

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM356652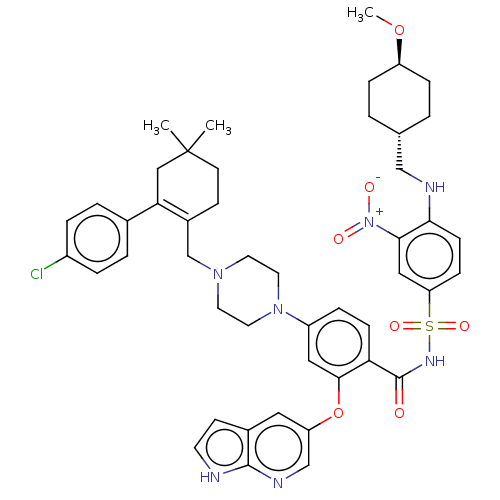 (US10213433, Compound 34 | US11369599, Compound 34 ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of Bcl2 (unknown origin) | J Med Chem 61: 2636-2651 (2018) Article DOI: 10.1021/acs.jmedchem.7b00717 BindingDB Entry DOI: 10.7270/Q2ZK5K94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM50162774 (ABT-199 | US11420968, Example ABT-199 | Venetoclax) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of Bcl2 (unknown origin) | J Med Chem 61: 2636-2651 (2018) Article DOI: 10.1021/acs.jmedchem.7b00717 BindingDB Entry DOI: 10.7270/Q2ZK5K94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM50270877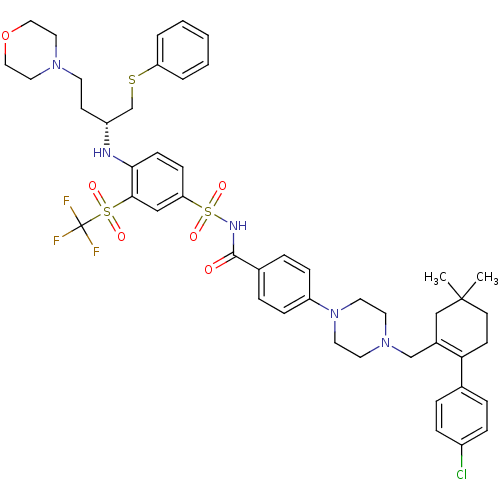 ((R)-4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of Bcl2 (unknown origin) | J Med Chem 61: 2636-2651 (2018) Article DOI: 10.1021/acs.jmedchem.7b00717 BindingDB Entry DOI: 10.7270/Q2ZK5K94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50270877 ((R)-4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of Bcl-xL (unknown origin) | J Med Chem 61: 2636-2651 (2018) Article DOI: 10.1021/acs.jmedchem.7b00717 BindingDB Entry DOI: 10.7270/Q2ZK5K94 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM21447 (4-(4-{[2-(4-chlorophenyl)phenyl]methyl}piperazin-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of Bcl2 (unknown origin) | J Med Chem 61: 2636-2651 (2018) Article DOI: 10.1021/acs.jmedchem.7b00717 BindingDB Entry DOI: 10.7270/Q2ZK5K94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM21447 (4-(4-{[2-(4-chlorophenyl)phenyl]methyl}piperazin-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of Bcl-xL (unknown origin) | J Med Chem 61: 2636-2651 (2018) Article DOI: 10.1021/acs.jmedchem.7b00717 BindingDB Entry DOI: 10.7270/Q2ZK5K94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM356652 (US10213433, Compound 34 | US11369599, Compound 34 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of Bcl-xL (unknown origin) | J Med Chem 61: 2636-2651 (2018) Article DOI: 10.1021/acs.jmedchem.7b00717 BindingDB Entry DOI: 10.7270/Q2ZK5K94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50267297 (CHEMBL507731 | Methyl (S)-1-((2S,4S,5S)-5-((S)-2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) assessed as midazolam 1'- hydroxylation | J Med Chem 52: 2571-86 (2009) Article DOI: 10.1021/jm900044w BindingDB Entry DOI: 10.7270/Q2G160Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50162774 (ABT-199 | US11420968, Example ABT-199 | Venetoclax) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of Bcl-xL (unknown origin) | J Med Chem 61: 2636-2651 (2018) Article DOI: 10.1021/acs.jmedchem.7b00717 BindingDB Entry DOI: 10.7270/Q2ZK5K94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50267295 (CHEMBL470508 | Methyl (S)-1-((2R,4S,5S)-4-Hydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) assessed as midazolam 1'- hydroxylation | J Med Chem 52: 2571-86 (2009) Article DOI: 10.1021/jm900044w BindingDB Entry DOI: 10.7270/Q2G160Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50267295 (CHEMBL470508 | Methyl (S)-1-((2R,4S,5S)-4-Hydroxy-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of cathepsin D (unknown origin) | J Med Chem 52: 2571-86 (2009) Article DOI: 10.1021/jm900044w BindingDB Entry DOI: 10.7270/Q2G160Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50267297 (CHEMBL507731 | Methyl (S)-1-((2S,4S,5S)-5-((S)-2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of renin (unknown origin) | J Med Chem 52: 2571-86 (2009) Article DOI: 10.1021/jm900044w BindingDB Entry DOI: 10.7270/Q2G160Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50267297 (CHEMBL507731 | Methyl (S)-1-((2S,4S,5S)-5-((S)-2-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of cathepsin D (unknown origin) | J Med Chem 52: 2571-86 (2009) Article DOI: 10.1021/jm900044w BindingDB Entry DOI: 10.7270/Q2G160Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50267295 (CHEMBL470508 | Methyl (S)-1-((2R,4S,5S)-4-Hydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of renin (unknown origin) | J Med Chem 52: 2571-86 (2009) Article DOI: 10.1021/jm900044w BindingDB Entry DOI: 10.7270/Q2G160Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM186331 (US9163017, 5) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.05 | n/a | n/a | n/a | n/a | n/a | n/a |
ABBVIE INC. US Patent | Assay Description The inhibitory effects of the compounds of the invention on HCV replication can be determined by measuring activity of the luciferase reporter gene. ... | US Patent US9163017 (2015) BindingDB Entry DOI: 10.7270/Q2F47MX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM186342 (US9163017, 16) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.05 | n/a | n/a | n/a | n/a | n/a | n/a |
ABBVIE INC. US Patent | Assay Description The inhibitory effects of the compounds of the invention on HCV replication can be determined by measuring activity of the luciferase reporter gene. ... | US Patent US9163017 (2015) BindingDB Entry DOI: 10.7270/Q2F47MX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM186339 (US9163017, 13) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.05 | n/a | n/a | n/a | n/a | n/a | n/a |
ABBVIE INC. US Patent | Assay Description The inhibitory effects of the compounds of the invention on HCV replication can be determined by measuring activity of the luciferase reporter gene. ... | US Patent US9163017 (2015) BindingDB Entry DOI: 10.7270/Q2F47MX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM186338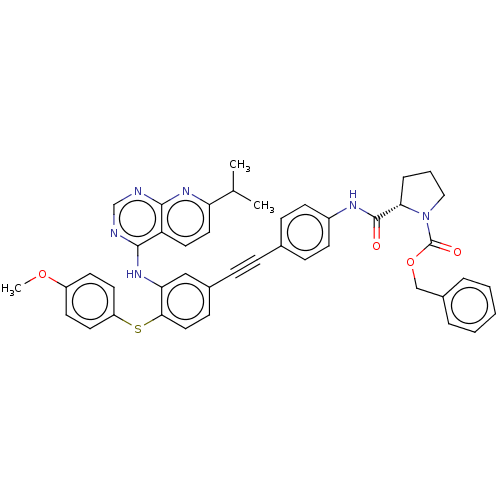 (US9163017, 12) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.05 | n/a | n/a | n/a | n/a | n/a | n/a |
ABBVIE INC. US Patent | Assay Description The inhibitory effects of the compounds of the invention on HCV replication can be determined by measuring activity of the luciferase reporter gene. ... | US Patent US9163017 (2015) BindingDB Entry DOI: 10.7270/Q2F47MX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM186337 (US9163017, 11) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.05 | n/a | n/a | n/a | n/a | n/a | n/a |
ABBVIE INC. US Patent | Assay Description The inhibitory effects of the compounds of the invention on HCV replication can be determined by measuring activity of the luciferase reporter gene. ... | US Patent US9163017 (2015) BindingDB Entry DOI: 10.7270/Q2F47MX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM186336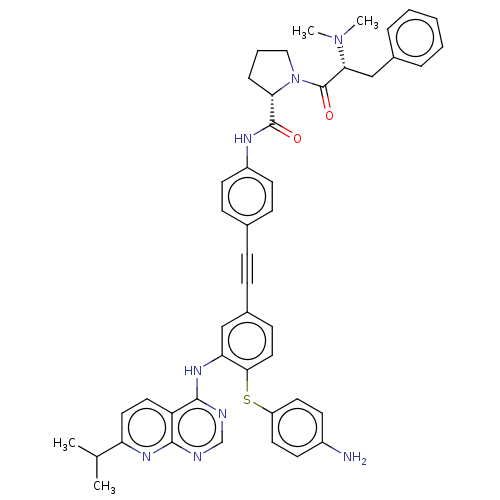 (US9163017, 10) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.05 | n/a | n/a | n/a | n/a | n/a | n/a |
ABBVIE INC. US Patent | Assay Description The inhibitory effects of the compounds of the invention on HCV replication can be determined by measuring activity of the luciferase reporter gene. ... | US Patent US9163017 (2015) BindingDB Entry DOI: 10.7270/Q2F47MX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM186335 (US9163017, 9) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.05 | n/a | n/a | n/a | n/a | n/a | n/a |
ABBVIE INC. US Patent | Assay Description The inhibitory effects of the compounds of the invention on HCV replication can be determined by measuring activity of the luciferase reporter gene. ... | US Patent US9163017 (2015) BindingDB Entry DOI: 10.7270/Q2F47MX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM186334 (US9163017, 8) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.05 | n/a | n/a | n/a | n/a | n/a | n/a |
ABBVIE INC. US Patent | Assay Description The inhibitory effects of the compounds of the invention on HCV replication can be determined by measuring activity of the luciferase reporter gene. ... | US Patent US9163017 (2015) BindingDB Entry DOI: 10.7270/Q2F47MX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM186333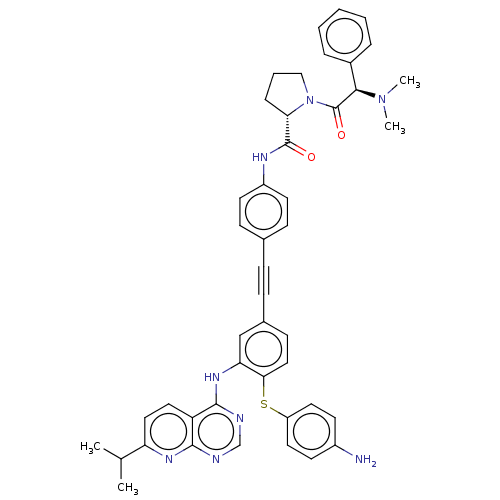 (US9163017, 7) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.05 | n/a | n/a | n/a | n/a | n/a | n/a |
ABBVIE INC. US Patent | Assay Description The inhibitory effects of the compounds of the invention on HCV replication can be determined by measuring activity of the luciferase reporter gene. ... | US Patent US9163017 (2015) BindingDB Entry DOI: 10.7270/Q2F47MX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM186332 (US9163017, 6) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.05 | n/a | n/a | n/a | n/a | n/a | n/a |
ABBVIE INC. US Patent | Assay Description The inhibitory effects of the compounds of the invention on HCV replication can be determined by measuring activity of the luciferase reporter gene. ... | US Patent US9163017 (2015) BindingDB Entry DOI: 10.7270/Q2F47MX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM186343 (US9163017, 17) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.05 | n/a | n/a | n/a | n/a | n/a | n/a |
ABBVIE INC. US Patent | Assay Description The inhibitory effects of the compounds of the invention on HCV replication can be determined by measuring activity of the luciferase reporter gene. ... | US Patent US9163017 (2015) BindingDB Entry DOI: 10.7270/Q2F47MX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM186324 (US9163017, 3) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.05 | n/a | n/a | n/a | n/a | n/a | n/a |
ABBVIE INC. US Patent | Assay Description The inhibitory effects of the compounds of the invention on HCV replication can be determined by measuring activity of the luciferase reporter gene. ... | US Patent US9163017 (2015) BindingDB Entry DOI: 10.7270/Q2F47MX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM186318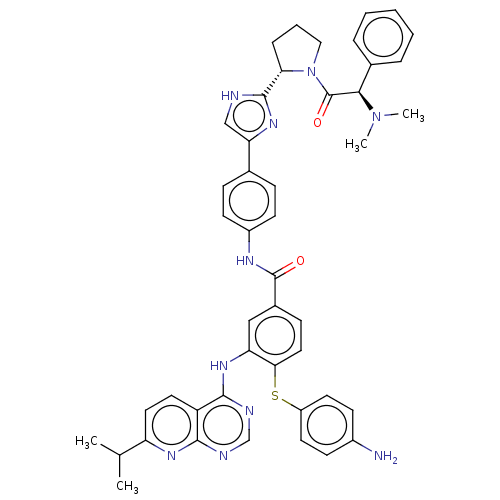 (US9163017, 2) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.05 | n/a | n/a | n/a | n/a | n/a | n/a |
ABBVIE INC. US Patent | Assay Description The inhibitory effects of the compounds of the invention on HCV replication can be determined by measuring activity of the luciferase reporter gene. ... | US Patent US9163017 (2015) BindingDB Entry DOI: 10.7270/Q2F47MX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM186329 (US9163017, 4) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
ABBVIE INC. US Patent | Assay Description The inhibitory effects of the compounds of the invention on HCV replication can be determined by measuring activity of the luciferase reporter gene. ... | US Patent US9163017 (2015) BindingDB Entry DOI: 10.7270/Q2F47MX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM186340 (US9163017, 14) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
ABBVIE INC. US Patent | Assay Description The inhibitory effects of the compounds of the invention on HCV replication can be determined by measuring activity of the luciferase reporter gene. ... | US Patent US9163017 (2015) BindingDB Entry DOI: 10.7270/Q2F47MX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM186341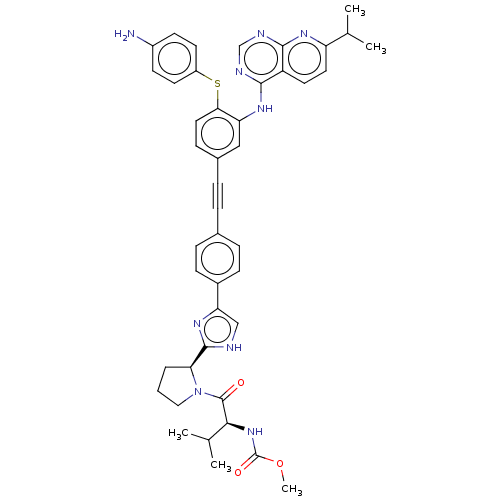 (US9163017, 15) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
ABBVIE INC. US Patent | Assay Description The inhibitory effects of the compounds of the invention on HCV replication can be determined by measuring activity of the luciferase reporter gene. ... | US Patent US9163017 (2015) BindingDB Entry DOI: 10.7270/Q2F47MX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM186305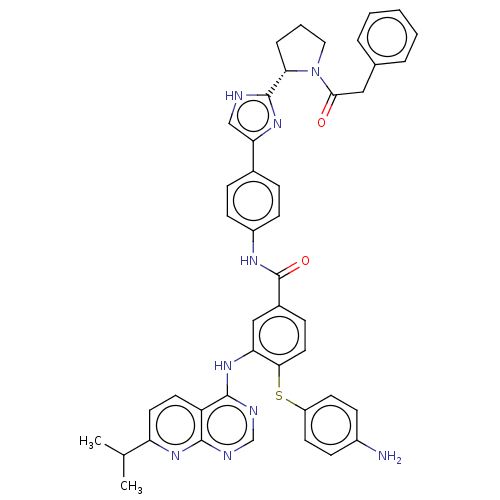 (US9163017, 1) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
ABBVIE INC. US Patent | Assay Description The inhibitory effects of the compounds of the invention on HCV replication can be determined by measuring activity of the luciferase reporter gene. ... | US Patent US9163017 (2015) BindingDB Entry DOI: 10.7270/Q2F47MX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50017659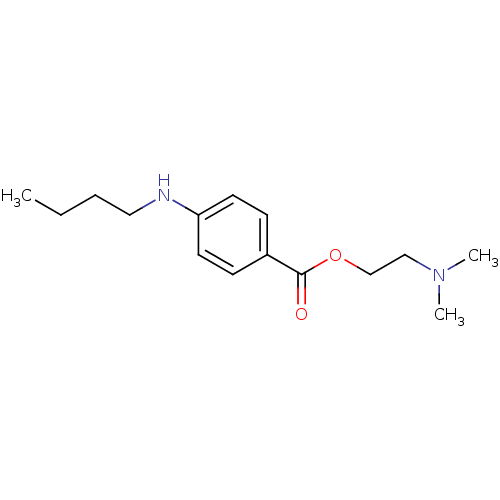 (2-(Dimethylamino)ethyl p-(butylamino)benzoate | 2-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Curated by ChEMBL | Assay Description Inhibition of recombinant human Nav1.7 expressed in HEK293F cells preincubated for 40 mins followed by DiSBAC2 substrate addition measured after 90 m... | J Med Chem 59: 3373-91 (2016) Article DOI: 10.1021/acs.jmedchem.6b00063 BindingDB Entry DOI: 10.7270/Q2C250GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 7 subunit alpha (Homo sapiens (Human)) | BDBM50017659 (2-(Dimethylamino)ethyl p-(butylamino)benzoate | 2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2022.116743 BindingDB Entry DOI: 10.7270/Q2BP06V9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 10 subunit alpha (Homo sapiens (Human)) | BDBM344702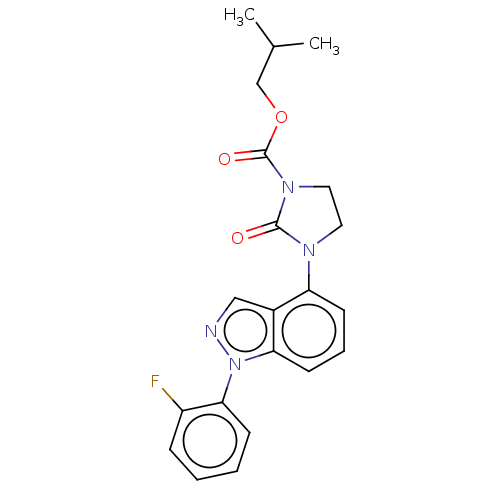 (US9783527, Example 5 | isobutyl 3-[1-(2-fluorophen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 57.2 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description Two days prior to the experiment, frozen HEK293 cells stably expressing recombinant human Nav1.8 (Essen, Ann Arbor, Mich.) were quickly thawed and pl... | US Patent US9783527 (2017) BindingDB Entry DOI: 10.7270/Q2S184MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 7 subunit alpha (Homo sapiens (Human)) | BDBM50600343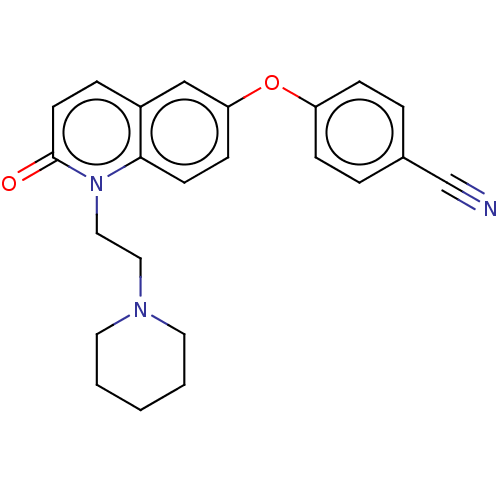 (CHEMBL5196338) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2022.116743 BindingDB Entry DOI: 10.7270/Q2BP06V9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 7 subunit alpha (Homo sapiens (Human)) | BDBM50600348 (CHEMBL5178152) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2022.116743 BindingDB Entry DOI: 10.7270/Q2BP06V9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 7 subunit alpha (Homo sapiens (Human)) | BDBM50600347 (CHEMBL5208962) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2022.116743 BindingDB Entry DOI: 10.7270/Q2BP06V9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 8 subunit alpha (Homo sapiens (Human)) | BDBM50600343 (CHEMBL5196338) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2022.116743 BindingDB Entry DOI: 10.7270/Q2BP06V9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 7 subunit alpha (Homo sapiens (Human)) | BDBM50600344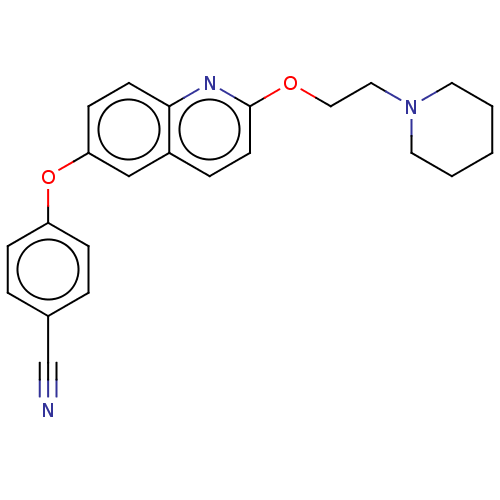 (CHEMBL5202508) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2022.116743 BindingDB Entry DOI: 10.7270/Q2BP06V9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50500962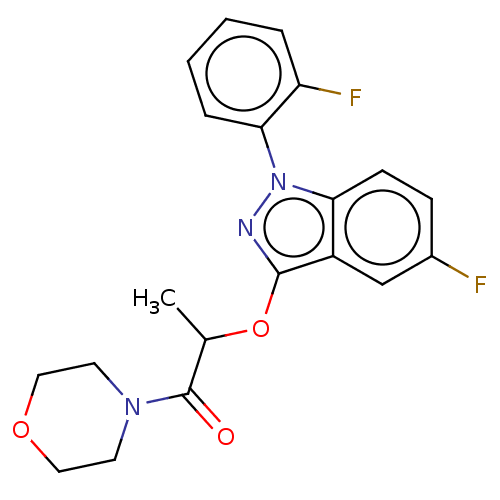 (CHEMBL3798242) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Curated by ChEMBL | Assay Description Inhibition of recombinant human Nav1.7 expressed in HEK293F cells preincubated for 40 mins followed by DiSBAC2 substrate addition measured after 90 m... | J Med Chem 59: 3373-91 (2016) Article DOI: 10.1021/acs.jmedchem.6b00063 BindingDB Entry DOI: 10.7270/Q2C250GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 10 subunit alpha (Homo sapiens (Human)) | BDBM250758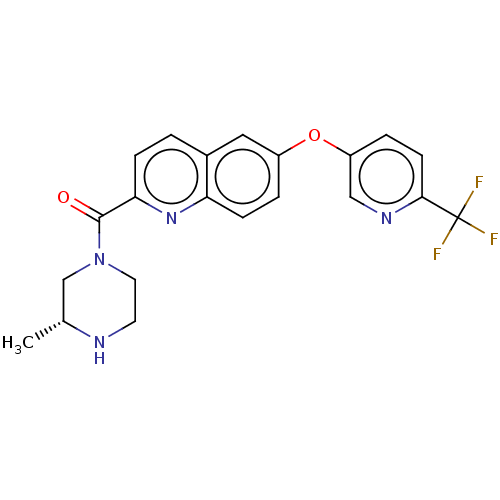 (US9452986, 62) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | 37 |
AbbVie Inc. US Patent | Assay Description Two days prior to the experiment, frozen HEK293 cells stably expressing recombinant human Nav1.8 (Essen, Ann Arbor, Mich.) were quickly thawed and pl... | US Patent US9452986 (2016) BindingDB Entry DOI: 10.7270/Q2MS3RQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50355055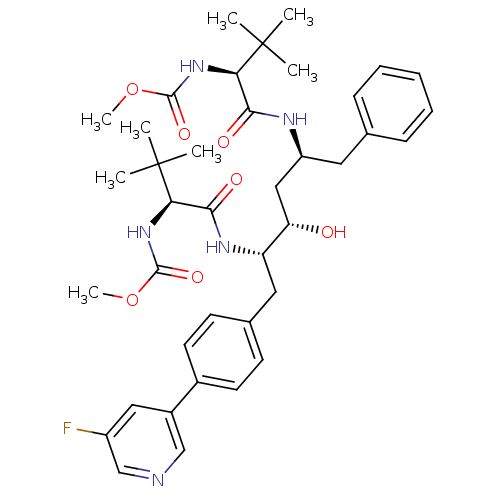 (CHEMBL1835487) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP3A4 | J Med Chem 54: 7094-104 (2011) Article DOI: 10.1021/jm201109t BindingDB Entry DOI: 10.7270/Q2D50NCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM251142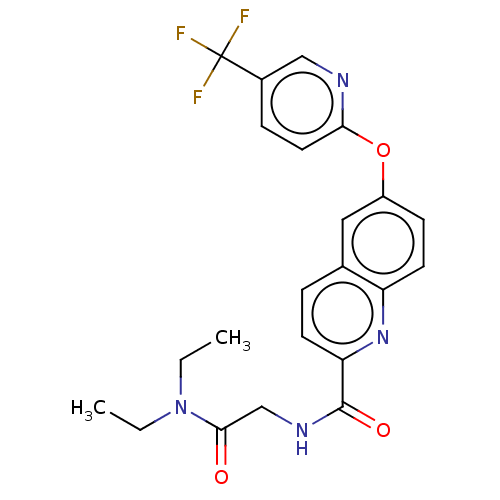 (US9452986, 446) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 166 | n/a | n/a | n/a | n/a | n/a | 37 |
AbbVie Inc. US Patent | Assay Description Two days prior to the experiment, frozen HEK293 cells stably expressing recombinant human Nav1.7 were quickly thawed and plated at 25,000 cells/well ... | US Patent US9452986 (2016) BindingDB Entry DOI: 10.7270/Q2MS3RQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM344702 (US9783527, Example 5 | isobutyl 3-[1-(2-fluorophen...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 166 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description Two days prior to the experiment, frozen HEK293 cells stably expressing recombinant human Nav1.7 were quickly thawed and plated at 25,000 cells/well ... | US Patent US9783527 (2017) BindingDB Entry DOI: 10.7270/Q2S184MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50500957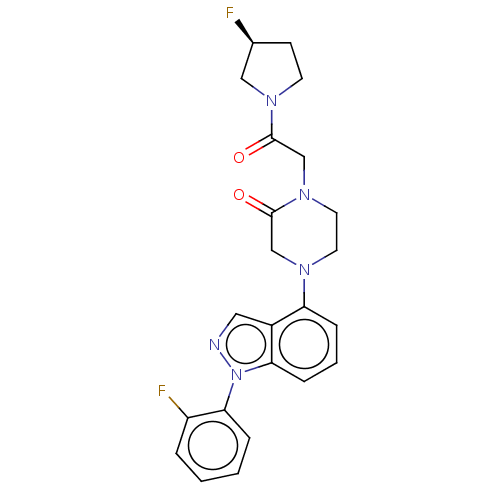 (CHEMBL3798039) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Curated by ChEMBL | Assay Description Inhibition of recombinant human Nav1.7 expressed in HEK293F cells preincubated for 40 mins followed by DiSBAC2 substrate addition measured after 90 m... | J Med Chem 59: 3373-91 (2016) Article DOI: 10.1021/acs.jmedchem.6b00063 BindingDB Entry DOI: 10.7270/Q2C250GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM250871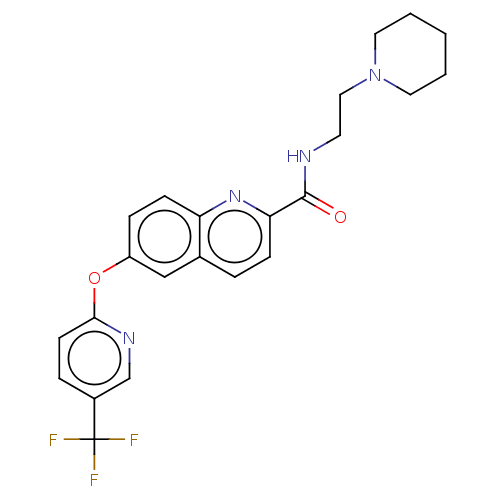 (US9452986, 175) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 206 | n/a | n/a | n/a | n/a | n/a | 37 |
AbbVie Inc. US Patent | Assay Description Two days prior to the experiment, frozen HEK293 cells stably expressing recombinant human Nav1.7 were quickly thawed and plated at 25,000 cells/well ... | US Patent US9452986 (2016) BindingDB Entry DOI: 10.7270/Q2MS3RQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM344943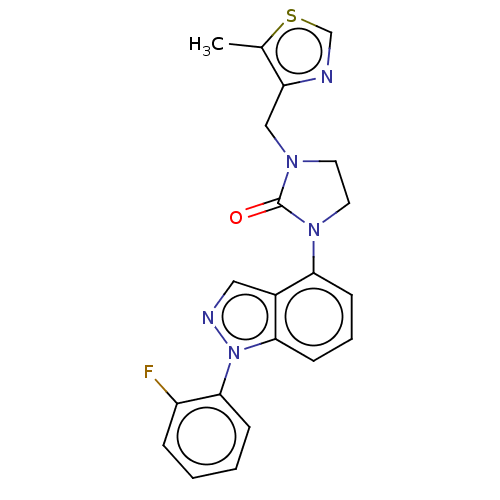 (1-[1-(2-fluorophenyl)-1H- | 1-[1-(2-fluorophenyl)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 207 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description Two days prior to the experiment, frozen HEK293 cells stably expressing recombinant human Nav1.7 were quickly thawed and plated at 25,000 cells/well ... | US Patent US9783527 (2017) BindingDB Entry DOI: 10.7270/Q2S184MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 10 subunit alpha (Homo sapiens (Human)) | BDBM251208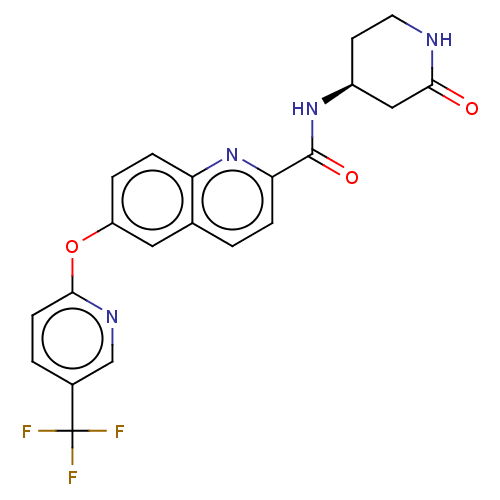 (US9452986, 512) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 213 | n/a | n/a | n/a | n/a | n/a | 37 |
AbbVie Inc. US Patent | Assay Description Two days prior to the experiment, frozen HEK293 cells stably expressing recombinant human Nav1.8 (Essen, Ann Arbor, Mich.) were quickly thawed and pl... | US Patent US9452986 (2016) BindingDB Entry DOI: 10.7270/Q2MS3RQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM251010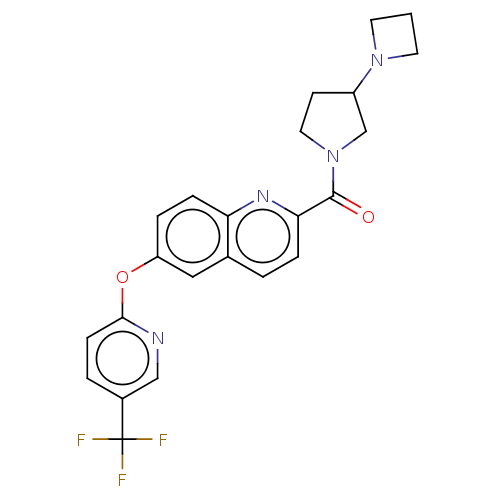 (US9452986, 314) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 218 | n/a | n/a | n/a | n/a | n/a | 37 |
AbbVie Inc. US Patent | Assay Description Two days prior to the experiment, frozen HEK293 cells stably expressing recombinant human Nav1.7 were quickly thawed and plated at 25,000 cells/well ... | US Patent US9452986 (2016) BindingDB Entry DOI: 10.7270/Q2MS3RQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM250796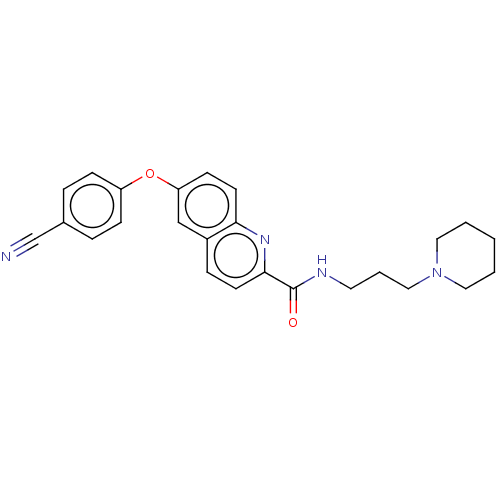 (US9452986, 100) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 221 | n/a | n/a | n/a | n/a | n/a | 37 |
AbbVie Inc. US Patent | Assay Description Two days prior to the experiment, frozen HEK293 cells stably expressing recombinant human Nav1.7 were quickly thawed and plated at 25,000 cells/well ... | US Patent US9452986 (2016) BindingDB Entry DOI: 10.7270/Q2MS3RQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1434 total ) | Next | Last >> |