

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine deaminase (Bos taurus (bovine)) | BDBM50451174 (CHEMBL4202457) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Inhibition of bovine adenosine deaminase pre-incubated for 5 mins before adenosine addition | Bioorg Med Chem 25: 5799-5819 (2017) Article DOI: 10.1016/j.bmc.2017.09.015 BindingDB Entry DOI: 10.7270/Q2M0482X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM50451177 (CHEMBL4210264) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Inhibition of bovine adenosine deaminase pre-incubated for 5 mins before adenosine addition | Bioorg Med Chem 25: 5799-5819 (2017) Article DOI: 10.1016/j.bmc.2017.09.015 BindingDB Entry DOI: 10.7270/Q2M0482X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM50451169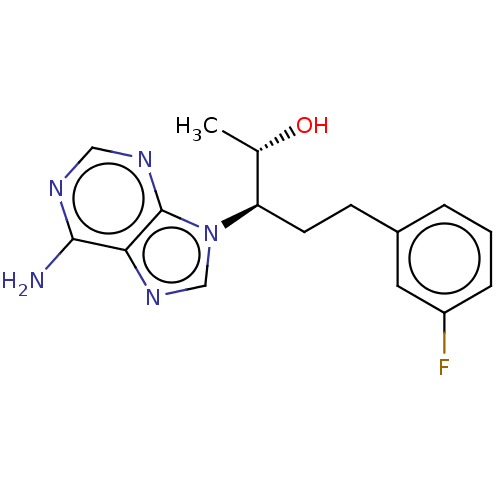 (CHEMBL4207346) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Inhibition of bovine adenosine deaminase pre-incubated for 5 mins before adenosine addition | Bioorg Med Chem 25: 5799-5819 (2017) Article DOI: 10.1016/j.bmc.2017.09.015 BindingDB Entry DOI: 10.7270/Q2M0482X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM50451139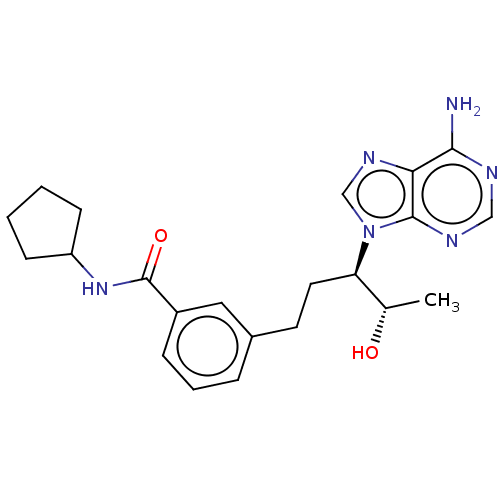 (CHEMBL4204605) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Inhibition of bovine adenosine deaminase pre-incubated for 5 mins before adenosine addition | Bioorg Med Chem 25: 5799-5819 (2017) Article DOI: 10.1016/j.bmc.2017.09.015 BindingDB Entry DOI: 10.7270/Q2M0482X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM50451140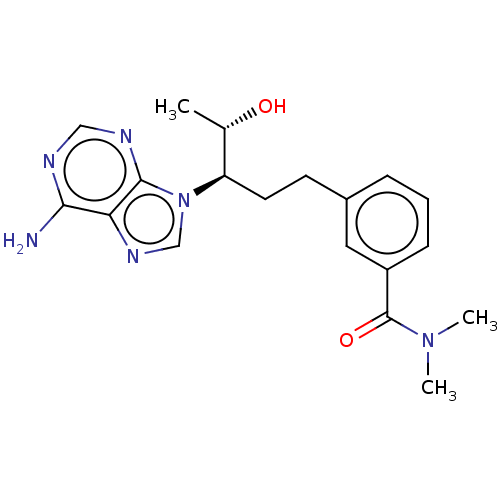 (CHEMBL4216271) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Inhibition of bovine adenosine deaminase pre-incubated for 5 mins before adenosine addition | Bioorg Med Chem 25: 5799-5819 (2017) Article DOI: 10.1016/j.bmc.2017.09.015 BindingDB Entry DOI: 10.7270/Q2M0482X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM50451167 (CHEMBL4206823) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Inhibition of bovine adenosine deaminase pre-incubated for 5 mins before adenosine addition | Bioorg Med Chem 25: 5799-5819 (2017) Article DOI: 10.1016/j.bmc.2017.09.015 BindingDB Entry DOI: 10.7270/Q2M0482X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM50451164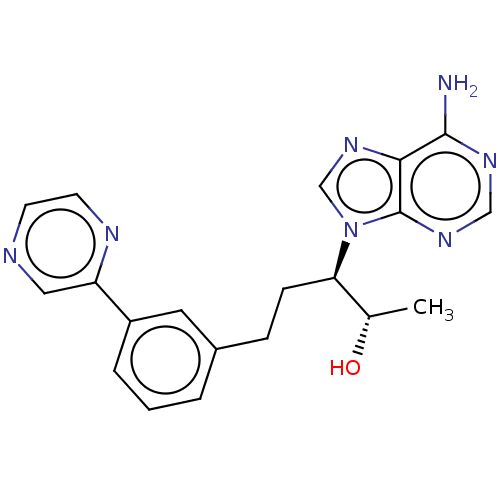 (CHEMBL4208941) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Inhibition of bovine adenosine deaminase pre-incubated for 5 mins before adenosine addition | Bioorg Med Chem 25: 5799-5819 (2017) Article DOI: 10.1016/j.bmc.2017.09.015 BindingDB Entry DOI: 10.7270/Q2M0482X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM50451168 (CHEMBL4211702) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Inhibition of bovine adenosine deaminase pre-incubated for 5 mins before adenosine addition | Bioorg Med Chem 25: 5799-5819 (2017) Article DOI: 10.1016/j.bmc.2017.09.015 BindingDB Entry DOI: 10.7270/Q2M0482X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM50451173 (CHEMBL4214254) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Inhibition of bovine adenosine deaminase pre-incubated for 5 mins before adenosine addition | Bioorg Med Chem 25: 5799-5819 (2017) Article DOI: 10.1016/j.bmc.2017.09.015 BindingDB Entry DOI: 10.7270/Q2M0482X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM28393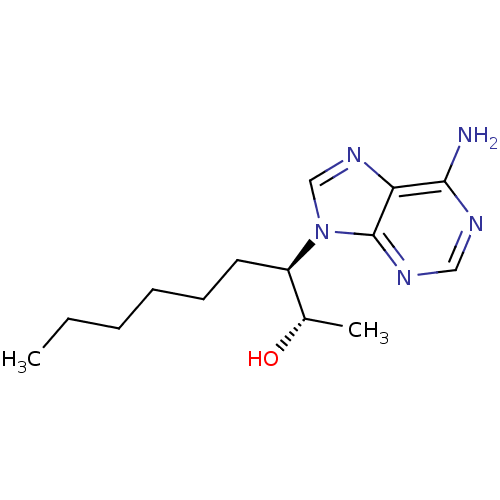 ((+)-EHNA | (2S,3R)-3-(6-amino-9H-purin-9-yl)nonan-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Inhibition of bovine adenosine deaminase pre-incubated for 5 mins before adenosine addition | Bioorg Med Chem 25: 5799-5819 (2017) Article DOI: 10.1016/j.bmc.2017.09.015 BindingDB Entry DOI: 10.7270/Q2M0482X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM50451138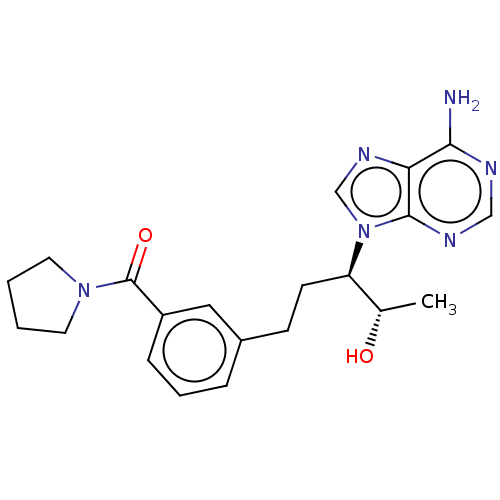 (CHEMBL4203116) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Inhibition of bovine adenosine deaminase pre-incubated for 5 mins before adenosine addition | Bioorg Med Chem 25: 5799-5819 (2017) Article DOI: 10.1016/j.bmc.2017.09.015 BindingDB Entry DOI: 10.7270/Q2M0482X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM50451165 (CHEMBL4204068) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Inhibition of bovine adenosine deaminase pre-incubated for 5 mins before adenosine addition | Bioorg Med Chem 25: 5799-5819 (2017) Article DOI: 10.1016/j.bmc.2017.09.015 BindingDB Entry DOI: 10.7270/Q2M0482X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM50451166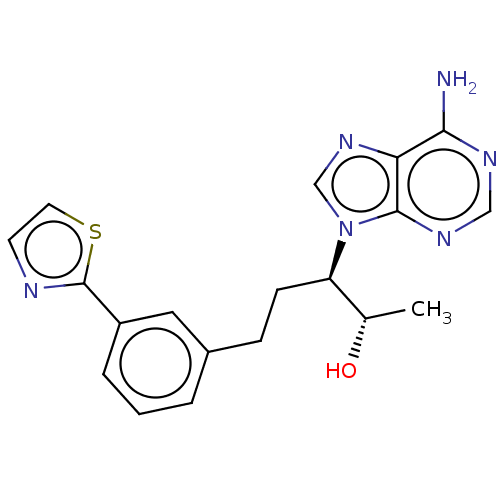 (CHEMBL4209345) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Inhibition of bovine adenosine deaminase pre-incubated for 5 mins before adenosine addition | Bioorg Med Chem 25: 5799-5819 (2017) Article DOI: 10.1016/j.bmc.2017.09.015 BindingDB Entry DOI: 10.7270/Q2M0482X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM50451141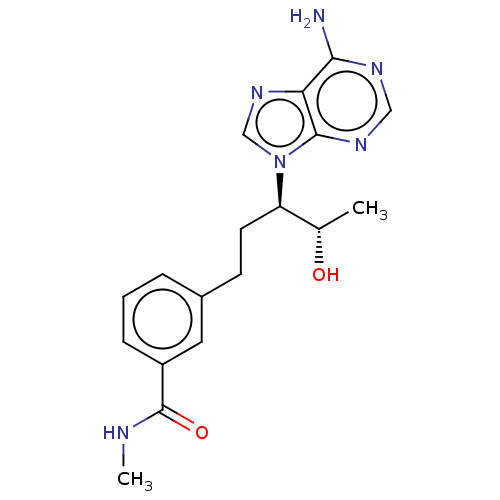 (CHEMBL4216791) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Inhibition of bovine adenosine deaminase pre-incubated for 5 mins before adenosine addition | Bioorg Med Chem 25: 5799-5819 (2017) Article DOI: 10.1016/j.bmc.2017.09.015 BindingDB Entry DOI: 10.7270/Q2M0482X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM50451142 (CHEMBL4211824) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Inhibition of bovine adenosine deaminase pre-incubated for 5 mins before adenosine addition | Bioorg Med Chem 25: 5799-5819 (2017) Article DOI: 10.1016/j.bmc.2017.09.015 BindingDB Entry DOI: 10.7270/Q2M0482X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM50451158 (CHEMBL4218202) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Inhibition of bovine adenosine deaminase pre-incubated for 5 mins before adenosine addition | Bioorg Med Chem 25: 5799-5819 (2017) Article DOI: 10.1016/j.bmc.2017.09.015 BindingDB Entry DOI: 10.7270/Q2M0482X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM50451171 (CHEMBL4214511) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Inhibition of bovine adenosine deaminase pre-incubated for 5 mins before adenosine addition | Bioorg Med Chem 25: 5799-5819 (2017) Article DOI: 10.1016/j.bmc.2017.09.015 BindingDB Entry DOI: 10.7270/Q2M0482X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM50451178 (CHEMBL4203469) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Inhibition of bovine adenosine deaminase pre-incubated for 5 mins before adenosine addition | Bioorg Med Chem 25: 5799-5819 (2017) Article DOI: 10.1016/j.bmc.2017.09.015 BindingDB Entry DOI: 10.7270/Q2M0482X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM50451170 (CHEMBL4214658) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Inhibition of bovine adenosine deaminase pre-incubated for 5 mins before adenosine addition | Bioorg Med Chem 25: 5799-5819 (2017) Article DOI: 10.1016/j.bmc.2017.09.015 BindingDB Entry DOI: 10.7270/Q2M0482X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50386376 (CHEMBL2046865) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]peptideYY from NPY1 receptor in human SK-N-MC cells after 1 hr by scintillation counting | ACS Med Chem Lett 3: 222-226 (2012) Article DOI: 10.1021/ml200265m BindingDB Entry DOI: 10.7270/Q2ZP475V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM50451133 (CHEMBL4218610) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Inhibition of bovine adenosine deaminase pre-incubated for 5 mins before adenosine addition | Bioorg Med Chem 25: 5799-5819 (2017) Article DOI: 10.1016/j.bmc.2017.09.015 BindingDB Entry DOI: 10.7270/Q2M0482X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM50451172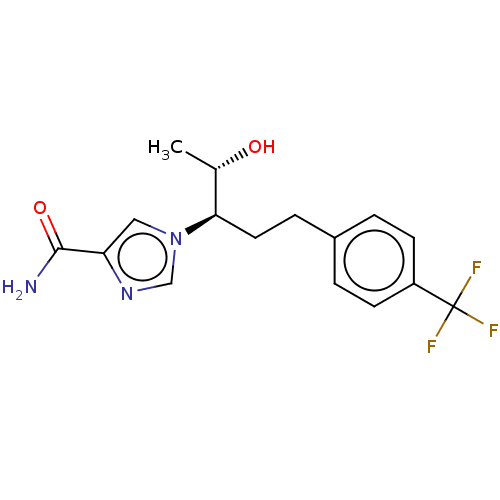 (CHEMBL4215142) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Inhibition of bovine adenosine deaminase pre-incubated for 5 mins before adenosine addition | Bioorg Med Chem 25: 5799-5819 (2017) Article DOI: 10.1016/j.bmc.2017.09.015 BindingDB Entry DOI: 10.7270/Q2M0482X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM50451134 (CHEMBL4206867) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Inhibition of bovine adenosine deaminase pre-incubated for 5 mins before adenosine addition | Bioorg Med Chem 25: 5799-5819 (2017) Article DOI: 10.1016/j.bmc.2017.09.015 BindingDB Entry DOI: 10.7270/Q2M0482X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM50451159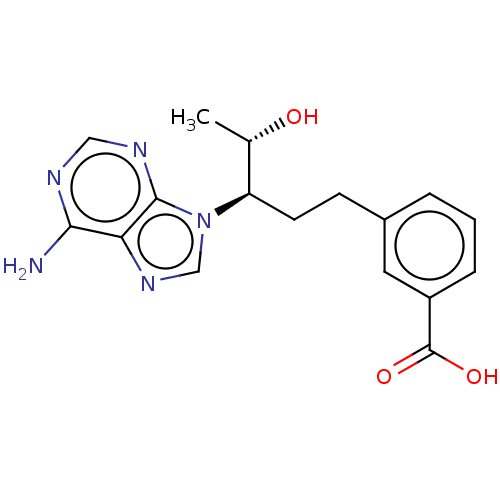 (CHEMBL4205307) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Inhibition of bovine adenosine deaminase pre-incubated for 5 mins before adenosine addition | Bioorg Med Chem 25: 5799-5819 (2017) Article DOI: 10.1016/j.bmc.2017.09.015 BindingDB Entry DOI: 10.7270/Q2M0482X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM50451137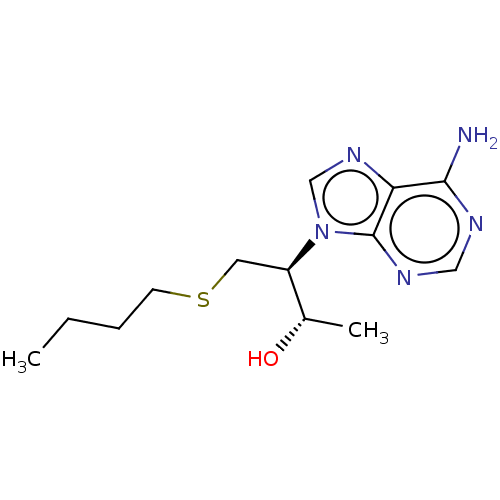 (CHEMBL4207993) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Inhibition of bovine adenosine deaminase pre-incubated for 5 mins before adenosine addition | Bioorg Med Chem 25: 5799-5819 (2017) Article DOI: 10.1016/j.bmc.2017.09.015 BindingDB Entry DOI: 10.7270/Q2M0482X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM50451135 (CHEMBL4206350) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Inhibition of bovine adenosine deaminase pre-incubated for 5 mins before adenosine addition | Bioorg Med Chem 25: 5799-5819 (2017) Article DOI: 10.1016/j.bmc.2017.09.015 BindingDB Entry DOI: 10.7270/Q2M0482X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM50451100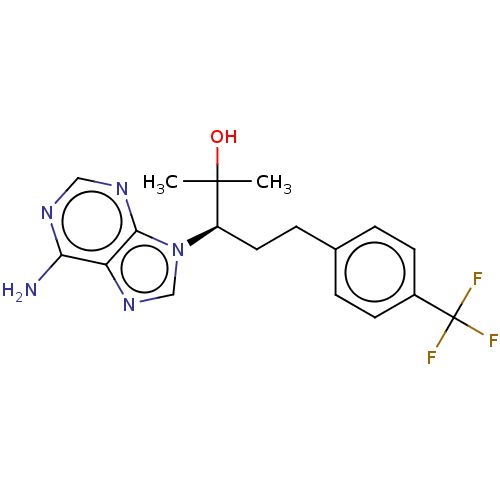 (CHEMBL4208733) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Inhibition of bovine adenosine deaminase pre-incubated for 5 mins before adenosine addition | Bioorg Med Chem 25: 5799-5819 (2017) Article DOI: 10.1016/j.bmc.2017.09.015 BindingDB Entry DOI: 10.7270/Q2M0482X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM50451136 (CHEMBL4211238) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 135 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Inhibition of bovine adenosine deaminase pre-incubated for 5 mins before adenosine addition | Bioorg Med Chem 25: 5799-5819 (2017) Article DOI: 10.1016/j.bmc.2017.09.015 BindingDB Entry DOI: 10.7270/Q2M0482X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kallikrein (Sus scrofa) | BDBM50004610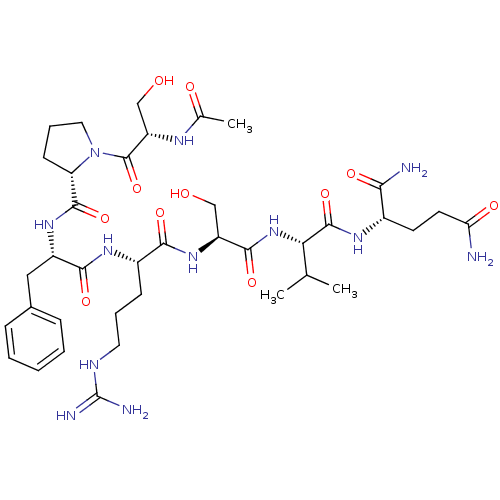 (2-(2-{2-[2-(2-{[1-(2-Acetylamino-3-hydroxy-propion...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.01E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Curated by ChEMBL | Assay Description Inhibitory activity against beta-kallikrein | J Med Chem 35: 3094-102 (1992) BindingDB Entry DOI: 10.7270/Q23R0RVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kallikrein (Sus scrofa) | BDBM50004613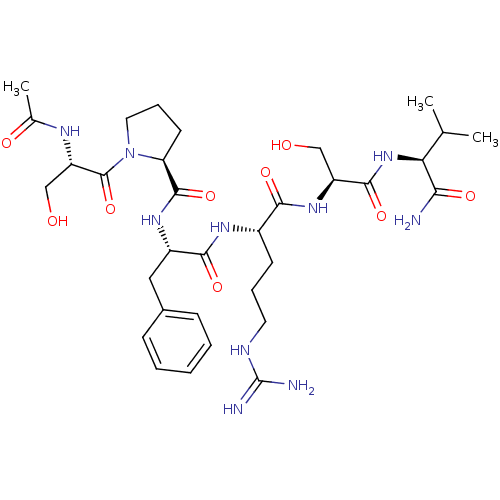 (1-(2-Acetylamino-3-hydroxy-propionyl)-pyrrolidine-...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.35E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Curated by ChEMBL | Assay Description Inhibitory activity against beta-kallikrein | J Med Chem 35: 3094-102 (1992) BindingDB Entry DOI: 10.7270/Q23R0RVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kallikrein (Sus scrofa) | BDBM50004609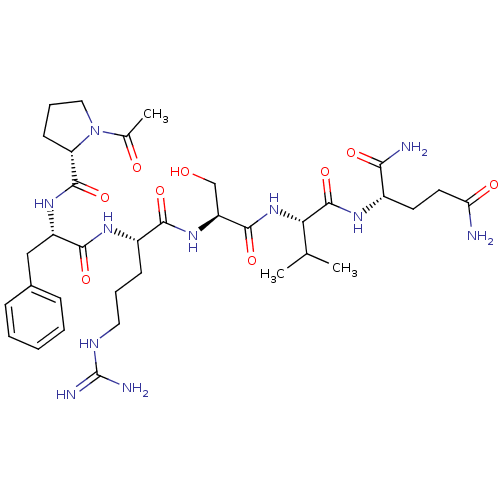 (2-{2-[2-(2-{2-[(1-Acetyl-pyrrolidine-2-carbonyl)-a...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 1.67E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Curated by ChEMBL | Assay Description Inhibitory activity against beta-kallikrein | J Med Chem 35: 3094-102 (1992) BindingDB Entry DOI: 10.7270/Q23R0RVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kallikrein (Sus scrofa) | BDBM50004605 (1-(2-Acetylamino-3-hydroxy-propionyl)-pyrrolidine-...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.23E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Curated by ChEMBL | Assay Description Inhibitory activity against beta-kallikrein | J Med Chem 35: 3094-102 (1992) BindingDB Entry DOI: 10.7270/Q23R0RVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kallikrein (Sus scrofa) | BDBM50004611 (2-(2-{2-[2-(2-Acetylamino-3-phenyl-propionylamino)...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Curated by ChEMBL | Assay Description Inhibitory activity against beta-kallikrein | J Med Chem 35: 3094-102 (1992) BindingDB Entry DOI: 10.7270/Q23R0RVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kallikrein (Sus scrofa) | BDBM50004607 (1-Acetyl-pyrrolidine-2-carboxylic acid (1-{1-[1-(1...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.66E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Curated by ChEMBL | Assay Description Inhibitory activity against beta-kallikrein | J Med Chem 35: 3094-102 (1992) BindingDB Entry DOI: 10.7270/Q23R0RVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kallikrein (Sus scrofa) | BDBM50004606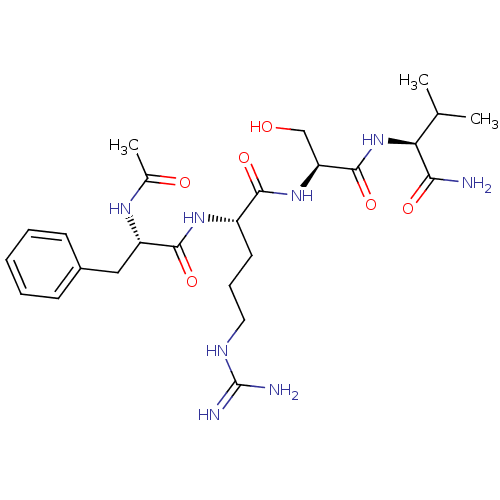 (2-(2-Acetylamino-3-phenyl-propionylamino)-5-guanid...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.79E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Curated by ChEMBL | Assay Description Inhibitory activity against beta-kallikrein | J Med Chem 35: 3094-102 (1992) BindingDB Entry DOI: 10.7270/Q23R0RVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kallikrein (Sus scrofa) | BDBM50004614 (1-Acetyl-pyrrolidine-2-carboxylic acid {1-[1-(1-ca...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.78E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Curated by ChEMBL | Assay Description Inhibitory activity against beta-kallikrein | J Med Chem 35: 3094-102 (1992) BindingDB Entry DOI: 10.7270/Q23R0RVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kallikrein (Sus scrofa) | BDBM50004604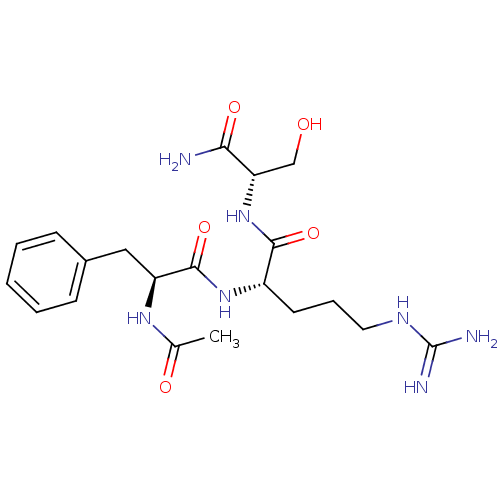 (2-(2-Acetylamino-3-phenyl-propionylamino)-5-guanid...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.18E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Curated by ChEMBL | Assay Description Inhibitory activity against beta-kallikrein | J Med Chem 35: 3094-102 (1992) BindingDB Entry DOI: 10.7270/Q23R0RVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kallikrein (Sus scrofa) | BDBM50004608 (2-{2-[2-(2-Acetylamino-5-guanidino-pentanoylamino)...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Curated by ChEMBL | Assay Description Inhibitory activity against beta-kallikrein | J Med Chem 35: 3094-102 (1992) BindingDB Entry DOI: 10.7270/Q23R0RVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kallikrein (Sus scrofa) | BDBM50004612 (1-(2-Acetylamino-3-hydroxy-propionyl)-pyrrolidine-...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.29E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Curated by ChEMBL | Assay Description Inhibitory activity against beta-kallikrein | J Med Chem 35: 3094-102 (1992) BindingDB Entry DOI: 10.7270/Q23R0RVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50476941 (CHEMBL233363) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of gamma secretase-mediated beta-APP cleavage in human H4 cells assessed by measuring Abeta40 production by ELISA | Bioorg Med Chem Lett 17: 4432-6 (2007) Article DOI: 10.1016/j.bmcl.2007.06.022 BindingDB Entry DOI: 10.7270/Q2708465 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50476939 (CHEMBL233364) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of gamma secretase-mediated beta-APP cleavage in human H4 cells assessed by measuring Abeta40 production by ELISA | Bioorg Med Chem Lett 17: 4432-6 (2007) Article DOI: 10.1016/j.bmcl.2007.06.022 BindingDB Entry DOI: 10.7270/Q2708465 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50476940 (CHEMBL233362) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of gamma secretase-mediated beta-APP cleavage in human H4 cells assessed by measuring Abeta40 production by ELISA | Bioorg Med Chem Lett 17: 4432-6 (2007) Article DOI: 10.1016/j.bmcl.2007.06.022 BindingDB Entry DOI: 10.7270/Q2708465 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50476947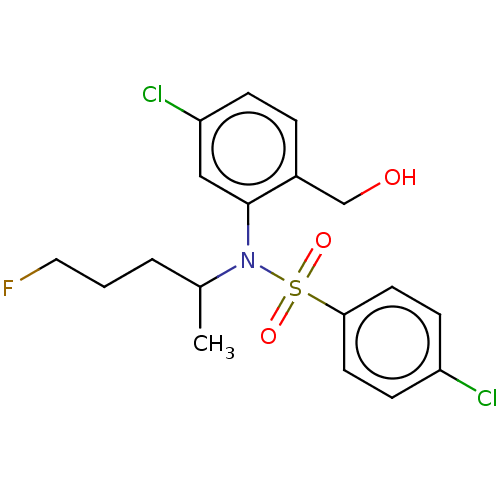 (CHEMBL394661) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of gamma secretase-mediated beta-APP cleavage in human H4 cells assessed by measuring Abeta40 production by ELISA | Bioorg Med Chem Lett 17: 4432-6 (2007) Article DOI: 10.1016/j.bmcl.2007.06.022 BindingDB Entry DOI: 10.7270/Q2708465 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50476919 (CHEMBL232948) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of gamma secretase-mediated beta-APP cleavage in human H4 cells assessed by measuring Abeta40 production by ELISA | Bioorg Med Chem Lett 17: 4432-6 (2007) Article DOI: 10.1016/j.bmcl.2007.06.022 BindingDB Entry DOI: 10.7270/Q2708465 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50476912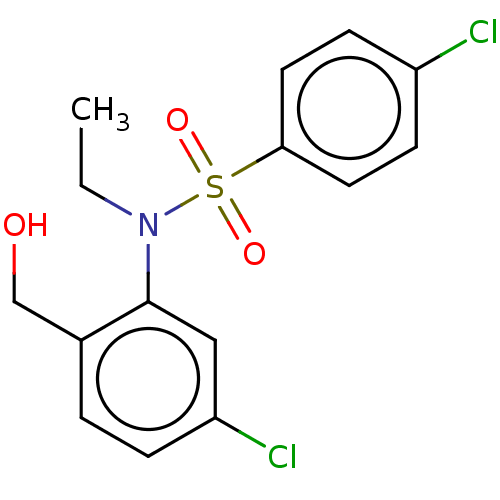 (CHEMBL231714) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of gamma secretase-mediated beta-APP cleavage in human H4 cells assessed by measuring Abeta40 production by ELISA | Bioorg Med Chem Lett 17: 4432-6 (2007) Article DOI: 10.1016/j.bmcl.2007.06.022 BindingDB Entry DOI: 10.7270/Q2708465 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50476953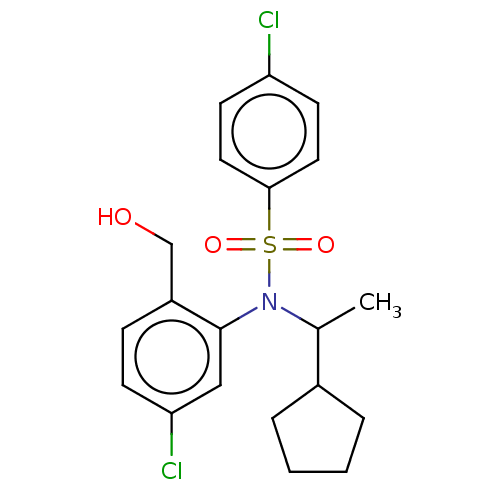 (CHEMBL232949) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of gamma secretase-mediated beta-APP cleavage in human H4 cells assessed by measuring Abeta40 production by ELISA | Bioorg Med Chem Lett 17: 4432-6 (2007) Article DOI: 10.1016/j.bmcl.2007.06.022 BindingDB Entry DOI: 10.7270/Q2708465 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50476922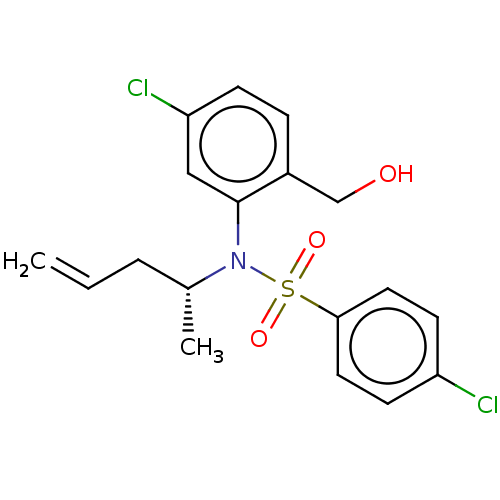 (CHEMBL391377) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of gamma secretase-mediated beta-APP cleavage in human H4 cells assessed by measuring Abeta40 production by ELISA | Bioorg Med Chem Lett 17: 4432-6 (2007) Article DOI: 10.1016/j.bmcl.2007.06.022 BindingDB Entry DOI: 10.7270/Q2708465 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50476957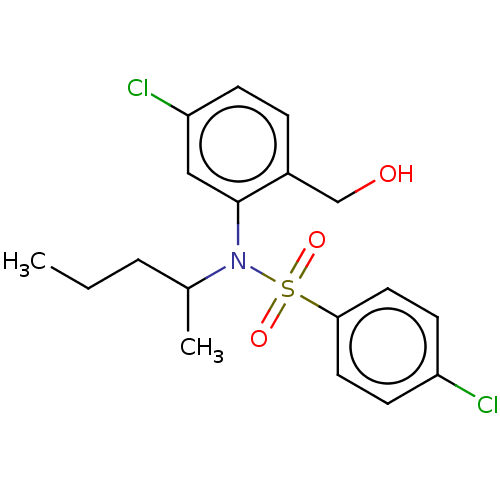 (CHEMBL231767) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of gamma secretase-mediated beta-APP cleavage in human H4 cells assessed by measuring Abeta40 production by ELISA | Bioorg Med Chem Lett 17: 4432-6 (2007) Article DOI: 10.1016/j.bmcl.2007.06.022 BindingDB Entry DOI: 10.7270/Q2708465 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50476917 (CHEMBL232152) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of gamma secretase-mediated beta-APP cleavage in human H4 cells assessed by measuring Abeta40 production by ELISA | Bioorg Med Chem Lett 17: 4432-6 (2007) Article DOI: 10.1016/j.bmcl.2007.06.022 BindingDB Entry DOI: 10.7270/Q2708465 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50476911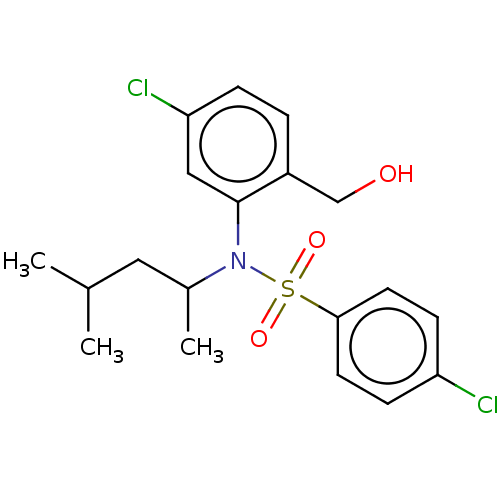 (CHEMBL394660) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of gamma secretase-mediated beta-APP cleavage in human H4 cells assessed by measuring Abeta40 production by ELISA | Bioorg Med Chem Lett 17: 4432-6 (2007) Article DOI: 10.1016/j.bmcl.2007.06.022 BindingDB Entry DOI: 10.7270/Q2708465 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 4051 total ) | Next | Last >> |