 Found 201 hits with Last Name = 'deuschle' and Initial = 'u'
Found 201 hits with Last Name = 'deuschle' and Initial = 'u' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Bile acid receptor
(Homo sapiens (Human)) | BDBM50323523
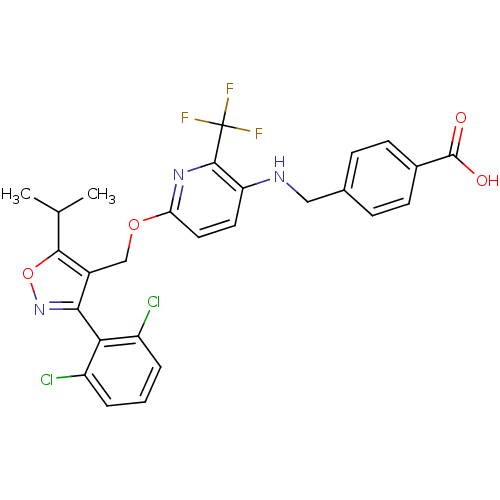
(4-((6-((3-(2,6-dichlorophenyl)-5-isopropylisoxazol...)Show SMILES CC(C)c1onc(c1COc1ccc(NCc2ccc(cc2)C(O)=O)c(n1)C(F)(F)F)-c1c(Cl)cccc1Cl |(5.95,-39.6,;5.33,-41.01,;3.8,-41.18,;6.24,-42.25,;7.79,-42.25,;8.27,-43.72,;7.02,-44.63,;5.77,-43.73,;4.44,-44.49,;3.1,-43.73,;1.77,-44.5,;.42,-43.74,;-.9,-44.52,;-.89,-46.05,;-2.22,-46.82,;-3.56,-46.06,;-4.89,-46.83,;-4.89,-48.38,;-6.22,-49.15,;-7.56,-48.38,;-7.55,-46.84,;-6.23,-46.07,;-8.89,-49.15,;-10.22,-48.38,;-8.89,-50.69,;.44,-46.82,;1.77,-46.04,;.44,-48.36,;-.89,-49.13,;1.78,-49.13,;.43,-49.89,;7.03,-46.16,;8.36,-46.92,;9.69,-46.14,;8.37,-48.46,;7.04,-49.24,;5.7,-48.48,;5.69,-46.94,;4.36,-46.17,)| Show InChI InChI=1S/C27H22Cl2F3N3O4/c1-14(2)24-17(23(35-39-24)22-18(28)4-3-5-19(22)29)13-38-21-11-10-20(25(34-21)27(30,31)32)33-12-15-6-8-16(9-7-15)26(36)37/h3-11,14,33H,12-13H2,1-2H3,(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 398 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at human GST-fused FXR LBD expressed in HEK293 cells coexpressing GAL4-DNA bindig domain and pFRluc by mammalian one-hybrid assay |
Bioorg Med Chem Lett 20: 4911-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.084
BindingDB Entry DOI: 10.7270/Q21V5F52 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50323524

(4-(((6-((3-(2,6-dichlorophenyl)-5-isopropylisoxazo...)Show SMILES CC(C)c1onc(c1COc1ccc(N(C)Cc2ccc(cc2)C(O)=O)c(n1)C(F)(F)F)-c1c(Cl)cccc1Cl |(29.01,-37.57,;28.39,-38.98,;26.86,-39.15,;29.3,-40.22,;30.85,-40.22,;31.33,-41.69,;30.08,-42.6,;28.83,-41.7,;27.5,-42.46,;26.16,-41.7,;24.83,-42.47,;23.48,-41.71,;22.16,-42.49,;22.17,-44.02,;20.84,-44.79,;20.84,-46.33,;19.5,-44.03,;18.17,-44.8,;18.18,-46.35,;16.84,-47.12,;15.5,-46.35,;15.51,-44.81,;16.84,-44.04,;14.17,-47.12,;12.84,-46.35,;14.17,-48.66,;23.5,-44.79,;24.83,-44.01,;23.5,-46.33,;22.17,-47.1,;24.84,-47.1,;23.49,-47.86,;30.09,-44.13,;31.42,-44.89,;32.75,-44.11,;31.43,-46.43,;30.1,-47.21,;28.76,-46.45,;28.75,-44.91,;27.42,-44.14,)| Show InChI InChI=1S/C28H24Cl2F3N3O4/c1-15(2)25-18(24(35-40-25)23-19(29)5-4-6-20(23)30)14-39-22-12-11-21(26(34-22)28(31,32)33)36(3)13-16-7-9-17(10-8-16)27(37)38/h4-12,15H,13-14H2,1-3H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 22 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at human GST-fused FXR LBD assessed as cofactor peptide interaction with receptor ligand binding domain by FRET assay |
Bioorg Med Chem Lett 20: 4911-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.084
BindingDB Entry DOI: 10.7270/Q21V5F52 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50323523

(4-((6-((3-(2,6-dichlorophenyl)-5-isopropylisoxazol...)Show SMILES CC(C)c1onc(c1COc1ccc(NCc2ccc(cc2)C(O)=O)c(n1)C(F)(F)F)-c1c(Cl)cccc1Cl |(5.95,-39.6,;5.33,-41.01,;3.8,-41.18,;6.24,-42.25,;7.79,-42.25,;8.27,-43.72,;7.02,-44.63,;5.77,-43.73,;4.44,-44.49,;3.1,-43.73,;1.77,-44.5,;.42,-43.74,;-.9,-44.52,;-.89,-46.05,;-2.22,-46.82,;-3.56,-46.06,;-4.89,-46.83,;-4.89,-48.38,;-6.22,-49.15,;-7.56,-48.38,;-7.55,-46.84,;-6.23,-46.07,;-8.89,-49.15,;-10.22,-48.38,;-8.89,-50.69,;.44,-46.82,;1.77,-46.04,;.44,-48.36,;-.89,-49.13,;1.78,-49.13,;.43,-49.89,;7.03,-46.16,;8.36,-46.92,;9.69,-46.14,;8.37,-48.46,;7.04,-49.24,;5.7,-48.48,;5.69,-46.94,;4.36,-46.17,)| Show InChI InChI=1S/C27H22Cl2F3N3O4/c1-14(2)24-17(23(35-39-24)22-18(28)4-3-5-19(22)29)13-38-21-11-10-20(25(34-21)27(30,31)32)33-12-15-6-8-16(9-7-15)26(36)37/h3-11,14,33H,12-13H2,1-2H3,(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 162 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at human GST-fused FXR LBD assessed as cofactor peptide interaction with receptor ligand binding domain by FRET assay |
Bioorg Med Chem Lett 20: 4911-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.084
BindingDB Entry DOI: 10.7270/Q21V5F52 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50323543
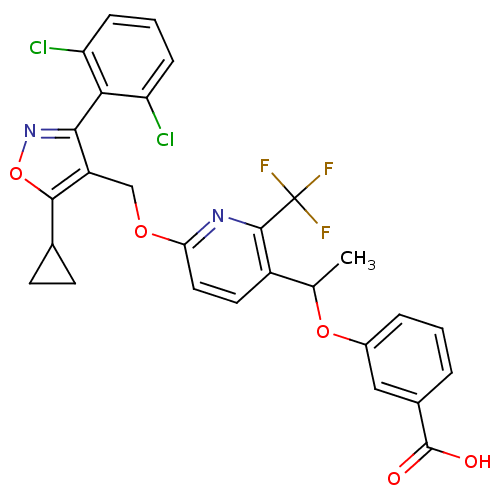
(3-(1-(6-((5-cyclopropyl-3-(2,6-dichlorophenyl)isox...)Show SMILES CC(Oc1cccc(c1)C(O)=O)c1ccc(OCc2c(onc2-c2c(Cl)cccc2Cl)C2CC2)nc1C(F)(F)F |(-2.38,-48.45,;-2.39,-46.91,;-3.72,-46.14,;-5.05,-46.92,;-5.05,-48.47,;-6.39,-49.24,;-7.72,-48.47,;-7.72,-46.92,;-6.39,-46.15,;-9.05,-46.15,;-10.39,-46.92,;-9.05,-44.61,;-1.06,-46.13,;-1.07,-44.6,;.26,-43.82,;1.6,-44.59,;2.94,-43.81,;4.28,-44.58,;5.6,-43.81,;6.07,-42.34,;7.62,-42.34,;8.11,-43.8,;6.86,-44.72,;6.86,-46.25,;8.2,-47.01,;9.52,-46.23,;8.21,-48.55,;6.87,-49.33,;5.54,-48.56,;5.53,-47.02,;4.19,-46.26,;5.16,-41.1,;4.99,-39.57,;3.75,-40.48,;1.61,-46.13,;.28,-46.9,;.28,-48.44,;-1.05,-49.21,;1.61,-49.21,;.26,-49.98,)| Show InChI InChI=1S/C28H21Cl2F3N2O5/c1-14(39-17-5-2-4-16(12-17)27(36)37)18-10-11-22(34-26(18)28(31,32)33)38-13-19-24(35-40-25(19)15-8-9-15)23-20(29)6-3-7-21(23)30/h2-7,10-12,14-15H,8-9,13H2,1H3,(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 107 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at human GST-fused FXR LBD expressed in HEK293 cells coexpressing GAL4-DNA bindig domain and pFRluc by mammalian one-hybrid assay |
Bioorg Med Chem Lett 20: 4911-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.084
BindingDB Entry DOI: 10.7270/Q21V5F52 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50323525
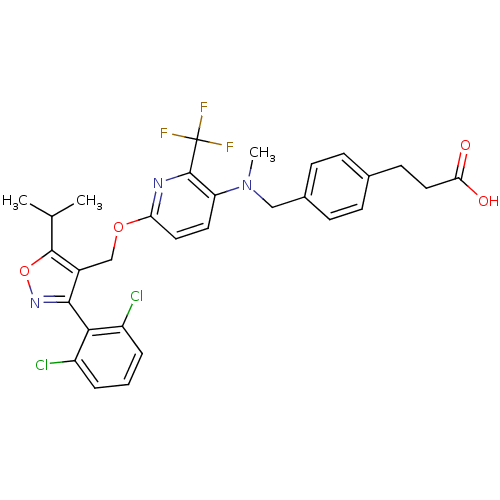
(3-(4-(((6-((3-(2,6-dichlorophenyl)-5-isopropylisox...)Show SMILES CC(C)c1onc(c1COc1ccc(N(C)Cc2ccc(CCC(O)=O)cc2)c(n1)C(F)(F)F)-c1c(Cl)cccc1Cl |(8.94,6.43,;8.32,5.03,;6.79,4.86,;9.23,3.78,;10.78,3.79,;11.26,2.32,;10.02,1.4,;8.76,2.31,;7.43,1.54,;6.1,2.31,;4.76,1.53,;3.41,2.3,;2.09,1.52,;2.1,-.01,;.77,-.78,;.78,-2.32,;-.57,-.02,;-1.9,-.79,;-3.23,-.03,;-4.56,-.8,;-4.56,-2.35,;-5.9,-3.11,;-7.23,-2.34,;-8.56,-3.11,;-9.9,-2.34,;-8.57,-4.65,;-3.23,-3.12,;-1.89,-2.34,;3.44,-.78,;4.77,-.01,;3.44,-2.32,;2.1,-3.09,;4.77,-3.09,;3.42,-3.86,;10.02,-.13,;11.35,-.89,;12.68,-.11,;11.37,-2.42,;10.03,-3.21,;8.69,-2.44,;8.69,-.9,;7.35,-.14,)| Show InChI InChI=1S/C30H28Cl2F3N3O4/c1-17(2)28-20(27(37-42-28)26-21(31)5-4-6-22(26)32)16-41-24-13-12-23(29(36-24)30(33,34)35)38(3)15-19-9-7-18(8-10-19)11-14-25(39)40/h4-10,12-13,17H,11,14-16H2,1-3H3,(H,39,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at human GST-fused FXR LBD assessed as cofactor peptide interaction with receptor ligand binding domain by FRET assay |
Bioorg Med Chem Lett 20: 4911-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.084
BindingDB Entry DOI: 10.7270/Q21V5F52 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50323544
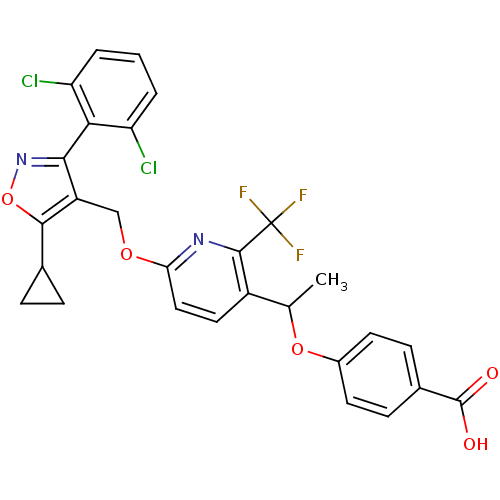
(4-(1-(6-((5-cyclopropyl-3-(2,6-dichlorophenyl)isox...)Show SMILES CC(Oc1ccc(cc1)C(O)=O)c1ccc(OCc2c(onc2-c2c(Cl)cccc2Cl)C2CC2)nc1C(F)(F)F |(19.89,-47.58,;19.89,-46.04,;18.55,-45.27,;17.22,-46.05,;17.23,-47.6,;15.89,-48.37,;14.56,-47.6,;14.56,-46.05,;15.89,-45.28,;13.22,-48.37,;11.89,-47.59,;13.22,-49.91,;21.22,-45.26,;21.21,-43.73,;22.53,-42.95,;23.88,-43.72,;25.22,-42.94,;26.55,-43.71,;27.88,-42.94,;28.35,-41.47,;29.9,-41.47,;30.38,-42.93,;29.13,-43.85,;29.14,-45.38,;30.47,-46.14,;31.8,-45.36,;30.48,-47.67,;29.15,-48.46,;27.81,-47.69,;27.81,-46.15,;26.47,-45.39,;27.43,-40.23,;27.27,-38.7,;26.02,-39.61,;23.88,-45.26,;22.55,-46.03,;22.56,-47.57,;21.22,-48.34,;23.89,-48.34,;22.54,-49.11,)| Show InChI InChI=1S/C28H21Cl2F3N2O5/c1-14(39-17-9-7-16(8-10-17)27(36)37)18-11-12-22(34-26(18)28(31,32)33)38-13-19-24(35-40-25(19)15-5-6-15)23-20(29)3-2-4-21(23)30/h2-4,7-12,14-15H,5-6,13H2,1H3,(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 189 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at human GST-fused FXR LBD expressed in HEK293 cells coexpressing GAL4-DNA bindig domain and pFRluc by mammalian one-hybrid assay |
Bioorg Med Chem Lett 20: 4911-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.084
BindingDB Entry DOI: 10.7270/Q21V5F52 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50323527
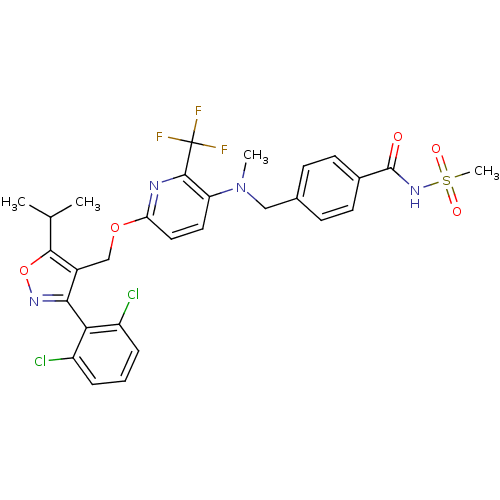
(4-(((6-((3-(2,6-dichlorophenyl)-5-isopropylisoxazo...)Show SMILES CC(C)c1onc(c1COc1ccc(N(C)Cc2ccc(cc2)C(=O)NS(C)(=O)=O)c(n1)C(F)(F)F)-c1c(Cl)cccc1Cl |(8.76,-5.27,;8.14,-6.68,;6.61,-6.85,;9.05,-7.92,;10.6,-7.92,;11.09,-9.39,;9.84,-10.3,;8.58,-9.4,;7.25,-10.16,;5.92,-9.4,;4.58,-10.17,;3.24,-9.41,;1.91,-10.18,;1.92,-11.71,;.59,-12.49,;.6,-14.03,;-.74,-11.72,;-2.08,-12.5,;-2.07,-14.05,;-3.41,-14.82,;-4.74,-14.05,;-4.74,-12.51,;-3.41,-11.74,;-6.08,-14.82,;-6.08,-16.36,;-7.41,-14.05,;-8.74,-14.82,;-10.08,-14.05,;-9.53,-16.15,;-7.99,-16.15,;3.26,-12.49,;4.59,-11.71,;3.26,-14.03,;1.93,-14.8,;4.59,-14.79,;3.24,-15.56,;9.84,-11.83,;11.18,-12.59,;12.5,-11.81,;11.19,-14.13,;9.85,-14.91,;8.51,-14.15,;8.51,-12.61,;7.17,-11.84,)| Show InChI InChI=1S/C29H27Cl2F3N4O5S/c1-16(2)26-19(25(36-43-26)24-20(30)6-5-7-21(24)31)15-42-23-13-12-22(27(35-23)29(32,33)34)38(3)14-17-8-10-18(11-9-17)28(39)37-44(4,40)41/h5-13,16H,14-15H2,1-4H3,(H,37,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at human GST-fused FXR LBD assessed as cofactor peptide interaction with receptor ligand binding domain by FRET assay |
Bioorg Med Chem Lett 20: 4911-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.084
BindingDB Entry DOI: 10.7270/Q21V5F52 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50323526
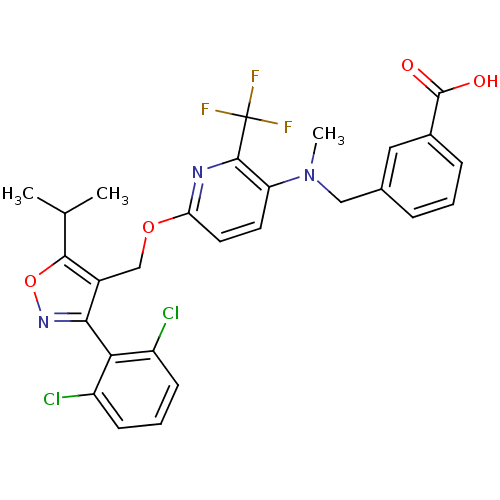
(3-(((6-((3-(2,6-dichlorophenyl)-5-isopropylisoxazo...)Show SMILES CC(C)c1onc(c1COc1ccc(N(C)Cc2cccc(c2)C(O)=O)c(n1)C(F)(F)F)-c1c(Cl)cccc1Cl |(27.52,7.37,;26.9,5.96,;25.37,5.79,;27.81,4.72,;29.36,4.72,;29.85,3.25,;28.6,2.34,;27.34,3.24,;26.01,2.48,;24.68,3.24,;23.34,2.47,;22,3.23,;20.67,2.45,;20.68,.92,;19.35,.15,;19.36,-1.39,;18.02,.91,;16.68,.14,;15.35,.9,;14.02,.13,;14.02,-1.41,;15.35,-2.18,;16.69,-1.41,;15.35,-3.72,;14.02,-4.49,;16.69,-4.49,;22.02,.15,;23.35,.93,;22.02,-1.39,;20.69,-2.16,;23.35,-2.16,;22,-2.92,;28.6,.81,;29.94,.05,;31.26,.83,;29.95,-1.49,;28.61,-2.27,;27.27,-1.51,;27.27,.03,;25.93,.8,)| Show InChI InChI=1S/C28H24Cl2F3N3O4/c1-15(2)25-18(24(35-40-25)23-19(29)8-5-9-20(23)30)14-39-22-11-10-21(26(34-22)28(31,32)33)36(3)13-16-6-4-7-17(12-16)27(37)38/h4-12,15H,13-14H2,1-3H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at human GST-fused FXR LBD assessed as cofactor peptide interaction with receptor ligand binding domain by FRET assay |
Bioorg Med Chem Lett 20: 4911-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.084
BindingDB Entry DOI: 10.7270/Q21V5F52 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50323525

(3-(4-(((6-((3-(2,6-dichlorophenyl)-5-isopropylisox...)Show SMILES CC(C)c1onc(c1COc1ccc(N(C)Cc2ccc(CCC(O)=O)cc2)c(n1)C(F)(F)F)-c1c(Cl)cccc1Cl |(8.94,6.43,;8.32,5.03,;6.79,4.86,;9.23,3.78,;10.78,3.79,;11.26,2.32,;10.02,1.4,;8.76,2.31,;7.43,1.54,;6.1,2.31,;4.76,1.53,;3.41,2.3,;2.09,1.52,;2.1,-.01,;.77,-.78,;.78,-2.32,;-.57,-.02,;-1.9,-.79,;-3.23,-.03,;-4.56,-.8,;-4.56,-2.35,;-5.9,-3.11,;-7.23,-2.34,;-8.56,-3.11,;-9.9,-2.34,;-8.57,-4.65,;-3.23,-3.12,;-1.89,-2.34,;3.44,-.78,;4.77,-.01,;3.44,-2.32,;2.1,-3.09,;4.77,-3.09,;3.42,-3.86,;10.02,-.13,;11.35,-.89,;12.68,-.11,;11.37,-2.42,;10.03,-3.21,;8.69,-2.44,;8.69,-.9,;7.35,-.14,)| Show InChI InChI=1S/C30H28Cl2F3N3O4/c1-17(2)28-20(27(37-42-28)26-21(31)5-4-6-22(26)32)16-41-24-13-12-23(29(36-24)30(33,34)35)38(3)15-19-9-7-18(8-10-19)11-14-25(39)40/h4-10,12-13,17H,11,14-16H2,1-3H3,(H,39,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 35 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at human GST-fused FXR LBD expressed in HEK293 cells coexpressing GAL4-DNA bindig domain and pFRluc by mammalian one-hybrid assay |
Bioorg Med Chem Lett 20: 4911-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.084
BindingDB Entry DOI: 10.7270/Q21V5F52 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50323527

(4-(((6-((3-(2,6-dichlorophenyl)-5-isopropylisoxazo...)Show SMILES CC(C)c1onc(c1COc1ccc(N(C)Cc2ccc(cc2)C(=O)NS(C)(=O)=O)c(n1)C(F)(F)F)-c1c(Cl)cccc1Cl |(8.76,-5.27,;8.14,-6.68,;6.61,-6.85,;9.05,-7.92,;10.6,-7.92,;11.09,-9.39,;9.84,-10.3,;8.58,-9.4,;7.25,-10.16,;5.92,-9.4,;4.58,-10.17,;3.24,-9.41,;1.91,-10.18,;1.92,-11.71,;.59,-12.49,;.6,-14.03,;-.74,-11.72,;-2.08,-12.5,;-2.07,-14.05,;-3.41,-14.82,;-4.74,-14.05,;-4.74,-12.51,;-3.41,-11.74,;-6.08,-14.82,;-6.08,-16.36,;-7.41,-14.05,;-8.74,-14.82,;-10.08,-14.05,;-9.53,-16.15,;-7.99,-16.15,;3.26,-12.49,;4.59,-11.71,;3.26,-14.03,;1.93,-14.8,;4.59,-14.79,;3.24,-15.56,;9.84,-11.83,;11.18,-12.59,;12.5,-11.81,;11.19,-14.13,;9.85,-14.91,;8.51,-14.15,;8.51,-12.61,;7.17,-11.84,)| Show InChI InChI=1S/C29H27Cl2F3N4O5S/c1-16(2)26-19(25(36-43-26)24-20(30)6-5-7-21(24)31)15-42-23-13-12-22(27(35-23)29(32,33)34)38(3)14-17-8-10-18(11-9-17)28(39)37-44(4,40)41/h5-13,16H,14-15H2,1-4H3,(H,37,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 221 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at human GST-fused FXR LBD expressed in HEK293 cells coexpressing GAL4-DNA bindig domain and pFRluc by mammalian one-hybrid assay |
Bioorg Med Chem Lett 20: 4911-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.084
BindingDB Entry DOI: 10.7270/Q21V5F52 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50323543

(3-(1-(6-((5-cyclopropyl-3-(2,6-dichlorophenyl)isox...)Show SMILES CC(Oc1cccc(c1)C(O)=O)c1ccc(OCc2c(onc2-c2c(Cl)cccc2Cl)C2CC2)nc1C(F)(F)F |(-2.38,-48.45,;-2.39,-46.91,;-3.72,-46.14,;-5.05,-46.92,;-5.05,-48.47,;-6.39,-49.24,;-7.72,-48.47,;-7.72,-46.92,;-6.39,-46.15,;-9.05,-46.15,;-10.39,-46.92,;-9.05,-44.61,;-1.06,-46.13,;-1.07,-44.6,;.26,-43.82,;1.6,-44.59,;2.94,-43.81,;4.28,-44.58,;5.6,-43.81,;6.07,-42.34,;7.62,-42.34,;8.11,-43.8,;6.86,-44.72,;6.86,-46.25,;8.2,-47.01,;9.52,-46.23,;8.21,-48.55,;6.87,-49.33,;5.54,-48.56,;5.53,-47.02,;4.19,-46.26,;5.16,-41.1,;4.99,-39.57,;3.75,-40.48,;1.61,-46.13,;.28,-46.9,;.28,-48.44,;-1.05,-49.21,;1.61,-49.21,;.26,-49.98,)| Show InChI InChI=1S/C28H21Cl2F3N2O5/c1-14(39-17-5-2-4-16(12-17)27(36)37)18-10-11-22(34-26(18)28(31,32)33)38-13-19-24(35-40-25(19)15-8-9-15)23-20(29)6-3-7-21(23)30/h2-7,10-12,14-15H,8-9,13H2,1H3,(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 155 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at human GST-fused FXR LBD assessed as cofactor peptide interaction with receptor ligand binding domain by FRET assay |
Bioorg Med Chem Lett 20: 4911-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.084
BindingDB Entry DOI: 10.7270/Q21V5F52 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50323524

(4-(((6-((3-(2,6-dichlorophenyl)-5-isopropylisoxazo...)Show SMILES CC(C)c1onc(c1COc1ccc(N(C)Cc2ccc(cc2)C(O)=O)c(n1)C(F)(F)F)-c1c(Cl)cccc1Cl |(29.01,-37.57,;28.39,-38.98,;26.86,-39.15,;29.3,-40.22,;30.85,-40.22,;31.33,-41.69,;30.08,-42.6,;28.83,-41.7,;27.5,-42.46,;26.16,-41.7,;24.83,-42.47,;23.48,-41.71,;22.16,-42.49,;22.17,-44.02,;20.84,-44.79,;20.84,-46.33,;19.5,-44.03,;18.17,-44.8,;18.18,-46.35,;16.84,-47.12,;15.5,-46.35,;15.51,-44.81,;16.84,-44.04,;14.17,-47.12,;12.84,-46.35,;14.17,-48.66,;23.5,-44.79,;24.83,-44.01,;23.5,-46.33,;22.17,-47.1,;24.84,-47.1,;23.49,-47.86,;30.09,-44.13,;31.42,-44.89,;32.75,-44.11,;31.43,-46.43,;30.1,-47.21,;28.76,-46.45,;28.75,-44.91,;27.42,-44.14,)| Show InChI InChI=1S/C28H24Cl2F3N3O4/c1-15(2)25-18(24(35-40-25)23-19(29)5-4-6-20(23)30)14-39-22-12-11-21(26(34-22)28(31,32)33)36(3)13-16-7-9-17(10-8-16)27(37)38/h4-12,15H,13-14H2,1-3H3,(H,37,38) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at LXRbeta-LBD expressed in HEK293 cells assessed as Gal4-DBD interaction by cellular mammalian one hybrid assay |
Bioorg Med Chem Lett 20: 4911-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.084
BindingDB Entry DOI: 10.7270/Q21V5F52 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50323526

(3-(((6-((3-(2,6-dichlorophenyl)-5-isopropylisoxazo...)Show SMILES CC(C)c1onc(c1COc1ccc(N(C)Cc2cccc(c2)C(O)=O)c(n1)C(F)(F)F)-c1c(Cl)cccc1Cl |(27.52,7.37,;26.9,5.96,;25.37,5.79,;27.81,4.72,;29.36,4.72,;29.85,3.25,;28.6,2.34,;27.34,3.24,;26.01,2.48,;24.68,3.24,;23.34,2.47,;22,3.23,;20.67,2.45,;20.68,.92,;19.35,.15,;19.36,-1.39,;18.02,.91,;16.68,.14,;15.35,.9,;14.02,.13,;14.02,-1.41,;15.35,-2.18,;16.69,-1.41,;15.35,-3.72,;14.02,-4.49,;16.69,-4.49,;22.02,.15,;23.35,.93,;22.02,-1.39,;20.69,-2.16,;23.35,-2.16,;22,-2.92,;28.6,.81,;29.94,.05,;31.26,.83,;29.95,-1.49,;28.61,-2.27,;27.27,-1.51,;27.27,.03,;25.93,.8,)| Show InChI InChI=1S/C28H24Cl2F3N3O4/c1-15(2)25-18(24(35-40-25)23-19(29)8-5-9-20(23)30)14-39-22-11-10-21(26(34-22)28(31,32)33)36(3)13-16-6-4-7-17(12-16)27(37)38/h4-12,15H,13-14H2,1-3H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 80 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at human GST-fused FXR LBD expressed in HEK293 cells coexpressing GAL4-DNA bindig domain and pFRluc by mammalian one-hybrid assay |
Bioorg Med Chem Lett 20: 4911-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.084
BindingDB Entry DOI: 10.7270/Q21V5F52 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50323528
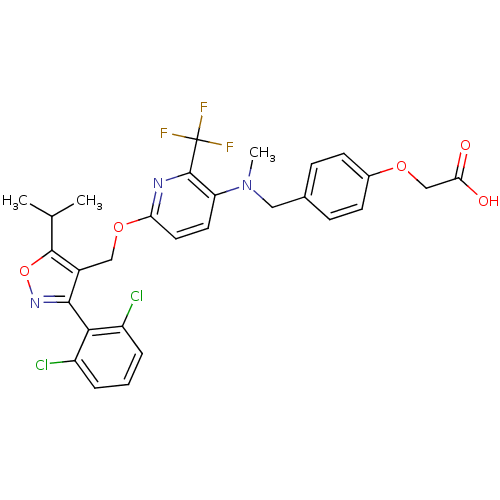
(2-(4-(((6-((3-(2,6-dichlorophenyl)-5-isopropylisox...)Show SMILES CC(C)c1onc(c1COc1ccc(N(C)Cc2ccc(OCC(O)=O)cc2)c(n1)C(F)(F)F)-c1c(Cl)cccc1Cl |(32.35,-3.82,;31.73,-5.23,;30.2,-5.4,;32.64,-6.48,;34.19,-6.47,;34.68,-7.94,;33.43,-8.85,;32.17,-7.95,;30.84,-8.72,;29.51,-7.95,;28.17,-8.72,;26.83,-7.96,;25.5,-8.74,;25.51,-10.27,;24.18,-11.04,;24.19,-12.58,;22.85,-10.28,;21.51,-11.05,;21.52,-12.6,;20.18,-13.38,;18.85,-12.6,;17.51,-13.37,;16.18,-12.6,;14.85,-13.37,;13.51,-12.6,;14.85,-14.91,;18.85,-11.06,;20.18,-10.29,;26.85,-11.04,;28.18,-10.27,;26.85,-12.58,;25.52,-13.35,;28.18,-13.35,;26.83,-14.12,;33.43,-10.39,;34.77,-11.15,;36.09,-10.37,;34.78,-12.68,;33.44,-13.47,;32.1,-12.7,;32.1,-11.16,;30.76,-10.4,)| Show InChI InChI=1S/C29H26Cl2F3N3O5/c1-16(2)27-19(26(36-42-27)25-20(30)5-4-6-21(25)31)14-41-23-12-11-22(28(35-23)29(32,33)34)37(3)13-17-7-9-18(10-8-17)40-15-24(38)39/h4-12,16H,13-15H2,1-3H3,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 214 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at human GST-fused FXR LBD expressed in HEK293 cells coexpressing GAL4-DNA bindig domain and pFRluc by mammalian one-hybrid assay |
Bioorg Med Chem Lett 20: 4911-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.084
BindingDB Entry DOI: 10.7270/Q21V5F52 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50323544

(4-(1-(6-((5-cyclopropyl-3-(2,6-dichlorophenyl)isox...)Show SMILES CC(Oc1ccc(cc1)C(O)=O)c1ccc(OCc2c(onc2-c2c(Cl)cccc2Cl)C2CC2)nc1C(F)(F)F |(19.89,-47.58,;19.89,-46.04,;18.55,-45.27,;17.22,-46.05,;17.23,-47.6,;15.89,-48.37,;14.56,-47.6,;14.56,-46.05,;15.89,-45.28,;13.22,-48.37,;11.89,-47.59,;13.22,-49.91,;21.22,-45.26,;21.21,-43.73,;22.53,-42.95,;23.88,-43.72,;25.22,-42.94,;26.55,-43.71,;27.88,-42.94,;28.35,-41.47,;29.9,-41.47,;30.38,-42.93,;29.13,-43.85,;29.14,-45.38,;30.47,-46.14,;31.8,-45.36,;30.48,-47.67,;29.15,-48.46,;27.81,-47.69,;27.81,-46.15,;26.47,-45.39,;27.43,-40.23,;27.27,-38.7,;26.02,-39.61,;23.88,-45.26,;22.55,-46.03,;22.56,-47.57,;21.22,-48.34,;23.89,-48.34,;22.54,-49.11,)| Show InChI InChI=1S/C28H21Cl2F3N2O5/c1-14(39-17-9-7-16(8-10-17)27(36)37)18-11-12-22(34-26(18)28(31,32)33)38-13-19-24(35-40-25(19)15-5-6-15)23-20(29)3-2-4-21(23)30/h2-4,7-12,14-15H,5-6,13H2,1H3,(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 352 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at human GST-fused FXR LBD assessed as cofactor peptide interaction with receptor ligand binding domain by FRET assay |
Bioorg Med Chem Lett 20: 4911-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.084
BindingDB Entry DOI: 10.7270/Q21V5F52 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50323540

(2-((6-((5-cyclopropyl-3-(2,6-dichlorophenyl)isoxaz...)Show SMILES OC(=O)c1ccccc1OCc1ccc(OCc2c(noc2C2CC2)-c2c(Cl)cccc2Cl)nc1C(F)(F)F |(13.66,-21.28,;14.99,-22.05,;16.33,-21.28,;15,-23.59,;13.67,-24.36,;13.67,-25.9,;15,-26.68,;16.34,-25.9,;16.33,-24.35,;17.67,-23.58,;19,-24.34,;20.33,-23.57,;20.32,-22.04,;21.65,-21.26,;22.99,-22.02,;24.33,-21.25,;25.66,-22.02,;26.99,-21.25,;28.25,-22.15,;29.5,-21.24,;29.01,-19.77,;27.46,-19.78,;26.55,-18.54,;26.38,-17.01,;25.14,-17.92,;28.25,-23.69,;29.59,-24.45,;30.91,-23.67,;29.6,-25.98,;28.26,-26.77,;26.92,-26,;26.92,-24.46,;25.58,-23.7,;23,-23.57,;21.67,-24.34,;21.67,-25.88,;20.34,-26.65,;23,-26.65,;21.65,-27.42,)| Show InChI InChI=1S/C27H19Cl2F3N2O5/c28-18-5-3-6-19(29)22(18)23-17(24(39-34-23)14-8-9-14)13-38-21-11-10-15(25(33-21)27(30,31)32)12-37-20-7-2-1-4-16(20)26(35)36/h1-7,10-11,14H,8-9,12-13H2,(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 611 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at human full length FXR transfected in HEK293 cells coexpressing pTRexDest/pGL2promotor assessed as luciferase activity by direct r... |
Bioorg Med Chem Lett 20: 4911-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.084
BindingDB Entry DOI: 10.7270/Q21V5F52 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50323542
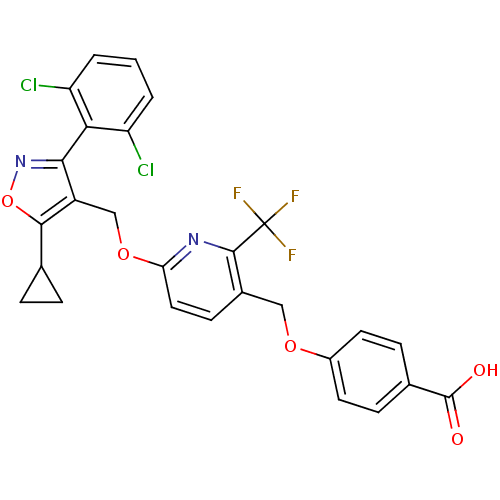
(4-((6-((5-cyclopropyl-3-(2,6-dichlorophenyl)isoxaz...)Show SMILES OC(=O)c1ccc(OCc2ccc(OCc3c(noc3C3CC3)-c3c(Cl)cccc3Cl)nc2C(F)(F)F)cc1 |(11.81,-36.69,;13.14,-37.46,;13.14,-39,;14.48,-36.69,;15.81,-37.46,;17.15,-36.69,;17.14,-35.14,;18.47,-34.37,;19.81,-35.13,;21.14,-34.36,;21.13,-32.83,;22.45,-32.05,;23.8,-32.81,;25.14,-32.04,;26.47,-32.8,;27.8,-32.04,;29.06,-32.94,;30.3,-32.03,;29.82,-30.56,;28.27,-30.56,;27.35,-29.33,;27.19,-27.8,;25.95,-28.71,;29.06,-34.47,;30.39,-35.23,;31.72,-34.45,;30.41,-36.77,;29.07,-37.55,;27.73,-36.79,;27.73,-35.25,;26.39,-34.48,;23.81,-34.35,;22.48,-35.13,;22.48,-36.67,;21.14,-37.44,;23.81,-37.44,;22.46,-38.2,;15.81,-34.38,;14.48,-35.15,)| Show InChI InChI=1S/C27H19Cl2F3N2O5/c28-19-2-1-3-20(29)22(19)23-18(24(39-34-23)14-4-5-14)13-38-21-11-8-16(25(33-21)27(30,31)32)12-37-17-9-6-15(7-10-17)26(35)36/h1-3,6-11,14H,4-5,12-13H2,(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 95 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at human GST-fused FXR LBD expressed in HEK293 cells coexpressing GAL4-DNA bindig domain and pFRluc by mammalian one-hybrid assay |
Bioorg Med Chem Lett 20: 4911-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.084
BindingDB Entry DOI: 10.7270/Q21V5F52 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50323541
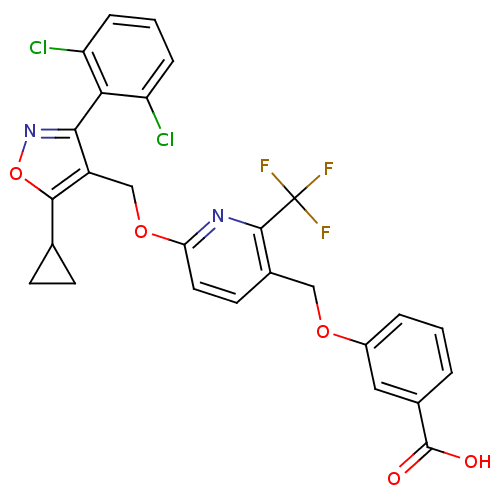
(3-((6-((5-cyclopropyl-3-(2,6-dichlorophenyl)isoxaz...)Show SMILES OC(=O)c1cccc(OCc2ccc(OCc3c(noc3C3CC3)-c3c(Cl)cccc3Cl)nc2C(F)(F)F)c1 |(-9.62,-34.92,;-8.29,-34.14,;-8.29,-32.6,;-6.96,-34.91,;-6.96,-36.46,;-5.62,-37.23,;-4.29,-36.46,;-4.29,-34.91,;-2.96,-34.13,;-1.62,-34.9,;-.29,-34.12,;-.31,-32.59,;1.02,-31.81,;2.36,-32.58,;3.7,-31.8,;5.04,-32.57,;6.36,-31.8,;7.62,-32.71,;8.87,-31.79,;8.39,-30.33,;6.83,-30.33,;5.92,-29.1,;5.75,-27.56,;4.51,-28.47,;7.63,-34.24,;8.96,-35,;10.29,-34.22,;8.97,-36.54,;7.64,-37.32,;6.3,-36.55,;6.29,-35.02,;4.96,-34.25,;2.37,-34.12,;1.04,-34.89,;1.04,-36.43,;-.29,-37.21,;2.38,-37.2,;1.03,-37.97,;-5.63,-34.14,)| Show InChI InChI=1S/C27H19Cl2F3N2O5/c28-19-5-2-6-20(29)22(19)23-18(24(39-34-23)14-7-8-14)13-38-21-10-9-16(25(33-21)27(30,31)32)12-37-17-4-1-3-15(11-17)26(35)36/h1-6,9-11,14H,7-8,12-13H2,(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 33 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at human full length FXR transfected in HEK293 cells coexpressing pTRexDest/pGL2promotor assessed as luciferase activity by direct r... |
Bioorg Med Chem Lett 20: 4911-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.084
BindingDB Entry DOI: 10.7270/Q21V5F52 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50323517
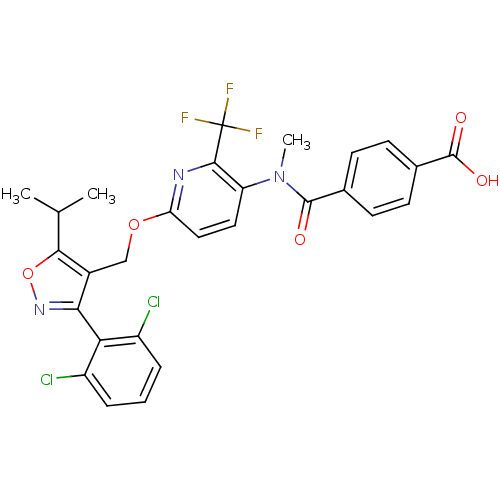
(4-((6-((3-(2,6-dichlorophenyl)-5-isopropylisoxazol...)Show SMILES CC(C)c1onc(c1COc1ccc(N(C)C(=O)c2ccc(cc2)C(O)=O)c(n1)C(F)(F)F)-c1c(Cl)cccc1Cl |(28.16,6.8,;27.54,5.39,;26.01,5.23,;28.45,4.15,;30,4.15,;30.48,2.69,;29.23,1.77,;27.98,2.68,;26.65,1.9,;25.31,2.66,;23.98,1.88,;22.63,2.65,;21.31,1.87,;21.32,.34,;19.99,-.43,;20,-1.97,;18.65,.33,;18.65,1.87,;17.32,-.44,;15.99,.32,;14.66,-.45,;14.66,-2,;15.99,-2.77,;17.33,-1.99,;13.32,-2.76,;11.99,-1.99,;13.32,-4.3,;22.65,-.43,;23.98,.34,;22.66,-1.97,;21.32,-2.74,;23.99,-2.74,;22.64,-3.51,;29.24,.24,;30.57,-.52,;31.9,.26,;30.58,-2.06,;29.25,-2.84,;27.91,-2.07,;27.9,-.54,;26.57,.23,)| Show InChI InChI=1S/C28H22Cl2F3N3O5/c1-14(2)24-17(23(35-41-24)22-18(29)5-4-6-19(22)30)13-40-21-12-11-20(25(34-21)28(31,32)33)36(3)26(37)15-7-9-16(10-8-15)27(38)39/h4-12,14H,13H2,1-3H3,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 208 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at human full length FXR transfected in HEK293 cells coexpressing pTRexDest/pGL2promotor assessed as luciferase activity by direct r... |
Bioorg Med Chem Lett 20: 4911-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.084
BindingDB Entry DOI: 10.7270/Q21V5F52 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50323519
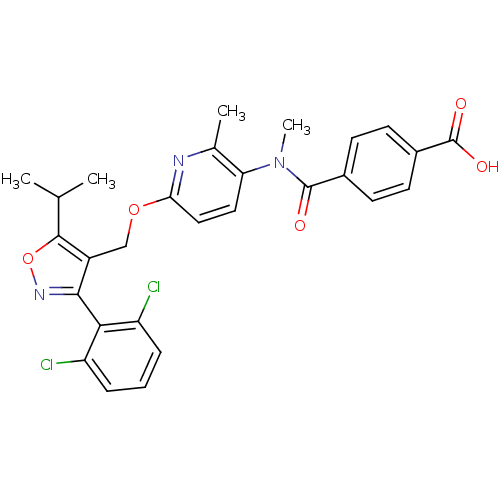
(4-((6-((3-(2,6-dichlorophenyl)-5-isopropylisoxazol...)Show SMILES CC(C)c1onc(c1COc1ccc(N(C)C(=O)c2ccc(cc2)C(O)=O)c(C)n1)-c1c(Cl)cccc1Cl |(29.05,-4.4,;28.42,-5.81,;26.89,-5.97,;29.33,-7.05,;30.89,-7.05,;31.37,-8.51,;30.12,-9.43,;28.86,-8.52,;27.53,-9.29,;26.2,-8.53,;24.87,-9.31,;23.52,-8.54,;22.2,-9.32,;22.21,-10.85,;20.88,-11.63,;20.88,-13.17,;19.54,-10.86,;19.53,-9.32,;18.21,-11.64,;16.87,-10.87,;15.55,-11.64,;15.54,-13.19,;16.88,-13.96,;18.21,-13.19,;14.21,-13.96,;12.88,-13.19,;14.21,-15.5,;23.54,-11.62,;23.54,-13.16,;24.87,-10.85,;30.13,-10.96,;31.46,-11.72,;32.79,-10.94,;31.47,-13.26,;30.13,-14.04,;28.8,-13.27,;28.79,-11.74,;27.46,-10.97,)| Show InChI InChI=1S/C28H25Cl2N3O5/c1-15(2)26-19(25(32-38-26)24-20(29)6-5-7-21(24)30)14-37-23-13-12-22(16(3)31-23)33(4)27(34)17-8-10-18(11-9-17)28(35)36/h5-13,15H,14H2,1-4H3,(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.03E+3 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at human GST-fused FXR LBD expressed in HEK293 cells coexpressing GAL4-DNA bindig domain and pFRluc by mammalian one-hybrid assay |
Bioorg Med Chem Lett 20: 4911-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.084
BindingDB Entry DOI: 10.7270/Q21V5F52 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50323518
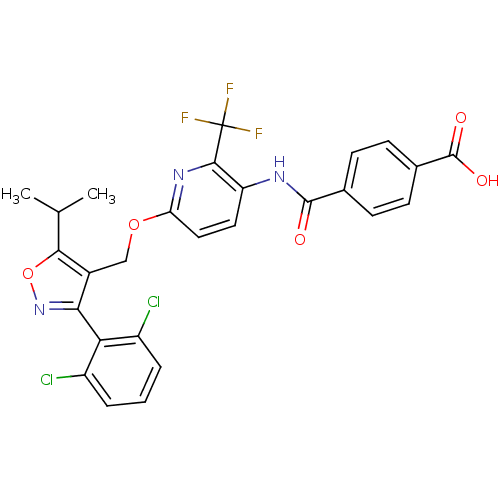
(4-(6-((3-(2,6-dichlorophenyl)-5-isopropylisoxazol-...)Show SMILES CC(C)c1onc(c1COc1ccc(NC(=O)c2ccc(cc2)C(O)=O)c(n1)C(F)(F)F)-c1c(Cl)cccc1Cl |(6.31,-3.35,;5.69,-4.76,;4.16,-4.92,;6.6,-6,;8.15,-6,;8.63,-7.46,;7.38,-8.38,;6.13,-7.47,;4.8,-8.25,;3.46,-7.49,;2.13,-8.27,;.79,-7.5,;-.54,-8.28,;-.53,-9.81,;-1.86,-10.58,;-3.19,-9.82,;-3.2,-8.28,;-4.53,-10.59,;-5.86,-9.83,;-7.19,-10.6,;-7.19,-12.15,;-5.86,-12.92,;-4.52,-12.14,;-8.53,-12.91,;-9.86,-12.14,;-8.53,-14.45,;.81,-10.58,;2.14,-9.81,;.81,-12.12,;-.52,-12.89,;2.14,-12.89,;.79,-13.66,;7.39,-9.91,;8.72,-10.67,;10.05,-9.89,;8.74,-12.21,;7.4,-12.99,;6.06,-12.22,;6.06,-10.69,;4.72,-9.92,)| Show InChI InChI=1S/C27H20Cl2F3N3O5/c1-13(2)23-16(22(35-40-23)21-17(28)4-3-5-18(21)29)12-39-20-11-10-19(24(34-20)27(30,31)32)33-25(36)14-6-8-15(9-7-14)26(37)38/h3-11,13H,12H2,1-2H3,(H,33,36)(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 474 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at human GST-fused FXR LBD expressed in HEK293 cells coexpressing GAL4-DNA bindig domain and pFRluc by mammalian one-hybrid assay |
Bioorg Med Chem Lett 20: 4911-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.084
BindingDB Entry DOI: 10.7270/Q21V5F52 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50323529
(2-(4-(((6-((3-(2,6-dichlorophenyl)-5-isopropylisox...)Show SMILES CC(C)c1onc(c1COc1ccc(N(C)Cc2ccc(OCCO)cc2)c(n1)C(F)(F)F)-c1c(Cl)cccc1Cl |(8.88,-16.84,;8.26,-18.25,;6.73,-18.41,;9.17,-19.49,;10.72,-19.49,;11.2,-20.95,;9.95,-21.87,;8.7,-20.96,;7.37,-21.73,;6.04,-20.96,;4.7,-21.74,;3.35,-20.97,;2.03,-21.75,;2.04,-23.28,;.71,-24.06,;.71,-25.6,;-.63,-23.29,;-1.96,-24.07,;-1.95,-25.62,;-3.29,-26.39,;-4.62,-25.62,;-5.96,-26.39,;-7.29,-25.61,;-8.63,-26.38,;-9.96,-25.61,;-4.62,-24.07,;-3.29,-23.3,;3.37,-24.05,;4.7,-23.28,;3.38,-25.59,;2.04,-26.36,;4.71,-26.36,;3.36,-27.13,;9.96,-23.4,;11.29,-24.16,;12.62,-23.38,;11.3,-25.69,;9.97,-26.48,;8.63,-25.71,;8.63,-24.17,;7.29,-23.41,)| Show InChI InChI=1S/C29H28Cl2F3N3O4/c1-17(2)27-20(26(36-41-27)25-21(30)5-4-6-22(25)31)16-40-24-12-11-23(28(35-24)29(32,33)34)37(3)15-18-7-9-19(10-8-18)39-14-13-38/h4-12,17,38H,13-16H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 155 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at human full length FXR transfected in HEK293 cells coexpressing pTRexDest/pGL2promotor assessed as luciferase activity by direct r... |
Bioorg Med Chem Lett 20: 4911-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.084
BindingDB Entry DOI: 10.7270/Q21V5F52 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50323533
(4-(((6-((5-cyclopropyl-3-(2,6-dichlorophenyl)isoxa...)Show SMILES CN(Cc1ccc(cc1)C(O)=O)c1ccc(OCc2c(onc2-c2c(Cl)cccc2Cl)C2CC2)nc1C(F)(F)F |(-1.96,-48.67,;-1.97,-47.13,;-3.3,-46.37,;-4.63,-47.14,;-4.63,-48.69,;-5.97,-49.46,;-7.3,-48.69,;-7.3,-47.15,;-5.97,-46.38,;-8.63,-49.46,;-9.97,-48.69,;-8.64,-51,;-.64,-46.36,;-.65,-44.83,;.68,-44.05,;2.02,-44.81,;3.36,-44.04,;4.7,-44.81,;6.02,-44.04,;6.49,-42.57,;8.04,-42.56,;8.53,-44.03,;7.28,-44.94,;7.28,-46.48,;8.62,-47.24,;9.94,-46.46,;8.63,-48.77,;7.29,-49.55,;5.96,-48.79,;5.95,-47.25,;4.61,-46.48,;5.58,-41.33,;5.41,-39.8,;4.17,-40.71,;2.03,-46.35,;.7,-47.13,;.7,-48.67,;-.63,-49.44,;2.03,-49.44,;.68,-50.21,)| Show InChI InChI=1S/C28H22Cl2F3N3O4/c1-36(13-15-5-7-17(8-6-15)27(37)38)21-11-12-22(34-26(21)28(31,32)33)39-14-18-24(35-40-25(18)16-9-10-16)23-19(29)3-2-4-20(23)30/h2-8,11-12,16H,9-10,13-14H2,1H3,(H,37,38) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at LXRbeta-LBD expressed in HEK293 cells assessed as Gal4-DBD interaction by cellular mammalian one hybrid assay |
Bioorg Med Chem Lett 20: 4911-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.084
BindingDB Entry DOI: 10.7270/Q21V5F52 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50323529

(2-(4-(((6-((3-(2,6-dichlorophenyl)-5-isopropylisox...)Show SMILES CC(C)c1onc(c1COc1ccc(N(C)Cc2ccc(OCCO)cc2)c(n1)C(F)(F)F)-c1c(Cl)cccc1Cl |(8.88,-16.84,;8.26,-18.25,;6.73,-18.41,;9.17,-19.49,;10.72,-19.49,;11.2,-20.95,;9.95,-21.87,;8.7,-20.96,;7.37,-21.73,;6.04,-20.96,;4.7,-21.74,;3.35,-20.97,;2.03,-21.75,;2.04,-23.28,;.71,-24.06,;.71,-25.6,;-.63,-23.29,;-1.96,-24.07,;-1.95,-25.62,;-3.29,-26.39,;-4.62,-25.62,;-5.96,-26.39,;-7.29,-25.61,;-8.63,-26.38,;-9.96,-25.61,;-4.62,-24.07,;-3.29,-23.3,;3.37,-24.05,;4.7,-23.28,;3.38,-25.59,;2.04,-26.36,;4.71,-26.36,;3.36,-27.13,;9.96,-23.4,;11.29,-24.16,;12.62,-23.38,;11.3,-25.69,;9.97,-26.48,;8.63,-25.71,;8.63,-24.17,;7.29,-23.41,)| Show InChI InChI=1S/C29H28Cl2F3N3O4/c1-17(2)27-20(26(36-41-27)25-21(30)5-4-6-22(25)31)16-40-24-12-11-23(28(35-24)29(32,33)34)37(3)15-18-7-9-19(10-8-18)39-14-13-38/h4-12,17,38H,13-16H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 164 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at human GST-fused FXR LBD assessed as cofactor peptide interaction with receptor ligand binding domain by FRET assay |
Bioorg Med Chem Lett 20: 4911-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.084
BindingDB Entry DOI: 10.7270/Q21V5F52 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM21724

(3-[(E)-2-(2-chloro-4-{[3-(2,6-dichlorophenyl)-5-(1...)Show SMILES CC(C)c1onc(c1COc1ccc(\C=C\c2cccc(c2)C(O)=O)c(Cl)c1)-c1c(Cl)cccc1Cl |(-5,9.13,;-4.28,7.76,;-2.74,7.7,;-5.11,6.46,;-6.65,6.36,;-7.03,4.87,;-5.73,4.05,;-4.54,5.03,;-3.21,4.26,;-1.87,5.03,;-.54,4.26,;.79,5.03,;2.13,4.26,;2.13,2.72,;3.46,1.95,;4.79,2.72,;6.13,1.95,;6.13,.41,;7.46,-.36,;8.79,.41,;8.79,1.95,;7.46,2.72,;10.13,2.72,;11.46,1.95,;10.13,4.26,;.79,1.95,;.79,.41,;-.54,2.72,;-5.63,2.51,;-4.28,1.78,;-2.97,2.59,;-4.23,.24,;-5.54,-.57,;-6.9,.16,;-6.94,1.7,;-8.3,2.43,)| Show InChI InChI=1S/C28H22Cl3NO4/c1-16(2)27-21(26(32-36-27)25-22(29)7-4-8-23(25)30)15-35-20-12-11-18(24(31)14-20)10-9-17-5-3-6-19(13-17)28(33)34/h3-14,16H,15H2,1-2H3,(H,33,34)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 35 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at human GST-fused FXR LBD expressed in HEK293 cells coexpressing GAL4-DNA bindig domain and pFRluc by mammalian one-hybrid assay |
Bioorg Med Chem Lett 20: 4911-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.084
BindingDB Entry DOI: 10.7270/Q21V5F52 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50323530
(3-(((6-((5-cyclopropyl-3-(2,6-dichlorophenyl)isoxa...)Show SMILES CN(Cc1cccc(c1)C(O)=O)c1ccc(OCc2c(onc2-c2c(Cl)cccc2Cl)C2CC2)nc1C(F)(F)F |(21.34,-24.3,;21.34,-22.76,;20,-21.99,;18.67,-22.77,;17.33,-22,;16,-22.77,;16,-24.32,;17.34,-25.09,;18.67,-24.32,;17.34,-26.63,;16,-27.4,;18.67,-27.4,;22.67,-21.98,;22.65,-20.45,;23.98,-19.67,;25.32,-20.44,;26.66,-19.66,;28,-20.43,;29.32,-19.66,;29.79,-18.19,;31.35,-18.19,;31.83,-19.65,;30.58,-20.57,;30.59,-22.1,;31.92,-22.86,;33.25,-22.08,;31.93,-24.4,;30.6,-25.18,;29.26,-24.41,;29.25,-22.87,;27.92,-22.11,;28.88,-16.95,;28.71,-15.42,;27.47,-16.33,;25.33,-21.98,;24,-22.75,;24,-24.29,;22.67,-25.06,;25.34,-25.06,;23.99,-25.83,)| Show InChI InChI=1S/C28H22Cl2F3N3O4/c1-36(13-15-4-2-5-17(12-15)27(37)38)21-10-11-22(34-26(21)28(31,32)33)39-14-18-24(35-40-25(18)16-8-9-16)23-19(29)6-3-7-20(23)30/h2-7,10-12,16H,8-9,13-14H2,1H3,(H,37,38) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at LXRbeta-LBD expressed in HEK293 cells assessed as Gal4-DBD interaction by cellular mammalian one hybrid assay |
Bioorg Med Chem Lett 20: 4911-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.084
BindingDB Entry DOI: 10.7270/Q21V5F52 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50323537
(3-(((3-chloro-5-((3-(2,6-dichlorophenyl)-5-isoprop...)Show SMILES CC(C)c1onc(c1COc1cnc(N(C)Cc2cccc(c2)C(O)=O)c(Cl)c1)-c1c(Cl)cccc1Cl |(4.6,-5.15,;3.98,-6.55,;2.45,-6.72,;4.89,-7.8,;6.44,-7.79,;6.92,-9.26,;5.68,-10.18,;4.42,-9.27,;3.09,-10.04,;1.76,-9.27,;.42,-10.05,;-.93,-9.28,;-2.25,-10.06,;-2.24,-11.59,;-3.57,-12.36,;-3.56,-13.9,;-4.91,-11.6,;-6.24,-12.37,;-7.57,-11.61,;-8.9,-12.38,;-8.9,-13.93,;-7.57,-14.7,;-6.23,-13.92,;-7.57,-16.24,;-8.9,-17.01,;-6.23,-17.01,;-.9,-12.36,;-.9,-13.9,;.43,-11.59,;5.68,-11.71,;7.01,-12.47,;8.34,-11.69,;7.03,-14,;5.69,-14.79,;4.35,-14.02,;4.35,-12.48,;3.01,-11.72,)| Show InChI InChI=1S/C27H24Cl3N3O4/c1-15(2)25-19(24(32-37-25)23-20(28)8-5-9-21(23)29)14-36-18-11-22(30)26(31-12-18)33(3)13-16-6-4-7-17(10-16)27(34)35/h4-12,15H,13-14H2,1-3H3,(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 61 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at human full length FXR transfected in HEK293 cells coexpressing pTRexDest/pGL2promotor assessed as luciferase activity by direct r... |
Bioorg Med Chem Lett 20: 4911-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.084
BindingDB Entry DOI: 10.7270/Q21V5F52 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50323536
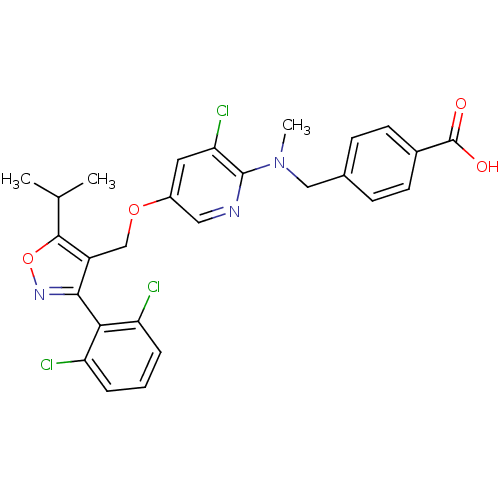
(4-(((3-chloro-5-((3-(2,6-dichlorophenyl)-5-isoprop...)Show SMILES CC(C)c1onc(c1COc1cnc(N(C)Cc2ccc(cc2)C(O)=O)c(Cl)c1)-c1c(Cl)cccc1Cl |(27.95,6.97,;27.33,5.56,;25.8,5.4,;28.24,4.32,;29.79,4.32,;30.27,2.86,;29.02,1.94,;27.77,2.85,;26.44,2.08,;25.11,2.85,;23.77,2.07,;22.42,2.84,;21.1,2.06,;21.11,.53,;19.78,-.25,;19.79,-1.79,;18.44,.52,;17.11,-.26,;17.12,-1.81,;15.78,-2.58,;14.45,-1.81,;14.45,-.26,;15.78,.51,;13.11,-2.58,;11.78,-1.81,;13.11,-4.12,;22.44,-.24,;22.45,-1.78,;23.77,.53,;29.03,.41,;30.36,-.35,;31.69,.43,;30.37,-1.89,;29.04,-2.67,;27.7,-1.9,;27.7,-.37,;26.36,.4,)| Show InChI InChI=1S/C27H24Cl3N3O4/c1-15(2)25-19(24(32-37-25)23-20(28)5-4-6-21(23)29)14-36-18-11-22(30)26(31-12-18)33(3)13-16-7-9-17(10-8-16)27(34)35/h4-12,15H,13-14H2,1-3H3,(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 242 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at human GST-fused FXR LBD assessed as cofactor peptide interaction with receptor ligand binding domain by FRET assay |
Bioorg Med Chem Lett 20: 4911-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.084
BindingDB Entry DOI: 10.7270/Q21V5F52 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50323530

(3-(((6-((5-cyclopropyl-3-(2,6-dichlorophenyl)isoxa...)Show SMILES CN(Cc1cccc(c1)C(O)=O)c1ccc(OCc2c(onc2-c2c(Cl)cccc2Cl)C2CC2)nc1C(F)(F)F |(21.34,-24.3,;21.34,-22.76,;20,-21.99,;18.67,-22.77,;17.33,-22,;16,-22.77,;16,-24.32,;17.34,-25.09,;18.67,-24.32,;17.34,-26.63,;16,-27.4,;18.67,-27.4,;22.67,-21.98,;22.65,-20.45,;23.98,-19.67,;25.32,-20.44,;26.66,-19.66,;28,-20.43,;29.32,-19.66,;29.79,-18.19,;31.35,-18.19,;31.83,-19.65,;30.58,-20.57,;30.59,-22.1,;31.92,-22.86,;33.25,-22.08,;31.93,-24.4,;30.6,-25.18,;29.26,-24.41,;29.25,-22.87,;27.92,-22.11,;28.88,-16.95,;28.71,-15.42,;27.47,-16.33,;25.33,-21.98,;24,-22.75,;24,-24.29,;22.67,-25.06,;25.34,-25.06,;23.99,-25.83,)| Show InChI InChI=1S/C28H22Cl2F3N3O4/c1-36(13-15-4-2-5-17(12-15)27(37)38)21-10-11-22(34-26(21)28(31,32)33)39-14-18-24(35-40-25(18)16-8-9-16)23-19(29)6-3-7-20(23)30/h2-7,10-12,16H,8-9,13-14H2,1H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 9 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at human GST-fused FXR LBD expressed in HEK293 cells coexpressing GAL4-DNA bindig domain and pFRluc by mammalian one-hybrid assay |
Bioorg Med Chem Lett 20: 4911-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.084
BindingDB Entry DOI: 10.7270/Q21V5F52 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50323534
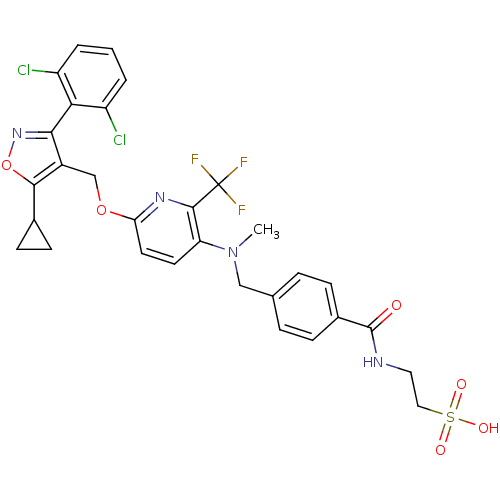
(2-(4-(((6-((5-cyclopropyl-3-(2,6-dichlorophenyl)is...)Show SMILES CN(Cc1ccc(cc1)C(=O)NCCS(O)(=O)=O)c1ccc(OCc2c(onc2-c2c(Cl)cccc2Cl)C2CC2)nc1C(F)(F)F |(25.42,-47.4,;25.41,-45.86,;24.07,-45.1,;22.74,-45.87,;22.75,-47.42,;21.41,-48.2,;20.08,-47.43,;20.08,-45.88,;21.41,-45.11,;18.74,-48.19,;18.74,-49.73,;17.41,-47.42,;16.08,-48.19,;14.74,-47.42,;13.41,-48.19,;12.08,-47.42,;12.62,-49.51,;14.16,-49.51,;26.74,-45.09,;26.73,-43.56,;28.05,-42.78,;29.4,-43.55,;30.74,-42.77,;32.07,-43.54,;33.4,-42.77,;33.87,-41.3,;35.42,-41.29,;35.9,-42.76,;34.66,-43.68,;34.66,-45.21,;35.99,-45.97,;37.32,-45.19,;36.01,-47.5,;34.67,-48.29,;33.33,-47.52,;33.33,-45.98,;31.99,-45.22,;32.95,-40.06,;32.79,-38.53,;31.55,-39.44,;29.41,-45.09,;28.08,-45.86,;28.08,-47.4,;26.74,-48.17,;29.41,-48.17,;28.06,-48.94,)| Show InChI InChI=1S/C30H27Cl2F3N4O6S/c1-39(15-17-5-7-19(8-6-17)29(40)36-13-14-46(41,42)43)23-11-12-24(37-28(23)30(33,34)35)44-16-20-26(38-45-27(20)18-9-10-18)25-21(31)3-2-4-22(25)32/h2-8,11-12,18H,9-10,13-16H2,1H3,(H,36,40)(H,41,42,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 925 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at human full length FXR transfected in HEK293 cells coexpressing pTRexDest/pGL2promotor assessed as luciferase activity by direct r... |
Bioorg Med Chem Lett 20: 4911-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.084
BindingDB Entry DOI: 10.7270/Q21V5F52 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50323533

(4-(((6-((5-cyclopropyl-3-(2,6-dichlorophenyl)isoxa...)Show SMILES CN(Cc1ccc(cc1)C(O)=O)c1ccc(OCc2c(onc2-c2c(Cl)cccc2Cl)C2CC2)nc1C(F)(F)F |(-1.96,-48.67,;-1.97,-47.13,;-3.3,-46.37,;-4.63,-47.14,;-4.63,-48.69,;-5.97,-49.46,;-7.3,-48.69,;-7.3,-47.15,;-5.97,-46.38,;-8.63,-49.46,;-9.97,-48.69,;-8.64,-51,;-.64,-46.36,;-.65,-44.83,;.68,-44.05,;2.02,-44.81,;3.36,-44.04,;4.7,-44.81,;6.02,-44.04,;6.49,-42.57,;8.04,-42.56,;8.53,-44.03,;7.28,-44.94,;7.28,-46.48,;8.62,-47.24,;9.94,-46.46,;8.63,-48.77,;7.29,-49.55,;5.96,-48.79,;5.95,-47.25,;4.61,-46.48,;5.58,-41.33,;5.41,-39.8,;4.17,-40.71,;2.03,-46.35,;.7,-47.13,;.7,-48.67,;-.63,-49.44,;2.03,-49.44,;.68,-50.21,)| Show InChI InChI=1S/C28H22Cl2F3N3O4/c1-36(13-15-5-7-17(8-6-15)27(37)38)21-11-12-22(34-26(21)28(31,32)33)39-14-18-24(35-40-25(18)16-9-10-16)23-19(29)3-2-4-20(23)30/h2-8,11-12,16H,9-10,13-14H2,1H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at human full length FXR transfected in HEK293 cells coexpressing pTRexDest/pGL2promotor assessed as luciferase activity by direct r... |
Bioorg Med Chem Lett 20: 4911-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.084
BindingDB Entry DOI: 10.7270/Q21V5F52 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50323532
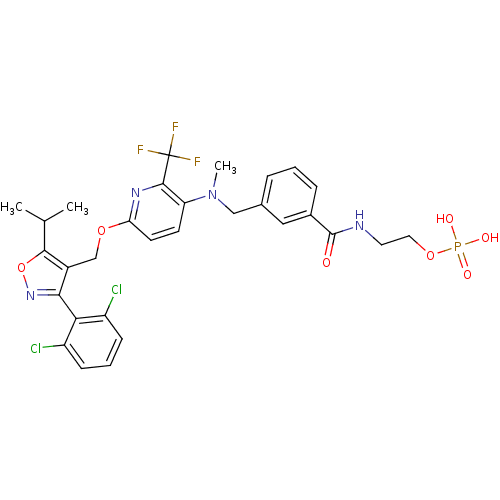
(2-(3-(((6-((3-(2,6-dichlorophenyl)-5-isopropylisox...)Show SMILES CC(C)c1onc(c1COc1ccc(N(C)Cc2cccc(c2)C(=O)NCCOP(O)(O)=O)c(n1)C(F)(F)F)-c1c(Cl)cccc1Cl |(29.58,-28.23,;28.96,-29.64,;27.43,-29.81,;29.87,-30.88,;31.42,-30.88,;31.91,-32.35,;30.66,-33.26,;29.4,-32.36,;28.08,-33.12,;26.74,-32.36,;25.4,-33.13,;24.06,-32.37,;22.73,-33.14,;22.74,-34.67,;21.41,-35.45,;21.42,-36.99,;20.08,-34.68,;18.75,-35.46,;17.41,-34.7,;16.08,-35.47,;16.08,-37.01,;17.41,-37.78,;18.75,-37.01,;17.41,-39.32,;18.75,-40.09,;16.08,-40.09,;14.75,-39.32,;13.41,-40.09,;12.08,-39.32,;10.74,-40.09,;9.96,-41.42,;11.5,-41.42,;9.41,-39.32,;24.08,-35.45,;25.41,-34.67,;24.08,-36.99,;22.75,-37.76,;25.41,-37.75,;24.06,-38.52,;30.66,-34.79,;32,-35.55,;33.32,-34.77,;32.01,-37.09,;30.67,-37.87,;29.34,-37.11,;29.33,-35.57,;27.99,-34.8,)| Show InChI InChI=1S/C30H30Cl2F3N4O7P/c1-17(2)27-20(26(38-46-27)25-21(31)8-5-9-22(25)32)16-44-24-11-10-23(28(37-24)30(33,34)35)39(3)15-18-6-4-7-19(14-18)29(40)36-12-13-45-47(41,42)43/h4-11,14,17H,12-13,15-16H2,1-3H3,(H,36,40)(H2,41,42,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 457 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at human GST-fused FXR LBD expressed in HEK293 cells coexpressing GAL4-DNA bindig domain and pFRluc by mammalian one-hybrid assay |
Bioorg Med Chem Lett 20: 4911-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.084
BindingDB Entry DOI: 10.7270/Q21V5F52 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50323532

(2-(3-(((6-((3-(2,6-dichlorophenyl)-5-isopropylisox...)Show SMILES CC(C)c1onc(c1COc1ccc(N(C)Cc2cccc(c2)C(=O)NCCOP(O)(O)=O)c(n1)C(F)(F)F)-c1c(Cl)cccc1Cl |(29.58,-28.23,;28.96,-29.64,;27.43,-29.81,;29.87,-30.88,;31.42,-30.88,;31.91,-32.35,;30.66,-33.26,;29.4,-32.36,;28.08,-33.12,;26.74,-32.36,;25.4,-33.13,;24.06,-32.37,;22.73,-33.14,;22.74,-34.67,;21.41,-35.45,;21.42,-36.99,;20.08,-34.68,;18.75,-35.46,;17.41,-34.7,;16.08,-35.47,;16.08,-37.01,;17.41,-37.78,;18.75,-37.01,;17.41,-39.32,;18.75,-40.09,;16.08,-40.09,;14.75,-39.32,;13.41,-40.09,;12.08,-39.32,;10.74,-40.09,;9.96,-41.42,;11.5,-41.42,;9.41,-39.32,;24.08,-35.45,;25.41,-34.67,;24.08,-36.99,;22.75,-37.76,;25.41,-37.75,;24.06,-38.52,;30.66,-34.79,;32,-35.55,;33.32,-34.77,;32.01,-37.09,;30.67,-37.87,;29.34,-37.11,;29.33,-35.57,;27.99,-34.8,)| Show InChI InChI=1S/C30H30Cl2F3N4O7P/c1-17(2)27-20(26(38-46-27)25-21(31)8-5-9-22(25)32)16-44-24-11-10-23(28(37-24)30(33,34)35)39(3)15-18-6-4-7-19(14-18)29(40)36-12-13-45-47(41,42)43/h4-11,14,17H,12-13,15-16H2,1-3H3,(H,36,40)(H2,41,42,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 216 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at human full length FXR transfected in HEK293 cells coexpressing pTRexDest/pGL2promotor assessed as luciferase activity by direct r... |
Bioorg Med Chem Lett 20: 4911-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.084
BindingDB Entry DOI: 10.7270/Q21V5F52 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50323531
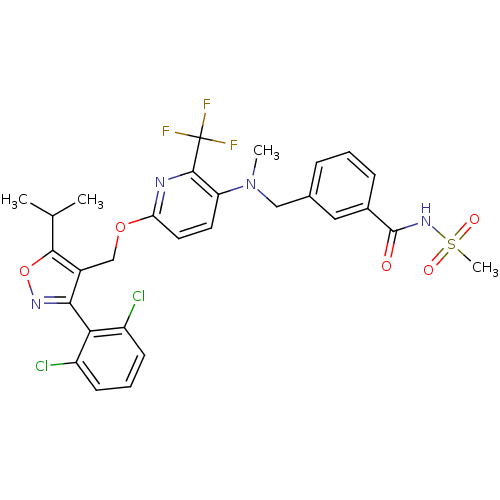
(3-(((6-((3-(2,6-dichlorophenyl)-5-isopropylisoxazo...)Show SMILES CC(C)c1onc(c1COc1ccc(N(C)Cc2cccc(c2)C(=O)NS(C)(=O)=O)c(n1)C(F)(F)F)-c1c(Cl)cccc1Cl |(6.62,-28.01,;6,-29.41,;4.47,-29.58,;6.91,-30.66,;8.46,-30.65,;8.95,-32.12,;7.7,-33.04,;6.44,-32.13,;5.12,-32.9,;3.78,-32.13,;2.44,-32.91,;1.1,-32.14,;-.23,-32.92,;-.22,-34.45,;-1.55,-35.22,;-1.54,-36.76,;-2.88,-34.46,;-4.21,-35.23,;-5.55,-34.47,;-6.88,-35.24,;-6.88,-36.79,;-5.55,-37.56,;-4.21,-36.78,;-5.55,-39.1,;-4.21,-39.87,;-6.88,-39.87,;-8.21,-39.1,;-9.54,-39.87,;-9,-37.75,;-7.46,-37.75,;1.12,-35.22,;2.45,-34.45,;1.12,-36.76,;-.21,-37.53,;2.45,-37.53,;1.1,-38.3,;7.7,-34.57,;9.04,-35.33,;10.36,-34.55,;9.05,-36.86,;7.71,-37.65,;6.38,-36.88,;6.37,-35.34,;5.03,-34.58,)| Show InChI InChI=1S/C29H27Cl2F3N4O5S/c1-16(2)26-19(25(36-43-26)24-20(30)9-6-10-21(24)31)15-42-23-12-11-22(27(35-23)29(32,33)34)38(3)14-17-7-5-8-18(13-17)28(39)37-44(4,40)41/h5-13,16H,14-15H2,1-4H3,(H,37,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 279 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at human GST-fused FXR LBD expressed in HEK293 cells coexpressing GAL4-DNA bindig domain and pFRluc by mammalian one-hybrid assay |
Bioorg Med Chem Lett 20: 4911-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.084
BindingDB Entry DOI: 10.7270/Q21V5F52 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50323531

(3-(((6-((3-(2,6-dichlorophenyl)-5-isopropylisoxazo...)Show SMILES CC(C)c1onc(c1COc1ccc(N(C)Cc2cccc(c2)C(=O)NS(C)(=O)=O)c(n1)C(F)(F)F)-c1c(Cl)cccc1Cl |(6.62,-28.01,;6,-29.41,;4.47,-29.58,;6.91,-30.66,;8.46,-30.65,;8.95,-32.12,;7.7,-33.04,;6.44,-32.13,;5.12,-32.9,;3.78,-32.13,;2.44,-32.91,;1.1,-32.14,;-.23,-32.92,;-.22,-34.45,;-1.55,-35.22,;-1.54,-36.76,;-2.88,-34.46,;-4.21,-35.23,;-5.55,-34.47,;-6.88,-35.24,;-6.88,-36.79,;-5.55,-37.56,;-4.21,-36.78,;-5.55,-39.1,;-4.21,-39.87,;-6.88,-39.87,;-8.21,-39.1,;-9.54,-39.87,;-9,-37.75,;-7.46,-37.75,;1.12,-35.22,;2.45,-34.45,;1.12,-36.76,;-.21,-37.53,;2.45,-37.53,;1.1,-38.3,;7.7,-34.57,;9.04,-35.33,;10.36,-34.55,;9.05,-36.86,;7.71,-37.65,;6.38,-36.88,;6.37,-35.34,;5.03,-34.58,)| Show InChI InChI=1S/C29H27Cl2F3N4O5S/c1-16(2)26-19(25(36-43-26)24-20(30)9-6-10-21(24)31)15-42-23-12-11-22(27(35-23)29(32,33)34)38(3)14-17-7-5-8-18(13-17)28(39)37-44(4,40)41/h5-13,16H,14-15H2,1-4H3,(H,37,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at human full length FXR transfected in HEK293 cells coexpressing pTRexDest/pGL2promotor assessed as luciferase activity by direct r... |
Bioorg Med Chem Lett 20: 4911-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.084
BindingDB Entry DOI: 10.7270/Q21V5F52 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50323534

(2-(4-(((6-((5-cyclopropyl-3-(2,6-dichlorophenyl)is...)Show SMILES CN(Cc1ccc(cc1)C(=O)NCCS(O)(=O)=O)c1ccc(OCc2c(onc2-c2c(Cl)cccc2Cl)C2CC2)nc1C(F)(F)F |(25.42,-47.4,;25.41,-45.86,;24.07,-45.1,;22.74,-45.87,;22.75,-47.42,;21.41,-48.2,;20.08,-47.43,;20.08,-45.88,;21.41,-45.11,;18.74,-48.19,;18.74,-49.73,;17.41,-47.42,;16.08,-48.19,;14.74,-47.42,;13.41,-48.19,;12.08,-47.42,;12.62,-49.51,;14.16,-49.51,;26.74,-45.09,;26.73,-43.56,;28.05,-42.78,;29.4,-43.55,;30.74,-42.77,;32.07,-43.54,;33.4,-42.77,;33.87,-41.3,;35.42,-41.29,;35.9,-42.76,;34.66,-43.68,;34.66,-45.21,;35.99,-45.97,;37.32,-45.19,;36.01,-47.5,;34.67,-48.29,;33.33,-47.52,;33.33,-45.98,;31.99,-45.22,;32.95,-40.06,;32.79,-38.53,;31.55,-39.44,;29.41,-45.09,;28.08,-45.86,;28.08,-47.4,;26.74,-48.17,;29.41,-48.17,;28.06,-48.94,)| Show InChI InChI=1S/C30H27Cl2F3N4O6S/c1-39(15-17-5-7-19(8-6-17)29(40)36-13-14-46(41,42)43)23-11-12-24(37-28(23)30(33,34)35)44-16-20-26(38-45-27(20)18-9-10-18)25-21(31)3-2-4-22(25)32/h2-8,11-12,18H,9-10,13-16H2,1H3,(H,36,40)(H,41,42,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.42E+3 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at human GST-fused FXR LBD expressed in HEK293 cells coexpressing GAL4-DNA bindig domain and pFRluc by mammalian one-hybrid assay |
Bioorg Med Chem Lett 20: 4911-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.084
BindingDB Entry DOI: 10.7270/Q21V5F52 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50323537

(3-(((3-chloro-5-((3-(2,6-dichlorophenyl)-5-isoprop...)Show SMILES CC(C)c1onc(c1COc1cnc(N(C)Cc2cccc(c2)C(O)=O)c(Cl)c1)-c1c(Cl)cccc1Cl |(4.6,-5.15,;3.98,-6.55,;2.45,-6.72,;4.89,-7.8,;6.44,-7.79,;6.92,-9.26,;5.68,-10.18,;4.42,-9.27,;3.09,-10.04,;1.76,-9.27,;.42,-10.05,;-.93,-9.28,;-2.25,-10.06,;-2.24,-11.59,;-3.57,-12.36,;-3.56,-13.9,;-4.91,-11.6,;-6.24,-12.37,;-7.57,-11.61,;-8.9,-12.38,;-8.9,-13.93,;-7.57,-14.7,;-6.23,-13.92,;-7.57,-16.24,;-8.9,-17.01,;-6.23,-17.01,;-.9,-12.36,;-.9,-13.9,;.43,-11.59,;5.68,-11.71,;7.01,-12.47,;8.34,-11.69,;7.03,-14,;5.69,-14.79,;4.35,-14.02,;4.35,-12.48,;3.01,-11.72,)| Show InChI InChI=1S/C27H24Cl3N3O4/c1-15(2)25-19(24(32-37-25)23-20(28)8-5-9-21(23)29)14-36-18-11-22(30)26(31-12-18)33(3)13-16-6-4-7-17(10-16)27(34)35/h4-12,15H,13-14H2,1-3H3,(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at human GST-fused FXR LBD expressed in HEK293 cells coexpressing GAL4-DNA bindig domain and pFRluc by mammalian one-hybrid assay |
Bioorg Med Chem Lett 20: 4911-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.084
BindingDB Entry DOI: 10.7270/Q21V5F52 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50323520
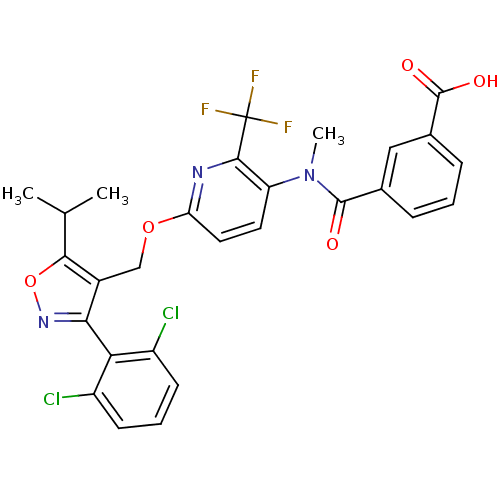
(3-((6-((3-(2,6-dichlorophenyl)-5-isopropylisoxazol...)Show SMILES CC(C)c1onc(c1COc1ccc(N(C)C(=O)c2cccc(c2)C(O)=O)c(n1)C(F)(F)F)-c1c(Cl)cccc1Cl |(4.21,-15.15,;3.59,-16.56,;2.06,-16.72,;4.5,-17.8,;6.05,-17.8,;6.54,-19.26,;5.29,-20.18,;4.03,-19.27,;2.7,-20.04,;1.37,-19.27,;.03,-20.05,;-1.31,-19.28,;-2.64,-20.06,;-2.63,-21.59,;-3.96,-22.37,;-3.95,-23.91,;-5.29,-21.6,;-5.3,-20.06,;-6.63,-22.38,;-7.96,-21.61,;-9.29,-22.38,;-9.29,-23.93,;-7.96,-24.7,;-6.62,-23.93,;-7.96,-26.24,;-6.62,-27.01,;-9.29,-27.01,;-1.29,-22.36,;.04,-21.59,;-1.29,-23.9,;-1.29,-25.44,;.04,-24.67,;-2.62,-24.68,;5.29,-21.71,;6.63,-22.47,;7.95,-21.69,;6.64,-24.01,;5.3,-24.79,;3.96,-24.02,;3.96,-22.49,;2.62,-21.72,)| Show InChI InChI=1S/C28H22Cl2F3N3O5/c1-14(2)24-17(23(35-41-24)22-18(29)8-5-9-19(22)30)13-40-21-11-10-20(25(34-21)28(31,32)33)36(3)26(37)15-6-4-7-16(12-15)27(38)39/h4-12,14H,13H2,1-3H3,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 537 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at human full length FXR transfected in HEK293 cells coexpressing pTRexDest/pGL2promotor assessed as luciferase activity by direct r... |
Bioorg Med Chem Lett 20: 4911-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.084
BindingDB Entry DOI: 10.7270/Q21V5F52 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50323533

(4-(((6-((5-cyclopropyl-3-(2,6-dichlorophenyl)isoxa...)Show SMILES CN(Cc1ccc(cc1)C(O)=O)c1ccc(OCc2c(onc2-c2c(Cl)cccc2Cl)C2CC2)nc1C(F)(F)F |(-1.96,-48.67,;-1.97,-47.13,;-3.3,-46.37,;-4.63,-47.14,;-4.63,-48.69,;-5.97,-49.46,;-7.3,-48.69,;-7.3,-47.15,;-5.97,-46.38,;-8.63,-49.46,;-9.97,-48.69,;-8.64,-51,;-.64,-46.36,;-.65,-44.83,;.68,-44.05,;2.02,-44.81,;3.36,-44.04,;4.7,-44.81,;6.02,-44.04,;6.49,-42.57,;8.04,-42.56,;8.53,-44.03,;7.28,-44.94,;7.28,-46.48,;8.62,-47.24,;9.94,-46.46,;8.63,-48.77,;7.29,-49.55,;5.96,-48.79,;5.95,-47.25,;4.61,-46.48,;5.58,-41.33,;5.41,-39.8,;4.17,-40.71,;2.03,-46.35,;.7,-47.13,;.7,-48.67,;-.63,-49.44,;2.03,-49.44,;.68,-50.21,)| Show InChI InChI=1S/C28H22Cl2F3N3O4/c1-36(13-15-5-7-17(8-6-15)27(37)38)21-11-12-22(34-26(21)28(31,32)33)39-14-18-24(35-40-25(18)16-9-10-16)23-19(29)3-2-4-20(23)30/h2-8,11-12,16H,9-10,13-14H2,1H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at human GST-fused FXR LBD expressed in HEK293 cells coexpressing GAL4-DNA bindig domain and pFRluc by mammalian one-hybrid assay |
Bioorg Med Chem Lett 20: 4911-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.084
BindingDB Entry DOI: 10.7270/Q21V5F52 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50323536

(4-(((3-chloro-5-((3-(2,6-dichlorophenyl)-5-isoprop...)Show SMILES CC(C)c1onc(c1COc1cnc(N(C)Cc2ccc(cc2)C(O)=O)c(Cl)c1)-c1c(Cl)cccc1Cl |(27.95,6.97,;27.33,5.56,;25.8,5.4,;28.24,4.32,;29.79,4.32,;30.27,2.86,;29.02,1.94,;27.77,2.85,;26.44,2.08,;25.11,2.85,;23.77,2.07,;22.42,2.84,;21.1,2.06,;21.11,.53,;19.78,-.25,;19.79,-1.79,;18.44,.52,;17.11,-.26,;17.12,-1.81,;15.78,-2.58,;14.45,-1.81,;14.45,-.26,;15.78,.51,;13.11,-2.58,;11.78,-1.81,;13.11,-4.12,;22.44,-.24,;22.45,-1.78,;23.77,.53,;29.03,.41,;30.36,-.35,;31.69,.43,;30.37,-1.89,;29.04,-2.67,;27.7,-1.9,;27.7,-.37,;26.36,.4,)| Show InChI InChI=1S/C27H24Cl3N3O4/c1-15(2)25-19(24(32-37-25)23-20(28)5-4-6-21(23)29)14-36-18-11-22(30)26(31-12-18)33(3)13-16-7-9-17(10-8-16)27(34)35/h4-12,15H,13-14H2,1-3H3,(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 107 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at human GST-fused FXR LBD expressed in HEK293 cells coexpressing GAL4-DNA bindig domain and pFRluc by mammalian one-hybrid assay |
Bioorg Med Chem Lett 20: 4911-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.084
BindingDB Entry DOI: 10.7270/Q21V5F52 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50323535
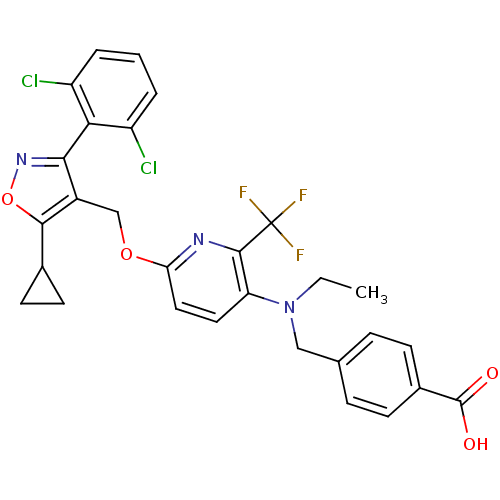
(4-(((6-((5-cyclopropyl-3-(2,6-dichlorophenyl)isoxa...)Show SMILES CCN(Cc1ccc(cc1)C(O)=O)c1ccc(OCc2c(onc2-c2c(Cl)cccc2Cl)C2CC2)nc1C(F)(F)F |(-3.51,-2.57,;-2.18,-1.8,;-2.18,-.26,;-3.52,.51,;-4.85,-.27,;-4.85,-1.82,;-6.18,-2.59,;-7.52,-1.82,;-7.52,-.27,;-6.19,.5,;-8.85,-2.59,;-10.19,-1.81,;-8.85,-4.13,;-.85,.52,;-.87,2.05,;.46,2.83,;1.8,2.06,;3.14,2.84,;4.48,2.07,;5.8,2.84,;6.27,4.31,;7.83,4.31,;8.31,2.85,;7.06,1.93,;7.07,.4,;8.4,-.36,;9.73,.42,;8.41,-1.89,;7.08,-2.68,;5.74,-1.91,;5.73,-.37,;4.4,.39,;5.36,5.55,;5.19,7.08,;3.95,6.17,;1.81,.52,;.48,-.25,;.48,-1.79,;-.85,-2.56,;1.82,-2.56,;.47,-3.33,)| Show InChI InChI=1S/C29H24Cl2F3N3O4/c1-2-37(14-16-6-8-18(9-7-16)28(38)39)22-12-13-23(35-27(22)29(32,33)34)40-15-19-25(36-41-26(19)17-10-11-17)24-20(30)4-3-5-21(24)31/h3-9,12-13,17H,2,10-11,14-15H2,1H3,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 180 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at human GST-fused FXR LBD expressed in HEK293 cells coexpressing GAL4-DNA bindig domain and pFRluc by mammalian one-hybrid assay |
Bioorg Med Chem Lett 20: 4911-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.084
BindingDB Entry DOI: 10.7270/Q21V5F52 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50323535

(4-(((6-((5-cyclopropyl-3-(2,6-dichlorophenyl)isoxa...)Show SMILES CCN(Cc1ccc(cc1)C(O)=O)c1ccc(OCc2c(onc2-c2c(Cl)cccc2Cl)C2CC2)nc1C(F)(F)F |(-3.51,-2.57,;-2.18,-1.8,;-2.18,-.26,;-3.52,.51,;-4.85,-.27,;-4.85,-1.82,;-6.18,-2.59,;-7.52,-1.82,;-7.52,-.27,;-6.19,.5,;-8.85,-2.59,;-10.19,-1.81,;-8.85,-4.13,;-.85,.52,;-.87,2.05,;.46,2.83,;1.8,2.06,;3.14,2.84,;4.48,2.07,;5.8,2.84,;6.27,4.31,;7.83,4.31,;8.31,2.85,;7.06,1.93,;7.07,.4,;8.4,-.36,;9.73,.42,;8.41,-1.89,;7.08,-2.68,;5.74,-1.91,;5.73,-.37,;4.4,.39,;5.36,5.55,;5.19,7.08,;3.95,6.17,;1.81,.52,;.48,-.25,;.48,-1.79,;-.85,-2.56,;1.82,-2.56,;.47,-3.33,)| Show InChI InChI=1S/C29H24Cl2F3N3O4/c1-2-37(14-16-6-8-18(9-7-16)28(38)39)22-12-13-23(35-27(22)29(32,33)34)40-15-19-25(36-41-26(19)17-10-11-17)24-20(30)4-3-5-21(24)31/h3-9,12-13,17H,2,10-11,14-15H2,1H3,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 113 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at human full length FXR transfected in HEK293 cells coexpressing pTRexDest/pGL2promotor assessed as luciferase activity by direct r... |
Bioorg Med Chem Lett 20: 4911-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.084
BindingDB Entry DOI: 10.7270/Q21V5F52 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50323520

(3-((6-((3-(2,6-dichlorophenyl)-5-isopropylisoxazol...)Show SMILES CC(C)c1onc(c1COc1ccc(N(C)C(=O)c2cccc(c2)C(O)=O)c(n1)C(F)(F)F)-c1c(Cl)cccc1Cl |(4.21,-15.15,;3.59,-16.56,;2.06,-16.72,;4.5,-17.8,;6.05,-17.8,;6.54,-19.26,;5.29,-20.18,;4.03,-19.27,;2.7,-20.04,;1.37,-19.27,;.03,-20.05,;-1.31,-19.28,;-2.64,-20.06,;-2.63,-21.59,;-3.96,-22.37,;-3.95,-23.91,;-5.29,-21.6,;-5.3,-20.06,;-6.63,-22.38,;-7.96,-21.61,;-9.29,-22.38,;-9.29,-23.93,;-7.96,-24.7,;-6.62,-23.93,;-7.96,-26.24,;-6.62,-27.01,;-9.29,-27.01,;-1.29,-22.36,;.04,-21.59,;-1.29,-23.9,;-1.29,-25.44,;.04,-24.67,;-2.62,-24.68,;5.29,-21.71,;6.63,-22.47,;7.95,-21.69,;6.64,-24.01,;5.3,-24.79,;3.96,-24.02,;3.96,-22.49,;2.62,-21.72,)| Show InChI InChI=1S/C28H22Cl2F3N3O5/c1-14(2)24-17(23(35-41-24)22-18(29)8-5-9-19(22)30)13-40-21-11-10-20(25(34-21)28(31,32)33)36(3)26(37)15-6-4-7-16(12-15)27(38)39/h4-12,14H,13H2,1-3H3,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 571 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at human GST-fused FXR LBD expressed in HEK293 cells coexpressing GAL4-DNA bindig domain and pFRluc by mammalian one-hybrid assay |
Bioorg Med Chem Lett 20: 4911-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.084
BindingDB Entry DOI: 10.7270/Q21V5F52 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50323522
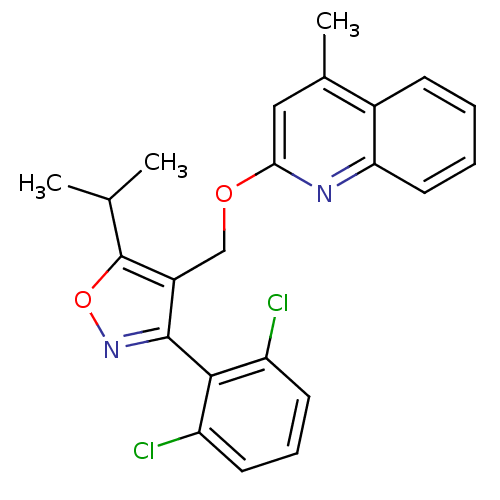
(3-(2,6-dichlorophenyl)-5-isopropyl-4-((4-methylqui...)Show SMILES CC(C)c1onc(c1COc1cc(C)c2ccccc2n1)-c1c(Cl)cccc1Cl |(-5.54,-39.13,;-4.92,-37.72,;-3.39,-37.56,;-5.83,-36.48,;-7.37,-36.48,;-7.84,-35.02,;-6.6,-34.12,;-5.35,-35.01,;-4.01,-34.26,;-2.69,-35.05,;-1.34,-34.31,;-1.33,-32.77,;.01,-32.02,;.03,-30.48,;1.34,-32.81,;2.68,-32.07,;4,-32.85,;3.98,-34.39,;2.63,-35.14,;1.32,-34.35,;-.03,-35.1,;-6.6,-32.58,;-7.93,-31.81,;-9.26,-32.58,;-7.93,-30.26,;-6.6,-29.49,;-5.26,-30.26,;-5.26,-31.81,;-3.92,-32.57,)| Show InChI InChI=1S/C23H20Cl2N2O2/c1-13(2)23-16(22(27-29-23)21-17(24)8-6-9-18(21)25)12-28-20-11-14(3)15-7-4-5-10-19(15)26-20/h4-11,13H,12H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 536 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at human GST-fused FXR LBD expressed in HEK293 cells coexpressing GAL4-DNA bindig domain and pFRluc by mammalian one-hybrid assay |
Bioorg Med Chem Lett 20: 4911-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.084
BindingDB Entry DOI: 10.7270/Q21V5F52 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50323522

(3-(2,6-dichlorophenyl)-5-isopropyl-4-((4-methylqui...)Show SMILES CC(C)c1onc(c1COc1cc(C)c2ccccc2n1)-c1c(Cl)cccc1Cl |(-5.54,-39.13,;-4.92,-37.72,;-3.39,-37.56,;-5.83,-36.48,;-7.37,-36.48,;-7.84,-35.02,;-6.6,-34.12,;-5.35,-35.01,;-4.01,-34.26,;-2.69,-35.05,;-1.34,-34.31,;-1.33,-32.77,;.01,-32.02,;.03,-30.48,;1.34,-32.81,;2.68,-32.07,;4,-32.85,;3.98,-34.39,;2.63,-35.14,;1.32,-34.35,;-.03,-35.1,;-6.6,-32.58,;-7.93,-31.81,;-9.26,-32.58,;-7.93,-30.26,;-6.6,-29.49,;-5.26,-30.26,;-5.26,-31.81,;-3.92,-32.57,)| Show InChI InChI=1S/C23H20Cl2N2O2/c1-13(2)23-16(22(27-29-23)21-17(24)8-6-9-18(21)25)12-28-20-11-14(3)15-7-4-5-10-19(15)26-20/h4-11,13H,12H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 878 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at human full length FXR transfected in HEK293 cells coexpressing pTRexDest/pGL2promotor assessed as luciferase activity by direct r... |
Bioorg Med Chem Lett 20: 4911-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.084
BindingDB Entry DOI: 10.7270/Q21V5F52 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50323530

(3-(((6-((5-cyclopropyl-3-(2,6-dichlorophenyl)isoxa...)Show SMILES CN(Cc1cccc(c1)C(O)=O)c1ccc(OCc2c(onc2-c2c(Cl)cccc2Cl)C2CC2)nc1C(F)(F)F |(21.34,-24.3,;21.34,-22.76,;20,-21.99,;18.67,-22.77,;17.33,-22,;16,-22.77,;16,-24.32,;17.34,-25.09,;18.67,-24.32,;17.34,-26.63,;16,-27.4,;18.67,-27.4,;22.67,-21.98,;22.65,-20.45,;23.98,-19.67,;25.32,-20.44,;26.66,-19.66,;28,-20.43,;29.32,-19.66,;29.79,-18.19,;31.35,-18.19,;31.83,-19.65,;30.58,-20.57,;30.59,-22.1,;31.92,-22.86,;33.25,-22.08,;31.93,-24.4,;30.6,-25.18,;29.26,-24.41,;29.25,-22.87,;27.92,-22.11,;28.88,-16.95,;28.71,-15.42,;27.47,-16.33,;25.33,-21.98,;24,-22.75,;24,-24.29,;22.67,-25.06,;25.34,-25.06,;23.99,-25.83,)| Show InChI InChI=1S/C28H22Cl2F3N3O4/c1-36(13-15-4-2-5-17(12-15)27(37)38)21-10-11-22(34-26(21)28(31,32)33)39-14-18-24(35-40-25(18)16-8-9-16)23-19(29)6-3-7-20(23)30/h2-7,10-12,16H,8-9,13-14H2,1H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at human GST-fused FXR LBD assessed as cofactor peptide interaction with receptor ligand binding domain by FRET assay |
Bioorg Med Chem Lett 20: 4911-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.084
BindingDB Entry DOI: 10.7270/Q21V5F52 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50323521
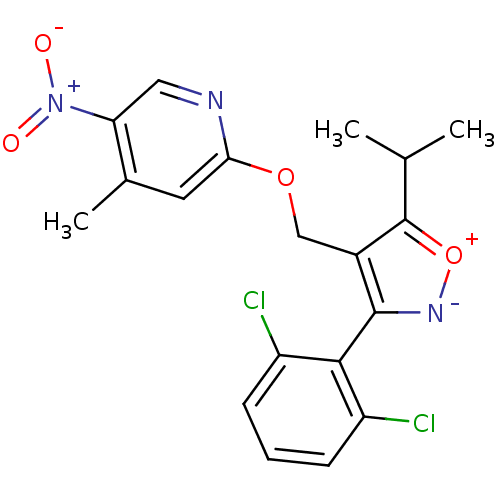
(3-(2,6-dichlorophenyl)-5-isopropyl-4-((4-methyl-5-...)Show SMILES CC(C)c1[o+][n-]c(c1COc1cc(C)c(cn1)[N+]([O-])=O)-c1c(Cl)cccc1Cl |(22.17,-16.64,;21.14,-17.78,;19.63,-17.46,;21.61,-19.25,;23.08,-19.73,;23.08,-21.27,;21.61,-21.74,;20.7,-20.5,;19.16,-20.5,;18.39,-21.83,;16.85,-21.83,;16.08,-23.16,;14.53,-23.16,;13.76,-24.49,;13.77,-21.82,;14.54,-20.49,;16.07,-20.49,;12.23,-21.82,;11.46,-20.48,;11.45,-23.15,;21.58,-23.28,;22.89,-24.07,;24.24,-23.32,;22.86,-25.61,;21.51,-26.35,;20.19,-25.55,;20.23,-24.01,;18.91,-23.21,)| Show InChI InChI=1S/C19H17Cl2N3O4/c1-10(2)19-12(9-27-16-7-11(3)15(8-22-16)24(25)26)18(23-28-19)17-13(20)5-4-6-14(17)21/h4-8,10H,9H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at human GST-fused FXR LBD expressed in HEK293 cells coexpressing GAL4-DNA bindig domain and pFRluc by mammalian one-hybrid assay |
Bioorg Med Chem Lett 20: 4911-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.084
BindingDB Entry DOI: 10.7270/Q21V5F52 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50323521

(3-(2,6-dichlorophenyl)-5-isopropyl-4-((4-methyl-5-...)Show SMILES CC(C)c1[o+][n-]c(c1COc1cc(C)c(cn1)[N+]([O-])=O)-c1c(Cl)cccc1Cl |(22.17,-16.64,;21.14,-17.78,;19.63,-17.46,;21.61,-19.25,;23.08,-19.73,;23.08,-21.27,;21.61,-21.74,;20.7,-20.5,;19.16,-20.5,;18.39,-21.83,;16.85,-21.83,;16.08,-23.16,;14.53,-23.16,;13.76,-24.49,;13.77,-21.82,;14.54,-20.49,;16.07,-20.49,;12.23,-21.82,;11.46,-20.48,;11.45,-23.15,;21.58,-23.28,;22.89,-24.07,;24.24,-23.32,;22.86,-25.61,;21.51,-26.35,;20.19,-25.55,;20.23,-24.01,;18.91,-23.21,)| Show InChI InChI=1S/C19H17Cl2N3O4/c1-10(2)19-12(9-27-16-7-11(3)15(8-22-16)24(25)26)18(23-28-19)17-13(20)5-4-6-14(17)21/h4-8,10H,9H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.14E+3 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at human full length FXR transfected in HEK293 cells coexpressing pTRexDest/pGL2promotor assessed as luciferase activity by direct r... |
Bioorg Med Chem Lett 20: 4911-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.084
BindingDB Entry DOI: 10.7270/Q21V5F52 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50323541

(3-((6-((5-cyclopropyl-3-(2,6-dichlorophenyl)isoxaz...)Show SMILES OC(=O)c1cccc(OCc2ccc(OCc3c(noc3C3CC3)-c3c(Cl)cccc3Cl)nc2C(F)(F)F)c1 |(-9.62,-34.92,;-8.29,-34.14,;-8.29,-32.6,;-6.96,-34.91,;-6.96,-36.46,;-5.62,-37.23,;-4.29,-36.46,;-4.29,-34.91,;-2.96,-34.13,;-1.62,-34.9,;-.29,-34.12,;-.31,-32.59,;1.02,-31.81,;2.36,-32.58,;3.7,-31.8,;5.04,-32.57,;6.36,-31.8,;7.62,-32.71,;8.87,-31.79,;8.39,-30.33,;6.83,-30.33,;5.92,-29.1,;5.75,-27.56,;4.51,-28.47,;7.63,-34.24,;8.96,-35,;10.29,-34.22,;8.97,-36.54,;7.64,-37.32,;6.3,-36.55,;6.29,-35.02,;4.96,-34.25,;2.37,-34.12,;1.04,-34.89,;1.04,-36.43,;-.29,-37.21,;2.38,-37.2,;1.03,-37.97,;-5.63,-34.14,)| Show InChI InChI=1S/C27H19Cl2F3N2O5/c28-19-5-2-6-20(29)22(19)23-18(24(39-34-23)14-7-8-14)13-38-21-10-9-16(25(33-21)27(30,31)32)12-37-17-4-1-3-15(11-17)26(35)36/h1-6,9-11,14H,7-8,12-13H2,(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 43 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at human GST-fused FXR LBD assessed as cofactor peptide interaction with receptor ligand binding domain by FRET assay |
Bioorg Med Chem Lett 20: 4911-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.084
BindingDB Entry DOI: 10.7270/Q21V5F52 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50323540

(2-((6-((5-cyclopropyl-3-(2,6-dichlorophenyl)isoxaz...)Show SMILES OC(=O)c1ccccc1OCc1ccc(OCc2c(noc2C2CC2)-c2c(Cl)cccc2Cl)nc1C(F)(F)F |(13.66,-21.28,;14.99,-22.05,;16.33,-21.28,;15,-23.59,;13.67,-24.36,;13.67,-25.9,;15,-26.68,;16.34,-25.9,;16.33,-24.35,;17.67,-23.58,;19,-24.34,;20.33,-23.57,;20.32,-22.04,;21.65,-21.26,;22.99,-22.02,;24.33,-21.25,;25.66,-22.02,;26.99,-21.25,;28.25,-22.15,;29.5,-21.24,;29.01,-19.77,;27.46,-19.78,;26.55,-18.54,;26.38,-17.01,;25.14,-17.92,;28.25,-23.69,;29.59,-24.45,;30.91,-23.67,;29.6,-25.98,;28.26,-26.77,;26.92,-26,;26.92,-24.46,;25.58,-23.7,;23,-23.57,;21.67,-24.34,;21.67,-25.88,;20.34,-26.65,;23,-26.65,;21.65,-27.42,)| Show InChI InChI=1S/C27H19Cl2F3N2O5/c28-18-5-3-6-19(29)22(18)23-17(24(39-34-23)14-8-9-14)13-38-21-11-10-15(25(33-21)27(30,31)32)12-37-20-7-2-1-4-16(20)26(35)36/h1-7,10-11,14H,8-9,12-13H2,(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 434 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at human GST-fused FXR LBD assessed as cofactor peptide interaction with receptor ligand binding domain by FRET assay |
Bioorg Med Chem Lett 20: 4911-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.084
BindingDB Entry DOI: 10.7270/Q21V5F52 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data


















































