 Found 212 hits with Last Name = 'delgado' and Initial = 'j'
Found 212 hits with Last Name = 'delgado' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM409005
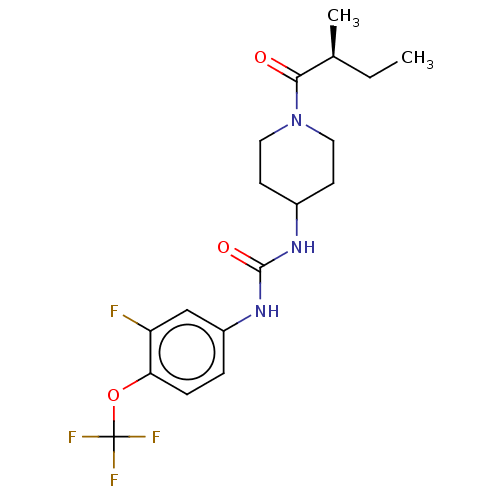
(US10377744, Compound No. 26 | US11123311, Compound...)Show SMILES CC[C@H](C)C(=O)N1CCC(CC1)NC(=O)Nc1ccc(OC(F)(F)F)c(F)c1 |r| Show InChI InChI=1S/C18H23F4N3O3/c1-3-11(2)16(26)25-8-6-12(7-9-25)23-17(27)24-13-4-5-15(14(19)10-13)28-18(20,21)22/h4-5,10-12H,3,6-9H2,1-2H3,(H2,23,24,27)/t11-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant sEH using CMNPC as substrate incubated for 5 mins by fluorescent based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01601
BindingDB Entry DOI: 10.7270/Q2H70KPP |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM50581725
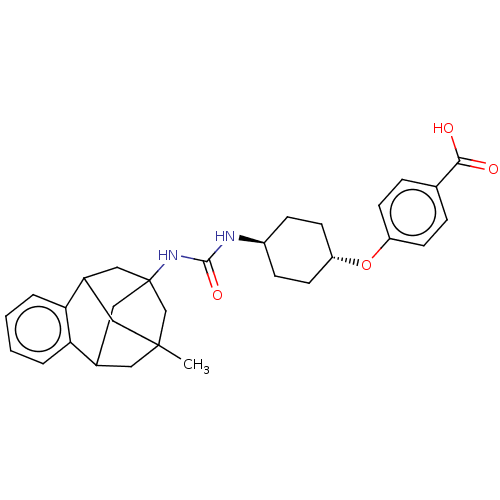
(CHEMBL5081862)Show SMILES CC12CC3CC(CC(C1)c1ccccc31)(C2)NC(=O)N[C@H]1CC[C@@H](CC1)Oc1ccc(cc1)C(O)=O |r,wU:23.30,wD:20.23,TLB:14:3:7.6.8:15,10:9:4:2.1.15,13:14:4:2.1.15,THB:8:7:4:2.1.15,8:1:4:14.7.6.9,9:7:3.4.2:15,0:1:4:14.7.6.9,(54.65,-28.03,;55.05,-29.51,;54.03,-30.74,;54.03,-32.3,;55.42,-32.86,;56.42,-31.6,;55.04,-31.94,;53.73,-31.46,;53.72,-29.99,;51.45,-31.64,;49.96,-32.05,;49.58,-33.55,;50.69,-34.63,;52.17,-34.21,;52.54,-32.72,;56.43,-30.09,;57.74,-32.36,;59.06,-31.6,;59.06,-30.07,;60.38,-32.36,;61.71,-31.59,;63.04,-32.37,;64.37,-31.61,;64.38,-30.07,;63.05,-29.29,;61.71,-30.06,;65.72,-29.3,;67.05,-30.08,;67.03,-31.61,;68.36,-32.39,;69.7,-31.63,;69.7,-30.08,;68.37,-29.31,;71.03,-32.4,;71.03,-33.94,;72.37,-31.64,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rat recombinant sEH using CMNPC as substrate incubated for 5 mins by fluorescent based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01601
BindingDB Entry DOI: 10.7270/Q2H70KPP |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM50581728
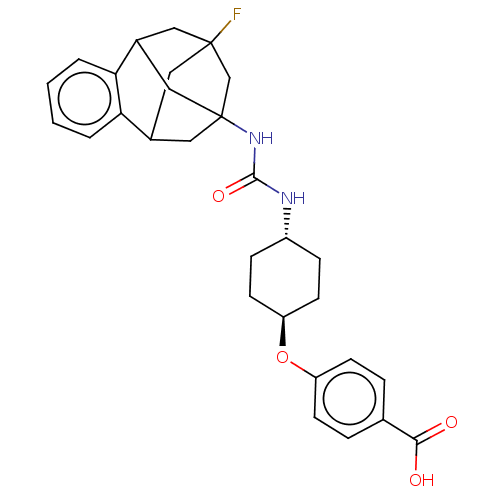
(CHEMBL5093683)Show SMILES OC(=O)c1ccc(O[C@H]2CC[C@@H](CC2)NC(=O)NC23CC4CC(F)(CC(C2)c2ccccc42)C3)cc1 |r,wU:8.7,wD:11.14,TLB:32:20:25.26.24:33,28:27:19:21.22.33,31:32:19:21.22.33,THB:24:25:19:21.22.33,24:22:19:32.25.26.27,27:25:20.19.21:33,23:22:19:32.25.26.27,(74.96,-46.38,;74.97,-44.84,;76.3,-44.08,;73.64,-44.07,;72.3,-44.83,;70.97,-44.06,;70.98,-42.52,;69.65,-41.74,;68.32,-42.51,;68.31,-44.05,;66.98,-44.81,;65.65,-44.03,;65.64,-42.5,;66.98,-41.73,;64.31,-44.8,;62.99,-44.04,;62.99,-42.52,;61.68,-44.8,;60.36,-44.04,;59.36,-45.3,;57.97,-44.75,;57.96,-43.18,;58.99,-41.95,;58.59,-40.47,;57.65,-42.43,;57.66,-43.9,;58.98,-44.38,;55.38,-44.08,;53.9,-44.49,;53.51,-45.99,;54.63,-47.08,;56.11,-46.65,;56.47,-45.16,;60.37,-42.53,;72.31,-41.75,;73.64,-42.52,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rat recombinant sEH using CMNPC as substrate incubated for 5 mins by fluorescent based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01601
BindingDB Entry DOI: 10.7270/Q2H70KPP |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50581727
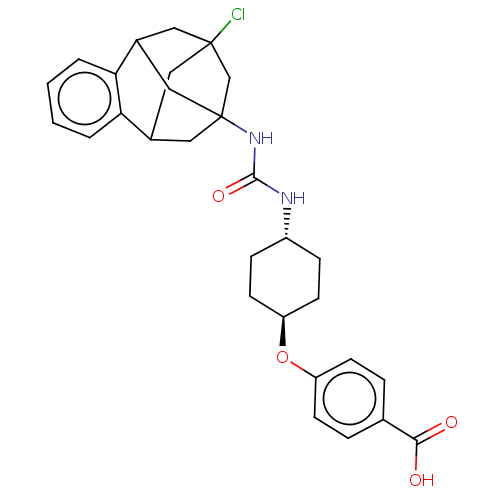
(CHEMBL5084744)Show SMILES OC(=O)c1ccc(O[C@H]2CC[C@@H](CC2)NC(=O)NC23CC4CC(Cl)(CC(C2)c2ccccc42)C3)cc1 |r,wU:8.7,wD:11.14,TLB:32:20:25.26.24:33,28:27:19:21.22.33,31:32:19:21.22.33,THB:24:25:19:21.22.33,24:22:19:32.25.26.27,27:25:20.19.21:33,23:22:19:32.25.26.27,(49.77,-46.61,;49.78,-45.07,;51.11,-44.3,;48.45,-44.3,;47.11,-45.06,;45.78,-44.28,;45.79,-42.75,;44.46,-41.97,;43.13,-42.73,;43.12,-44.28,;41.79,-45.04,;40.46,-44.26,;40.45,-42.72,;41.79,-41.96,;39.12,-45.03,;37.8,-44.27,;37.8,-42.74,;36.49,-45.03,;35.17,-44.27,;34.17,-45.53,;32.78,-44.97,;32.77,-43.4,;33.8,-42.18,;33.4,-40.69,;32.46,-42.65,;32.47,-44.12,;33.79,-44.61,;30.19,-44.31,;28.71,-44.72,;28.32,-46.22,;29.44,-47.3,;30.92,-46.88,;31.28,-45.39,;35.18,-42.76,;47.12,-41.98,;48.45,-42.75,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant sEH using CMNPC as substrate incubated for 5 mins by fluorescent based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01601
BindingDB Entry DOI: 10.7270/Q2H70KPP |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM50581727

(CHEMBL5084744)Show SMILES OC(=O)c1ccc(O[C@H]2CC[C@@H](CC2)NC(=O)NC23CC4CC(Cl)(CC(C2)c2ccccc42)C3)cc1 |r,wU:8.7,wD:11.14,TLB:32:20:25.26.24:33,28:27:19:21.22.33,31:32:19:21.22.33,THB:24:25:19:21.22.33,24:22:19:32.25.26.27,27:25:20.19.21:33,23:22:19:32.25.26.27,(49.77,-46.61,;49.78,-45.07,;51.11,-44.3,;48.45,-44.3,;47.11,-45.06,;45.78,-44.28,;45.79,-42.75,;44.46,-41.97,;43.13,-42.73,;43.12,-44.28,;41.79,-45.04,;40.46,-44.26,;40.45,-42.72,;41.79,-41.96,;39.12,-45.03,;37.8,-44.27,;37.8,-42.74,;36.49,-45.03,;35.17,-44.27,;34.17,-45.53,;32.78,-44.97,;32.77,-43.4,;33.8,-42.18,;33.4,-40.69,;32.46,-42.65,;32.47,-44.12,;33.79,-44.61,;30.19,-44.31,;28.71,-44.72,;28.32,-46.22,;29.44,-47.3,;30.92,-46.88,;31.28,-45.39,;35.18,-42.76,;47.12,-41.98,;48.45,-42.75,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rat recombinant sEH using CMNPC as substrate incubated for 5 mins by fluorescent based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01601
BindingDB Entry DOI: 10.7270/Q2H70KPP |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Mus musculus (Mouse)) | BDBM50581727

(CHEMBL5084744)Show SMILES OC(=O)c1ccc(O[C@H]2CC[C@@H](CC2)NC(=O)NC23CC4CC(Cl)(CC(C2)c2ccccc42)C3)cc1 |r,wU:8.7,wD:11.14,TLB:32:20:25.26.24:33,28:27:19:21.22.33,31:32:19:21.22.33,THB:24:25:19:21.22.33,24:22:19:32.25.26.27,27:25:20.19.21:33,23:22:19:32.25.26.27,(49.77,-46.61,;49.78,-45.07,;51.11,-44.3,;48.45,-44.3,;47.11,-45.06,;45.78,-44.28,;45.79,-42.75,;44.46,-41.97,;43.13,-42.73,;43.12,-44.28,;41.79,-45.04,;40.46,-44.26,;40.45,-42.72,;41.79,-41.96,;39.12,-45.03,;37.8,-44.27,;37.8,-42.74,;36.49,-45.03,;35.17,-44.27,;34.17,-45.53,;32.78,-44.97,;32.77,-43.4,;33.8,-42.18,;33.4,-40.69,;32.46,-42.65,;32.47,-44.12,;33.79,-44.61,;30.19,-44.31,;28.71,-44.72,;28.32,-46.22,;29.44,-47.3,;30.92,-46.88,;31.28,-45.39,;35.18,-42.76,;47.12,-41.98,;48.45,-42.75,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mouse recombinant sEH using CMNPC as substrate incubated for 5 mins by fluorescent based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01601
BindingDB Entry DOI: 10.7270/Q2H70KPP |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Mus musculus (Mouse)) | BDBM50581728

(CHEMBL5093683)Show SMILES OC(=O)c1ccc(O[C@H]2CC[C@@H](CC2)NC(=O)NC23CC4CC(F)(CC(C2)c2ccccc42)C3)cc1 |r,wU:8.7,wD:11.14,TLB:32:20:25.26.24:33,28:27:19:21.22.33,31:32:19:21.22.33,THB:24:25:19:21.22.33,24:22:19:32.25.26.27,27:25:20.19.21:33,23:22:19:32.25.26.27,(74.96,-46.38,;74.97,-44.84,;76.3,-44.08,;73.64,-44.07,;72.3,-44.83,;70.97,-44.06,;70.98,-42.52,;69.65,-41.74,;68.32,-42.51,;68.31,-44.05,;66.98,-44.81,;65.65,-44.03,;65.64,-42.5,;66.98,-41.73,;64.31,-44.8,;62.99,-44.04,;62.99,-42.52,;61.68,-44.8,;60.36,-44.04,;59.36,-45.3,;57.97,-44.75,;57.96,-43.18,;58.99,-41.95,;58.59,-40.47,;57.65,-42.43,;57.66,-43.9,;58.98,-44.38,;55.38,-44.08,;53.9,-44.49,;53.51,-45.99,;54.63,-47.08,;56.11,-46.65,;56.47,-45.16,;60.37,-42.53,;72.31,-41.75,;73.64,-42.52,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mouse recombinant sEH using CMNPC as substrate incubated for 5 mins by fluorescent based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01601
BindingDB Entry DOI: 10.7270/Q2H70KPP |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50581728

(CHEMBL5093683)Show SMILES OC(=O)c1ccc(O[C@H]2CC[C@@H](CC2)NC(=O)NC23CC4CC(F)(CC(C2)c2ccccc42)C3)cc1 |r,wU:8.7,wD:11.14,TLB:32:20:25.26.24:33,28:27:19:21.22.33,31:32:19:21.22.33,THB:24:25:19:21.22.33,24:22:19:32.25.26.27,27:25:20.19.21:33,23:22:19:32.25.26.27,(74.96,-46.38,;74.97,-44.84,;76.3,-44.08,;73.64,-44.07,;72.3,-44.83,;70.97,-44.06,;70.98,-42.52,;69.65,-41.74,;68.32,-42.51,;68.31,-44.05,;66.98,-44.81,;65.65,-44.03,;65.64,-42.5,;66.98,-41.73,;64.31,-44.8,;62.99,-44.04,;62.99,-42.52,;61.68,-44.8,;60.36,-44.04,;59.36,-45.3,;57.97,-44.75,;57.96,-43.18,;58.99,-41.95,;58.59,-40.47,;57.65,-42.43,;57.66,-43.9,;58.98,-44.38,;55.38,-44.08,;53.9,-44.49,;53.51,-45.99,;54.63,-47.08,;56.11,-46.65,;56.47,-45.16,;60.37,-42.53,;72.31,-41.75,;73.64,-42.52,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant sEH using CMNPC as substrate incubated for 5 mins by fluorescent based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01601
BindingDB Entry DOI: 10.7270/Q2H70KPP |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50217448
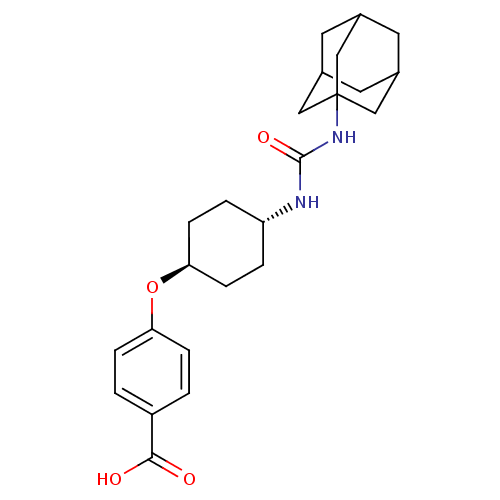
(CHEMBL242459 | US9029401, 1471 (t-AUCB) | trans-4-...)Show SMILES OC(=O)c1ccc(O[C@H]2CC[C@@H](CC2)NC(=O)NC23CC4CC(CC(C4)C2)C3)cc1 |wU:8.7,wD:11.14,TLB:17:18:21.20.25:23,THB:19:20:23:27.18.26,19:18:21.20.25:23,26:18:21:25.24.23,26:24:21:27.19.18,17:18:21:25.24.23,(19.63,-26.42,;18.81,-25.12,;19.53,-23.76,;17.27,-25.17,;16.54,-26.54,;15.01,-26.59,;14.19,-25.28,;12.65,-25.34,;11.93,-26.7,;12.75,-28.01,;12.03,-29.36,;10.48,-29.42,;9.67,-28.12,;10.39,-26.76,;9.77,-30.78,;8.23,-30.84,;7.41,-29.54,;7.51,-32.21,;5.97,-32.27,;4.96,-33.54,;3.55,-32.98,;2.06,-33.4,;3.25,-32.13,;3.24,-30.64,;4.59,-30.16,;3.55,-31.39,;5.99,-30.74,;4.58,-32.61,;14.91,-23.93,;16.44,-23.87,)| Show InChI InChI=1S/C24H32N2O4/c27-22(28)18-1-5-20(6-2-18)30-21-7-3-19(4-8-21)25-23(29)26-24-12-15-9-16(13-24)11-17(10-15)14-24/h1-2,5-6,15-17,19,21H,3-4,7-14H2,(H,27,28)(H2,25,26,29)/t15?,16?,17?,19-,21-,24? | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant sEH using CMNPC as substrate incubated for 5 mins by fluorescent based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01601
BindingDB Entry DOI: 10.7270/Q2H70KPP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50581725

(CHEMBL5081862)Show SMILES CC12CC3CC(CC(C1)c1ccccc31)(C2)NC(=O)N[C@H]1CC[C@@H](CC1)Oc1ccc(cc1)C(O)=O |r,wU:23.30,wD:20.23,TLB:14:3:7.6.8:15,10:9:4:2.1.15,13:14:4:2.1.15,THB:8:7:4:2.1.15,8:1:4:14.7.6.9,9:7:3.4.2:15,0:1:4:14.7.6.9,(54.65,-28.03,;55.05,-29.51,;54.03,-30.74,;54.03,-32.3,;55.42,-32.86,;56.42,-31.6,;55.04,-31.94,;53.73,-31.46,;53.72,-29.99,;51.45,-31.64,;49.96,-32.05,;49.58,-33.55,;50.69,-34.63,;52.17,-34.21,;52.54,-32.72,;56.43,-30.09,;57.74,-32.36,;59.06,-31.6,;59.06,-30.07,;60.38,-32.36,;61.71,-31.59,;63.04,-32.37,;64.37,-31.61,;64.38,-30.07,;63.05,-29.29,;61.71,-30.06,;65.72,-29.3,;67.05,-30.08,;67.03,-31.61,;68.36,-32.39,;69.7,-31.63,;69.7,-30.08,;68.37,-29.31,;71.03,-32.4,;71.03,-33.94,;72.37,-31.64,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant sEH using CMNPC as substrate incubated for 5 mins by fluorescent based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01601
BindingDB Entry DOI: 10.7270/Q2H70KPP |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50581720
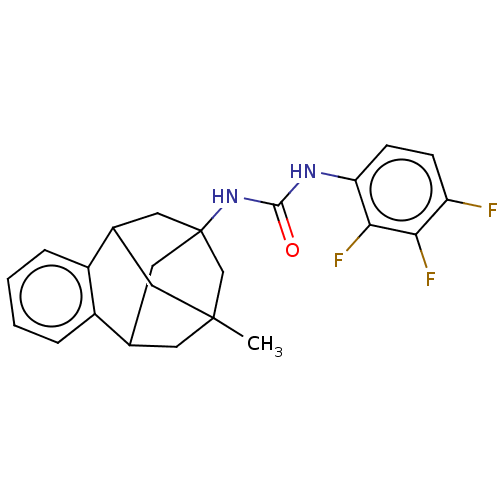
(CHEMBL5080024)Show SMILES CC12CC3CC(CC(C1)c1ccccc31)(C2)NC(=O)Nc1ccc(F)c(F)c1F |TLB:14:3:7.6.8:15,10:9:4:2.1.15,13:14:4:2.1.15,THB:8:7:4:2.1.15,8:1:4:14.7.6.9,9:7:3.4.2:15,0:1:4:14.7.6.9| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant sEH using CMNPC as substrate incubated for 5 mins by fluorescent based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01601
BindingDB Entry DOI: 10.7270/Q2H70KPP |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Mus musculus (Mouse)) | BDBM50217448

(CHEMBL242459 | US9029401, 1471 (t-AUCB) | trans-4-...)Show SMILES OC(=O)c1ccc(O[C@H]2CC[C@@H](CC2)NC(=O)NC23CC4CC(CC(C4)C2)C3)cc1 |wU:8.7,wD:11.14,TLB:17:18:21.20.25:23,THB:19:20:23:27.18.26,19:18:21.20.25:23,26:18:21:25.24.23,26:24:21:27.19.18,17:18:21:25.24.23,(19.63,-26.42,;18.81,-25.12,;19.53,-23.76,;17.27,-25.17,;16.54,-26.54,;15.01,-26.59,;14.19,-25.28,;12.65,-25.34,;11.93,-26.7,;12.75,-28.01,;12.03,-29.36,;10.48,-29.42,;9.67,-28.12,;10.39,-26.76,;9.77,-30.78,;8.23,-30.84,;7.41,-29.54,;7.51,-32.21,;5.97,-32.27,;4.96,-33.54,;3.55,-32.98,;2.06,-33.4,;3.25,-32.13,;3.24,-30.64,;4.59,-30.16,;3.55,-31.39,;5.99,-30.74,;4.58,-32.61,;14.91,-23.93,;16.44,-23.87,)| Show InChI InChI=1S/C24H32N2O4/c27-22(28)18-1-5-20(6-2-18)30-21-7-3-19(4-8-21)25-23(29)26-24-12-15-9-16(13-24)11-17(10-15)14-24/h1-2,5-6,15-17,19,21H,3-4,7-14H2,(H,27,28)(H2,25,26,29)/t15?,16?,17?,19-,21-,24? | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mouse recombinant sEH using CMNPC as substrate incubated for 5 mins by fluorescent based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01601
BindingDB Entry DOI: 10.7270/Q2H70KPP |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50581722
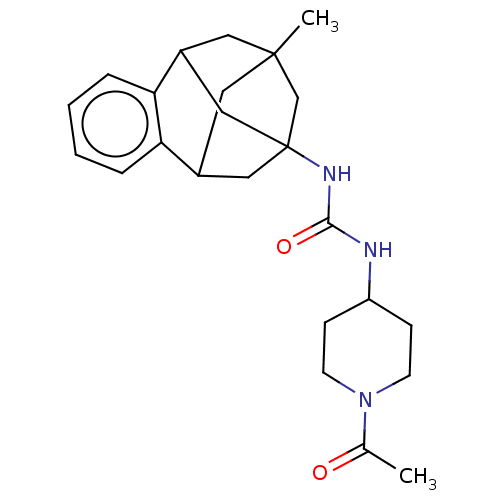
(CHEMBL5081815)Show SMILES CC(=O)N1CCC(CC1)NC(=O)NC12CC3CC(C)(CC(C1)c1ccccc31)C2 |TLB:27:15:20.21.19:28,23:22:14:16.17.28,26:27:14:16.17.28,THB:19:20:14:16.17.28,19:17:14:27.20.21.22,22:20:15.14.16:28,18:17:14:27.20.21.22| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant sEH using CMNPC as substrate incubated for 5 mins by fluorescent based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01601
BindingDB Entry DOI: 10.7270/Q2H70KPP |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50351247
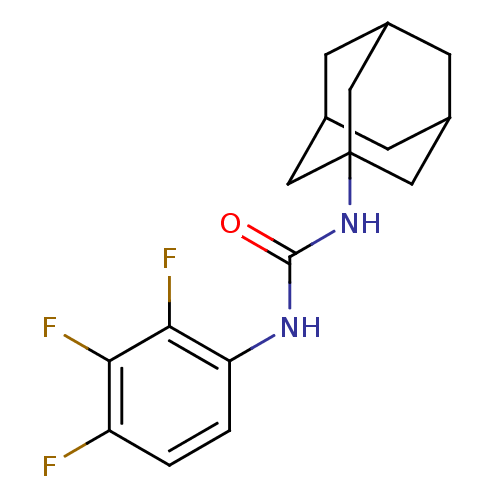
(CHEMBL1818385)Show SMILES Fc1ccc(NC(=O)NC23CC4CC(CC(C4)C2)C3)c(F)c1F |TLB:8:9:12:16.14.15,THB:14:13:10:16.15.17,14:15:12.13.18:10,17:15:12:18.9.10,17:9:12:16.14.15| Show InChI InChI=1S/C17H19F3N2O/c18-12-1-2-13(15(20)14(12)19)21-16(23)22-17-6-9-3-10(7-17)5-11(4-9)8-17/h1-2,9-11H,3-8H2,(H2,21,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant sEH using CMNPC as substrate incubated for 5 mins by fluorescent based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01601
BindingDB Entry DOI: 10.7270/Q2H70KPP |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50191854
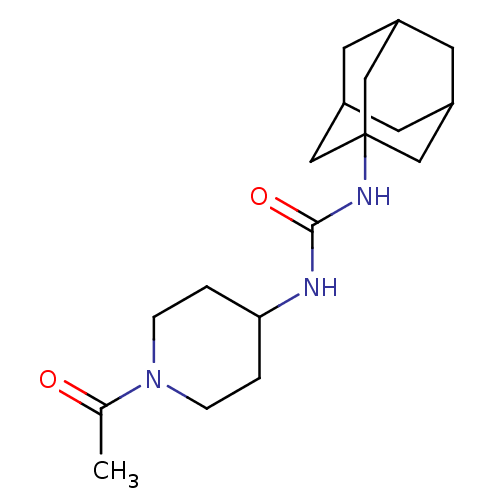
(CHEMBL436774 | N-(1-acetyl-piperidin-4-yl)-N'-(ada...)Show SMILES CC(=O)N1CCC(CC1)NC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:12:13:16.15.20:18,THB:14:15:18:22.13.21,14:13:16.15.20:18,21:13:16:20.19.18,21:19:16:22.14.13,12:13:16:20.19.18| Show InChI InChI=1S/C18H29N3O2/c1-12(22)21-4-2-16(3-5-21)19-17(23)20-18-9-13-6-14(10-18)8-15(7-13)11-18/h13-16H,2-11H2,1H3,(H2,19,20,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant sEH using CMNPC as substrate incubated for 5 mins by fluorescent based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01601
BindingDB Entry DOI: 10.7270/Q2H70KPP |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM50217448

(CHEMBL242459 | US9029401, 1471 (t-AUCB) | trans-4-...)Show SMILES OC(=O)c1ccc(O[C@H]2CC[C@@H](CC2)NC(=O)NC23CC4CC(CC(C4)C2)C3)cc1 |wU:8.7,wD:11.14,TLB:17:18:21.20.25:23,THB:19:20:23:27.18.26,19:18:21.20.25:23,26:18:21:25.24.23,26:24:21:27.19.18,17:18:21:25.24.23,(19.63,-26.42,;18.81,-25.12,;19.53,-23.76,;17.27,-25.17,;16.54,-26.54,;15.01,-26.59,;14.19,-25.28,;12.65,-25.34,;11.93,-26.7,;12.75,-28.01,;12.03,-29.36,;10.48,-29.42,;9.67,-28.12,;10.39,-26.76,;9.77,-30.78,;8.23,-30.84,;7.41,-29.54,;7.51,-32.21,;5.97,-32.27,;4.96,-33.54,;3.55,-32.98,;2.06,-33.4,;3.25,-32.13,;3.24,-30.64,;4.59,-30.16,;3.55,-31.39,;5.99,-30.74,;4.58,-32.61,;14.91,-23.93,;16.44,-23.87,)| Show InChI InChI=1S/C24H32N2O4/c27-22(28)18-1-5-20(6-2-18)30-21-7-3-19(4-8-21)25-23(29)26-24-12-15-9-16(13-24)11-17(10-15)14-24/h1-2,5-6,15-17,19,21H,3-4,7-14H2,(H,27,28)(H2,25,26,29)/t15?,16?,17?,19-,21-,24? | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rat recombinant sEH using CMNPC as substrate incubated for 5 mins by fluorescent based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01601
BindingDB Entry DOI: 10.7270/Q2H70KPP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ZAP-70
(Homo sapiens (Human)) | BDBM50083639
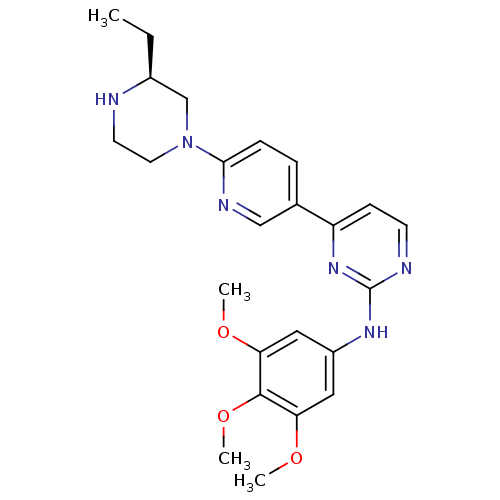
(CHEMBL112346 | {4-[6-((S)-3-Ethyl-piperazin-1-yl)-...)Show SMILES CC[C@H]1CN(CCN1)c1ccc(cn1)-c1ccnc(Nc2cc(OC)c(OC)c(OC)c2)n1 Show InChI InChI=1S/C24H30N6O3/c1-5-17-15-30(11-10-25-17)22-7-6-16(14-27-22)19-8-9-26-24(29-19)28-18-12-20(31-2)23(33-4)21(13-18)32-3/h6-9,12-14,17,25H,5,10-11,15H2,1-4H3,(H,26,28,29)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech Therapeutics Limited
Curated by ChEMBL
| Assay Description
Inhibition of Zeta-chain (TCR) associated protein kinase 70 kDa phosphorylation of polyGly-Tyr. |
Bioorg Med Chem Lett 9: 3351-6 (2000)
BindingDB Entry DOI: 10.7270/Q29Z943G |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50295759
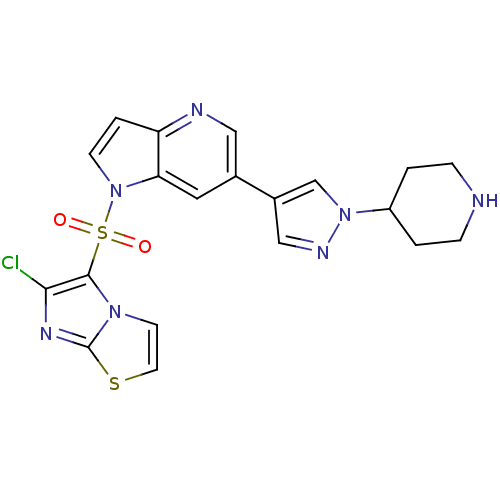
(1-(6-chloroimidazo[2,1-b]thiazol-5-ylsulfonyl)-6-(...)Show SMILES Clc1nc2sccn2c1S(=O)(=O)n1ccc2ncc(cc12)-c1cnn(c1)C1CCNCC1 Show InChI InChI=1S/C20H18ClN7O2S2/c21-18-19(26-7-8-31-20(26)25-18)32(29,30)28-6-3-16-17(28)9-13(10-23-16)14-11-24-27(12-14)15-1-4-22-5-2-15/h3,6-12,15,22H,1-2,4-5H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Celltech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-tagged c-Met by TR-FRET assay |
Bioorg Med Chem Lett 19: 2780-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.110
BindingDB Entry DOI: 10.7270/Q2BV7GN9 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50295752
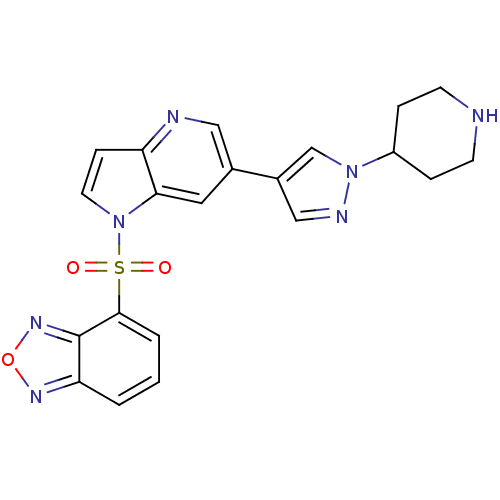
(4-(6-(1-(piperidin-4-yl)-1H-pyrazol-4-yl)-1H-pyrro...)Show SMILES O=S(=O)(c1cccc2nonc12)n1ccc2ncc(cc12)-c1cnn(c1)C1CCNCC1 Show InChI InChI=1S/C21H19N7O3S/c29-32(30,20-3-1-2-18-21(20)26-31-25-18)28-9-6-17-19(28)10-14(11-23-17)15-12-24-27(13-15)16-4-7-22-8-5-16/h1-3,6,9-13,16,22H,4-5,7-8H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Celltech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-tagged c-Met by TR-FRET assay |
Bioorg Med Chem Lett 19: 2780-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.110
BindingDB Entry DOI: 10.7270/Q2BV7GN9 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50560744
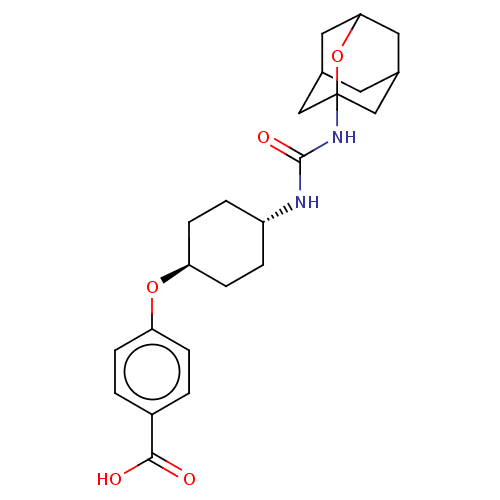
(CHEMBL4779073)Show SMILES OC(=O)c1ccc(O[C@H]2CC[C@@H](CC2)NC(=O)NC23CC4CC(CC(C4)O2)C3)cc1 |r,wU:8.7,wD:11.14,TLB:17:18:21.20.25:23,THB:19:20:23:27.18.26,19:18:21.20.25:23,26:18:21:25.24.23,26:24:21:27.19.18,17:18:21:25.24.23,(20.44,-38.18,;19.62,-36.87,;20.33,-35.51,;18.08,-36.93,;17.36,-38.29,;15.82,-38.34,;15,-37.03,;13.46,-37.09,;12.74,-38.45,;13.56,-39.76,;12.84,-41.11,;11.3,-41.16,;10.47,-39.87,;11.2,-38.51,;10.58,-42.52,;9.04,-42.59,;8.23,-41.29,;8.33,-43.95,;6.79,-44.01,;5.77,-45.29,;4.37,-44.73,;2.87,-45.14,;4.07,-43.87,;4.06,-42.38,;5.41,-41.91,;4.36,-43.14,;6.8,-42.48,;5.39,-44.36,;15.71,-35.68,;17.25,-35.62,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant sEH using CMNPC as substrate incubated for 5 mins by fluorescent based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01601
BindingDB Entry DOI: 10.7270/Q2H70KPP |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Mus musculus (Mouse)) | BDBM50581725

(CHEMBL5081862)Show SMILES CC12CC3CC(CC(C1)c1ccccc31)(C2)NC(=O)N[C@H]1CC[C@@H](CC1)Oc1ccc(cc1)C(O)=O |r,wU:23.30,wD:20.23,TLB:14:3:7.6.8:15,10:9:4:2.1.15,13:14:4:2.1.15,THB:8:7:4:2.1.15,8:1:4:14.7.6.9,9:7:3.4.2:15,0:1:4:14.7.6.9,(54.65,-28.03,;55.05,-29.51,;54.03,-30.74,;54.03,-32.3,;55.42,-32.86,;56.42,-31.6,;55.04,-31.94,;53.73,-31.46,;53.72,-29.99,;51.45,-31.64,;49.96,-32.05,;49.58,-33.55,;50.69,-34.63,;52.17,-34.21,;52.54,-32.72,;56.43,-30.09,;57.74,-32.36,;59.06,-31.6,;59.06,-30.07,;60.38,-32.36,;61.71,-31.59,;63.04,-32.37,;64.37,-31.61,;64.38,-30.07,;63.05,-29.29,;61.71,-30.06,;65.72,-29.3,;67.05,-30.08,;67.03,-31.61,;68.36,-32.39,;69.7,-31.63,;69.7,-30.08,;68.37,-29.31,;71.03,-32.4,;71.03,-33.94,;72.37,-31.64,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mouse recombinant sEH using CMNPC as substrate incubated for 5 mins by fluorescent based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01601
BindingDB Entry DOI: 10.7270/Q2H70KPP |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50295760
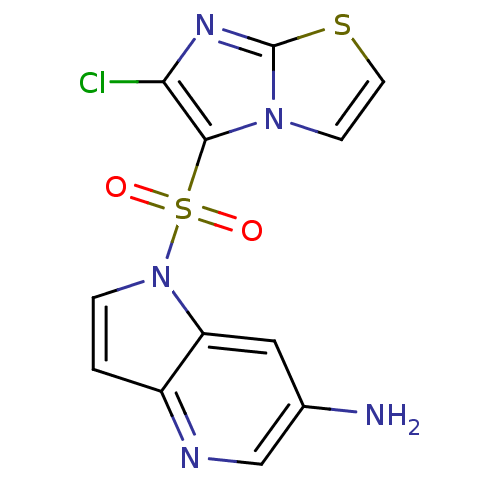
(1-(6-chloroimidazo[2,1-b]thiazol-5-ylsulfonyl)-1H-...)Show InChI InChI=1S/C12H8ClN5O2S2/c13-10-11(17-3-4-21-12(17)16-10)22(19,20)18-2-1-8-9(18)5-7(14)6-15-8/h1-6H,14H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Celltech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-tagged c-Met by TR-FRET assay |
Bioorg Med Chem Lett 19: 2780-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.110
BindingDB Entry DOI: 10.7270/Q2BV7GN9 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50295754
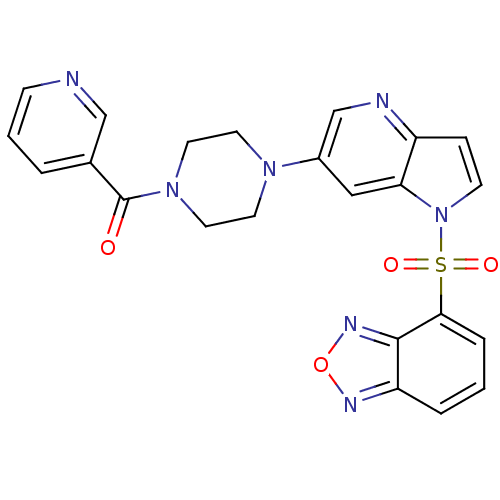
((4-(1-(benzo[c][1,2,5]oxadiazol-4-ylsulfonyl)-1H-p...)Show SMILES O=C(N1CCN(CC1)c1cnc2ccn(c2c1)S(=O)(=O)c1cccc2nonc12)c1cccnc1 Show InChI InChI=1S/C23H19N7O4S/c31-23(16-3-2-7-24-14-16)29-11-9-28(10-12-29)17-13-20-18(25-15-17)6-8-30(20)35(32,33)21-5-1-4-19-22(21)27-34-26-19/h1-8,13-15H,9-12H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Celltech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-tagged c-Met by TR-FRET assay |
Bioorg Med Chem Lett 19: 2780-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.110
BindingDB Entry DOI: 10.7270/Q2BV7GN9 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ZAP-70
(Homo sapiens (Human)) | BDBM50083638
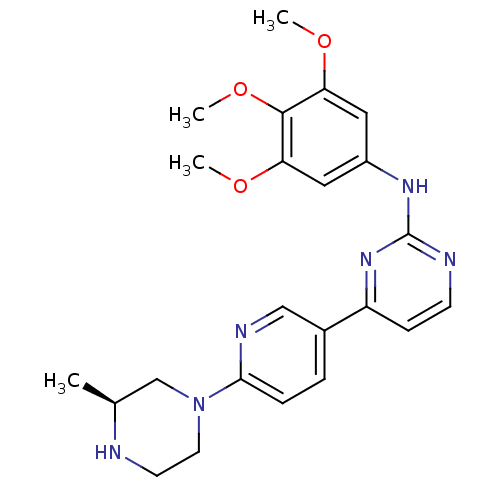
((S)-4-(6-(3-methylpiperazin-1-yl)pyridin-3-yl)-N-(...)Show SMILES COc1cc(Nc2nccc(n2)-c2ccc(nc2)N2CCN[C@@H](C)C2)cc(OC)c1OC |r| Show InChI InChI=1S/C23H28N6O3/c1-15-14-29(10-9-24-15)21-6-5-16(13-26-21)18-7-8-25-23(28-18)27-17-11-19(30-2)22(32-4)20(12-17)31-3/h5-8,11-13,15,24H,9-10,14H2,1-4H3,(H,25,27,28)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech Therapeutics Limited
Curated by ChEMBL
| Assay Description
Inhibition of Zeta-chain (TCR) associated protein kinase 70 kDa phosphorylation of polyGly-Tyr. |
Bioorg Med Chem Lett 9: 3351-6 (2000)
BindingDB Entry DOI: 10.7270/Q29Z943G |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50295755
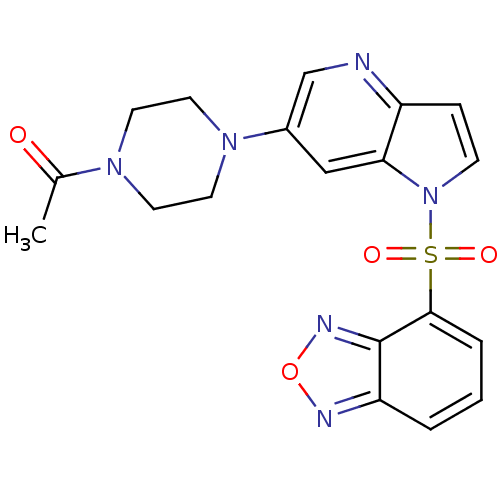
(1-(4-(1-(benzo[c][1,2,5]oxadiazol-4-ylsulfonyl)-1H...)Show SMILES CC(=O)N1CCN(CC1)c1cnc2ccn(c2c1)S(=O)(=O)c1cccc2nonc12 Show InChI InChI=1S/C19H18N6O4S/c1-13(26)23-7-9-24(10-8-23)14-11-17-15(20-12-14)5-6-25(17)30(27,28)18-4-2-3-16-19(18)22-29-21-16/h2-6,11-12H,7-10H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Celltech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-tagged c-Met by TR-FRET assay |
Bioorg Med Chem Lett 19: 2780-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.110
BindingDB Entry DOI: 10.7270/Q2BV7GN9 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50295756
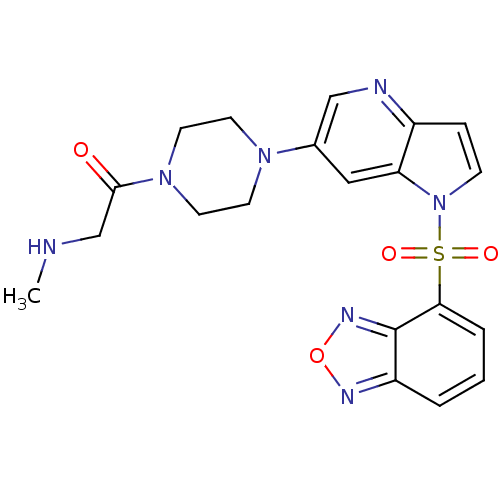
(1-(4-(1-(benzo[c][1,2,5]oxadiazol-4-ylsulfonyl)-1H...)Show SMILES CNCC(=O)N1CCN(CC1)c1cnc2ccn(c2c1)S(=O)(=O)c1cccc2nonc12 Show InChI InChI=1S/C20H21N7O4S/c1-21-13-19(28)26-9-7-25(8-10-26)14-11-17-15(22-12-14)5-6-27(17)32(29,30)18-4-2-3-16-20(18)24-31-23-16/h2-6,11-12,21H,7-10,13H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Celltech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-tagged c-Met by TR-FRET assay |
Bioorg Med Chem Lett 19: 2780-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.110
BindingDB Entry DOI: 10.7270/Q2BV7GN9 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50295750
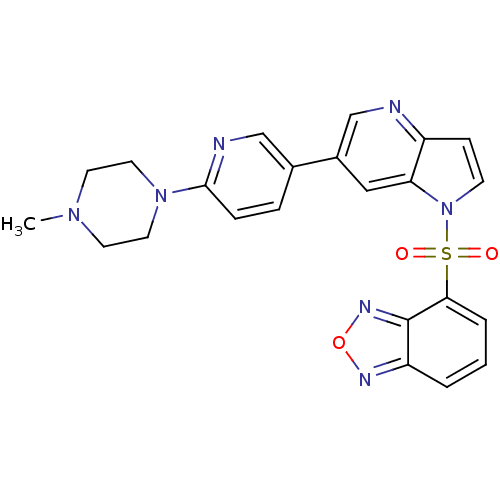
(4-(6-(6-(4-methylpiperazin-1-yl)pyridin-3-yl)-1H-p...)Show SMILES CN1CCN(CC1)c1ccc(cn1)-c1cnc2ccn(c2c1)S(=O)(=O)c1cccc2nonc12 Show InChI InChI=1S/C23H21N7O3S/c1-28-9-11-29(12-10-28)22-6-5-16(14-25-22)17-13-20-18(24-15-17)7-8-30(20)34(31,32)21-4-2-3-19-23(21)27-33-26-19/h2-8,13-15H,9-12H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Celltech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-tagged c-Met by TR-FRET assay |
Bioorg Med Chem Lett 19: 2780-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.110
BindingDB Entry DOI: 10.7270/Q2BV7GN9 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50295761
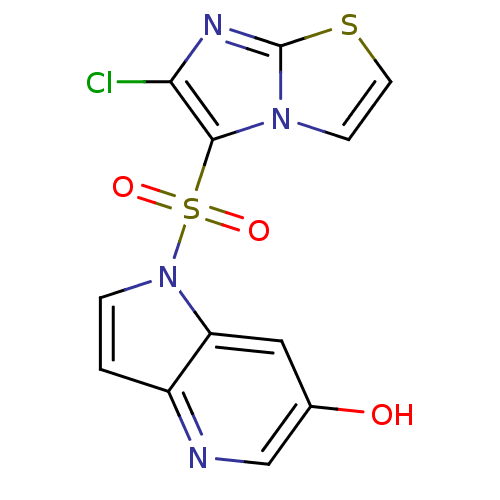
(1-(6-chloroimidazo[2,1-b]thiazol-5-ylsulfonyl)-1H-...)Show InChI InChI=1S/C12H7ClN4O3S2/c13-10-11(16-3-4-21-12(16)15-10)22(19,20)17-2-1-8-9(17)5-7(18)6-14-8/h1-6,18H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Celltech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-tagged c-Met by TR-FRET assay |
Bioorg Med Chem Lett 19: 2780-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.110
BindingDB Entry DOI: 10.7270/Q2BV7GN9 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50295753
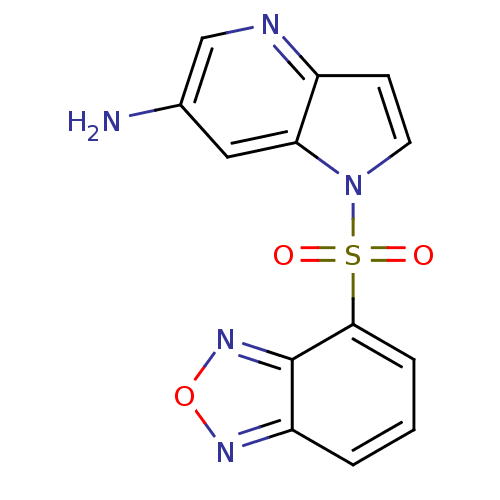
(1-(benzo[c][1,2,5]oxadiazol-4-ylsulfonyl)-1H-pyrro...)Show InChI InChI=1S/C13H9N5O3S/c14-8-6-11-9(15-7-8)4-5-18(11)22(19,20)12-3-1-2-10-13(12)17-21-16-10/h1-7H,14H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Celltech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-tagged c-Met by TR-FRET assay |
Bioorg Med Chem Lett 19: 2780-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.110
BindingDB Entry DOI: 10.7270/Q2BV7GN9 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50265989
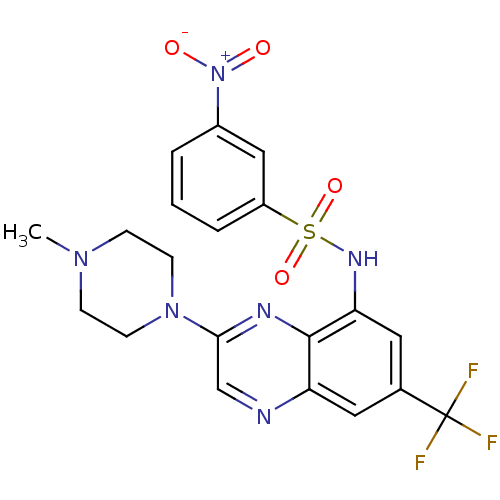
(CHEMBL462707 | N-(3-(4-methylpiperazin-1-yl)-7-(tr...)Show SMILES CN1CCN(CC1)c1cnc2cc(cc(NS(=O)(=O)c3cccc(c3)[N+]([O-])=O)c2n1)C(F)(F)F Show InChI InChI=1S/C20H19F3N6O4S/c1-27-5-7-28(8-6-27)18-12-24-16-9-13(20(21,22)23)10-17(19(16)25-18)26-34(32,33)15-4-2-3-14(11-15)29(30)31/h2-4,9-12,26H,5-8H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Celltech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant his-tagged c-Met by TR-FRET assay |
Bioorg Med Chem Lett 19: 397-400 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.062
BindingDB Entry DOI: 10.7270/Q2ZC82QC |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50295764
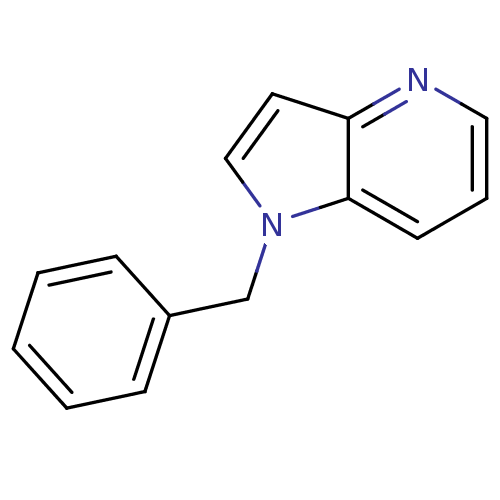
(1-benzyl-1H-pyrrolo[3,2-b]pyridine | CHEMBL561256)Show InChI InChI=1S/C14H12N2/c1-2-5-12(6-3-1)11-16-10-8-13-14(16)7-4-9-15-13/h1-10H,11H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Celltech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-tagged c-Met by TR-FRET assay |
Bioorg Med Chem Lett 19: 2780-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.110
BindingDB Entry DOI: 10.7270/Q2BV7GN9 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50295767
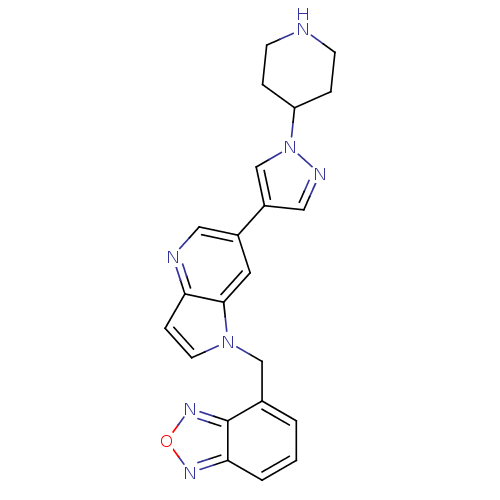
(4-((6-(1-(piperidin-4-yl)-1H-pyrazol-4-yl)-1H-pyrr...)Show SMILES C(c1cccc2nonc12)n1ccc2ncc(cc12)-c1cnn(c1)C1CCNCC1 Show InChI InChI=1S/C22H21N7O/c1-2-15(22-20(3-1)26-30-27-22)13-28-9-6-19-21(28)10-16(11-24-19)17-12-25-29(14-17)18-4-7-23-8-5-18/h1-3,6,9-12,14,18,23H,4-5,7-8,13H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Celltech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-tagged c-Met by TR-FRET assay |
Bioorg Med Chem Lett 19: 2780-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.110
BindingDB Entry DOI: 10.7270/Q2BV7GN9 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50295763
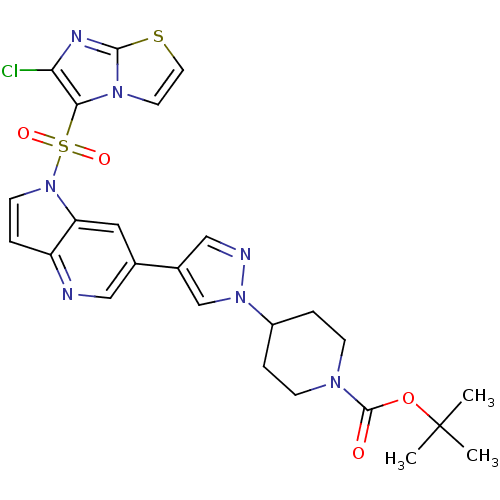
(CHEMBL564440 | tert-butyl 4-(4-(1-(6-chloroimidazo...)Show SMILES CC(C)(C)OC(=O)N1CCC(CC1)n1cc(cn1)-c1cnc2ccn(c2c1)S(=O)(=O)c1c(Cl)nc2sccn12 Show InChI InChI=1S/C25H26ClN7O4S2/c1-25(2,3)37-24(34)30-7-4-18(5-8-30)32-15-17(14-28-32)16-12-20-19(27-13-16)6-9-33(20)39(35,36)22-21(26)29-23-31(22)10-11-38-23/h6,9-15,18H,4-5,7-8H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Celltech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-tagged c-Met by TR-FRET assay |
Bioorg Med Chem Lett 19: 2780-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.110
BindingDB Entry DOI: 10.7270/Q2BV7GN9 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50581721
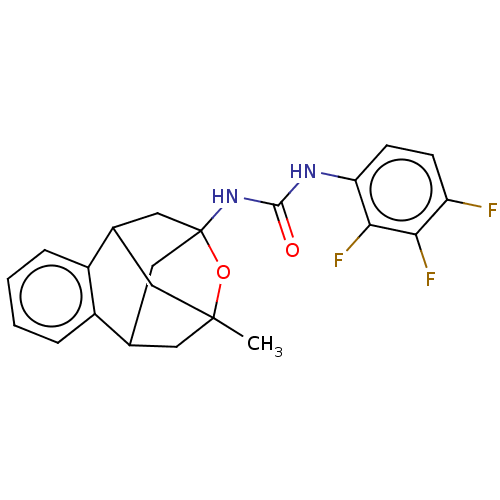
(CHEMBL5083623)Show SMILES CC12CC3CC(CC(C1)c1ccccc31)(NC(=O)Nc1ccc(F)c(F)c1F)O2 |TLB:14:3:7.6.8:28,10:9:4:2.1.28,13:14:4:2.1.28,THB:8:7:4:2.1.28,8:1:4:14.7.6.9,9:7:3.4.2:28,0:1:4:14.7.6.9| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant sEH using CMNPC as substrate incubated for 5 mins by fluorescent based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01601
BindingDB Entry DOI: 10.7270/Q2H70KPP |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50295735
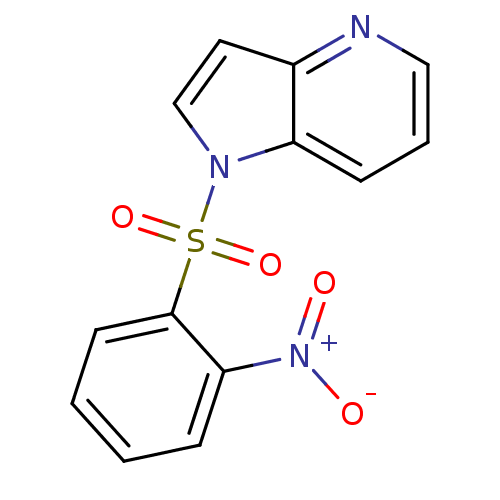
(1-(2-nitrophenylsulfonyl)-1H-pyrrolo[3,2-b]pyridin...)Show InChI InChI=1S/C13H9N3O4S/c17-16(18)12-4-1-2-6-13(12)21(19,20)15-9-7-10-11(15)5-3-8-14-10/h1-9H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Celltech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-tagged c-Met by TR-FRET assay |
Bioorg Med Chem Lett 19: 2780-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.110
BindingDB Entry DOI: 10.7270/Q2BV7GN9 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50295747
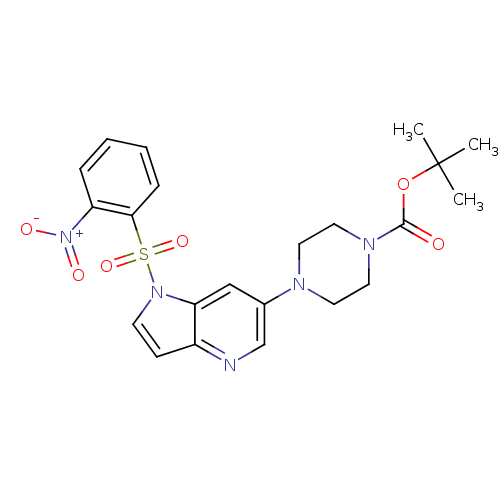
(CHEMBL556627 | tert-butyl 4-(1-(2-nitrophenylsulfo...)Show SMILES CC(C)(C)OC(=O)N1CCN(CC1)c1cnc2ccn(c2c1)S(=O)(=O)c1ccccc1[N+]([O-])=O Show InChI InChI=1S/C22H25N5O6S/c1-22(2,3)33-21(28)25-12-10-24(11-13-25)16-14-19-17(23-15-16)8-9-26(19)34(31,32)20-7-5-4-6-18(20)27(29)30/h4-9,14-15H,10-13H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Celltech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-tagged c-Met by TR-FRET assay |
Bioorg Med Chem Lett 19: 2780-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.110
BindingDB Entry DOI: 10.7270/Q2BV7GN9 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50295748
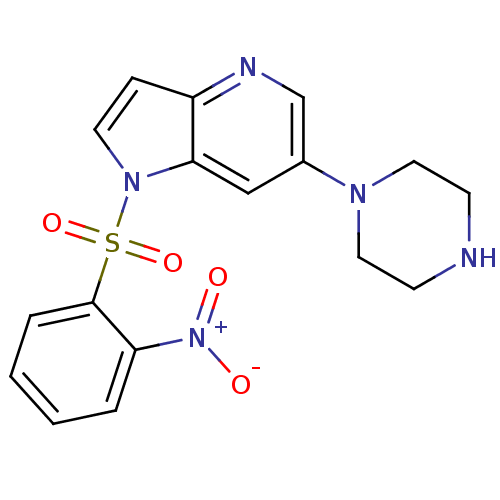
(1-(2-nitrophenylsulfonyl)-6-(piperazin-1-yl)-1H-py...)Show SMILES [O-][N+](=O)c1ccccc1S(=O)(=O)n1ccc2ncc(cc12)N1CCNCC1 Show InChI InChI=1S/C17H17N5O4S/c23-22(24)15-3-1-2-4-17(15)27(25,26)21-8-5-14-16(21)11-13(12-19-14)20-9-6-18-7-10-20/h1-5,8,11-12,18H,6-7,9-10H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Celltech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-tagged c-Met by TR-FRET assay |
Bioorg Med Chem Lett 19: 2780-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.110
BindingDB Entry DOI: 10.7270/Q2BV7GN9 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50560745
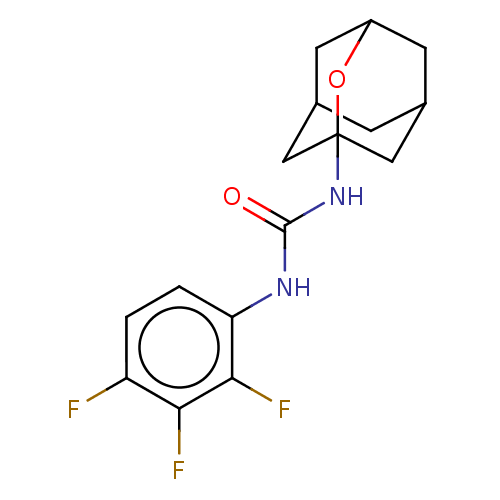
(CHEMBL4797443)Show SMILES Fc1ccc(NC(=O)NC23CC4CC(CC(C4)O2)C3)c(F)c1F |TLB:8:9:11.16.12:14,THB:17:9:12:16.15.14,17:15:18.10.9:12,10:11:17.18.9:14,10:9:11.16.12:14,8:9:12:16.15.14| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant sEH using CMNPC as substrate incubated for 5 mins by fluorescent based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01601
BindingDB Entry DOI: 10.7270/Q2H70KPP |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50295749
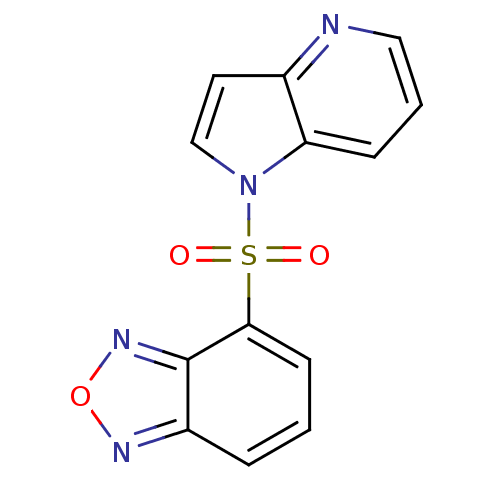
(4-(1H-pyrrolo[3,2-b]pyridin-1-ylsulfonyl)benzo[c][...)Show InChI InChI=1S/C13H8N4O3S/c18-21(19,12-5-1-3-10-13(12)16-20-15-10)17-8-6-9-11(17)4-2-7-14-9/h1-8H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Celltech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-tagged c-Met by TR-FRET assay |
Bioorg Med Chem Lett 19: 2780-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.110
BindingDB Entry DOI: 10.7270/Q2BV7GN9 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50295751
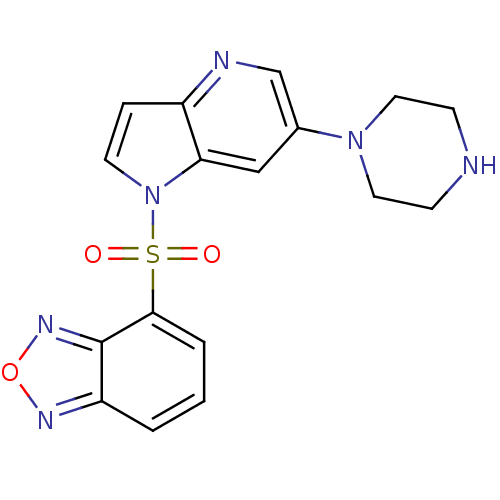
(4-(6-(piperazin-1-yl)-1H-pyrrolo[3,2-b]pyridin-1-y...)Show SMILES O=S(=O)(c1cccc2nonc12)n1ccc2ncc(cc12)N1CCNCC1 Show InChI InChI=1S/C17H16N6O3S/c24-27(25,16-3-1-2-14-17(16)21-26-20-14)23-7-4-13-15(23)10-12(11-19-13)22-8-5-18-6-9-22/h1-4,7,10-11,18H,5-6,8-9H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Celltech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-tagged c-Met by TR-FRET assay |
Bioorg Med Chem Lett 19: 2780-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.110
BindingDB Entry DOI: 10.7270/Q2BV7GN9 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50295758
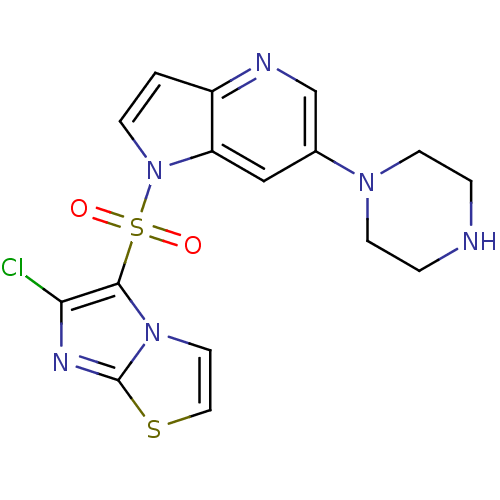
(1-(6-chloroimidazo[2,1-b]thiazol-5-ylsulfonyl)-6-(...)Show SMILES Clc1nc2sccn2c1S(=O)(=O)n1ccc2ncc(cc12)N1CCNCC1 Show InChI InChI=1S/C16H15ClN6O2S2/c17-14-15(22-7-8-26-16(22)20-14)27(24,25)23-4-1-12-13(23)9-11(10-19-12)21-5-2-18-3-6-21/h1,4,7-10,18H,2-3,5-6H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Celltech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-tagged c-Met by TR-FRET assay |
Bioorg Med Chem Lett 19: 2780-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.110
BindingDB Entry DOI: 10.7270/Q2BV7GN9 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ZAP-70
(Homo sapiens (Human)) | BDBM50083649
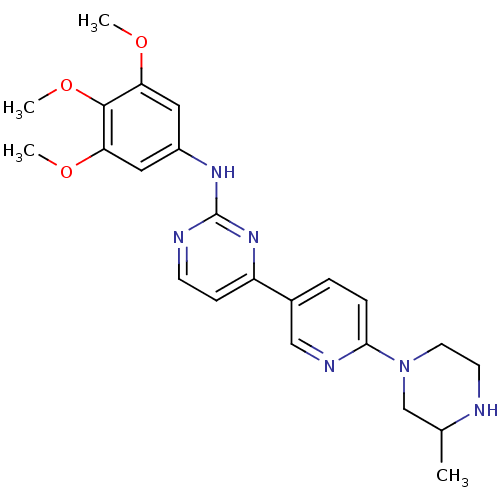
(CHEMBL112518 | {4-[6-(3-Methyl-piperazin-1-yl)-pyr...)Show SMILES COc1cc(Nc2nccc(n2)-c2ccc(nc2)N2CCNC(C)C2)cc(OC)c1OC Show InChI InChI=1S/C23H28N6O3/c1-15-14-29(10-9-24-15)21-6-5-16(13-26-21)18-7-8-25-23(28-18)27-17-11-19(30-2)22(32-4)20(12-17)31-3/h5-8,11-13,15,24H,9-10,14H2,1-4H3,(H,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech Therapeutics Limited
Curated by ChEMBL
| Assay Description
Inhibition of Zeta-chain (TCR) associated protein kinase 70 kDa phosphorylation of polyGly-Tyr. |
Bioorg Med Chem Lett 9: 3351-6 (2000)
BindingDB Entry DOI: 10.7270/Q29Z943G |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50295762
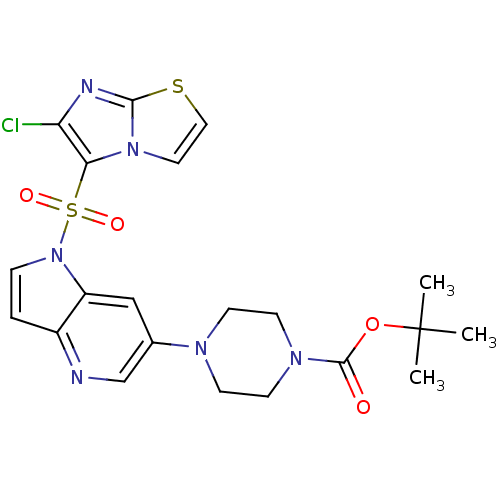
(CHEMBL550158 | tert-butyl 4-(1-(6-chloroimidazo[2,...)Show SMILES CC(C)(C)OC(=O)N1CCN(CC1)c1cnc2ccn(c2c1)S(=O)(=O)c1c(Cl)nc2sccn12 Show InChI InChI=1S/C21H23ClN6O4S2/c1-21(2,3)32-20(29)26-8-6-25(7-9-26)14-12-16-15(23-13-14)4-5-28(16)34(30,31)18-17(22)24-19-27(18)10-11-33-19/h4-5,10-13H,6-9H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Celltech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-tagged c-Met by TR-FRET assay |
Bioorg Med Chem Lett 19: 2780-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.110
BindingDB Entry DOI: 10.7270/Q2BV7GN9 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50581726
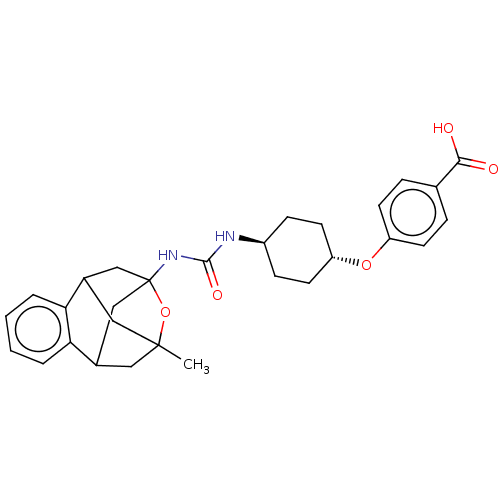
(CHEMBL5084924)Show SMILES CC12CC3CC(CC(C1)c1ccccc31)(NC(=O)N[C@H]1CC[C@@H](CC1)Oc1ccc(cc1)C(O)=O)O2 |r,wU:22.28,wD:19.21,TLB:14:3:7.6.8:35,10:9:4:2.1.35,13:14:4:2.1.35,THB:8:7:4:2.1.35,8:1:4:14.7.6.9,9:7:3.4.2:35,0:1:4:14.7.6.9,(7.35,-41.62,;7.75,-43.1,;6.73,-44.33,;6.73,-45.9,;8.12,-46.45,;9.12,-45.19,;7.74,-45.53,;6.43,-45.05,;6.42,-43.58,;4.15,-45.23,;2.66,-45.64,;2.27,-47.14,;3.39,-48.23,;4.87,-47.8,;5.24,-46.31,;10.44,-45.95,;11.76,-45.19,;11.76,-43.66,;13.08,-45.95,;14.41,-45.18,;15.74,-45.96,;17.07,-45.2,;17.08,-43.66,;15.75,-42.88,;14.4,-43.65,;18.42,-42.89,;19.75,-43.67,;19.73,-45.21,;21.06,-45.98,;22.4,-45.22,;22.4,-43.67,;21.07,-42.9,;23.73,-45.99,;23.73,-47.53,;25.07,-45.23,;9.13,-43.68,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant sEH using CMNPC as substrate incubated for 5 mins by fluorescent based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01601
BindingDB Entry DOI: 10.7270/Q2H70KPP |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50560743

(CHEMBL4759592)Show SMILES CC(=O)N1CCC(CC1)NC(=O)NC12CC3CC(CC(C3)O1)C2 |TLB:12:13:16.15.20:18,THB:14:15:18:22.13.21,14:13:16.15.20:18,21:13:16:20.19.18,21:19:16:22.14.13,12:13:16:20.19.18| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant sEH using CMNPC as substrate incubated for 5 mins by fluorescent based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01601
BindingDB Entry DOI: 10.7270/Q2H70KPP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ZAP-70
(Homo sapiens (Human)) | BDBM50083640
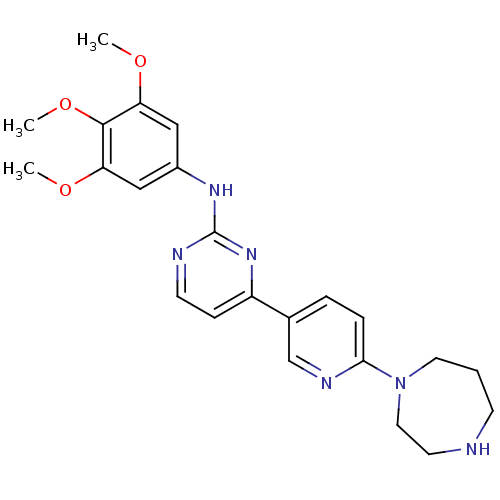
(CHEMBL112172 | [4-(6-[1,4]Diazepan-1-yl-pyridin-3-...)Show SMILES COc1cc(Nc2nccc(n2)-c2ccc(nc2)N2CCCNCC2)cc(OC)c1OC Show InChI InChI=1S/C23H28N6O3/c1-30-19-13-17(14-20(31-2)22(19)32-3)27-23-25-9-7-18(28-23)16-5-6-21(26-15-16)29-11-4-8-24-10-12-29/h5-7,9,13-15,24H,4,8,10-12H2,1-3H3,(H,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech Therapeutics Limited
Curated by ChEMBL
| Assay Description
Inhibition of Zeta-chain (TCR) associated protein kinase 70 kDa phosphorylation of polyGly-Tyr. |
Bioorg Med Chem Lett 9: 3351-6 (2000)
BindingDB Entry DOI: 10.7270/Q29Z943G |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50266023
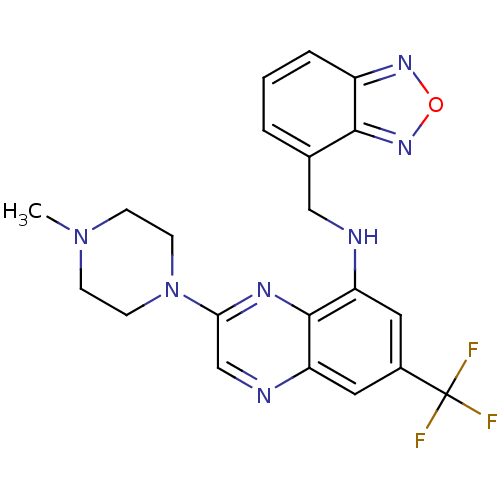
(CHEMBL515659 | N-(benzo[c][1,2,5]oxadiazol-4-ylmet...)Show SMILES CN1CCN(CC1)c1cnc2cc(cc(NCc3cccc4nonc34)c2n1)C(F)(F)F Show InChI InChI=1S/C21H20F3N7O/c1-30-5-7-31(8-6-30)18-12-26-17-10-14(21(22,23)24)9-16(20(17)27-18)25-11-13-3-2-4-15-19(13)29-32-28-15/h2-4,9-10,12,25H,5-8,11H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Celltech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant his-tagged c-Met by TR-FRET assay |
Bioorg Med Chem Lett 19: 397-400 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.062
BindingDB Entry DOI: 10.7270/Q2ZC82QC |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50295769
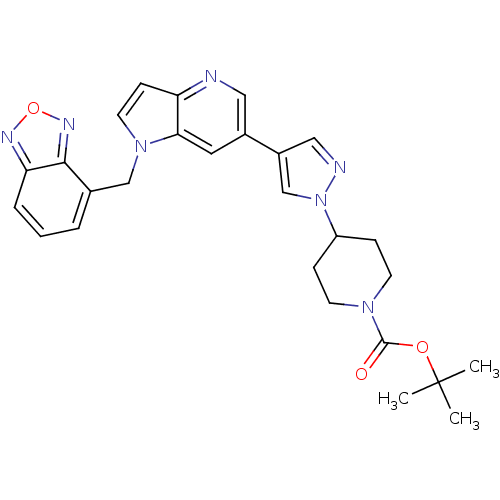
(CHEMBL564827 | tert-butyl 4-(4-(1-(benzo[c][1,2,5]...)Show SMILES CC(C)(C)OC(=O)N1CCC(CC1)n1cc(cn1)-c1cnc2ccn(Cc3cccc4nonc34)c2c1 Show InChI InChI=1S/C27H29N7O3/c1-27(2,3)36-26(35)32-10-7-21(8-11-32)34-17-20(15-29-34)19-13-24-22(28-14-19)9-12-33(24)16-18-5-4-6-23-25(18)31-37-30-23/h4-6,9,12-15,17,21H,7-8,10-11,16H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Celltech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-tagged c-Met by TR-FRET assay |
Bioorg Med Chem Lett 19: 2780-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.110
BindingDB Entry DOI: 10.7270/Q2BV7GN9 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50295757
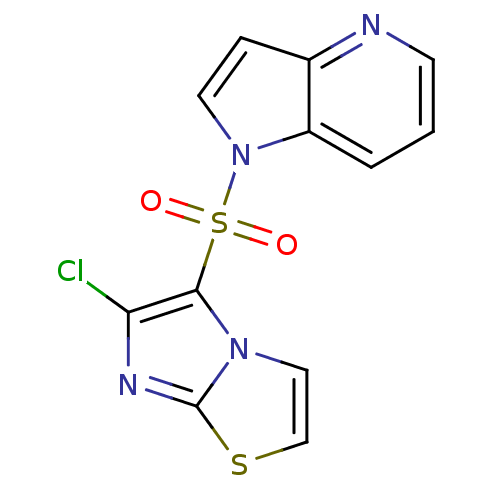
(1-(6-chloroimidazo[2,1-b]thiazol-5-ylsulfonyl)-1H-...)Show InChI InChI=1S/C12H7ClN4O2S2/c13-10-11(16-6-7-20-12(16)15-10)21(18,19)17-5-3-8-9(17)2-1-4-14-8/h1-7H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Celltech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-tagged c-Met by TR-FRET assay |
Bioorg Med Chem Lett 19: 2780-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.110
BindingDB Entry DOI: 10.7270/Q2BV7GN9 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50265954
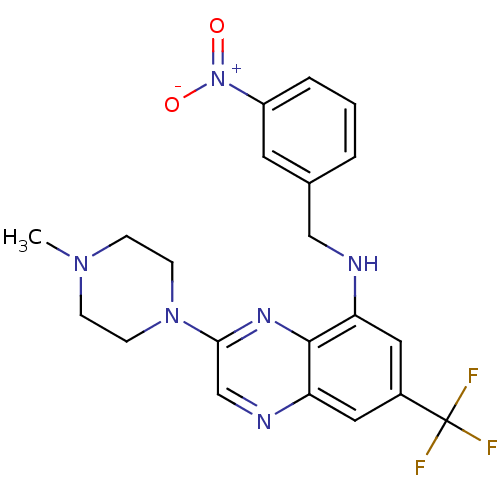
(3-(4-methylpiperazin-1-yl)-N-(3-nitrobenzyl)-7-(tr...)Show SMILES CN1CCN(CC1)c1cnc2cc(cc(NCc3cccc(c3)[N+]([O-])=O)c2n1)C(F)(F)F Show InChI InChI=1S/C21H21F3N6O2/c1-28-5-7-29(8-6-28)19-13-26-18-11-15(21(22,23)24)10-17(20(18)27-19)25-12-14-3-2-4-16(9-14)30(31)32/h2-4,9-11,13,25H,5-8,12H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Celltech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant his-tagged c-Met by TR-FRET assay |
Bioorg Med Chem Lett 19: 397-400 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.062
BindingDB Entry DOI: 10.7270/Q2ZC82QC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data