 Found 154 hits with Last Name = 'di micco' and Initial = 's'
Found 154 hits with Last Name = 'di micco' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50317647
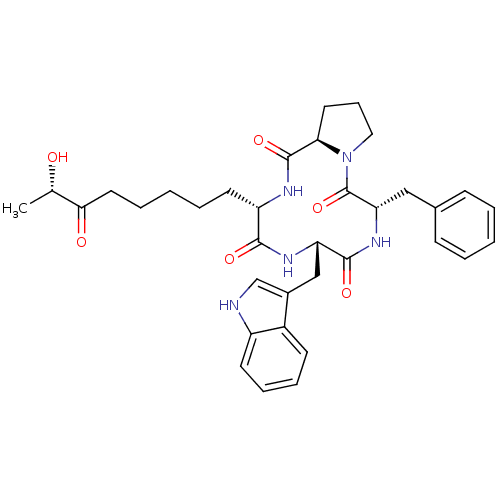
((3S,6S,9S,14aR)-6-((1H-indol-3-yl)methyl)-9-benzyl...)Show SMILES C[C@H](O)C(=O)CCCCC[C@@H]1NC(=O)[C@H]2CCCN2C(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O |r| Show InChI InChI=1S/C35H43N5O6/c1-22(41)31(42)17-7-3-6-15-27-32(43)38-28(20-24-21-36-26-14-9-8-13-25(24)26)33(44)39-29(19-23-11-4-2-5-12-23)35(46)40-18-10-16-30(40)34(45)37-27/h2,4-5,8-9,11-14,21-22,27-30,36,41H,3,6-7,10,15-20H2,1H3,(H,37,45)(H,38,43)(H,39,44)/t22-,27-,28-,29-,30+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno
Curated by ChEMBL
| Assay Description
Inhibition of full length HDAC3 expressed in HEK293 cells after 15 mins by fluorescence assay |
Bioorg Med Chem 18: 3252-60 (2010)
Article DOI: 10.1016/j.bmc.2010.03.022
BindingDB Entry DOI: 10.7270/Q2BC3ZP6 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50591532

(CHEMBL4590950) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00817
BindingDB Entry DOI: 10.7270/Q2GT5S5W |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50317647

((3S,6S,9S,14aR)-6-((1H-indol-3-yl)methyl)-9-benzyl...)Show SMILES C[C@H](O)C(=O)CCCCC[C@@H]1NC(=O)[C@H]2CCCN2C(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O |r| Show InChI InChI=1S/C35H43N5O6/c1-22(41)31(42)17-7-3-6-15-27-32(43)38-28(20-24-21-36-26-14-9-8-13-25(24)26)33(44)39-29(19-23-11-4-2-5-12-23)35(46)40-18-10-16-30(40)34(45)37-27/h2,4-5,8-9,11-14,21-22,27-30,36,41H,3,6-7,10,15-20H2,1H3,(H,37,45)(H,38,43)(H,39,44)/t22-,27-,28-,29-,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno
Curated by ChEMBL
| Assay Description
Inhibition of full length HDAC1 expressed in HEK293 cells after 15 mins by fluorescence assay |
Bioorg Med Chem 18: 3252-60 (2010)
Article DOI: 10.1016/j.bmc.2010.03.022
BindingDB Entry DOI: 10.7270/Q2BC3ZP6 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50591533
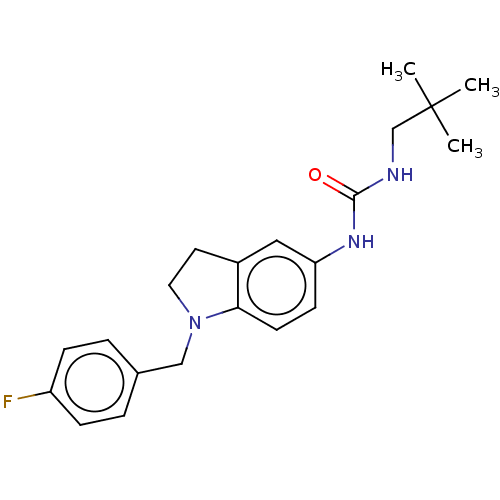
(CHEMBL4449485)Show SMILES CC(C)(C)CNC(=O)Nc1ccc2N(Cc3ccc(F)cc3)CCc2c1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00817
BindingDB Entry DOI: 10.7270/Q2GT5S5W |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50591535
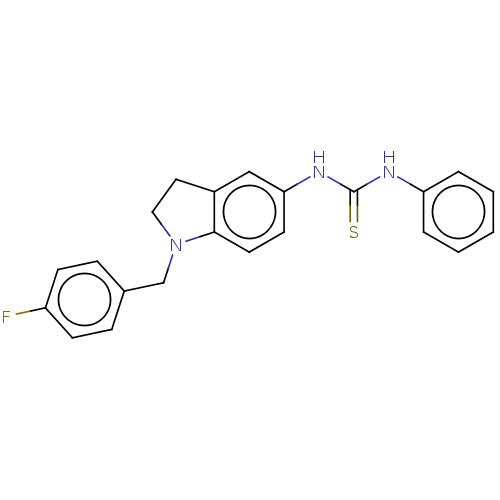
(CHEMBL5185907) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00817
BindingDB Entry DOI: 10.7270/Q2GT5S5W |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50317647

((3S,6S,9S,14aR)-6-((1H-indol-3-yl)methyl)-9-benzyl...)Show SMILES C[C@H](O)C(=O)CCCCC[C@@H]1NC(=O)[C@H]2CCCN2C(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O |r| Show InChI InChI=1S/C35H43N5O6/c1-22(41)31(42)17-7-3-6-15-27-32(43)38-28(20-24-21-36-26-14-9-8-13-25(24)26)33(44)39-29(19-23-11-4-2-5-12-23)35(46)40-18-10-16-30(40)34(45)37-27/h2,4-5,8-9,11-14,21-22,27-30,36,41H,3,6-7,10,15-20H2,1H3,(H,37,45)(H,38,43)(H,39,44)/t22-,27-,28-,29-,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 179 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno
Curated by ChEMBL
| Assay Description
Inhibition of full length HDAC2 expressed in HEK293 cells after 15 mins by fluorescence assay |
Bioorg Med Chem 18: 3252-60 (2010)
Article DOI: 10.1016/j.bmc.2010.03.022
BindingDB Entry DOI: 10.7270/Q2BC3ZP6 |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50464480
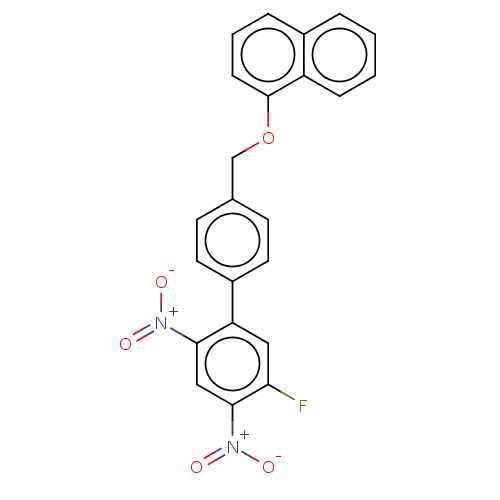
(CHEMBL4282628)Show SMILES [O-][N+](=O)c1cc(c(cc1F)-c1ccc(COc2cccc3ccccc23)cc1)[N+]([O-])=O Show InChI InChI=1S/C23H15FN2O5/c24-20-12-19(21(25(27)28)13-22(20)26(29)30)17-10-8-15(9-11-17)14-31-23-7-3-5-16-4-1-2-6-18(16)23/h1-13H,14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno
Curated by ChEMBL
| Assay Description
Inhibition of mPGES1 in IL-1beta-stimulated human A549 cell microsomes using PGH2 as substrate assessed as reduction in PGE2 production preincubated ... |
Eur J Med Chem 143: 1419-1427 (2018)
Article DOI: 10.1016/j.ejmech.2017.10.039
BindingDB Entry DOI: 10.7270/Q2G44SXG |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50591533

(CHEMBL4449485)Show SMILES CC(C)(C)CNC(=O)Nc1ccc2N(Cc3ccc(F)cc3)CCc2c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00817
BindingDB Entry DOI: 10.7270/Q2GT5S5W |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50464484
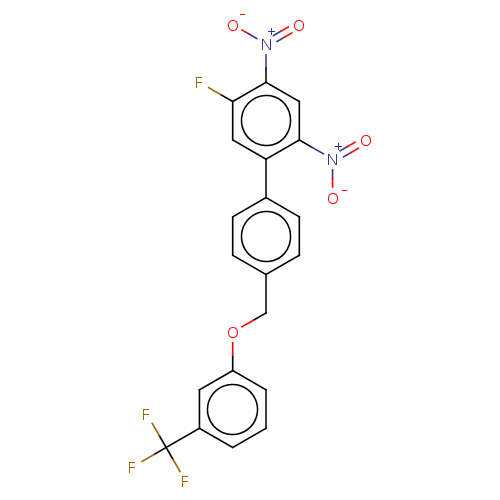
(CHEMBL4290910)Show SMILES [O-][N+](=O)c1cc(c(cc1F)-c1ccc(COc2cccc(c2)C(F)(F)F)cc1)[N+]([O-])=O Show InChI InChI=1S/C20H12F4N2O5/c21-17-9-16(18(25(27)28)10-19(17)26(29)30)13-6-4-12(5-7-13)11-31-15-3-1-2-14(8-15)20(22,23)24/h1-10H,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno
Curated by ChEMBL
| Assay Description
Inhibition of mPGES1 in IL-1beta-stimulated human A549 cell microsomes using PGH2 as substrate assessed as reduction in PGE2 production preincubated ... |
Eur J Med Chem 143: 1419-1427 (2018)
Article DOI: 10.1016/j.ejmech.2017.10.039
BindingDB Entry DOI: 10.7270/Q2G44SXG |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50591532

(CHEMBL4590950) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00817
BindingDB Entry DOI: 10.7270/Q2GT5S5W |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50591524
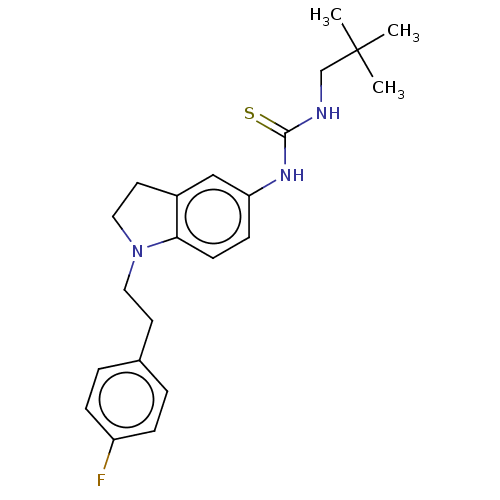
(CHEMBL5202375)Show SMILES CC(C)(C)CNC(=S)Nc1ccc2N(CCc3ccc(F)cc3)CCc2c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00817
BindingDB Entry DOI: 10.7270/Q2GT5S5W |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50591539
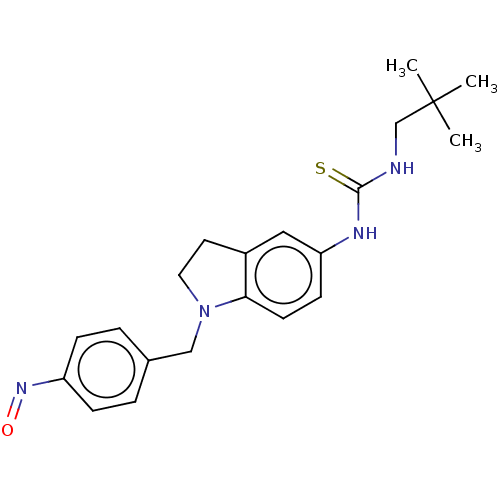
(CHEMBL5170803)Show SMILES CC(C)(C)CNC(=S)Nc1ccc2N(Cc3ccc(cc3)N=O)CCc2c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00817
BindingDB Entry DOI: 10.7270/Q2GT5S5W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50591539

(CHEMBL5170803)Show SMILES CC(C)(C)CNC(=S)Nc1ccc2N(Cc3ccc(cc3)N=O)CCc2c1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00817
BindingDB Entry DOI: 10.7270/Q2GT5S5W |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50591522
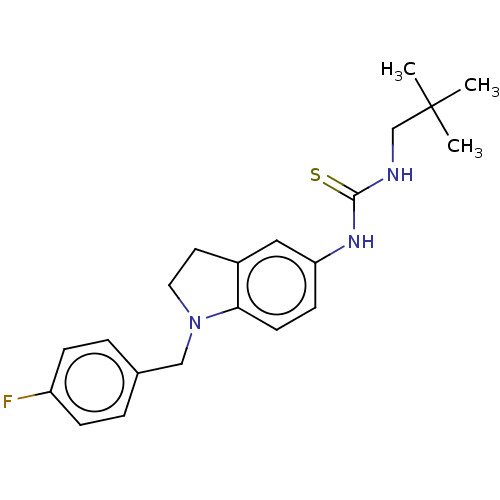
(CHEMBL4473010)Show SMILES CC(C)(C)CNC(=S)Nc1ccc2N(Cc3ccc(F)cc3)CCc2c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00817
BindingDB Entry DOI: 10.7270/Q2GT5S5W |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi del Piemonte Orientale A. Avogadro
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human SHSY5Y cells by fluorimetric cellular activity assay |
J Med Chem 52: 2776-85 (2009)
Article DOI: 10.1021/jm801529c
BindingDB Entry DOI: 10.7270/Q2D221F1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50464486
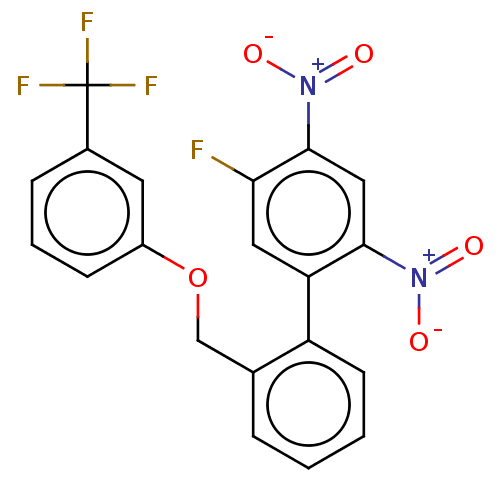
(CHEMBL4293214)Show SMILES [O-][N+](=O)c1cc(c(cc1F)-c1ccccc1COc1cccc(c1)C(F)(F)F)[N+]([O-])=O Show InChI InChI=1S/C20H12F4N2O5/c21-17-9-16(18(25(27)28)10-19(17)26(29)30)15-7-2-1-4-12(15)11-31-14-6-3-5-13(8-14)20(22,23)24/h1-10H,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno
Curated by ChEMBL
| Assay Description
Inhibition of mPGES1 in IL-1beta-stimulated human A549 cell microsomes using PGH2 as substrate assessed as reduction in PGE2 production preincubated ... |
Eur J Med Chem 143: 1419-1427 (2018)
Article DOI: 10.1016/j.ejmech.2017.10.039
BindingDB Entry DOI: 10.7270/Q2G44SXG |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50591534
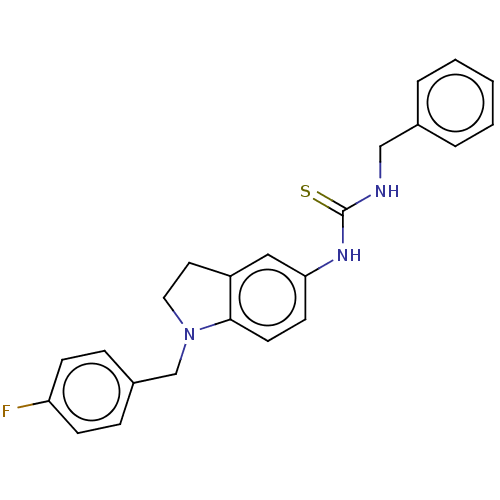
(CHEMBL5199450)Show SMILES Fc1ccc(CN2CCc3cc(NC(=S)NCc4ccccc4)ccc23)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00817
BindingDB Entry DOI: 10.7270/Q2GT5S5W |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50591539

(CHEMBL5170803)Show SMILES CC(C)(C)CNC(=S)Nc1ccc2N(Cc3ccc(cc3)N=O)CCc2c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00817
BindingDB Entry DOI: 10.7270/Q2GT5S5W |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50464479
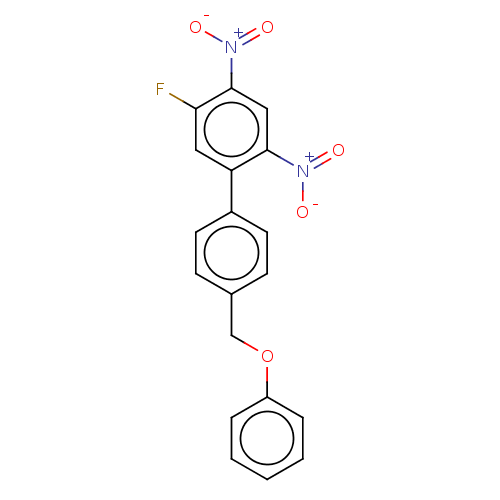
(CHEMBL4283041)Show SMILES [O-][N+](=O)c1cc(c(cc1F)-c1ccc(COc2ccccc2)cc1)[N+]([O-])=O Show InChI InChI=1S/C19H13FN2O5/c20-17-10-16(18(21(23)24)11-19(17)22(25)26)14-8-6-13(7-9-14)12-27-15-4-2-1-3-5-15/h1-11H,12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno
Curated by ChEMBL
| Assay Description
Inhibition of mPGES1 in IL-1beta-stimulated human A549 cell microsomes using PGH2 as substrate assessed as reduction in PGE2 production preincubated ... |
Eur J Med Chem 143: 1419-1427 (2018)
Article DOI: 10.1016/j.ejmech.2017.10.039
BindingDB Entry DOI: 10.7270/Q2G44SXG |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50591531
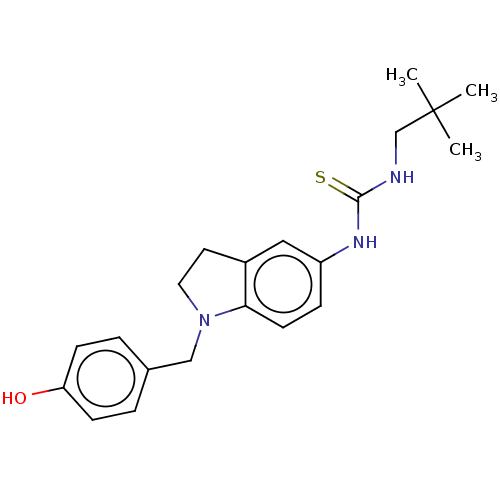
(CHEMBL5193145)Show SMILES CC(C)(C)CNC(=S)Nc1ccc2N(Cc3ccc(O)cc3)CCc2c1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00817
BindingDB Entry DOI: 10.7270/Q2GT5S5W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50591526

(CHEMBL5169630)Show SMILES CC(C)(C)CNC(=S)Nc1ccc2N(Cc3ccc4ccccc4c3)CCc2c1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00817
BindingDB Entry DOI: 10.7270/Q2GT5S5W |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50591534

(CHEMBL5199450)Show SMILES Fc1ccc(CN2CCc3cc(NC(=S)NCc4ccccc4)ccc23)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00817
BindingDB Entry DOI: 10.7270/Q2GT5S5W |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50591526

(CHEMBL5169630)Show SMILES CC(C)(C)CNC(=S)Nc1ccc2N(Cc3ccc4ccccc4c3)CCc2c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00817
BindingDB Entry DOI: 10.7270/Q2GT5S5W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50591524

(CHEMBL5202375)Show SMILES CC(C)(C)CNC(=S)Nc1ccc2N(CCc3ccc(F)cc3)CCc2c1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00817
BindingDB Entry DOI: 10.7270/Q2GT5S5W |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 6B
(Homo sapiens (Human)) | BDBM50535443

(CHEMBL4591907)Show InChI InChI=1S/C13H9NO3/c15-8-5-6-11(16)9(7-8)13-14-10-3-1-2-4-12(10)17-13/h1-7,15-16H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biomolecular Chemistry (ICB)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human JMJD3 expressed in baculovirus infected Sf9 cells using biotinylated histone H3 peptide as substrate incubated for 15... |
ACS Med Chem Lett 10: 601-605 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00589
BindingDB Entry DOI: 10.7270/Q2GF0Z01 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50480332
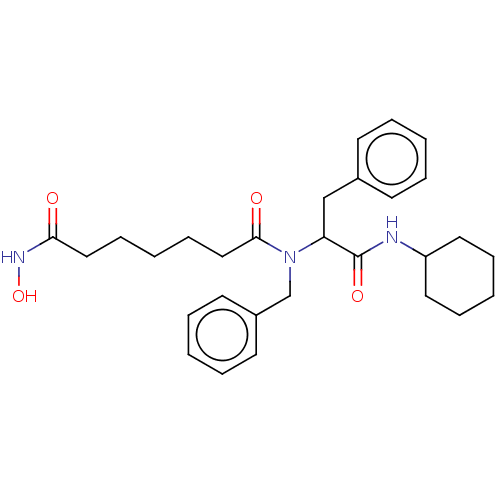
(CHEMBL519853)Show SMILES ONC(=O)CCCCCC(=O)N(Cc1ccccc1)C(Cc1ccccc1)C(=O)NC1CCCCC1 Show InChI InChI=1S/C29H39N3O4/c33-27(31-36)19-11-4-12-20-28(34)32(22-24-15-7-2-8-16-24)26(21-23-13-5-1-6-14-23)29(35)30-25-17-9-3-10-18-25/h1-2,5-8,13-16,25-26,36H,3-4,9-12,17-22H2,(H,30,35)(H,31,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi del Piemonte Orientale A. Avogadro
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human SHSY5Y cells by fluorimetric cellular activity assay |
J Med Chem 52: 2776-85 (2009)
Article DOI: 10.1021/jm801529c
BindingDB Entry DOI: 10.7270/Q2D221F1 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50591522

(CHEMBL4473010)Show SMILES CC(C)(C)CNC(=S)Nc1ccc2N(Cc3ccc(F)cc3)CCc2c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00817
BindingDB Entry DOI: 10.7270/Q2GT5S5W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50591522

(CHEMBL4473010)Show SMILES CC(C)(C)CNC(=S)Nc1ccc2N(Cc3ccc(F)cc3)CCc2c1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00817
BindingDB Entry DOI: 10.7270/Q2GT5S5W |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50591529
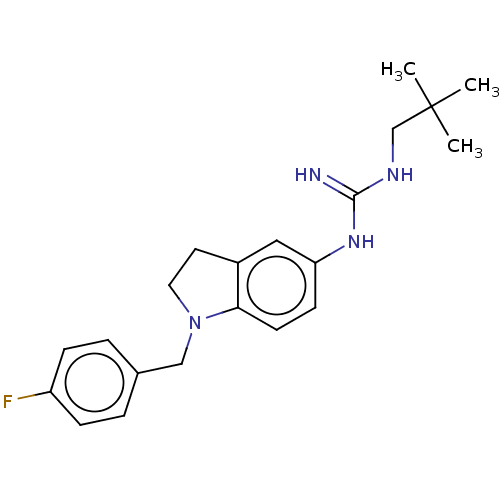
(CHEMBL4458075)Show SMILES CC(C)(C)CNC(=N)Nc1ccc2N(Cc3ccc(F)cc3)CCc2c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00817
BindingDB Entry DOI: 10.7270/Q2GT5S5W |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50591525
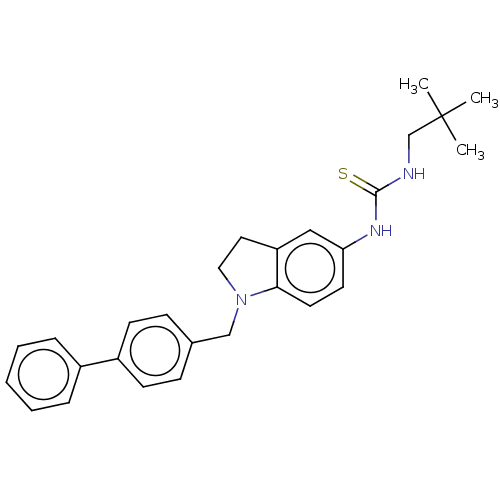
(CHEMBL5185080)Show SMILES CC(C)(C)CNC(=S)Nc1ccc2N(Cc3ccc(cc3)-c3ccccc3)CCc2c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00817
BindingDB Entry DOI: 10.7270/Q2GT5S5W |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50464485
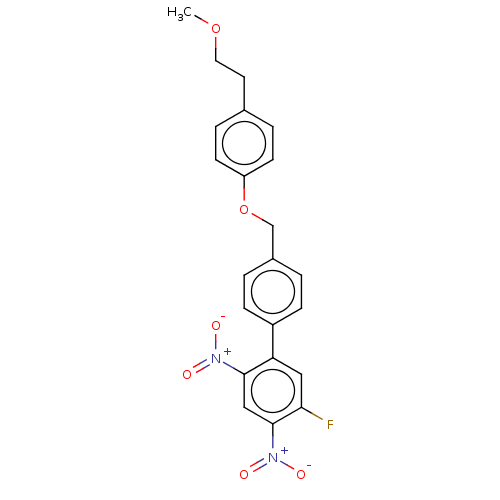
(CHEMBL4279158)Show SMILES COCCc1ccc(OCc2ccc(cc2)-c2cc(F)c(cc2[N+]([O-])=O)[N+]([O-])=O)cc1 Show InChI InChI=1S/C22H19FN2O6/c1-30-11-10-15-4-8-18(9-5-15)31-14-16-2-6-17(7-3-16)19-12-20(23)22(25(28)29)13-21(19)24(26)27/h2-9,12-13H,10-11,14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno
Curated by ChEMBL
| Assay Description
Inhibition of mPGES1 in IL-1beta-stimulated human A549 cell microsomes using PGH2 as substrate assessed as reduction in PGE2 production preincubated ... |
Eur J Med Chem 143: 1419-1427 (2018)
Article DOI: 10.1016/j.ejmech.2017.10.039
BindingDB Entry DOI: 10.7270/Q2G44SXG |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50591535

(CHEMBL5185907) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00817
BindingDB Entry DOI: 10.7270/Q2GT5S5W |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50317649

(6-((3S,6S,9S,14aR)-6-((1H-indol-3-yl)methyl)-9-ben...)Show SMILES ONC(=O)CCCCC[C@@H]1NC(=O)[C@H]2CCCN2C(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O |r| Show InChI InChI=1S/C33H40N6O6/c40-29(38-45)16-6-2-5-14-25-30(41)36-26(19-22-20-34-24-13-8-7-12-23(22)24)31(42)37-27(18-21-10-3-1-4-11-21)33(44)39-17-9-15-28(39)32(43)35-25/h1,3-4,7-8,10-13,20,25-28,34,45H,2,5-6,9,14-19H2,(H,35,43)(H,36,41)(H,37,42)(H,38,40)/t25-,26-,27-,28+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno
Curated by ChEMBL
| Assay Description
Inhibition of full length HDAC6 expressed in HEK293 cells after 15 mins by fluorescence assay |
Bioorg Med Chem 18: 3252-60 (2010)
Article DOI: 10.1016/j.bmc.2010.03.022
BindingDB Entry DOI: 10.7270/Q2BC3ZP6 |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50140689
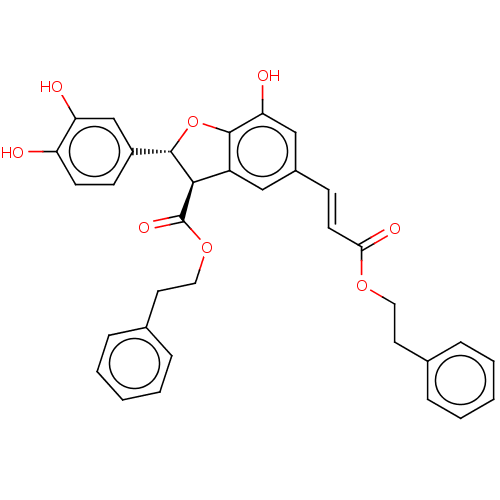
(CHEMBL3752809)Show SMILES Oc1cc(\C=C\C(=O)OCCc2ccccc2)cc2[C@H]([C@@H](Oc12)c1ccc(O)c(O)c1)C(=O)OCCc1ccccc1 |r| Show InChI InChI=1S/C34H30O8/c35-27-13-12-25(21-28(27)36)32-31(34(39)41-18-16-23-9-5-2-6-10-23)26-19-24(20-29(37)33(26)42-32)11-14-30(38)40-17-15-22-7-3-1-4-8-22/h1-14,19-21,31-32,35-37H,15-18H2/b14-11+/t31-,32+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Salerno
Curated by ChEMBL
| Assay Description
Inhibition of PGES-1 in IL-1beta stimulated human A549 cell microsomes using PGH2 as substrate assessed as suppression of PGE2 formation preincubated... |
Bioorg Med Chem 24: 820-6 (2016)
Article DOI: 10.1016/j.bmc.2016.01.002
BindingDB Entry DOI: 10.7270/Q2JH3P1M |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50140688

(CHEMBL3754377)Show SMILES CCCCOC(=O)\C=C\c1cc2[C@H]([C@@H](Oc2c(O)c1)c1ccc(O)c(O)c1)C(=O)OCCCC |r| Show InChI InChI=1S/C26H30O8/c1-3-5-11-32-22(30)10-7-16-13-18-23(26(31)33-12-6-4-2)24(34-25(18)21(29)14-16)17-8-9-19(27)20(28)15-17/h7-10,13-15,23-24,27-29H,3-6,11-12H2,1-2H3/b10-7+/t23-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Salerno
Curated by ChEMBL
| Assay Description
Inhibition of PGES-1 in IL-1beta stimulated human A549 cell microsomes using PGH2 as substrate assessed as suppression of PGE2 formation preincubated... |
Bioorg Med Chem 24: 820-6 (2016)
Article DOI: 10.1016/j.bmc.2016.01.002
BindingDB Entry DOI: 10.7270/Q2JH3P1M |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50006805

(3-(1-(4-chlorobenzyl)-3-(tert-butylthio)-5-isoprop...)Show SMILES CC(C)c1ccc2n(Cc3ccc(Cl)cc3)c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c2c1 Show InChI InChI=1S/C27H34ClNO2S/c1-17(2)19-10-13-22-21(14-19)24(32-26(3,4)5)23(15-27(6,7)25(30)31)29(22)16-18-8-11-20(28)12-9-18/h8-14,17H,15-16H2,1-7H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Salerno
Curated by ChEMBL
| Assay Description
Inhibition of PGES-1 in IL-1beta stimulated human A549 cell microsomes using PGH2 as substrate assessed as suppression of PGE2 formation preincubated... |
Bioorg Med Chem 24: 820-6 (2016)
Article DOI: 10.1016/j.bmc.2016.01.002
BindingDB Entry DOI: 10.7270/Q2JH3P1M |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50140689

(CHEMBL3752809)Show SMILES Oc1cc(\C=C\C(=O)OCCc2ccccc2)cc2[C@H]([C@@H](Oc12)c1ccc(O)c(O)c1)C(=O)OCCc1ccccc1 |r| Show InChI InChI=1S/C34H30O8/c35-27-13-12-25(21-28(27)36)32-31(34(39)41-18-16-23-9-5-2-6-10-23)26-19-24(20-29(37)33(26)42-32)11-14-30(38)40-17-15-22-7-3-1-4-8-22/h1-14,19-21,31-32,35-37H,15-18H2/b14-11+/t31-,32+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Salerno
Curated by ChEMBL
| Assay Description
Inhibition of PGES-1 in IL-1beta stimulated human A549 cell microsomes using PGH2 as substrate assessed as suppression of PGE2 formation preincubated... |
Bioorg Med Chem 24: 820-6 (2016)
Article DOI: 10.1016/j.bmc.2016.01.002
BindingDB Entry DOI: 10.7270/Q2JH3P1M |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50591540
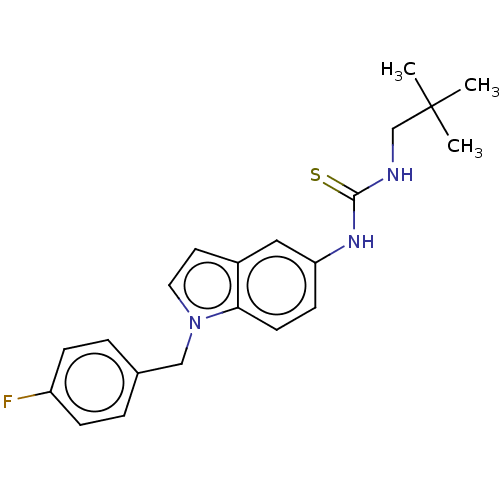
(CHEMBL5204046)Show SMILES CC(C)(C)CNC(=S)Nc1ccc2n(Cc3ccc(F)cc3)ccc2c1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00817
BindingDB Entry DOI: 10.7270/Q2GT5S5W |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50591536
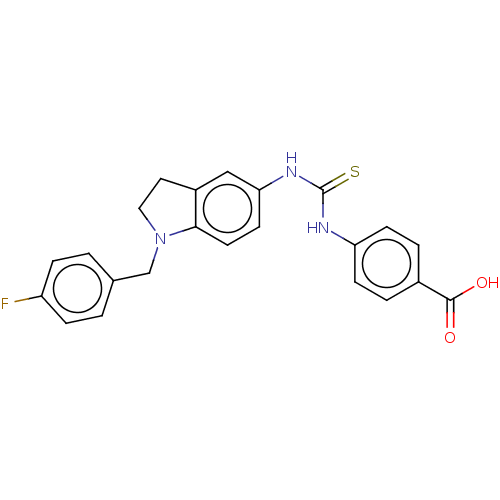
(CHEMBL5191521)Show SMILES OC(=O)c1ccc(NC(=S)Nc2ccc3N(Cc4ccc(F)cc4)CCc3c2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00817
BindingDB Entry DOI: 10.7270/Q2GT5S5W |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50612860
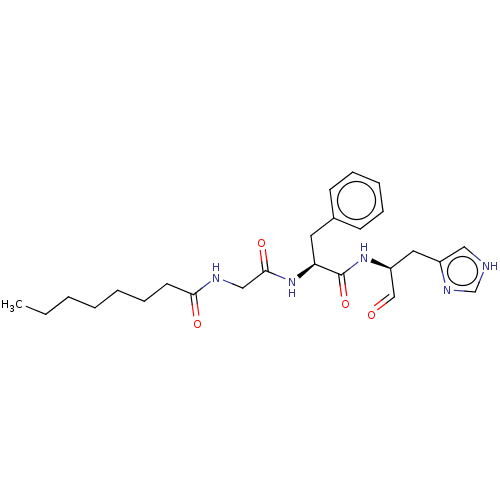
(CHEMBL5287352)Show SMILES CCCCCCCC(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1c[nH]cn1)C=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 2.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50591528
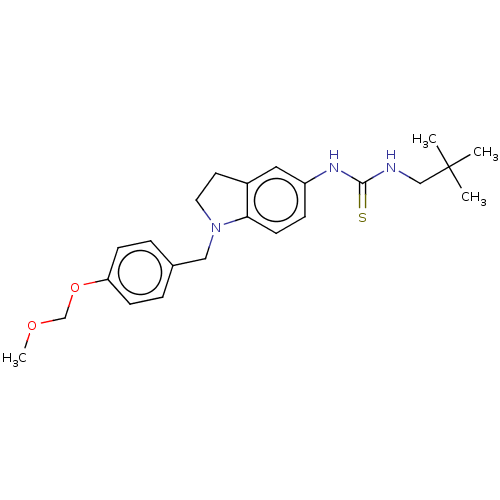
(CHEMBL5207672)Show SMILES COCOc1ccc(CN2CCc3cc(NC(=S)NCC(C)(C)C)ccc23)cc1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00817
BindingDB Entry DOI: 10.7270/Q2GT5S5W |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50084655
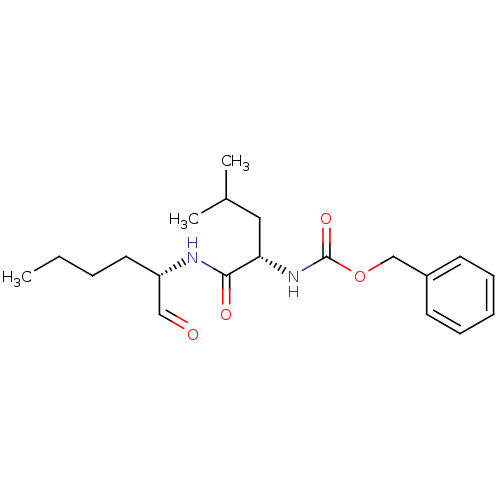
(CHEMBL92708 | Calpeptin | Z-Leu-Nle-CHO | [(S)-1-(...)Show SMILES CCCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1)C=O |r| Show InChI InChI=1S/C20H30N2O4/c1-4-5-11-17(13-23)21-19(24)18(12-15(2)3)22-20(25)26-14-16-9-7-6-8-10-16/h6-10,13,15,17-18H,4-5,11-12,14H2,1-3H3,(H,21,24)(H,22,25)/t17-,18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 2.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50612853
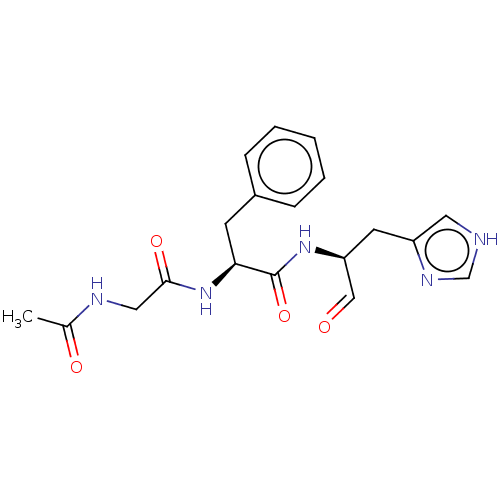
(CHEMBL5286815)Show SMILES CC(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1c[nH]cn1)C=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50140690

(CHEMBL3752963)Show SMILES Oc1cc(\C=C\C(=O)OCCc2ccccc2)cc2[C@@H]([C@H](Oc12)c1ccc(O)c(O)c1)C(=O)OCCc1ccccc1 |r| Show InChI InChI=1S/C34H30O8/c35-27-13-12-25(21-28(27)36)32-31(34(39)41-18-16-23-9-5-2-6-10-23)26-19-24(20-29(37)33(26)42-32)11-14-30(38)40-17-15-22-7-3-1-4-8-22/h1-14,19-21,31-32,35-37H,15-18H2/b14-11+/t31-,32+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Salerno
Curated by ChEMBL
| Assay Description
Inhibition of PGES-1 in IL-1beta stimulated human A549 cell microsomes using PGH2 as substrate assessed as suppression of PGE2 formation preincubated... |
Bioorg Med Chem 24: 820-6 (2016)
Article DOI: 10.1016/j.bmc.2016.01.002
BindingDB Entry DOI: 10.7270/Q2JH3P1M |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50591531

(CHEMBL5193145)Show SMILES CC(C)(C)CNC(=S)Nc1ccc2N(Cc3ccc(O)cc3)CCc2c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00817
BindingDB Entry DOI: 10.7270/Q2GT5S5W |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50591528

(CHEMBL5207672)Show SMILES COCOc1ccc(CN2CCc3cc(NC(=S)NCC(C)(C)C)ccc23)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00817
BindingDB Entry DOI: 10.7270/Q2GT5S5W |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50464486

(CHEMBL4293214)Show SMILES [O-][N+](=O)c1cc(c(cc1F)-c1ccccc1COc1cccc(c1)C(F)(F)F)[N+]([O-])=O Show InChI InChI=1S/C20H12F4N2O5/c21-17-9-16(18(25(27)28)10-19(17)26(29)30)15-7-2-1-4-12(15)11-31-14-6-3-5-13(8-14)20(22,23)24/h1-10H,11H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition and measured after 5 mins by UPLC... |
Eur J Med Chem 143: 1419-1427 (2018)
Article DOI: 10.1016/j.ejmech.2017.10.039
BindingDB Entry DOI: 10.7270/Q2G44SXG |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50140687
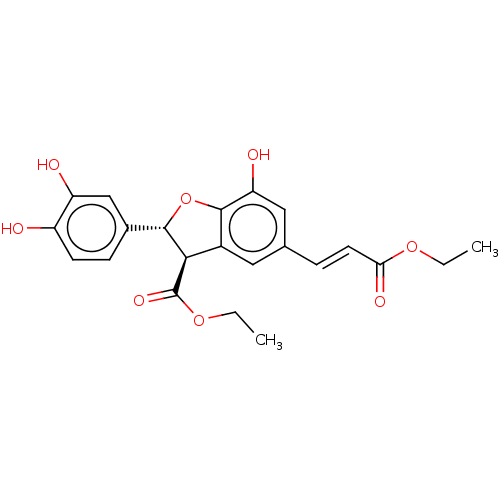
(CHEMBL3752388)Show SMILES CCOC(=O)\C=C\c1cc2[C@H]([C@@H](Oc2c(O)c1)c1ccc(O)c(O)c1)C(=O)OCC |r| Show InChI InChI=1S/C22H22O8/c1-3-28-18(26)8-5-12-9-14-19(22(27)29-4-2)20(30-21(14)17(25)10-12)13-6-7-15(23)16(24)11-13/h5-11,19-20,23-25H,3-4H2,1-2H3/b8-5+/t19-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Salerno
Curated by ChEMBL
| Assay Description
Inhibition of PGES-1 in IL-1beta stimulated human A549 cell microsomes using PGH2 as substrate assessed as suppression of PGE2 formation preincubated... |
Bioorg Med Chem 24: 820-6 (2016)
Article DOI: 10.1016/j.bmc.2016.01.002
BindingDB Entry DOI: 10.7270/Q2JH3P1M |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50591534

(CHEMBL5199450)Show SMILES Fc1ccc(CN2CCc3cc(NC(=S)NCc4ccccc4)ccc23)cc1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00817
BindingDB Entry DOI: 10.7270/Q2GT5S5W |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50480333
(CHEMBL503268)Show SMILES ONC(=O)CCCCCCC(=O)N(Cc1ccccc1)C(Cc1ccc(cc1)-c1ccccc1)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C38H43N3O4/c42-36(40-45)20-12-1-2-13-21-37(43)41(29-32-16-8-4-9-17-32)35(38(44)39-27-26-30-14-6-3-7-15-30)28-31-22-24-34(25-23-31)33-18-10-5-11-19-33/h3-11,14-19,22-25,35,45H,1-2,12-13,20-21,26-29H2,(H,39,44)(H,40,42) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi del Piemonte Orientale A. Avogadro
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human SHSY5Y cells by fluorimetric cellular activity assay |
J Med Chem 52: 2776-85 (2009)
Article DOI: 10.1021/jm801529c
BindingDB Entry DOI: 10.7270/Q2D221F1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data