 Found 371 hits with Last Name = 'di paolo' and Initial = 'j'
Found 371 hits with Last Name = 'di paolo' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50015453
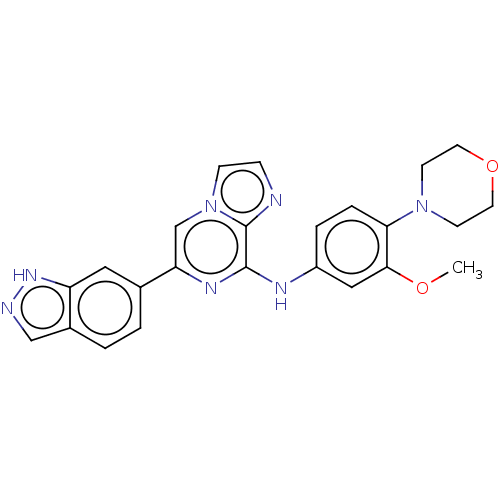
(CHEMBL3265037)Show SMILES COc1cc(Nc2nc(cn3ccnc23)-c2ccc3cn[nH]c3c2)ccc1N1CCOCC1 Show InChI InChI=1S/C24H23N7O2/c1-32-22-13-18(4-5-21(22)30-8-10-33-11-9-30)27-23-24-25-6-7-31(24)15-20(28-23)16-2-3-17-14-26-29-19(17)12-16/h2-7,12-15H,8-11H2,1H3,(H,26,29)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full length Syk (unknown origin) using biotinylated peptide substrate |
J Med Chem 57: 3856-73 (2014)
Article DOI: 10.1021/jm500228a
BindingDB Entry DOI: 10.7270/Q2B27WV1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50538456
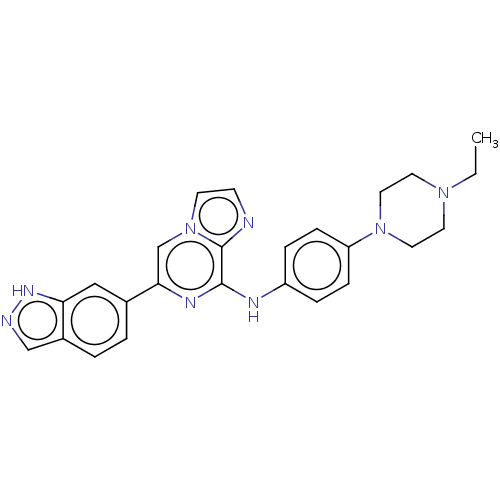
(CHEMBL4649800)Show SMILES CCN1CCN(CC1)c1ccc(Nc2nc(cn3ccnc23)-c2ccc3cn[nH]c3c2)cc1 Show InChI InChI=1S/C25H26N8/c1-2-31-11-13-32(14-12-31)21-7-5-20(6-8-21)28-24-25-26-9-10-33(25)17-23(29-24)18-3-4-19-16-27-30-22(19)15-18/h3-10,15-17H,2,11-14H2,1H3,(H,27,30)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) using XL665-labeled peptide as substrate in presence of ATP measured after 30 mins by TR-FRET assay |
ACS Med Chem Lett 11: 506-513 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00621
BindingDB Entry DOI: 10.7270/Q2ZG6WR1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50015454
(CHEMBL3264995)Show SMILES COc1ccc(Nc2nc(cn3ccnc23)-c2cccc(c2)C(=O)Nc2ccc(cc2)C(O)=O)cc1OC Show InChI InChI=1S/C28H23N5O5/c1-37-23-11-10-21(15-24(23)38-2)30-25-26-29-12-13-33(26)16-22(32-25)18-4-3-5-19(14-18)27(34)31-20-8-6-17(7-9-20)28(35)36/h3-16H,1-2H3,(H,30,32)(H,31,34)(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full length Syk (unknown origin) using biotinylated peptide substrate |
J Med Chem 57: 3856-73 (2014)
Article DOI: 10.1021/jm500228a
BindingDB Entry DOI: 10.7270/Q2B27WV1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50538457

(CHEMBL4638089)Show SMILES COCCN1CCN(CC1)c1ccc(Nc2nc(cn3ccnc23)-c2ccc3cn[nH]c3c2)cc1 Show InChI InChI=1S/C26H28N8O/c1-35-15-14-32-10-12-33(13-11-32)22-6-4-21(5-7-22)29-25-26-27-8-9-34(26)18-24(30-25)19-2-3-20-17-28-31-23(20)16-19/h2-9,16-18H,10-15H2,1H3,(H,28,31)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) using XL665-labeled peptide as substrate in presence of ATP measured after 30 mins by TR-FRET assay |
ACS Med Chem Lett 11: 506-513 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00621
BindingDB Entry DOI: 10.7270/Q2ZG6WR1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50538455
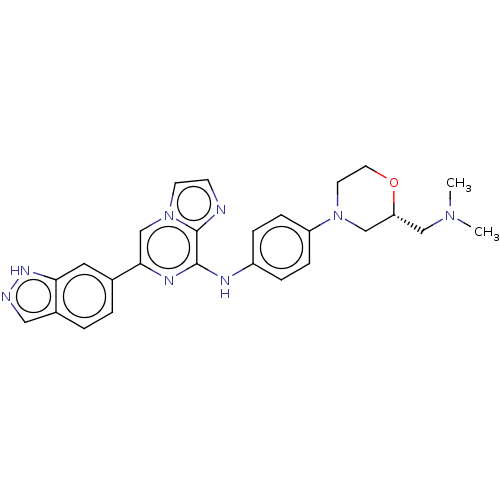
(CHEMBL4637943)Show SMILES CN(C)C[C@@H]1CN(CCO1)c1ccc(Nc2nc(cn3ccnc23)-c2ccc3cn[nH]c3c2)cc1 |r| Show InChI InChI=1S/C26H28N8O/c1-32(2)15-22-16-33(11-12-35-22)21-7-5-20(6-8-21)29-25-26-27-9-10-34(26)17-24(30-25)18-3-4-19-14-28-31-23(19)13-18/h3-10,13-14,17,22H,11-12,15-16H2,1-2H3,(H,28,31)(H,29,30)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) using XL665-labeled peptide as substrate in presence of ATP measured after 30 mins by TR-FRET assay |
ACS Med Chem Lett 11: 506-513 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00621
BindingDB Entry DOI: 10.7270/Q2ZG6WR1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50134365
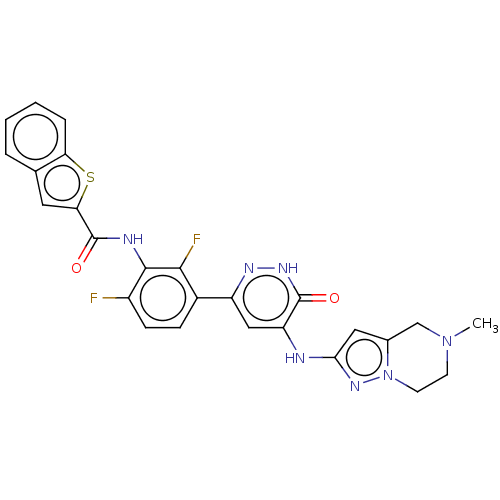
(CHEMBL3745935)Show SMILES CN1CCn2nc(Nc3cc(n[nH]c3=O)-c3ccc(F)c(NC(=O)c4cc5ccccc5s4)c3F)cc2C1 Show InChI InChI=1S/C26H21F2N7O2S/c1-34-8-9-35-15(13-34)11-22(33-35)29-19-12-18(31-32-25(19)36)16-6-7-17(27)24(23(16)28)30-26(37)21-10-14-4-2-3-5-20(14)38-21/h2-7,10-12H,8-9,13H2,1H3,(H,30,37)(H,32,36)(H,29,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of BTK (unknown origin) by Lanthascreen assay |
Bioorg Med Chem Lett 26: 575-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.076
BindingDB Entry DOI: 10.7270/Q2222WMH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50538454
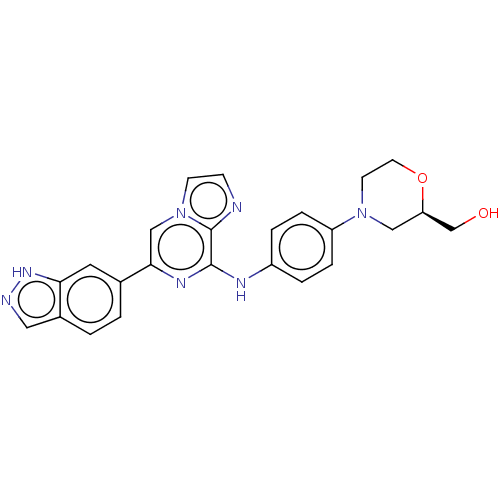
(CHEMBL4643354)Show SMILES OC[C@H]1CN(CCO1)c1ccc(Nc2nc(cn3ccnc23)-c2ccc3cn[nH]c3c2)cc1 |r| Show InChI InChI=1S/C24H23N7O2/c32-15-20-13-30(9-10-33-20)19-5-3-18(4-6-19)27-23-24-25-7-8-31(24)14-22(28-23)16-1-2-17-12-26-29-21(17)11-16/h1-8,11-12,14,20,32H,9-10,13,15H2,(H,26,29)(H,27,28)/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) using XL665-labeled peptide as substrate in presence of ATP measured after 30 mins by TR-FRET assay |
ACS Med Chem Lett 11: 506-513 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00621
BindingDB Entry DOI: 10.7270/Q2ZG6WR1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50538453
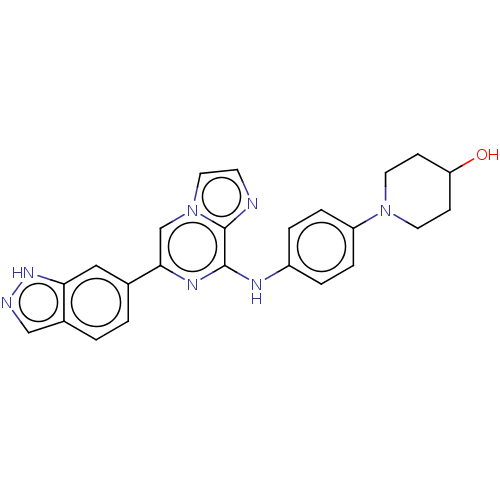
(CHEMBL4647927)Show SMILES OC1CCN(CC1)c1ccc(Nc2nc(cn3ccnc23)-c2ccc3cn[nH]c3c2)cc1 Show InChI InChI=1S/C24H23N7O/c32-20-7-10-30(11-8-20)19-5-3-18(4-6-19)27-23-24-25-9-12-31(24)15-22(28-23)16-1-2-17-14-26-29-21(17)13-16/h1-6,9,12-15,20,32H,7-8,10-11H2,(H,26,29)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) using XL665-labeled peptide as substrate in presence of ATP measured after 30 mins by TR-FRET assay |
ACS Med Chem Lett 11: 506-513 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00621
BindingDB Entry DOI: 10.7270/Q2ZG6WR1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50015447

(CHEMBL3265031)Show SMILES CC1(O)CCN(CC1)c1ccc(Nc2nc(cn3ccnc23)-c2ccc3cn[nH]c3c2)cc1 Show InChI InChI=1S/C25H25N7O/c1-25(33)8-11-31(12-9-25)20-6-4-19(5-7-20)28-23-24-26-10-13-32(24)16-22(29-23)17-2-3-18-15-27-30-21(18)14-17/h2-7,10,13-16,33H,8-9,11-12H2,1H3,(H,27,30)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full length Syk (unknown origin) using biotinylated peptide substrate |
J Med Chem 57: 3856-73 (2014)
Article DOI: 10.1021/jm500228a
BindingDB Entry DOI: 10.7270/Q2B27WV1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50015460
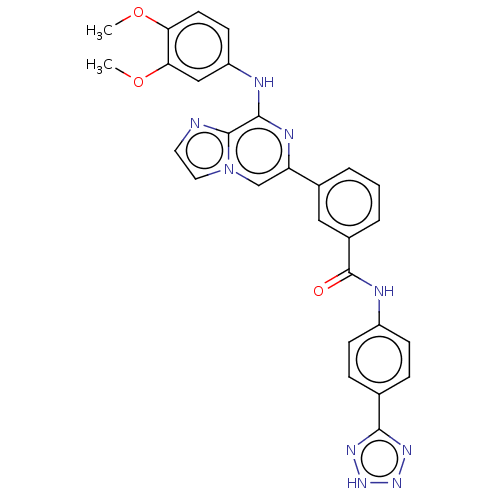
(CHEMBL3265001)Show SMILES COc1ccc(Nc2nc(cn3ccnc23)-c2cccc(c2)C(=O)Nc2ccc(cc2)-c2nn[nH]n2)cc1OC Show InChI InChI=1S/C28H23N9O3/c1-39-23-11-10-21(15-24(23)40-2)30-26-27-29-12-13-37(27)16-22(32-26)18-4-3-5-19(14-18)28(38)31-20-8-6-17(7-9-20)25-33-35-36-34-25/h3-16H,1-2H3,(H,30,32)(H,31,38)(H,33,34,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full length Syk (unknown origin) using biotinylated peptide substrate |
J Med Chem 57: 3856-73 (2014)
Article DOI: 10.1021/jm500228a
BindingDB Entry DOI: 10.7270/Q2B27WV1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50538469
(CHEMBL4643695)Show SMILES C1OCC1N1CCN(CC1)c1ccc(Nc2nc(cn3ccnc23)-c2cnc3cc[nH]c3n2)cc1 Show InChI InChI=1S/C25H25N9O/c1-3-18(32-9-11-33(12-10-32)19-15-35-16-19)4-2-17(1)29-24-25-27-7-8-34(25)14-22(31-24)21-13-28-20-5-6-26-23(20)30-21/h1-8,13-14,19H,9-12,15-16H2,(H,26,30)(H,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) using XL665-labeled peptide as substrate in presence of ATP measured after 30 mins by TR-FRET assay |
ACS Med Chem Lett 11: 506-513 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00621
BindingDB Entry DOI: 10.7270/Q2ZG6WR1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM36516
(4-(tert-Butyl)-N-(2-methyl-3-(4-methyl-6-((4-(morp...)Show SMILES Cc1c(NC(=O)c2ccc(cc2)C(C)(C)C)cccc1-c1cn(C)c(=O)c(Nc2ccc(cc2)C(=O)N2CCOCC2)n1 Show InChI InChI=1S/C34H37N5O4/c1-22-27(7-6-8-28(22)37-31(40)23-9-13-25(14-10-23)34(2,3)4)29-21-38(5)33(42)30(36-29)35-26-15-11-24(12-16-26)32(41)39-17-19-43-20-18-39/h6-16,21H,17-20H2,1-5H3,(H,35,36)(H,37,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.90 | 1.5 | n/a | n/a | n/a | 7.5 | 23 |
CGI Pharmaceuticals
| Assay Description
Biochemical assay using Lanthascreen (human, full-lenght, C-terminal v5-His6 expressed in Sf9 cell) assay from Invitrogen. |
Nat Chem Biol 7: 41-50 (2011)
Article DOI: 10.1038/nchembio.481
BindingDB Entry DOI: 10.7270/Q2B56H2T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50015449

(CHEMBL3265033)Show SMILES O=S1(=O)CCN(CC1)c1ccc(Nc2nc(cn3ccnc23)-c2ccc3cn[nH]c3c2)cc1 Show InChI InChI=1S/C23H21N7O2S/c31-33(32)11-9-29(10-12-33)19-5-3-18(4-6-19)26-22-23-24-7-8-30(23)15-21(27-22)16-1-2-17-14-25-28-20(17)13-16/h1-8,13-15H,9-12H2,(H,25,28)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full length Syk (unknown origin) using biotinylated peptide substrate |
J Med Chem 57: 3856-73 (2014)
Article DOI: 10.1021/jm500228a
BindingDB Entry DOI: 10.7270/Q2B27WV1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50015459
(CHEMBL3265000)Show SMILES COc1ccc(Nc2nc(cn3ccnc23)-c2cccc(c2)C(=O)Nc2ccc(cc2)C(=O)NS(C)(=O)=O)cc1OC Show InChI InChI=1S/C29H26N6O6S/c1-40-24-12-11-22(16-25(24)41-2)31-26-27-30-13-14-35(27)17-23(33-26)19-5-4-6-20(15-19)28(36)32-21-9-7-18(8-10-21)29(37)34-42(3,38)39/h4-17H,1-3H3,(H,31,33)(H,32,36)(H,34,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full length Syk (unknown origin) using biotinylated peptide substrate |
J Med Chem 57: 3856-73 (2014)
Article DOI: 10.1021/jm500228a
BindingDB Entry DOI: 10.7270/Q2B27WV1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50015439

(CHEMBL3265024)Show SMILES COc1ccc(Nc2nc(cn3ccnc23)-c2ccc3OCC(=O)Nc3c2)cc1OC Show InChI InChI=1S/C22H19N5O4/c1-29-18-6-4-14(10-19(18)30-2)24-21-22-23-7-8-27(22)11-16(26-21)13-3-5-17-15(9-13)25-20(28)12-31-17/h3-11H,12H2,1-2H3,(H,24,26)(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full length Syk (unknown origin) using biotinylated peptide substrate |
J Med Chem 57: 3856-73 (2014)
Article DOI: 10.1021/jm500228a
BindingDB Entry DOI: 10.7270/Q2B27WV1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50015457

(CHEMBL3264998)Show SMILES COc1ccc(Nc2nc(cn3ccnc23)-c2cccc(c2)C(=O)Nc2ccc(CC(O)=O)cc2)cc1OC Show InChI InChI=1S/C29H25N5O5/c1-38-24-11-10-22(16-25(24)39-2)31-27-28-30-12-13-34(28)17-23(33-27)19-4-3-5-20(15-19)29(37)32-21-8-6-18(7-9-21)14-26(35)36/h3-13,15-17H,14H2,1-2H3,(H,31,33)(H,32,37)(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full length Syk (unknown origin) using biotinylated peptide substrate |
J Med Chem 57: 3856-73 (2014)
Article DOI: 10.1021/jm500228a
BindingDB Entry DOI: 10.7270/Q2B27WV1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50015425
(CHEMBL3265016)Show SMILES COc1ccc(Nc2nc(cn3ccnc23)-c2ccc3cc[nH]c3c2)cc1OC Show InChI InChI=1S/C22H19N5O2/c1-28-19-6-5-16(12-20(19)29-2)25-21-22-24-9-10-27(22)13-18(26-21)15-4-3-14-7-8-23-17(14)11-15/h3-13,23H,1-2H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full length Syk (unknown origin) using biotinylated peptide substrate |
J Med Chem 57: 3856-73 (2014)
Article DOI: 10.1021/jm500228a
BindingDB Entry DOI: 10.7270/Q2B27WV1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50538460

(CHEMBL4634466)Show SMILES C1OCC1N1CCN(CC1)c1ccc(Nc2nc(cn3ccnc23)-c2cnc3cn[nH]c3c2)cc1 Show InChI InChI=1S/C25H25N9O/c1-3-19(32-7-9-33(10-8-32)20-15-35-16-20)4-2-18(1)29-24-25-26-5-6-34(25)14-23(30-24)17-11-21-22(27-12-17)13-28-31-21/h1-6,11-14,20H,7-10,15-16H2,(H,28,31)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) using XL665-labeled peptide as substrate in presence of ATP measured after 30 mins by TR-FRET assay |
ACS Med Chem Lett 11: 506-513 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00621
BindingDB Entry DOI: 10.7270/Q2ZG6WR1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50134377
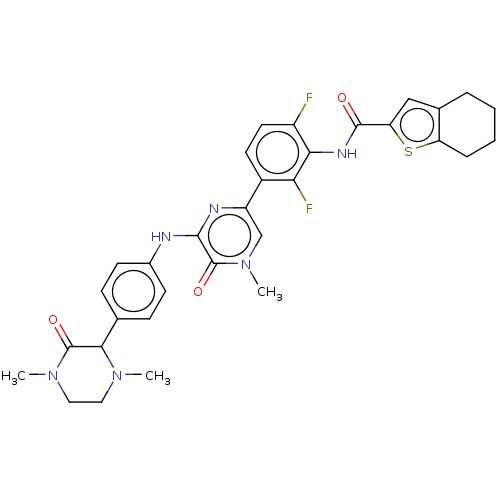
(CHEMBL3745934)Show SMILES CN1CCN(C)C(=O)C1c1ccc(Nc2nc(cn(C)c2=O)-c2ccc(F)c(NC(=O)c3cc4CCCCc4s3)c2F)cc1 Show InChI InChI=1S/C32H32F2N6O3S/c1-38-14-15-39(2)31(42)28(38)18-8-10-20(11-9-18)35-29-32(43)40(3)17-23(36-29)21-12-13-22(33)27(26(21)34)37-30(41)25-16-19-6-4-5-7-24(19)44-25/h8-13,16-17,28H,4-7,14-15H2,1-3H3,(H,35,36)(H,37,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of BTK (unknown origin) by Lanthascreen assay |
Bioorg Med Chem Lett 26: 575-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.076
BindingDB Entry DOI: 10.7270/Q2222WMH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50134377

(CHEMBL3745934)Show SMILES CN1CCN(C)C(=O)C1c1ccc(Nc2nc(cn(C)c2=O)-c2ccc(F)c(NC(=O)c3cc4CCCCc4s3)c2F)cc1 Show InChI InChI=1S/C32H32F2N6O3S/c1-38-14-15-39(2)31(42)28(38)18-8-10-20(11-9-18)35-29-32(43)40(3)17-23(36-29)21-12-13-22(33)27(26(21)34)37-30(41)25-16-19-6-4-5-7-24(19)44-25/h8-13,16-17,28H,4-7,14-15H2,1-3H3,(H,35,36)(H,37,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of BTK (unknown origin) by Lanthascreen assay |
Bioorg Med Chem Lett 26: 575-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.076
BindingDB Entry DOI: 10.7270/Q2222WMH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50134320
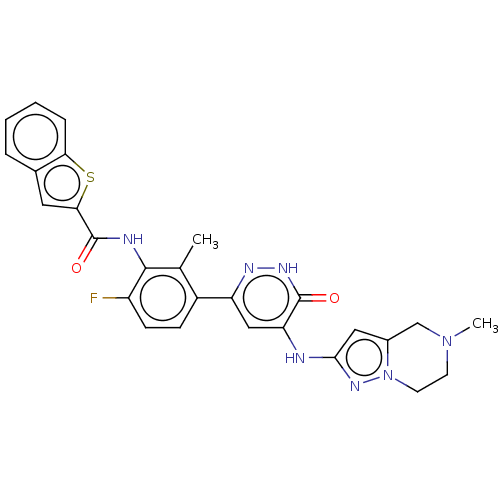
(CHEMBL3746293)Show SMILES CN1CCn2nc(Nc3cc(n[nH]c3=O)-c3ccc(F)c(NC(=O)c4cc5ccccc5s4)c3C)cc2C1 Show InChI InChI=1S/C27H24FN7O2S/c1-15-18(7-8-19(28)25(15)30-27(37)23-11-16-5-3-4-6-22(16)38-23)20-13-21(26(36)32-31-20)29-24-12-17-14-34(2)9-10-35(17)33-24/h3-8,11-13H,9-10,14H2,1-2H3,(H,30,37)(H,32,36)(H,29,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of BTK (unknown origin) by Lanthascreen assay |
Bioorg Med Chem Lett 26: 575-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.076
BindingDB Entry DOI: 10.7270/Q2222WMH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50538461

(CHEMBL4637326)Show SMILES C1OCC1N1CCN(CC1)c1ccc(Nc2nc(cn3ccnc23)-c2cnc3cn[nH]c3n2)cc1 Show InChI InChI=1S/C24H24N10O/c1-3-17(32-7-9-33(10-8-32)18-14-35-15-18)4-2-16(1)28-23-24-25-5-6-34(24)13-21(30-23)19-11-26-20-12-27-31-22(20)29-19/h1-6,11-13,18H,7-10,14-15H2,(H,28,30)(H,27,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) using XL665-labeled peptide as substrate in presence of ATP measured after 30 mins by TR-FRET assay |
ACS Med Chem Lett 11: 506-513 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00621
BindingDB Entry DOI: 10.7270/Q2ZG6WR1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50015427
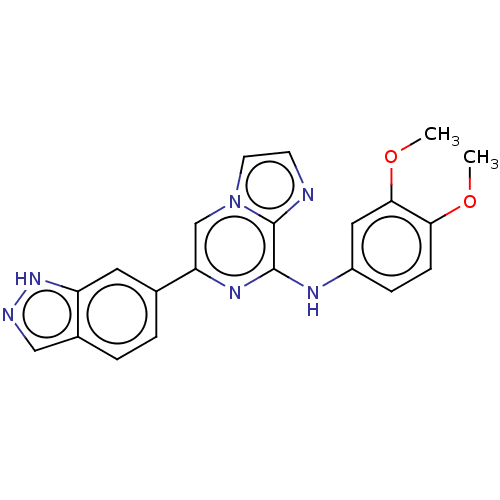
(CHEMBL3265017)Show SMILES COc1ccc(Nc2nc(cn3ccnc23)-c2ccc3cn[nH]c3c2)cc1OC Show InChI InChI=1S/C21H18N6O2/c1-28-18-6-5-15(10-19(18)29-2)24-20-21-22-7-8-27(21)12-17(25-20)13-3-4-14-11-23-26-16(14)9-13/h3-12H,1-2H3,(H,23,26)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full length Syk (unknown origin) using biotinylated peptide substrate |
J Med Chem 57: 3856-73 (2014)
Article DOI: 10.1021/jm500228a
BindingDB Entry DOI: 10.7270/Q2B27WV1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50015450
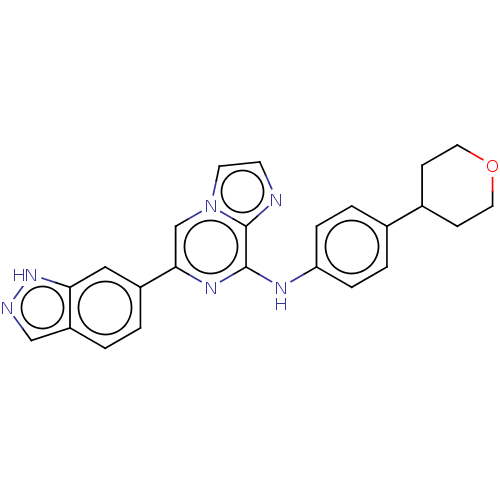
(CHEMBL3265034)Show SMILES C1CC(CCO1)c1ccc(Nc2nc(cn3ccnc23)-c2ccc3cn[nH]c3c2)cc1 Show InChI InChI=1S/C24H22N6O/c1-2-19-14-26-29-21(19)13-18(1)22-15-30-10-9-25-24(30)23(28-22)27-20-5-3-16(4-6-20)17-7-11-31-12-8-17/h1-6,9-10,13-15,17H,7-8,11-12H2,(H,26,29)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full length Syk (unknown origin) using biotinylated peptide substrate |
J Med Chem 57: 3856-73 (2014)
Article DOI: 10.1021/jm500228a
BindingDB Entry DOI: 10.7270/Q2B27WV1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50015446
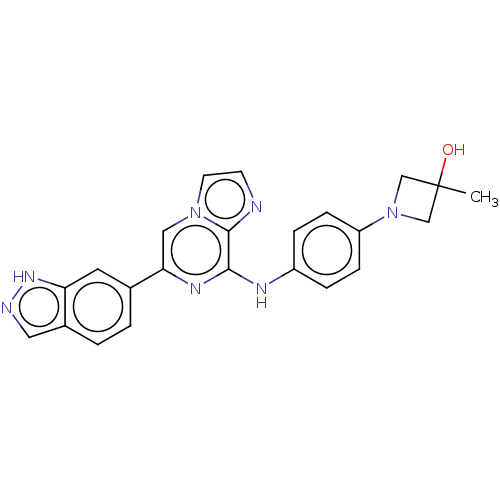
(CHEMBL3265030)Show SMILES CC1(O)CN(C1)c1ccc(Nc2nc(cn3ccnc23)-c2ccc3cn[nH]c3c2)cc1 Show InChI InChI=1S/C23H21N7O/c1-23(31)13-30(14-23)18-6-4-17(5-7-18)26-21-22-24-8-9-29(22)12-20(27-21)15-2-3-16-11-25-28-19(16)10-15/h2-12,31H,13-14H2,1H3,(H,25,28)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full length Syk (unknown origin) using biotinylated peptide substrate |
J Med Chem 57: 3856-73 (2014)
Article DOI: 10.1021/jm500228a
BindingDB Entry DOI: 10.7270/Q2B27WV1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50134366
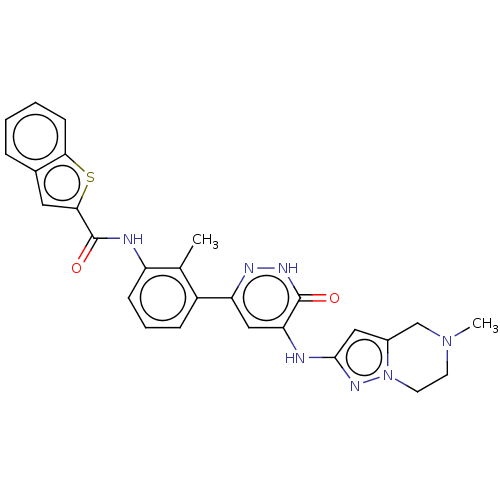
(CHEMBL3746115)Show SMILES CN1CCn2nc(Nc3cc(n[nH]c3=O)-c3cccc(NC(=O)c4cc5ccccc5s4)c3C)cc2C1 Show InChI InChI=1S/C27H25N7O2S/c1-16-19(7-5-8-20(16)29-27(36)24-12-17-6-3-4-9-23(17)37-24)21-14-22(26(35)31-30-21)28-25-13-18-15-33(2)10-11-34(18)32-25/h3-9,12-14H,10-11,15H2,1-2H3,(H,29,36)(H,31,35)(H,28,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of BTK (unknown origin) by Lanthascreen assay |
Bioorg Med Chem Lett 26: 575-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.076
BindingDB Entry DOI: 10.7270/Q2222WMH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50134368

(CHEMBL3746333)Show SMILES CN1CCn2nc(Nc3cc(n[nH]c3=O)-c3ccc(F)c(NC(=O)c4cnc(cn4)C(C)(C)C)c3C)cc2C1 Show InChI InChI=1S/C27H30FN9O2/c1-15-17(6-7-18(28)24(15)32-25(38)21-12-30-22(13-29-21)27(2,3)4)19-11-20(26(39)34-33-19)31-23-10-16-14-36(5)8-9-37(16)35-23/h6-7,10-13H,8-9,14H2,1-5H3,(H,32,38)(H,34,39)(H,31,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of BTK (unknown origin) by Lanthascreen assay |
Bioorg Med Chem Lett 26: 575-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.076
BindingDB Entry DOI: 10.7270/Q2222WMH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50134369
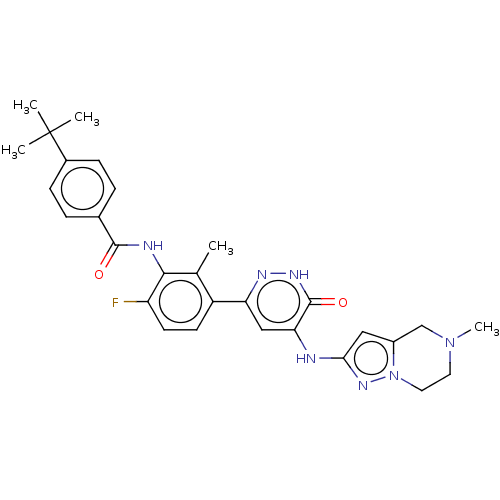
(CHEMBL3747417)Show SMILES CN1CCn2nc(Nc3cc(n[nH]c3=O)-c3ccc(F)c(NC(=O)c4ccc(cc4)C(C)(C)C)c3C)cc2C1 Show InChI InChI=1S/C29H32FN7O2/c1-17-21(10-11-22(30)26(17)32-27(38)18-6-8-19(9-7-18)29(2,3)4)23-15-24(28(39)34-33-23)31-25-14-20-16-36(5)12-13-37(20)35-25/h6-11,14-15H,12-13,16H2,1-5H3,(H,32,38)(H,34,39)(H,31,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of BTK (unknown origin) by Lanthascreen assay |
Bioorg Med Chem Lett 26: 575-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.076
BindingDB Entry DOI: 10.7270/Q2222WMH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50015458
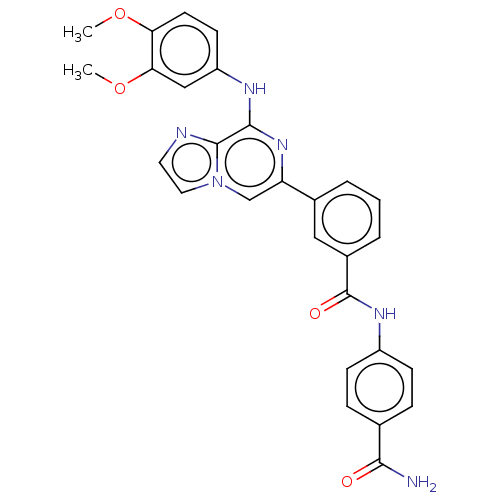
(CHEMBL3264999)Show SMILES COc1ccc(Nc2nc(cn3ccnc23)-c2cccc(c2)C(=O)Nc2ccc(cc2)C(N)=O)cc1OC Show InChI InChI=1S/C28H24N6O4/c1-37-23-11-10-21(15-24(23)38-2)31-26-27-30-12-13-34(27)16-22(33-26)18-4-3-5-19(14-18)28(36)32-20-8-6-17(7-9-20)25(29)35/h3-16H,1-2H3,(H2,29,35)(H,31,33)(H,32,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full length Syk (unknown origin) using biotinylated peptide substrate |
J Med Chem 57: 3856-73 (2014)
Article DOI: 10.1021/jm500228a
BindingDB Entry DOI: 10.7270/Q2B27WV1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50015442

(CHEMBL3265026)Show SMILES COc1ccc(Nc2nc(cn3ccnc23)-c2ccc3OCCNc3c2)cc1OC Show InChI InChI=1S/C22H21N5O3/c1-28-19-6-4-15(12-20(19)29-2)25-21-22-24-7-9-27(22)13-17(26-21)14-3-5-18-16(11-14)23-8-10-30-18/h3-7,9,11-13,23H,8,10H2,1-2H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full length Syk (unknown origin) using biotinylated peptide substrate |
J Med Chem 57: 3856-73 (2014)
Article DOI: 10.1021/jm500228a
BindingDB Entry DOI: 10.7270/Q2B27WV1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50538451
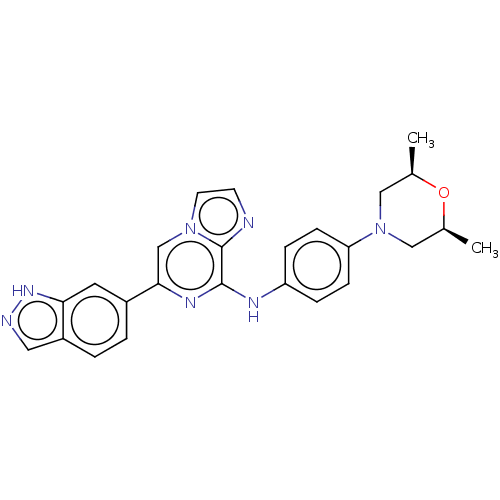
(CHEMBL4642016)Show SMILES C[C@H]1CN(C[C@@H](C)O1)c1ccc(Nc2nc(cn3ccnc23)-c2ccc3cn[nH]c3c2)cc1 |r| Show InChI InChI=1S/C25H25N7O/c1-16-13-32(14-17(2)33-16)21-7-5-20(6-8-21)28-24-25-26-9-10-31(25)15-23(29-24)18-3-4-19-12-27-30-22(19)11-18/h3-12,15-17H,13-14H2,1-2H3,(H,27,30)(H,28,29)/t16-,17+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) using XL665-labeled peptide as substrate in presence of ATP measured after 30 mins by TR-FRET assay |
ACS Med Chem Lett 11: 506-513 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00621
BindingDB Entry DOI: 10.7270/Q2ZG6WR1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50538449
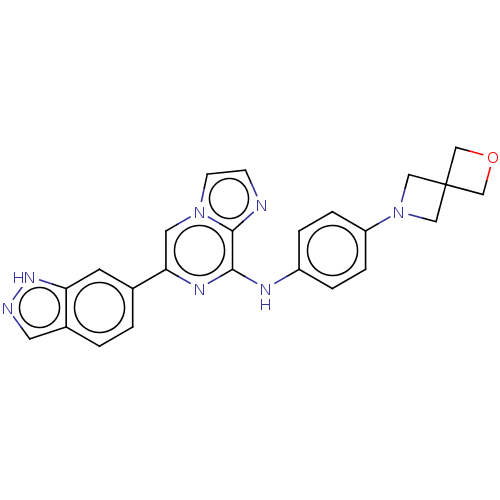
(CHEMBL4633338)Show SMILES C1OCC11CN(C1)c1ccc(Nc2nc(cn3ccnc23)-c2ccc3cn[nH]c3c2)cc1 Show InChI InChI=1S/C24H21N7O/c1-2-17-10-26-29-20(17)9-16(1)21-11-30-8-7-25-23(30)22(28-21)27-18-3-5-19(6-4-18)31-12-24(13-31)14-32-15-24/h1-11H,12-15H2,(H,26,29)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) using XL665-labeled peptide as substrate in presence of ATP measured after 30 mins by TR-FRET assay |
ACS Med Chem Lett 11: 506-513 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00621
BindingDB Entry DOI: 10.7270/Q2ZG6WR1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50134371
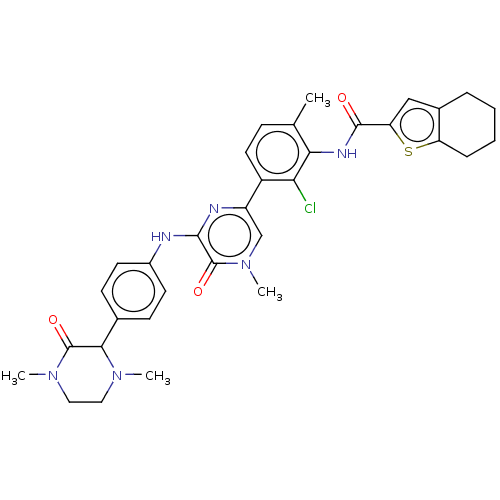
(CHEMBL3747742)Show SMILES CN1CCN(C)C(=O)C1c1ccc(Nc2nc(cn(C)c2=O)-c2ccc(C)c(NC(=O)c3cc4CCCCc4s3)c2Cl)cc1 Show InChI InChI=1S/C33H35ClN6O3S/c1-19-9-14-23(27(34)28(19)37-31(41)26-17-21-7-5-6-8-25(21)44-26)24-18-40(4)33(43)30(36-24)35-22-12-10-20(11-13-22)29-32(42)39(3)16-15-38(29)2/h9-14,17-18,29H,5-8,15-16H2,1-4H3,(H,35,36)(H,37,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of BTK (unknown origin) by Lanthascreen assay |
Bioorg Med Chem Lett 26: 575-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.076
BindingDB Entry DOI: 10.7270/Q2222WMH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50538445

(CHEMBL4634723)Show SMILES [H][C@]12CN(c3ccc(Nc4nc(cn5ccnc45)-c4ccc5cn[nH]c5c4)cc3)[C@]([H])(CO1)C2 |r| Show InChI InChI=1S/C24H21N7O/c1-2-16-11-26-29-21(16)9-15(1)22-13-30-8-7-25-24(30)23(28-22)27-17-3-5-18(6-4-17)31-12-20-10-19(31)14-32-20/h1-9,11,13,19-20H,10,12,14H2,(H,26,29)(H,27,28)/t19-,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) using XL665-labeled peptide as substrate in presence of ATP measured after 30 mins by TR-FRET assay |
ACS Med Chem Lett 11: 506-513 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00621
BindingDB Entry DOI: 10.7270/Q2ZG6WR1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50134371

(CHEMBL3747742)Show SMILES CN1CCN(C)C(=O)C1c1ccc(Nc2nc(cn(C)c2=O)-c2ccc(C)c(NC(=O)c3cc4CCCCc4s3)c2Cl)cc1 Show InChI InChI=1S/C33H35ClN6O3S/c1-19-9-14-23(27(34)28(19)37-31(41)26-17-21-7-5-6-8-25(21)44-26)24-18-40(4)33(43)30(36-24)35-22-12-10-20(11-13-22)29-32(42)39(3)16-15-38(29)2/h9-14,17-18,29H,5-8,15-16H2,1-4H3,(H,35,36)(H,37,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of BTK (unknown origin) by Lanthascreen assay |
Bioorg Med Chem Lett 26: 575-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.076
BindingDB Entry DOI: 10.7270/Q2222WMH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50538444

(CHEMBL4640287)Show SMILES [H][C@]12CC[C@]([H])(CN(C1)c1ccc(Nc3nc(cn4ccnc34)-c3ccc4cn[nH]c4c3)cc1)O2 |r| Show InChI InChI=1S/C25H23N7O/c1-2-17-12-27-30-22(17)11-16(1)23-15-31-10-9-26-25(31)24(29-23)28-18-3-5-19(6-4-18)32-13-20-7-8-21(14-32)33-20/h1-6,9-12,15,20-21H,7-8,13-14H2,(H,27,30)(H,28,29)/t20-,21+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) using XL665-labeled peptide as substrate in presence of ATP measured after 30 mins by TR-FRET assay |
ACS Med Chem Lett 11: 506-513 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00621
BindingDB Entry DOI: 10.7270/Q2ZG6WR1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50134373
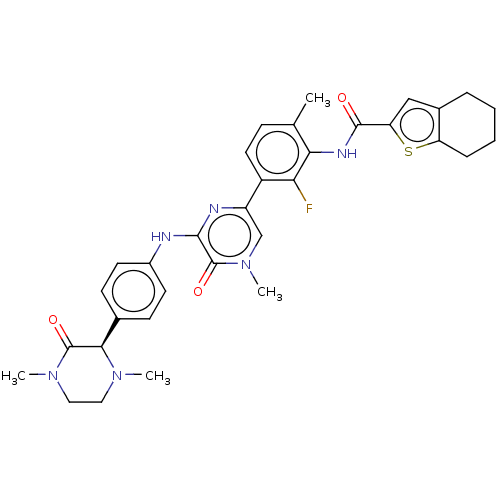
(CHEMBL3747315)Show SMILES CN1CCN(C)C(=O)[C@H]1c1ccc(Nc2nc(cn(C)c2=O)-c2ccc(C)c(NC(=O)c3cc4CCCCc4s3)c2F)cc1 |r| Show InChI InChI=1S/C33H35FN6O3S/c1-19-9-14-23(27(34)28(19)37-31(41)26-17-21-7-5-6-8-25(21)44-26)24-18-40(4)33(43)30(36-24)35-22-12-10-20(11-13-22)29-32(42)39(3)16-15-38(29)2/h9-14,17-18,29H,5-8,15-16H2,1-4H3,(H,35,36)(H,37,41)/t29-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of BTK (unknown origin) by Lanthascreen assay |
Bioorg Med Chem Lett 26: 575-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.076
BindingDB Entry DOI: 10.7270/Q2222WMH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50134374

(CHEMBL3747161)Show SMILES CN1CCN(C)C(=O)[C@@H]1c1ccc(Nc2nc(cn(C)c2=O)-c2ccc(C)c(NC(=O)c3cc4CCCCc4s3)c2F)cc1 |r| Show InChI InChI=1S/C33H35FN6O3S/c1-19-9-14-23(27(34)28(19)37-31(41)26-17-21-7-5-6-8-25(21)44-26)24-18-40(4)33(43)30(36-24)35-22-12-10-20(11-13-22)29-32(42)39(3)16-15-38(29)2/h9-14,17-18,29H,5-8,15-16H2,1-4H3,(H,35,36)(H,37,41)/t29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of BTK (unknown origin) by Lanthascreen assay |
Bioorg Med Chem Lett 26: 575-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.076
BindingDB Entry DOI: 10.7270/Q2222WMH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50134385
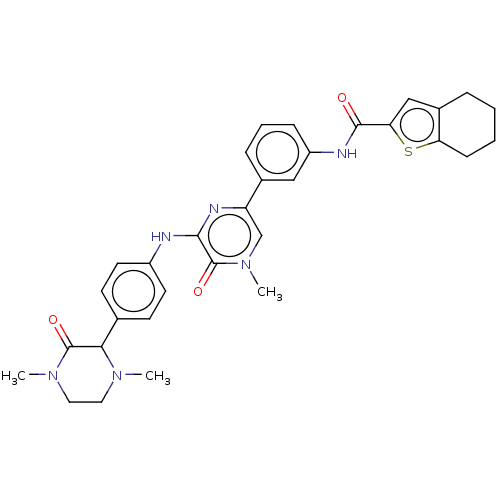
(CHEMBL3745795)Show SMILES CN1CCN(C)C(=O)C1c1ccc(Nc2nc(cn(C)c2=O)-c2cccc(NC(=O)c3cc4CCCCc4s3)c2)cc1 Show InChI InChI=1S/C32H34N6O3S/c1-36-15-16-37(2)31(40)28(36)20-11-13-23(14-12-20)33-29-32(41)38(3)19-25(35-29)21-8-6-9-24(17-21)34-30(39)27-18-22-7-4-5-10-26(22)42-27/h6,8-9,11-14,17-19,28H,4-5,7,10,15-16H2,1-3H3,(H,33,35)(H,34,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of BTK (unknown origin) by Lanthascreen assay |
Bioorg Med Chem Lett 26: 575-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.076
BindingDB Entry DOI: 10.7270/Q2222WMH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50388183
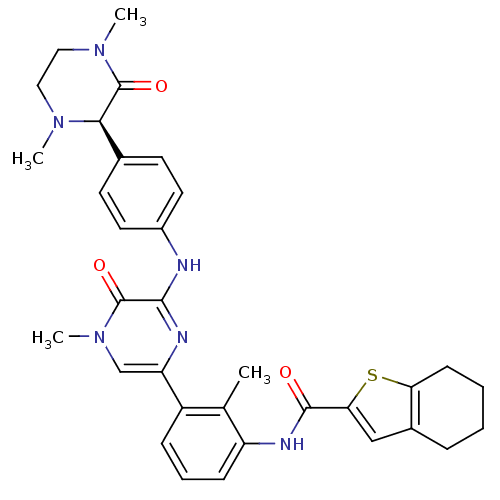
(CHEMBL2057915)Show SMILES CN1CCN(C)C(=O)[C@H]1c1ccc(Nc2nc(cn(C)c2=O)-c2cccc(NC(=O)c3cc4CCCCc4s3)c2C)cc1 |r| Show InChI InChI=1S/C33H36N6O3S/c1-20-24(9-7-10-25(20)36-31(40)28-18-22-8-5-6-11-27(22)43-28)26-19-39(4)33(42)30(35-26)34-23-14-12-21(13-15-23)29-32(41)38(3)17-16-37(29)2/h7,9-10,12-15,18-19,29H,5-6,8,11,16-17H2,1-4H3,(H,34,35)(H,36,40)/t29-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of BTK (unknown origin) by Lanthascreen method |
Bioorg Med Chem Lett 25: 1333-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.01.032
BindingDB Entry DOI: 10.7270/Q26Q1ZXS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50134383

(CHEMBL3747554)Show SMILES CN1CCN(C)C(=O)C1c1ccc(Nc2nc(cn(C)c2=O)-c2cccc(NC(=O)c3cc4CCCCc4s3)c2Cl)cc1 Show InChI InChI=1S/C32H33ClN6O3S/c1-37-15-16-38(2)31(41)28(37)19-11-13-21(14-12-19)34-29-32(42)39(3)18-24(35-29)22-8-6-9-23(27(22)33)36-30(40)26-17-20-7-4-5-10-25(20)43-26/h6,8-9,11-14,17-18,28H,4-5,7,10,15-16H2,1-3H3,(H,34,35)(H,36,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of BTK (unknown origin) by Lanthascreen assay |
Bioorg Med Chem Lett 26: 575-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.076
BindingDB Entry DOI: 10.7270/Q2222WMH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM212271
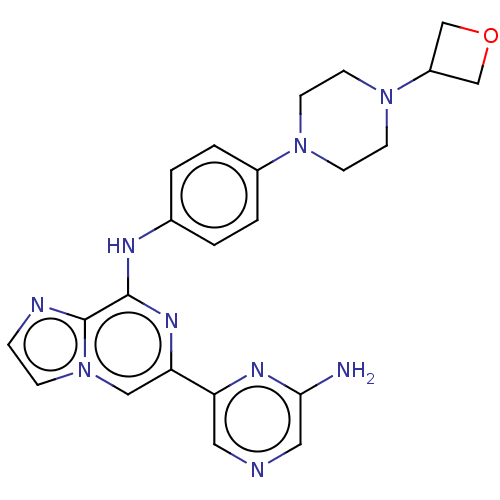
(6-(6-aminopyrazin-2-yl)-N-(4-(4-(oxetan-3-yl)piper...)Show SMILES Nc1cncc(n1)-c1cn2ccnc2c(Nc2ccc(cc2)N2CCN(CC2)C2COC2)n1 Show InChI InChI=1S/C23H25N9O/c24-21-12-25-11-19(28-21)20-13-32-6-5-26-23(32)22(29-20)27-16-1-3-17(4-2-16)30-7-9-31(10-8-30)18-14-33-15-18/h1-6,11-13,18H,7-10,14-15H2,(H2,24,28)(H,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) using XL665-labeled peptide as substrate in presence of ATP measured after 30 mins by TR-FRET assay |
ACS Med Chem Lett 11: 506-513 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00621
BindingDB Entry DOI: 10.7270/Q2ZG6WR1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM212270
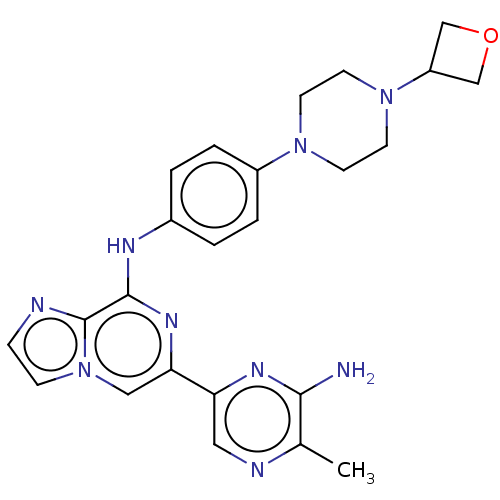
(6-(6-amino-5-methylpyrazin-2-yl)-N-(4-(4-(oxetan-3...)Show SMILES Cc1ncc(nc1N)-c1cn2ccnc2c(Nc2ccc(cc2)N2CCN(CC2)C2COC2)n1 Show InChI InChI=1S/C24H27N9O/c1-16-22(25)29-20(12-27-16)21-13-33-7-6-26-24(33)23(30-21)28-17-2-4-18(5-3-17)31-8-10-32(11-9-31)19-14-34-15-19/h2-7,12-13,19H,8-11,14-15H2,1H3,(H2,25,29)(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee
| Assay Description
Syk activity was measured using KinEASE (Cisbio), a time-resolved fluorescence resonance energy transfer (TR-FRET) immunoassay. In this assay, Syk-ca... |
Bioorg Med Chem 16: 8853-62 (2008)
BindingDB Entry DOI: 10.7270/Q2K076M3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM212270

(6-(6-amino-5-methylpyrazin-2-yl)-N-(4-(4-(oxetan-3...)Show SMILES Cc1ncc(nc1N)-c1cn2ccnc2c(Nc2ccc(cc2)N2CCN(CC2)C2COC2)n1 Show InChI InChI=1S/C24H27N9O/c1-16-22(25)29-20(12-27-16)21-13-33-7-6-26-24(33)23(30-21)28-17-2-4-18(5-3-17)31-8-10-32(11-9-31)19-14-34-15-19/h2-7,12-13,19H,8-11,14-15H2,1H3,(H2,25,29)(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Gilead Sciences, Inc.
US Patent
| Assay Description
Syk activity was measured using KinEASE (Cisbio), a time-resolved fluorescence resonance energy transfer (TR-FRET) immunoassay. In this assay, Syk-ca... |
US Patent US9504684 (2016)
BindingDB Entry DOI: 10.7270/Q2XS5TB7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50015428
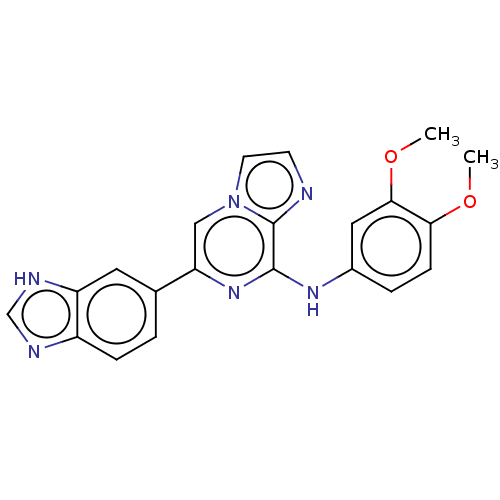
(CHEMBL3265018)Show SMILES COc1ccc(Nc2nc(cn3ccnc23)-c2ccc3nc[nH]c3c2)cc1OC Show InChI InChI=1S/C21H18N6O2/c1-28-18-6-4-14(10-19(18)29-2)25-20-21-22-7-8-27(21)11-17(26-20)13-3-5-15-16(9-13)24-12-23-15/h3-12H,1-2H3,(H,23,24)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full length Syk (unknown origin) using biotinylated peptide substrate |
J Med Chem 57: 3856-73 (2014)
Article DOI: 10.1021/jm500228a
BindingDB Entry DOI: 10.7270/Q2B27WV1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50538459

(CHEMBL4644977)Show SMILES C1OCC1N1CCN(CC1)c1ccc(Nc2nc(cn3ccnc23)-c2ccc3cn[nH]c3n2)cc1 Show InChI InChI=1S/C25H25N9O/c1-6-21(29-23-17(1)13-27-31-23)22-14-34-8-7-26-25(34)24(30-22)28-18-2-4-19(5-3-18)32-9-11-33(12-10-32)20-15-35-16-20/h1-8,13-14,20H,9-12,15-16H2,(H,28,30)(H,27,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) using XL665-labeled peptide as substrate in presence of ATP measured after 30 mins by TR-FRET assay |
ACS Med Chem Lett 11: 506-513 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00621
BindingDB Entry DOI: 10.7270/Q2ZG6WR1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50538468

(CHEMBL4640138)Show SMILES C1OCC1N1CCN(CC1)c1ccc(Nc2nc(cn3ccnc23)-c2cnc3cc[nH]c3c2)cc1 Show InChI InChI=1S/C26H26N8O/c1-3-20(32-9-11-33(12-10-32)21-16-35-17-21)4-2-19(1)30-25-26-28-7-8-34(26)15-24(31-25)18-13-23-22(29-14-18)5-6-27-23/h1-8,13-15,21,27H,9-12,16-17H2,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) using XL665-labeled peptide as substrate in presence of ATP measured after 30 mins by TR-FRET assay |
ACS Med Chem Lett 11: 506-513 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00621
BindingDB Entry DOI: 10.7270/Q2ZG6WR1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50015445
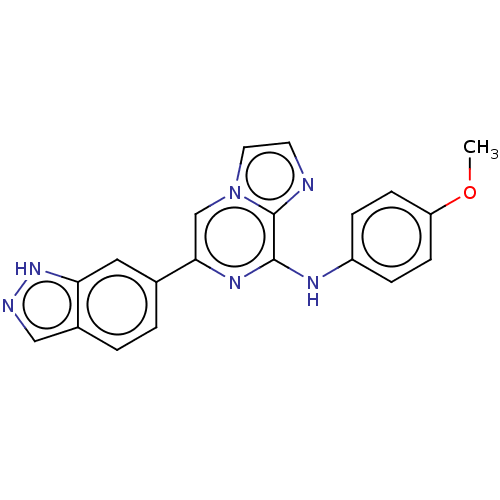
(CHEMBL3265029)Show SMILES COc1ccc(Nc2nc(cn3ccnc23)-c2ccc3cn[nH]c3c2)cc1 Show InChI InChI=1S/C20H16N6O/c1-27-16-6-4-15(5-7-16)23-19-20-21-8-9-26(20)12-18(24-19)13-2-3-14-11-22-25-17(14)10-13/h2-12H,1H3,(H,22,25)(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full length Syk (unknown origin) using biotinylated peptide substrate |
J Med Chem 57: 3856-73 (2014)
Article DOI: 10.1021/jm500228a
BindingDB Entry DOI: 10.7270/Q2B27WV1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50015451
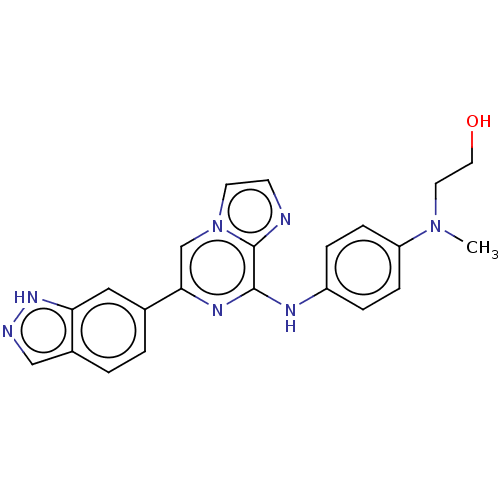
(CHEMBL3265035)Show SMILES CN(CCO)c1ccc(Nc2nc(cn3ccnc23)-c2ccc3cn[nH]c3c2)cc1 Show InChI InChI=1S/C22H21N7O/c1-28(10-11-30)18-6-4-17(5-7-18)25-21-22-23-8-9-29(22)14-20(26-21)15-2-3-16-13-24-27-19(16)12-15/h2-9,12-14,30H,10-11H2,1H3,(H,24,27)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full length Syk (unknown origin) using biotinylated peptide substrate |
J Med Chem 57: 3856-73 (2014)
Article DOI: 10.1021/jm500228a
BindingDB Entry DOI: 10.7270/Q2B27WV1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50015448
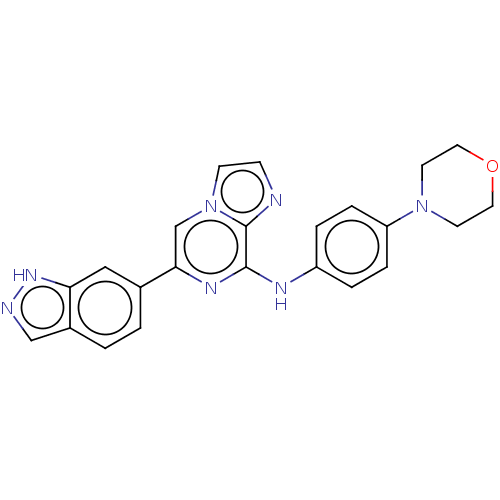
(CHEMBL3265032)Show SMILES C1CN(CCO1)c1ccc(Nc2nc(cn3ccnc23)-c2ccc3cn[nH]c3c2)cc1 Show InChI InChI=1S/C23H21N7O/c1-2-17-14-25-28-20(17)13-16(1)21-15-30-8-7-24-23(30)22(27-21)26-18-3-5-19(6-4-18)29-9-11-31-12-10-29/h1-8,13-15H,9-12H2,(H,25,28)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full length Syk (unknown origin) using biotinylated peptide substrate |
J Med Chem 57: 3856-73 (2014)
Article DOI: 10.1021/jm500228a
BindingDB Entry DOI: 10.7270/Q2B27WV1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data