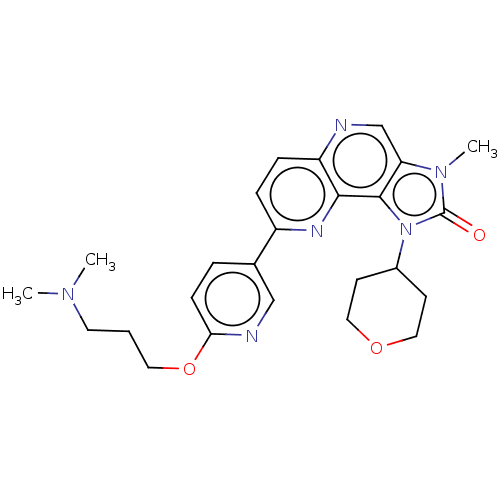
(CHEMBL4163629)Show SMILES CN(C)CCCOc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n(C4CCOCC4)c3c2n1 Show InChI InChI=1S/C25H30N6O3/c1-29(2)11-4-12-34-22-8-5-17(15-27-22)19-6-7-20-23(28-19)24-21(16-26-20)30(3)25(32)31(24)18-9-13-33-14-10-18/h5-8,15-16,18H,4,9-14H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATM autophosphorylation at Ser1981 in human HT29 cells preincubated for 1 hr followed by X ray irradiation and measured after 1 hr by H... |
J Med Chem 61: 3823-3841 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01896
BindingDB Entry DOI: 10.7270/Q2RB775P |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50246761
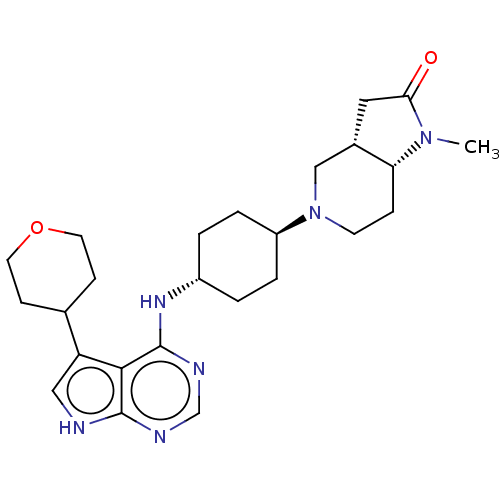
(CHEMBL4102501)Show SMILES [H][C@@]12CC(=O)N(C)[C@]1([H])CCN(C2)[C@H]1CC[C@@H](CC1)Nc1ncnc2[nH]cc(C3CCOCC3)c12 |r,wU:16.21,1.0,7.8,wD:13.14,(14.1,-14.84,;15.43,-15.62,;15.76,-14.11,;17.29,-13.95,;18.06,-12.62,;17.91,-15.36,;19.41,-15.68,;16.76,-16.39,;18.09,-17.15,;16.75,-17.93,;15.42,-18.69,;14.1,-17.92,;14.09,-16.38,;12.76,-18.68,;11.43,-17.91,;10.09,-18.68,;10.1,-20.22,;11.43,-20.99,;12.75,-20.22,;8.76,-20.99,;8.77,-22.53,;10.1,-23.29,;10.11,-24.84,;8.77,-25.61,;7.44,-24.84,;5.97,-25.33,;5.05,-24.09,;5.95,-22.83,;5.47,-21.37,;6.48,-20.21,;6,-18.76,;4.5,-18.44,;3.47,-19.59,;3.95,-21.06,;7.43,-23.3,)| Show InChI InChI=1S/C25H36N6O2/c1-30-21-6-9-31(14-17(21)12-22(30)32)19-4-2-18(3-5-19)29-25-23-20(16-7-10-33-11-8-16)13-26-24(23)27-15-28-25/h13,15-19,21H,2-12,14H2,1H3,(H2,26,27,28,29)/t17-,18-,19-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length His-tagged human IRAK4 expressed in baculovirus expression system using 5-FAM-IPTSPITTTYFFFKKK-COOH as substrat... |
J Med Chem 60: 10071-10091 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01290
BindingDB Entry DOI: 10.7270/Q2T72KWX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593706

(CHEMBL5190750)Show SMILES C[C@H](F)Cn1ccc2nc(Nc3cnn(c3)C3CCN(C)CC3)nc(NC3(C)CC3)c2c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593704
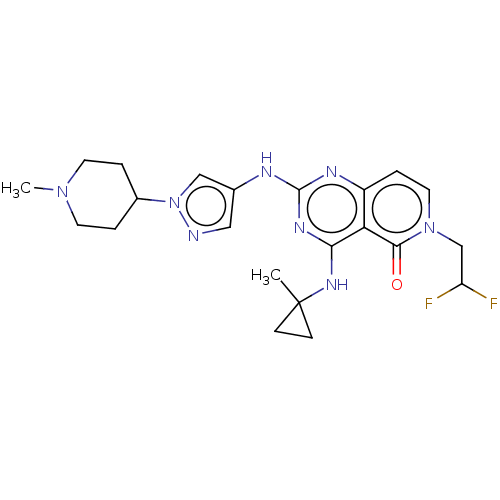
(CHEMBL5186874)Show SMILES CN1CCC(CC1)n1cc(Nc2nc(NC3(C)CC3)c3c(ccn(CC(F)F)c3=O)n2)cn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Oxidized purine nucleoside triphosphate hydrolase
(Homo sapiens (Human)) | BDBM50548119
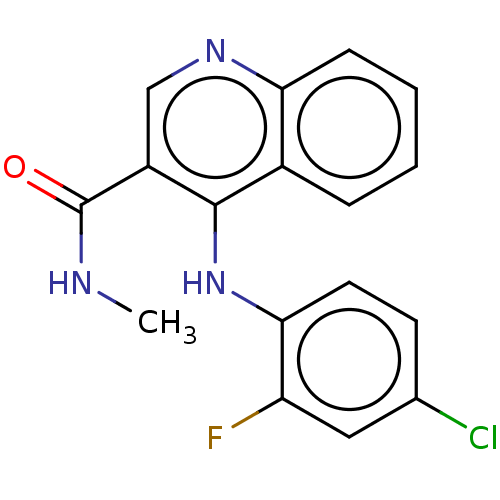
(CHEMBL4749068) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate preincubated for 15 mins followed by substrate addition and measured after 20 mins ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.5b01760
BindingDB Entry DOI: 10.7270/Q2TB1BHR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50573135

(CHEMBL4873217)Show SMILES CN(Cc1ccc2nccn2c1)c1nnc(o1)-c1ccc(cc1C)-c1cnn(C)c1C | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MER (unknown origin) by LC-MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00920
BindingDB Entry DOI: 10.7270/Q2S18686 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data