 Found 905 hits with Last Name = 'dawson' and Initial = 'j'
Found 905 hits with Last Name = 'dawson' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50184767
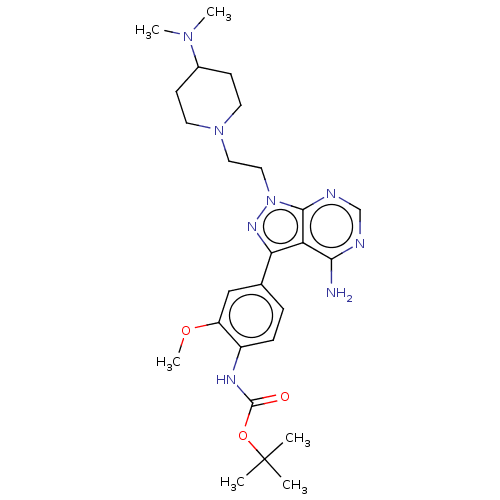
(CHEMBL3824089 | US10294227, Code 506)Show SMILES COc1cc(ccc1NC(=O)OC(C)(C)C)-c1nn(CCN2CCC(CC2)N(C)C)c2ncnc(N)c12 Show InChI InChI=1S/C26H38N8O3/c1-26(2,3)37-25(35)30-19-8-7-17(15-20(19)36-6)22-21-23(27)28-16-29-24(21)34(31-22)14-13-33-11-9-18(10-12-33)32(4)5/h7-8,15-16,18H,9-14H2,1-6H3,(H,30,35)(H2,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Competitive inhibition of C-terminal His-tagged full length human SRC expressed in insect cells preincubated for 20 mins using poly[Glu,Tyr]4:1 as su... |
J Med Chem 59: 4697-710 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00065
BindingDB Entry DOI: 10.7270/Q2B85B2D |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50415001
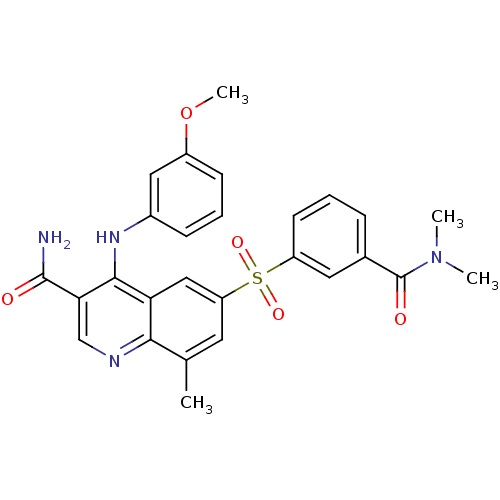
(CHEMBL570015 | GSK-256066 | GSK-256066 (3))Show SMILES COc1cccc(Nc2c(cnc3c(C)cc(cc23)S(=O)(=O)c2cccc(c2)C(=O)N(C)C)C(N)=O)c1 Show InChI InChI=1S/C27H26N4O5S/c1-16-11-21(37(34,35)20-10-5-7-17(12-20)27(33)31(2)3)14-22-24(16)29-15-23(26(28)32)25(22)30-18-8-6-9-19(13-18)36-4/h5-15H,1-4H3,(H2,28,32)(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.00794 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5261-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.012
BindingDB Entry DOI: 10.7270/Q2K075HB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50414999

(CHEMBL569791)Show SMILES COc1cccc(Nc2c(cnc3ccc(cc23)S(=O)(=O)c2cccc(c2)C(=O)N(C)C)C(N)=O)c1 Show InChI InChI=1S/C26H24N4O5S/c1-30(2)26(32)16-6-4-9-19(12-16)36(33,34)20-10-11-23-21(14-20)24(22(15-28-23)25(27)31)29-17-7-5-8-18(13-17)35-3/h4-15H,1-3H3,(H2,27,31)(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5261-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.012
BindingDB Entry DOI: 10.7270/Q2K075HB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50546172

(CHEMBL4762397)Show SMILES CN(Cc1c[nH]c2ncnc(-c3cccc(NC(=O)c4ccc(cc4)C(C)(C)C)c3C)c12)C(=O)C=C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full length human unphosphorylated BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate incubated for 60 mins in presence of ATP at... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00317
BindingDB Entry DOI: 10.7270/Q20K2D40 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50415009
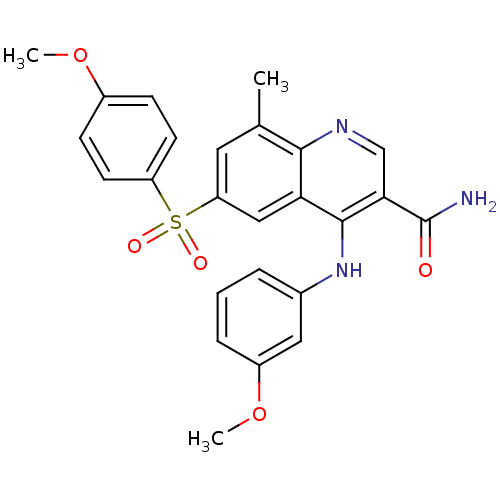
(CHEMBL571381)Show SMILES COc1ccc(cc1)S(=O)(=O)c1cc(C)c2ncc(C(N)=O)c(Nc3cccc(OC)c3)c2c1 Show InChI InChI=1S/C25H23N3O5S/c1-15-11-20(34(30,31)19-9-7-17(32-2)8-10-19)13-21-23(15)27-14-22(25(26)29)24(21)28-16-5-4-6-18(12-16)33-3/h4-14H,1-3H3,(H2,26,29)(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5261-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.012
BindingDB Entry DOI: 10.7270/Q2K075HB |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50415008
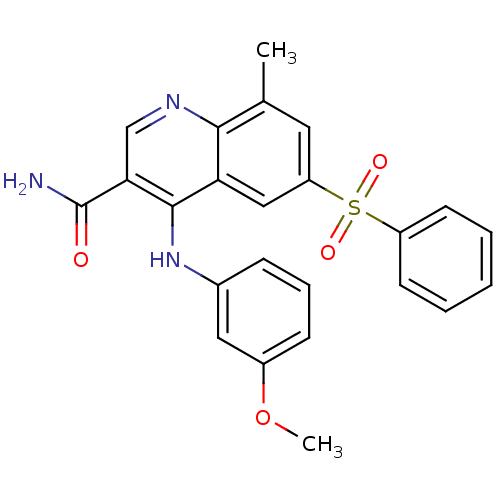
(CHEMBL584327)Show SMILES COc1cccc(Nc2c(cnc3c(C)cc(cc23)S(=O)(=O)c2ccccc2)C(N)=O)c1 Show InChI InChI=1S/C24H21N3O4S/c1-15-11-19(32(29,30)18-9-4-3-5-10-18)13-20-22(15)26-14-21(24(25)28)23(20)27-16-7-6-8-17(12-16)31-2/h3-14H,1-2H3,(H2,25,28)(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5261-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.012
BindingDB Entry DOI: 10.7270/Q2K075HB |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50575488

(CHEMBL4852381)Show SMILES N[C@@H](CC(O)=O)Cn1nnc(n1)-c1ccc(Oc2ncc(Cl)cc2F)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant full length N-terminal His6-tagged LTA4H (unknown origin) expressed in Escherichia coli BL21 DE3 cells at enzyme concentrat... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01955
BindingDB Entry DOI: 10.7270/Q2B85CZG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50414998
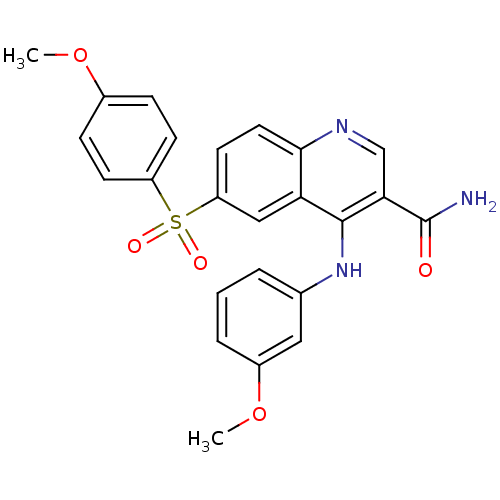
(CHEMBL569556)Show SMILES COc1ccc(cc1)S(=O)(=O)c1ccc2ncc(C(N)=O)c(Nc3cccc(OC)c3)c2c1 Show InChI InChI=1S/C24H21N3O5S/c1-31-16-6-8-18(9-7-16)33(29,30)19-10-11-22-20(13-19)23(21(14-26-22)24(25)28)27-15-4-3-5-17(12-15)32-2/h3-14H,1-2H3,(H2,25,28)(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5261-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.012
BindingDB Entry DOI: 10.7270/Q2K075HB |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM14774

(3-(cyclopropylmethoxy)-N-(3,5-dichloropyridin-4-yl...)Show SMILES FC(F)Oc1ccc(cc1OCC1CC1)C(=O)Nc1c(Cl)cncc1Cl Show InChI InChI=1S/C17H14Cl2F2N2O3/c18-11-6-22-7-12(19)15(11)23-16(24)10-3-4-13(26-17(20)21)14(5-10)25-8-9-1-2-9/h3-7,9,17H,1-2,8H2,(H,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5261-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.012
BindingDB Entry DOI: 10.7270/Q2K075HB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50184767

(CHEMBL3824089 | US10294227, Code 506)Show SMILES COc1cc(ccc1NC(=O)OC(C)(C)C)-c1nn(CCN2CCC(CC2)N(C)C)c2ncnc(N)c12 Show InChI InChI=1S/C26H38N8O3/c1-26(2,3)37-25(35)30-19-8-7-17(15-20(19)36-6)22-21-23(27)28-16-29-24(21)34(31-22)14-13-33-11-9-18(10-12-33)32(4)5/h7-8,15-16,18H,9-14H2,1-6H3,(H,30,35)(H2,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged full length human SRC expressed in insect cells preincubated for 20 mins using poly[Glu,Tyr]4:1 as substrate meas... |
J Med Chem 59: 4697-710 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00065
BindingDB Entry DOI: 10.7270/Q2B85B2D |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50184776
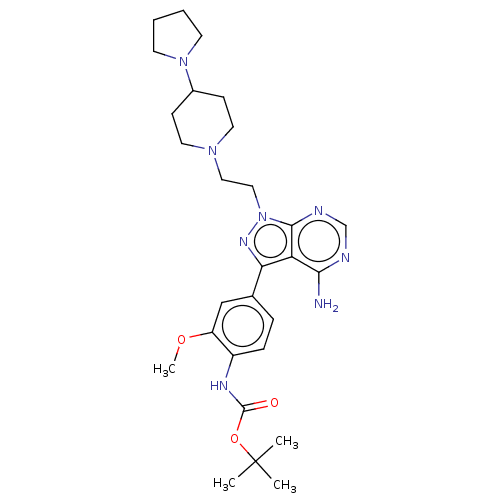
(CHEMBL3824233 | US10294227, Code 518)Show SMILES COc1cc(ccc1NC(=O)OC(C)(C)C)-c1nn(CCN2CCC(CC2)N2CCCC2)c2ncnc(N)c12 Show InChI InChI=1S/C28H40N8O3/c1-28(2,3)39-27(37)32-21-8-7-19(17-22(21)38-4)24-23-25(29)30-18-31-26(23)36(33-24)16-15-34-13-9-20(10-14-34)35-11-5-6-12-35/h7-8,17-18,20H,5-6,9-16H2,1-4H3,(H,32,37)(H2,29,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged full length human SRC expressed in insect cells preincubated for 20 mins using poly[Glu,Tyr]4:1 as substrate meas... |
J Med Chem 59: 4697-710 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00065
BindingDB Entry DOI: 10.7270/Q2B85B2D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fyn
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human FYN using poly[Glu,Tyr]4:1 as substrate in presence of [gamma-33P]ATP |
J Med Chem 59: 4697-710 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00065
BindingDB Entry DOI: 10.7270/Q2B85B2D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human ABL using EAIYAAPFAKKK as substrate in presence of [gamma-33P]ATP |
J Med Chem 59: 4697-710 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00065
BindingDB Entry DOI: 10.7270/Q2B85B2D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human YES using poly[Glu,Tyr]4:1 as substrate in presence of [gamma-33P] ATP |
J Med Chem 59: 4697-710 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00065
BindingDB Entry DOI: 10.7270/Q2B85B2D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM50184767

(CHEMBL3824089 | US10294227, Code 506)Show SMILES COc1cc(ccc1NC(=O)OC(C)(C)C)-c1nn(CCN2CCC(CC2)N(C)C)c2ncnc(N)c12 Show InChI InChI=1S/C26H38N8O3/c1-26(2,3)37-25(35)30-19-8-7-17(15-20(19)36-6)22-21-23(27)28-16-29-24(21)34(31-22)14-13-33-11-9-18(10-12-33)32(4)5/h7-8,15-16,18H,9-14H2,1-6H3,(H,30,35)(H2,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human YES using poly[Glu,Tyr]4:1 as substrate in presence of [gamma-33P] ATP |
J Med Chem 59: 4697-710 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00065
BindingDB Entry DOI: 10.7270/Q2B85B2D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged full length human SRC expressed in insect cells preincubated for 20 mins using poly[Glu,Tyr]4:1 as substrate meas... |
J Med Chem 59: 4697-710 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00065
BindingDB Entry DOI: 10.7270/Q2B85B2D |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50085135
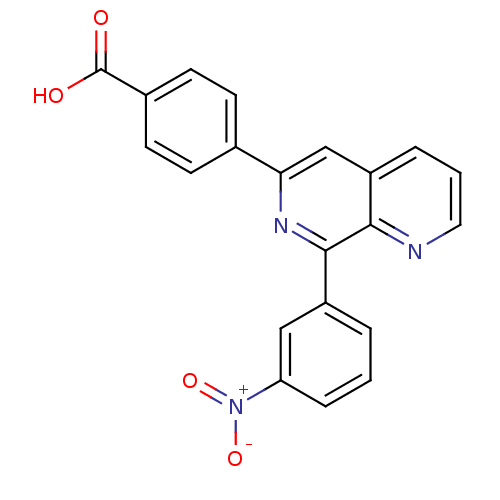
(4-(8-(3-nitrophenyl)-1,7-naphthyridin-6-yl)benzoic...)Show SMILES OC(=O)c1ccc(cc1)-c1cc2cccnc2c(n1)-c1cccc(c1)[N+]([O-])=O Show InChI InChI=1S/C21H13N3O4/c25-21(26)14-8-6-13(7-9-14)18-12-16-4-2-10-22-19(16)20(23-18)15-3-1-5-17(11-15)24(27)28/h1-12H,(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of [3H]rolipram binding in human peripheral blood mononuclear cells |
Bioorg Med Chem Lett 12: 233-5 (2001)
BindingDB Entry DOI: 10.7270/Q2MG7Q1T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50546180
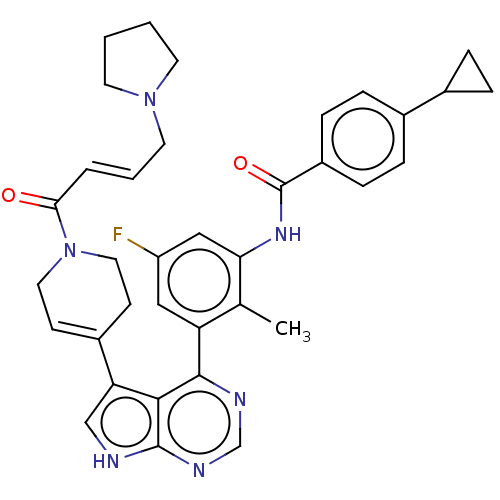
(CHEMBL4749522)Show SMILES Cc1c(NC(=O)c2ccc(cc2)C2CC2)cc(F)cc1-c1ncnc2[nH]cc(C3=CCN(CC3)C(=O)\C=C\CN3CCCC3)c12 |t:31| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full length human unphosphorylated BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate incubated for 60 mins in presence of ATP at... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00317
BindingDB Entry DOI: 10.7270/Q20K2D40 |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50281849

(CHEMBL4175305)Show SMILES CCS(=O)(=O)c1ccc(CC(=O)Nc2cnc(O[C@@H](C)c3ccccc3)c(Cl)c2)cc1 |r| Show InChI InChI=1S/C23H23ClN2O4S/c1-3-31(28,29)20-11-9-17(10-12-20)13-22(27)26-19-14-21(24)23(25-15-19)30-16(2)18-7-5-4-6-8-18/h4-12,14-16H,3,13H2,1-2H3,(H,26,27)/t16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human His6-tagged RORgammat LBD (264 to 518 residues) assessed as reduction in biotinylated RIP140 co-activator recruitme... |
J Med Chem 61: 6724-6735 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00529
BindingDB Entry DOI: 10.7270/Q2Z60RK3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50184777
(CHEMBL3823104 | US10294227, Code 519)Show SMILES COc1cc(ccc1NC(=O)OC(C)(C)C)-c1nn(CCN2CCC(CC2)N2CCCCC2)c2ncnc(N)c12 Show InChI InChI=1S/C29H42N8O3/c1-29(2,3)40-28(38)33-22-9-8-20(18-23(22)39-4)25-24-26(30)31-19-32-27(24)37(34-25)17-16-35-14-10-21(11-15-35)36-12-6-5-7-13-36/h8-9,18-19,21H,5-7,10-17H2,1-4H3,(H,33,38)(H2,30,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged full length human SRC expressed in insect cells preincubated for 20 mins using poly[Glu,Tyr]4:1 as substrate meas... |
J Med Chem 59: 4697-710 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00065
BindingDB Entry DOI: 10.7270/Q2B85B2D |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50415007
(CHEMBL571171)Show SMILES COc1cccc(Nc2c(cnc3ccc(cc23)S(=O)(=O)c2ccc(cc2)C(C)(C)C)C(N)=O)c1 Show InChI InChI=1S/C27H27N3O4S/c1-27(2,3)17-8-10-20(11-9-17)35(32,33)21-12-13-24-22(15-21)25(23(16-29-24)26(28)31)30-18-6-5-7-19(14-18)34-4/h5-16H,1-4H3,(H2,28,31)(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5261-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.012
BindingDB Entry DOI: 10.7270/Q2K075HB |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50414992
(CHEMBL576479)Show SMILES COc1cccc(Nc2c(cnc3ccc(cc23)S(=O)(=O)N(C)C)C(N)=O)c1 Show InChI InChI=1S/C19H20N4O4S/c1-23(2)28(25,26)14-7-8-17-15(10-14)18(16(11-21-17)19(20)24)22-12-5-4-6-13(9-12)27-3/h4-11H,1-3H3,(H2,20,24)(H,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5261-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.012
BindingDB Entry DOI: 10.7270/Q2K075HB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM259415
(US10457647, Example 14 | US11180460, Example 14 | ...)Show SMILES CCN(CCOc1c(N)ncnc1-c1cc(F)cc(NC(=O)c2ccc(cc2F)C2CC2)c1C)C(=O)C=C Show InChI InChI=1S/C28H29F2N5O3/c1-4-24(36)35(5-2)10-11-38-26-25(32-15-33-27(26)31)21-13-19(29)14-23(16(21)3)34-28(37)20-9-8-18(12-22(20)30)17-6-7-17/h4,8-9,12-15,17H,1,5-7,10-11H2,2-3H3,(H,34,37)(H2,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full-length human recombinant BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate measured after 60 mins by caliper assay |
J Med Chem 63: 5102-5118 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01916
BindingDB Entry DOI: 10.7270/Q2GB27FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50514642

(CHEMBL4593663)Show SMILES Cc1c(NC(=O)c2ccc(cc2)C2CC2)cc(F)cc1-c1ncnc2[nH]cc(C3=CCN(CC3)C(=O)C=C)c12 |t:31| Show InChI InChI=1S/C31H28FN5O2/c1-3-27(38)37-12-10-21(11-13-37)25-16-33-30-28(25)29(34-17-35-30)24-14-23(32)15-26(18(24)2)36-31(39)22-8-6-20(7-9-22)19-4-5-19/h3,6-10,14-17,19H,1,4-5,11-13H2,2H3,(H,36,39)(H,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full length human unphosphorylated BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate incubated for 60 mins in presence of ATP at... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00317
BindingDB Entry DOI: 10.7270/Q20K2D40 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50514642

(CHEMBL4593663)Show SMILES Cc1c(NC(=O)c2ccc(cc2)C2CC2)cc(F)cc1-c1ncnc2[nH]cc(C3=CCN(CC3)C(=O)C=C)c12 |t:31| Show InChI InChI=1S/C31H28FN5O2/c1-3-27(38)37-12-10-21(11-13-37)25-16-33-30-28(25)29(34-17-35-30)24-14-23(32)15-26(18(24)2)36-31(39)22-8-6-20(7-9-22)19-4-5-19/h3,6-10,14-17,19H,1,4-5,11-13H2,2H3,(H,36,39)(H,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full-length human recombinant BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate measured after 60 mins by caliper assay |
J Med Chem 63: 5102-5118 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01916
BindingDB Entry DOI: 10.7270/Q2GB27FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50546179

(CHEMBL4739958)Show SMILES Cc1c(NC(=O)c2ccc(cc2)C2CC2)cc(F)cc1-c1ncnc2[nH]cc(C3CCN(CC3)C(=O)C=C)c12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full length human unphosphorylated BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate incubated for 60 mins in presence of ATP at... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00317
BindingDB Entry DOI: 10.7270/Q20K2D40 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50108504
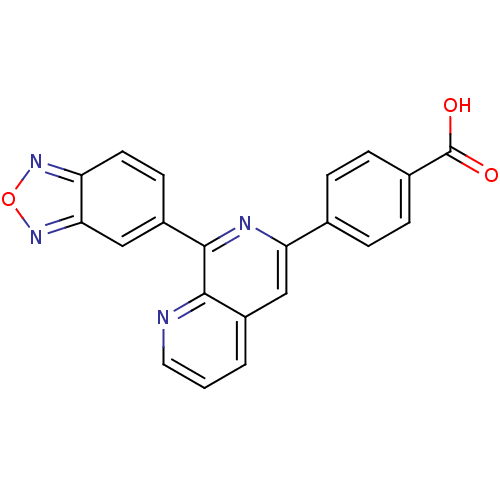
(4-(8-(benzo[c][1,2,5]oxadiazol-5-yl)-1,7-naphthyri...)Show SMILES OC(=O)c1ccc(cc1)-c1cc2cccnc2c(n1)-c1ccc2nonc2c1 Show InChI InChI=1S/C21H12N4O3/c26-21(27)13-5-3-12(4-6-13)17-10-14-2-1-9-22-19(14)20(23-17)15-7-8-16-18(11-15)25-28-24-16/h1-11H,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of [3H]rolipram binding in human peripheral blood mononuclear cells |
Bioorg Med Chem Lett 12: 233-5 (2001)
BindingDB Entry DOI: 10.7270/Q2MG7Q1T |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50184785
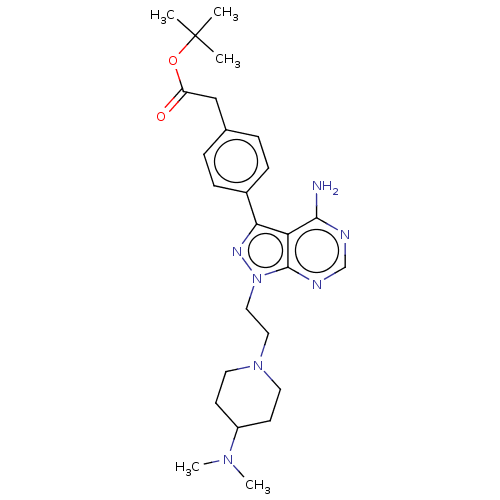
(CHEMBL3823620 | US10294227, Code 553)Show SMILES CN(C)C1CCN(CCn2nc(-c3ccc(CC(=O)OC(C)(C)C)cc3)c3c(N)ncnc23)CC1 Show InChI InChI=1S/C26H37N7O2/c1-26(2,3)35-21(34)16-18-6-8-19(9-7-18)23-22-24(27)28-17-29-25(22)33(30-23)15-14-32-12-10-20(11-13-32)31(4)5/h6-9,17,20H,10-16H2,1-5H3,(H2,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged full length human SRC expressed in insect cells preincubated for 20 mins using poly[Glu,Tyr]4:1 as substrate meas... |
J Med Chem 59: 4697-710 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00065
BindingDB Entry DOI: 10.7270/Q2B85B2D |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50184786
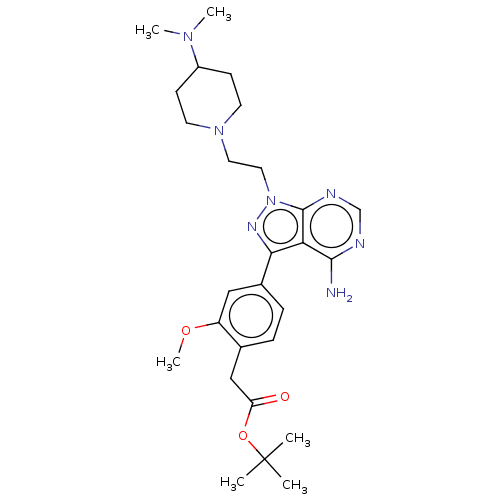
(CHEMBL3824116 | US10294227, Code 565)Show SMILES COc1cc(ccc1CC(=O)OC(C)(C)C)-c1nn(CCN2CCC(CC2)N(C)C)c2ncnc(N)c12 Show InChI InChI=1S/C27H39N7O3/c1-27(2,3)37-22(35)16-18-7-8-19(15-21(18)36-6)24-23-25(28)29-17-30-26(23)34(31-24)14-13-33-11-9-20(10-12-33)32(4)5/h7-8,15,17,20H,9-14,16H2,1-6H3,(H2,28,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged full length human SRC expressed in insect cells preincubated for 20 mins using poly[Glu,Tyr]4:1 as substrate meas... |
J Med Chem 59: 4697-710 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00065
BindingDB Entry DOI: 10.7270/Q2B85B2D |
More data for this
Ligand-Target Pair | |
Cytoplasmic tyrosine-protein kinase BMX
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BMX (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00317
BindingDB Entry DOI: 10.7270/Q20K2D40 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50085135

(4-(8-(3-nitrophenyl)-1,7-naphthyridin-6-yl)benzoic...)Show SMILES OC(=O)c1ccc(cc1)-c1cc2cccnc2c(n1)-c1cccc(c1)[N+]([O-])=O Show InChI InChI=1S/C21H13N3O4/c25-21(26)14-8-6-13(7-9-14)18-12-16-4-2-10-22-19(16)20(23-18)15-3-1-5-17(11-15)24(27)28/h1-12H,(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of human Phosphodiesterase 4D from peripheral blood mononuclear cells |
Bioorg Med Chem Lett 12: 233-5 (2001)
BindingDB Entry DOI: 10.7270/Q2MG7Q1T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin K
(Homo sapiens (Human)) | BDBM50260351
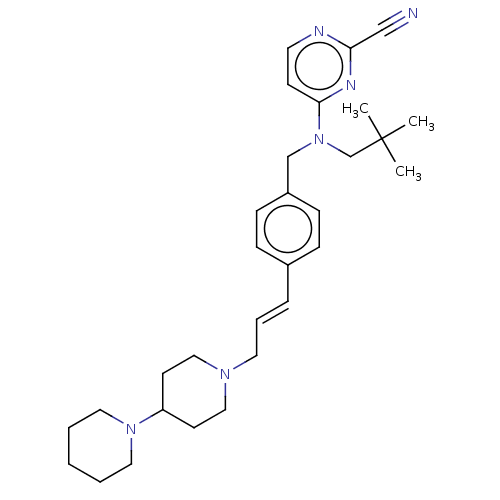
(CHEMBL4086667)Show SMILES CC(C)(C)CN(Cc1ccc(\C=C\CN2CCC(CC2)N2CCCCC2)cc1)c1ccnc(n1)C#N Show InChI InChI=1S/C30H42N6/c1-30(2,3)24-36(29-13-16-32-28(22-31)33-29)23-26-11-9-25(10-12-26)8-7-17-34-20-14-27(15-21-34)35-18-5-4-6-19-35/h7-13,16,27H,4-6,14-15,17-21,23-24H2,1-3H3/b8-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin K |
Bioorg Med Chem 25: 4512-4525 (2017)
Article DOI: 10.1016/j.bmc.2017.06.050
BindingDB Entry DOI: 10.7270/Q2NS0XB3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50546175

(CHEMBL4795673)Show SMILES CN(C(=O)Cc1c[nH]c2ncnc(-c3cc(F)cc(NC(=O)c4ccc(cc4)C4CC4)c3C)c12)C(=O)C=C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full length human unphosphorylated BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate incubated for 60 mins in presence of ATP at... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00317
BindingDB Entry DOI: 10.7270/Q20K2D40 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM259407
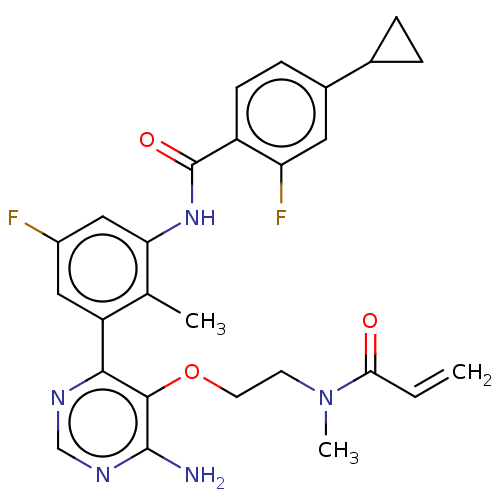
(US10457647, Example 6 | US11180460, Example 6 | US...)Show SMILES CN(CCOc1c(N)ncnc1-c1cc(F)cc(NC(=O)c2ccc(cc2F)C2CC2)c1C)C(=O)C=C Show InChI InChI=1S/C27H27F2N5O3/c1-4-23(35)34(3)9-10-37-25-24(31-14-32-26(25)30)20-12-18(28)13-22(15(20)2)33-27(36)19-8-7-17(11-21(19)29)16-5-6-16/h4,7-8,11-14,16H,1,5-6,9-10H2,2-3H3,(H,33,36)(H2,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full-length human recombinant BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate measured after 60 mins by caliper assay |
J Med Chem 63: 5102-5118 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01916
BindingDB Entry DOI: 10.7270/Q2GB27FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM259423
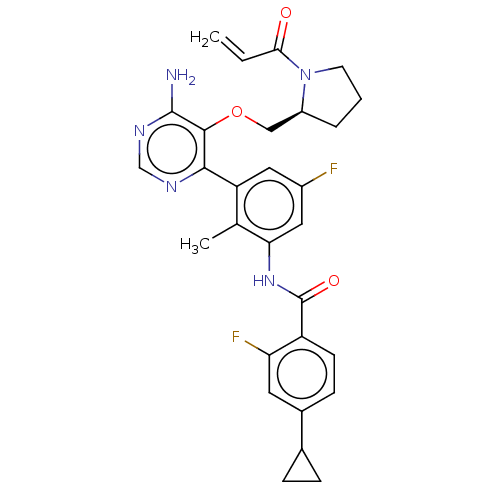
(US10457647, Example 22 | US11180460, Example 22 | ...)Show SMILES Cc1c(NC(=O)c2ccc(cc2F)C2CC2)cc(F)cc1-c1ncnc(N)c1OC[C@@H]1CCCN1C(=O)C=C |r| Show InChI InChI=1S/C29H29F2N5O3/c1-3-25(37)36-10-4-5-20(36)14-39-27-26(33-15-34-28(27)32)22-12-19(30)13-24(16(22)2)35-29(38)21-9-8-18(11-23(21)31)17-6-7-17/h3,8-9,11-13,15,17,20H,1,4-7,10,14H2,2H3,(H,35,38)(H2,32,33,34)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full-length human recombinant BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate measured after 60 mins by caliper assay |
J Med Chem 63: 5102-5118 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01916
BindingDB Entry DOI: 10.7270/Q2GB27FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50546176

(CHEMBL4746262)Show SMILES Cc1c(NC(=O)c2ccc(cc2)C2CC2)cc(F)cc1-c1ncnc2[nH]cc(c12)C1(O)CN(C1)C(=O)C=C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full length human unphosphorylated BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate incubated for 60 mins in presence of ATP at... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00317
BindingDB Entry DOI: 10.7270/Q20K2D40 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50108504

(4-(8-(benzo[c][1,2,5]oxadiazol-5-yl)-1,7-naphthyri...)Show SMILES OC(=O)c1ccc(cc1)-c1cc2cccnc2c(n1)-c1ccc2nonc2c1 Show InChI InChI=1S/C21H12N4O3/c26-21(27)13-5-3-12(4-6-13)17-10-14-2-1-9-22-19(14)20(23-17)15-7-8-16-18(11-15)25-28-24-16/h1-11H,(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of human Phosphodiesterase 4D from peripheral blood mononuclear cells |
Bioorg Med Chem Lett 12: 233-5 (2001)
BindingDB Entry DOI: 10.7270/Q2MG7Q1T |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50413291
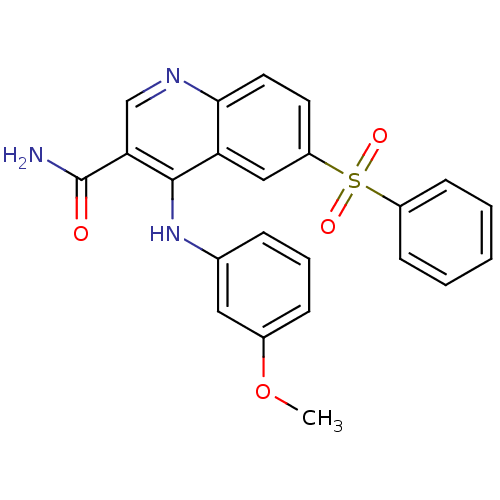
(CHEMBL515240)Show SMILES COc1cccc(Nc2c(cnc3ccc(cc23)S(=O)(=O)c2ccccc2)C(N)=O)c1 Show InChI InChI=1S/C23H19N3O4S/c1-30-16-7-5-6-15(12-16)26-22-19-13-18(31(28,29)17-8-3-2-4-9-17)10-11-21(19)25-14-20(22)23(24)27/h2-14H,1H3,(H2,24,27)(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5261-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.012
BindingDB Entry DOI: 10.7270/Q2K075HB |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50415000
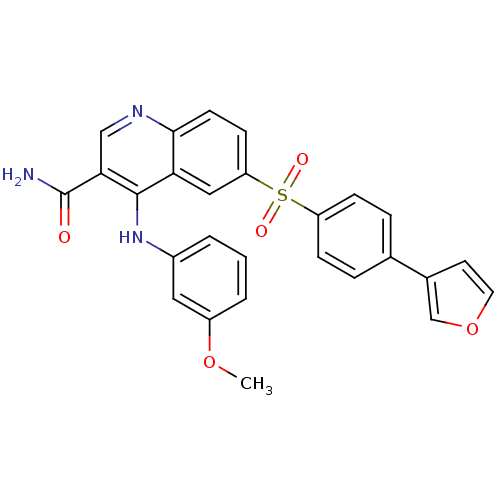
(CHEMBL570029)Show SMILES COc1cccc(Nc2c(cnc3ccc(cc23)S(=O)(=O)c2ccc(cc2)-c2ccoc2)C(N)=O)c1 Show InChI InChI=1S/C27H21N3O5S/c1-34-20-4-2-3-19(13-20)30-26-23-14-22(9-10-25(23)29-15-24(26)27(28)31)36(32,33)21-7-5-17(6-8-21)18-11-12-35-16-18/h2-16H,1H3,(H2,28,31)(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5261-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.012
BindingDB Entry DOI: 10.7270/Q2K075HB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50514634

(CHEMBL4450082)Show SMILES CN(CC\C=C\c1c(N)ncnc1-c1cc(F)cc(NC(=O)c2ccc(cc2)C2CC2)c1C)C(=O)C=C Show InChI InChI=1S/C29H30FN5O2/c1-4-26(36)35(3)14-6-5-7-23-27(32-17-33-28(23)31)24-15-22(30)16-25(18(24)2)34-29(37)21-12-10-20(11-13-21)19-8-9-19/h4-5,7,10-13,15-17,19H,1,6,8-9,14H2,2-3H3,(H,34,37)(H2,31,32,33)/b7-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full-length human recombinant BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate measured after 60 mins by caliper assay |
J Med Chem 63: 5102-5118 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01916
BindingDB Entry DOI: 10.7270/Q2GB27FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM259409
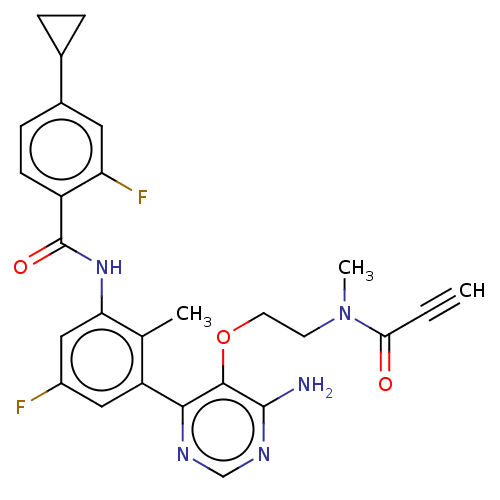
(US10457647, Example 8 | US11180460, Example 8 | US...)Show SMILES CN(CCOc1c(N)ncnc1-c1cc(F)cc(NC(=O)c2ccc(cc2F)C2CC2)c1C)C(=O)C#C Show InChI InChI=1S/C27H25F2N5O3/c1-4-23(35)34(3)9-10-37-25-24(31-14-32-26(25)30)20-12-18(28)13-22(15(20)2)33-27(36)19-8-7-17(11-21(19)29)16-5-6-16/h1,7-8,11-14,16H,5-6,9-10H2,2-3H3,(H,33,36)(H2,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full-length human recombinant BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate measured after 60 mins by caliper assay |
J Med Chem 63: 5102-5118 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01916
BindingDB Entry DOI: 10.7270/Q2GB27FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
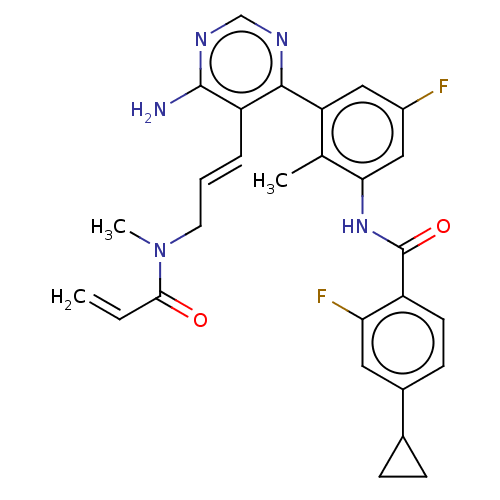
(Homo sapiens (Human)) | BDBM50514645
(CHEMBL4546324)Show SMILES CN(C\C=C\c1c(N)ncnc1-c1cc(F)cc(NC(=O)c2ccc(cc2F)C2CC2)c1C)C(=O)C=C Show InChI InChI=1S/C28H27F2N5O2/c1-4-25(36)35(3)11-5-6-21-26(32-15-33-27(21)31)22-13-19(29)14-24(16(22)2)34-28(37)20-10-9-18(12-23(20)30)17-7-8-17/h4-6,9-10,12-15,17H,1,7-8,11H2,2-3H3,(H,34,37)(H2,31,32,33)/b6-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full-length human recombinant BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate measured after 60 mins by caliper assay |
J Med Chem 63: 5102-5118 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01916
BindingDB Entry DOI: 10.7270/Q2GB27FW |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50575492

(CHEMBL4784496)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)NC(C)=O)N2Cc1ccc(Oc2nc3ncccc3s2)cc1 |THB:14:13:2.3:7.8.6| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant full length N-terminal His6-tagged LTA4H (unknown origin) expressed in Escherichia coli BL21 DE3 cells at enzyme concentrat... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01955
BindingDB Entry DOI: 10.7270/Q2B85CZG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50415004
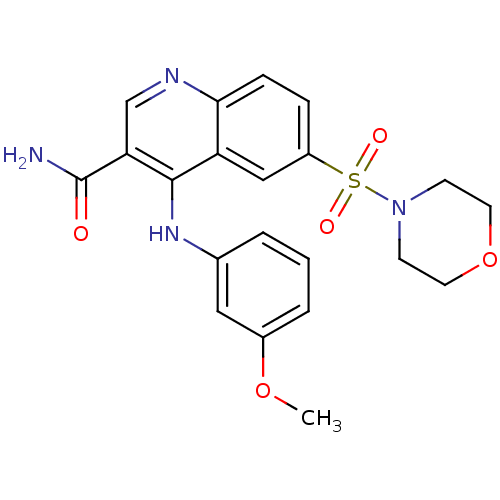
(CHEMBL571593)Show SMILES COc1cccc(Nc2c(cnc3ccc(cc23)S(=O)(=O)N2CCOCC2)C(N)=O)c1 Show InChI InChI=1S/C21H22N4O5S/c1-29-15-4-2-3-14(11-15)24-20-17-12-16(31(27,28)25-7-9-30-10-8-25)5-6-19(17)23-13-18(20)21(22)26/h2-6,11-13H,7-10H2,1H3,(H2,22,26)(H,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5261-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.012
BindingDB Entry DOI: 10.7270/Q2K075HB |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50506051
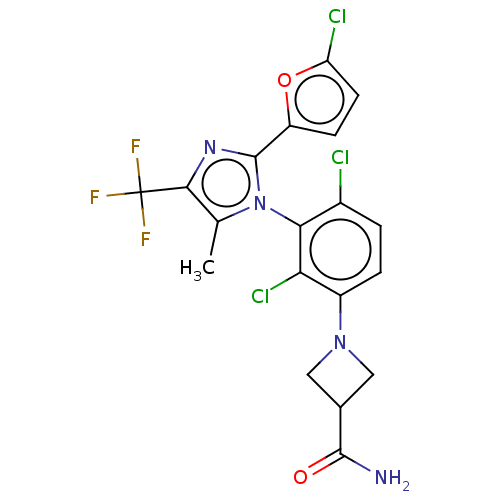
(CHEMBL4587988)Show SMILES Cc1c(nc(-c2ccc(Cl)o2)n1-c1c(Cl)ccc(N2CC(C2)C(N)=O)c1Cl)C(F)(F)F |(37.14,-25.15,;36.82,-26.66,;37.85,-27.8,;37.08,-29.13,;35.57,-28.81,;34.43,-29.85,;34.6,-31.38,;33.19,-32.01,;32.16,-30.87,;30.63,-31.04,;32.92,-29.54,;35.4,-27.28,;34.07,-26.51,;34.08,-24.97,;35.42,-24.21,;32.75,-24.2,;31.41,-24.97,;31.42,-26.52,;30.09,-27.29,;28.6,-26.9,;28.21,-28.39,;29.7,-28.78,;26.88,-29.16,;25.54,-28.4,;26.88,-30.7,;32.75,-27.28,;31.41,-28.04,;39.39,-27.8,;40.16,-29.13,;40.15,-26.46,;40.93,-27.8,)| Show InChI InChI=1S/C19H14Cl3F3N4O2/c1-8-16(19(23,24)25)27-18(12-4-5-13(21)31-12)29(8)15-10(20)2-3-11(14(15)22)28-6-9(7-28)17(26)30/h2-5,9H,6-7H2,1H3,(H2,26,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Discovery Chemistry
Curated by ChEMBL
| Assay Description
Displacement of RIP140 cofactor peptide from human His6-tagged RORgammat LBD (264 to 518 residues) by TR-FRET assay |
J Med Chem 62: 10816-10832 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01291
BindingDB Entry DOI: 10.7270/Q2CF9TD7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fyn
(Homo sapiens (Human)) | BDBM50184767

(CHEMBL3824089 | US10294227, Code 506)Show SMILES COc1cc(ccc1NC(=O)OC(C)(C)C)-c1nn(CCN2CCC(CC2)N(C)C)c2ncnc(N)c12 Show InChI InChI=1S/C26H38N8O3/c1-26(2,3)37-25(35)30-19-8-7-17(15-20(19)36-6)22-21-23(27)28-16-29-24(21)34(31-22)14-13-33-11-9-18(10-12-33)32(4)5/h7-8,15-16,18H,9-14H2,1-6H3,(H,30,35)(H2,27,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of human FYN using poly[Glu,Tyr]4:1 as substrate in presence of [gamma-33P]ATP |
J Med Chem 59: 4697-710 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00065
BindingDB Entry DOI: 10.7270/Q2B85B2D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50514637

(CHEMBL4463185)Show SMILES Cc1c(NC(=O)c2ccc(c(F)c2F)C(C)(C)O)cc(F)cc1-c1ncnc(N)c1OC1CN(C1)C(=O)C=C Show InChI InChI=1S/C27H26F3N5O4/c1-5-20(36)35-10-15(11-35)39-24-23(32-12-33-25(24)31)17-8-14(28)9-19(13(17)2)34-26(37)16-6-7-18(27(3,4)38)22(30)21(16)29/h5-9,12,15,38H,1,10-11H2,2-4H3,(H,34,37)(H2,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full-length human recombinant BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate measured after 60 mins by caliper assay |
J Med Chem 63: 5102-5118 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01916
BindingDB Entry DOI: 10.7270/Q2GB27FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full-length human recombinant BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate measured after 60 mins by caliper assay |
J Med Chem 63: 5102-5118 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01916
BindingDB Entry DOI: 10.7270/Q2GB27FW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM259407

(US10457647, Example 6 | US11180460, Example 6 | US...)Show SMILES CN(CCOc1c(N)ncnc1-c1cc(F)cc(NC(=O)c2ccc(cc2F)C2CC2)c1C)C(=O)C=C Show InChI InChI=1S/C27H27F2N5O3/c1-4-23(35)34(3)9-10-37-25-24(31-14-32-26(25)30)20-12-18(28)13-22(15(20)2)33-27(36)19-8-7-17(11-21(19)29)16-5-6-16/h4,7-8,11-14,16H,1,5-6,9-10H2,2-3H3,(H,33,36)(H2,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of BTK in vitamin D3 differentiated human THP1 cells assessed as inhibition of FCgammaR-induced IL8 production measured after 24 hrs by HT... |
J Med Chem 63: 5102-5118 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01916
BindingDB Entry DOI: 10.7270/Q2GB27FW |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50415006
(CHEMBL585528)Show SMILES NC(=O)c1cnc2ccc(cc2c1Nc1ccc2ncsc2c1)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C23H16N4O3S2/c24-23(28)18-12-25-19-9-7-16(32(29,30)15-4-2-1-3-5-15)11-17(19)22(18)27-14-6-8-20-21(10-14)31-13-26-20/h1-13H,(H2,24,28)(H,25,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4B by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5261-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.012
BindingDB Entry DOI: 10.7270/Q2K075HB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data