 Found 18 hits with Last Name = 'dixit' and Initial = 'v'
Found 18 hits with Last Name = 'dixit' and Initial = 'v' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50497604
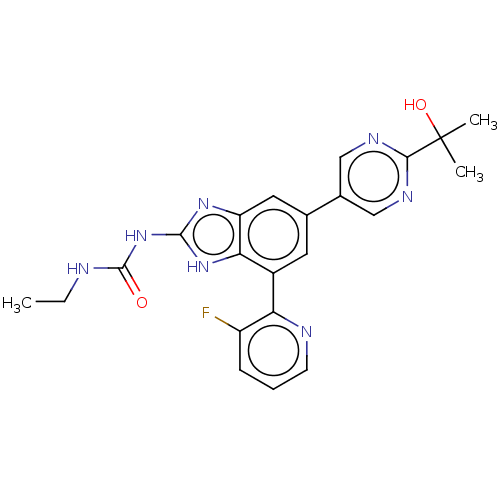
(CHEMBL3264033)Show SMILES CCNC(=O)Nc1nc2cc(cc(-c3ncccc3F)c2[nH]1)-c1cnc(nc1)C(C)(C)O Show InChI InChI=1S/C22H22FN7O2/c1-4-24-21(31)30-20-28-16-9-12(13-10-26-19(27-11-13)22(2,3)32)8-14(18(16)29-20)17-15(23)6-5-7-25-17/h5-11,32H,4H2,1-3H3,(H3,24,28,29,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of aureus Escherichia coli DNA gyrase A2B2 using pBR322 plasmid DNA as substrate by coupled enzyme reaction assay |
J Med Chem 57: 8792-816 (2014)
Article DOI: 10.1021/jm500563g
BindingDB Entry DOI: 10.7270/Q2TF01BF |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50497603
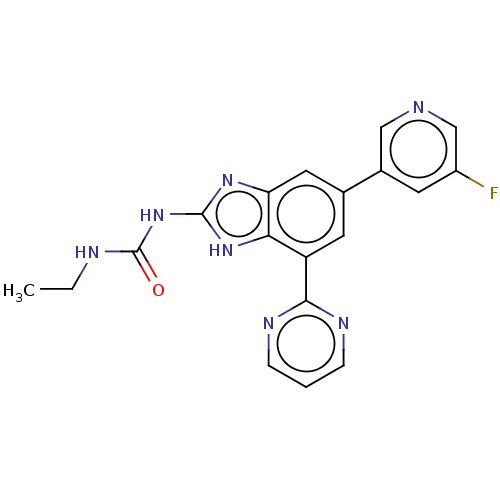
(CHEMBL3356986)Show SMILES CCNC(=O)Nc1nc2cc(cc(-c3ncccn3)c2[nH]1)-c1cncc(F)c1 Show InChI InChI=1S/C19H16FN7O/c1-2-22-19(28)27-18-25-15-8-11(12-6-13(20)10-21-9-12)7-14(16(15)26-18)17-23-4-3-5-24-17/h3-10H,2H2,1H3,(H3,22,25,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of aureus Escherichia coli DNA gyrase A2B2 using pBR322 plasmid DNA as substrate by coupled enzyme reaction assay |
J Med Chem 57: 8792-816 (2014)
Article DOI: 10.1021/jm500563g
BindingDB Entry DOI: 10.7270/Q2TF01BF |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50497602
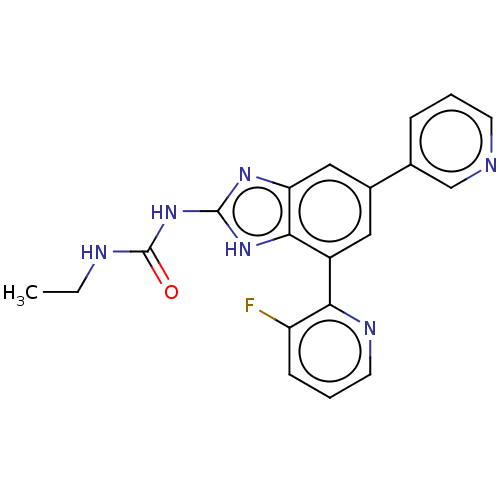
(CHEMBL222333 | VRT-752586)Show SMILES CCNC(=O)Nc1nc2cc(cc(-c3ncccc3F)c2[nH]1)-c1cccnc1 Show InChI InChI=1S/C20H17FN6O/c1-2-23-20(28)27-19-25-16-10-13(12-5-3-7-22-11-12)9-14(18(16)26-19)17-15(21)6-4-8-24-17/h3-11H,2H2,1H3,(H3,23,25,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of aureus Escherichia coli DNA gyrase A2B2 using pBR322 plasmid DNA as substrate by coupled enzyme reaction assay |
J Med Chem 57: 8792-816 (2014)
Article DOI: 10.1021/jm500563g
BindingDB Entry DOI: 10.7270/Q2TF01BF |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Staphylococcus aureus) | BDBM50393079
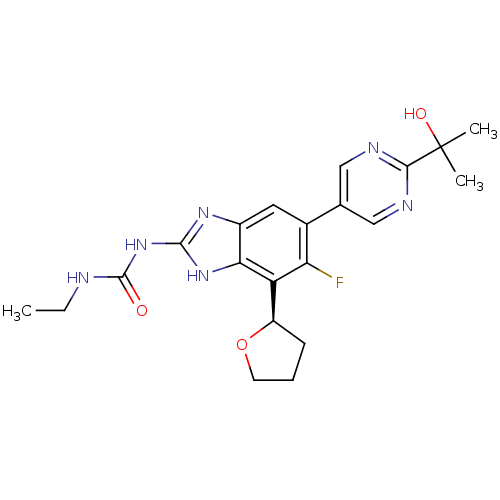
(CHEMBL2152855 | US9040542, 23)Show SMILES CCNC(=O)Nc1nc2cc(c(F)c([C@H]3CCCO3)c2[nH]1)-c1cnc(nc1)C(C)(C)O |r| Show InChI InChI=1S/C21H25FN6O3/c1-4-23-20(29)28-19-26-13-8-12(11-9-24-18(25-10-11)21(2,3)30)16(22)15(17(13)27-19)14-6-5-7-31-14/h8-10,14,30H,4-7H2,1-3H3,(H3,23,26,27,28,29)/t14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus DNA gyrase using pBR322 plasmid DNA as substrate by coupled enzyme reaction assay |
J Med Chem 57: 8792-816 (2014)
Article DOI: 10.1021/jm500563g
BindingDB Entry DOI: 10.7270/Q2TF01BF |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Staphylococcus aureus) | BDBM50112815
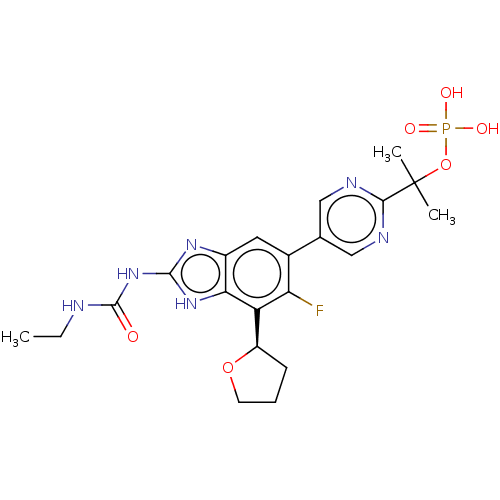
(CHEMBL2221212)Show SMILES CCNC(=O)Nc1nc2cc(c(F)c([C@H]3CCCO3)c2[nH]1)-c1cnc(nc1)C(C)(C)OP(O)(O)=O |r| Show InChI InChI=1S/C21H26FN6O6P/c1-4-23-20(29)28-19-26-13-8-12(16(22)15(17(13)27-19)14-6-5-7-33-14)11-9-24-18(25-10-11)21(2,3)34-35(30,31)32/h8-10,14H,4-7H2,1-3H3,(H2,30,31,32)(H3,23,26,27,28,29)/t14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <17.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus DNA gyrase |
ACS Med Chem Lett 6: 822-6 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00196
BindingDB Entry DOI: 10.7270/Q2J67JPW |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Staphylococcus aureus) | BDBM50393079

(CHEMBL2152855 | US9040542, 23)Show SMILES CCNC(=O)Nc1nc2cc(c(F)c([C@H]3CCCO3)c2[nH]1)-c1cnc(nc1)C(C)(C)O |r| Show InChI InChI=1S/C21H25FN6O3/c1-4-23-20(29)28-19-26-13-8-12(11-9-24-18(25-10-11)21(2,3)30)16(22)15(17(13)27-19)14-6-5-7-31-14/h8-10,14,30H,4-7H2,1-3H3,(H3,23,26,27,28,29)/t14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus DNA gyrase |
ACS Med Chem Lett 6: 822-6 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00196
BindingDB Entry DOI: 10.7270/Q2J67JPW |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 4 subunit A/B
(Staphylococcus aureus) | BDBM50393079

(CHEMBL2152855 | US9040542, 23)Show SMILES CCNC(=O)Nc1nc2cc(c(F)c([C@H]3CCCO3)c2[nH]1)-c1cnc(nc1)C(C)(C)O |r| Show InChI InChI=1S/C21H25FN6O3/c1-4-23-20(29)28-19-26-13-8-12(11-9-24-18(25-10-11)21(2,3)30)16(22)15(17(13)27-19)14-6-5-7-31-14/h8-10,14,30H,4-7H2,1-3H3,(H3,23,26,27,28,29)/t14-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus DNA topoisomerase 4 |
ACS Med Chem Lett 6: 822-6 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00196
BindingDB Entry DOI: 10.7270/Q2J67JPW |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 4 subunit A/B
(Staphylococcus aureus) | BDBM50112815

(CHEMBL2221212)Show SMILES CCNC(=O)Nc1nc2cc(c(F)c([C@H]3CCCO3)c2[nH]1)-c1cnc(nc1)C(C)(C)OP(O)(O)=O |r| Show InChI InChI=1S/C21H26FN6O6P/c1-4-23-20(29)28-19-26-13-8-12(16(22)15(17(13)27-19)14-6-5-7-33-14)11-9-24-18(25-10-11)21(2,3)34-35(30,31)32/h8-10,14H,4-7H2,1-3H3,(H2,30,31,32)(H3,23,26,27,28,29)/t14-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus DNA topoisomerase 4 |
ACS Med Chem Lett 6: 822-6 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00196
BindingDB Entry DOI: 10.7270/Q2J67JPW |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50030474
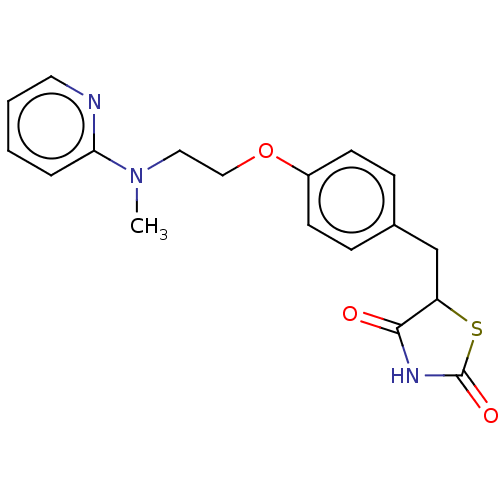
(Avandamet | Avandaryl | Avandia | BRL-49653 | CHEB...)Show InChI InChI=1S/C18H19N3O3S/c1-21(16-4-2-3-9-19-16)10-11-24-14-7-5-13(6-8-14)12-15-17(22)20-18(23)25-15/h2-9,15H,10-12H2,1H3,(H,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged human PPAR-gamma receptor by FRET assay |
Eur J Med Chem 108: 423-35 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.030
BindingDB Entry DOI: 10.7270/Q2KP841X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50137109
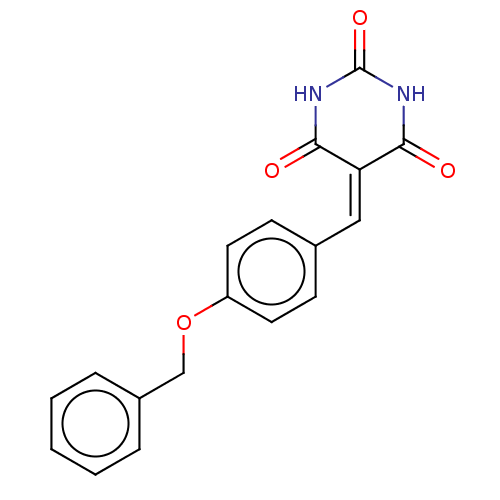
(CHEMBL3754507)Show SMILES O=[#6]-1-[#7]-[#6](=O)\[#6](=[#6]/c2ccc(-[#8]-[#6]-c3ccccc3)cc2)-[#6](=O)-[#7]-1 Show InChI InChI=1S/C18H14N2O4/c21-16-15(17(22)20-18(23)19-16)10-12-6-8-14(9-7-12)24-11-13-4-2-1-3-5-13/h1-10H,11H2,(H2,19,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged human PPAR-gamma receptor by FRET assay |
Eur J Med Chem 108: 423-35 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.030
BindingDB Entry DOI: 10.7270/Q2KP841X |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50137108
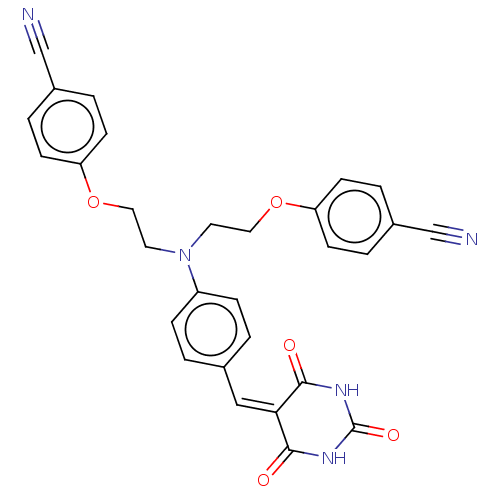
(CHEMBL3753473)Show SMILES O=[#6]-1-[#7]-[#6](=O)\[#6](=[#6]/c2ccc(cc2)-[#7](-[#6]-[#6]-[#8]-c2ccc(cc2)C#N)-[#6]-[#6]-[#8]-c2ccc(cc2)C#N)-[#6](=O)-[#7]-1 Show InChI InChI=1S/C29H23N5O5/c30-18-21-3-9-24(10-4-21)38-15-13-34(14-16-39-25-11-5-22(19-31)6-12-25)23-7-1-20(2-8-23)17-26-27(35)32-29(37)33-28(26)36/h1-12,17H,13-16H2,(H2,32,33,35,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged human PPAR-gamma receptor by FRET assay |
Eur J Med Chem 108: 423-35 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.030
BindingDB Entry DOI: 10.7270/Q2KP841X |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50137110
(CHEMBL3753490)Show SMILES [#8-]-[#7+](=O)-c1ccc(-[#8]-[#6]-[#6]-[#7](-[#6]-[#6]-[#8]-c2ccc(cc2)-[#7+](-[#8-])=O)-c2ccc(\[#6]=[#6]-3/[#6](=O)-[#7]-[#6](=O)-[#7]-[#6]-3=O)cc2)cc1 Show InChI InChI=1S/C27H23N5O9/c33-25-24(26(34)29-27(35)28-25)17-18-1-3-19(4-2-18)30(13-15-40-22-9-5-20(6-10-22)31(36)37)14-16-41-23-11-7-21(8-12-23)32(38)39/h1-12,17H,13-16H2,(H2,28,29,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged human PPAR-gamma receptor by FRET assay |
Eur J Med Chem 108: 423-35 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.030
BindingDB Entry DOI: 10.7270/Q2KP841X |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50137111
(CHEMBL3752848)Show SMILES [#8]-[#6]-[#6]-[#7](-[#6]-[#6]-[#8]C(c1ccccc1)(c1ccccc1)c1ccccc1)-c1ccc(\[#6]=[#6]-2/[#6](=O)-[#7]-[#6](=O)-[#7]-[#6]-2=O)cc1 Show InChI InChI=1S/C34H31N3O5/c38-22-20-37(29-18-16-25(17-19-29)24-30-31(39)35-33(41)36-32(30)40)21-23-42-34(26-10-4-1-5-11-26,27-12-6-2-7-13-27)28-14-8-3-9-15-28/h1-19,24,38H,20-23H2,(H2,35,36,39,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged human PPAR-gamma receptor by FRET assay |
Eur J Med Chem 108: 423-35 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.030
BindingDB Entry DOI: 10.7270/Q2KP841X |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50137104
(CHEMBL3753560)Show SMILES [#6]-[#8]-c1ccc(-[#6]-[#8]-[#6]-[#6]-[#7](-[#6]-[#6]-[#8]-[#6]-c2ccc(-[#8]-[#6])cc2)-c2ccc(\[#6]=[#6]-3/[#6](=O)-[#7]-[#6](=O)-[#7]-[#6]-3=O)cc2)cc1 Show InChI InChI=1S/C31H33N3O7/c1-38-26-11-5-23(6-12-26)20-40-17-15-34(16-18-41-21-24-7-13-27(39-2)14-8-24)25-9-3-22(4-10-25)19-28-29(35)32-31(37)33-30(28)36/h3-14,19H,15-18,20-21H2,1-2H3,(H2,32,33,35,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.91E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged human PPAR-gamma receptor by FRET assay |
Eur J Med Chem 108: 423-35 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.030
BindingDB Entry DOI: 10.7270/Q2KP841X |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50137107

(CHEMBL3754461)Show SMILES O=[#6]-1-[#7]-[#6](=O)\[#6](=[#6]/c2ccc(cc2)-[#7](-[#6]-[#6]-[#8]-[#6]-c2ccccc2)-[#6]-[#6]-[#8]-[#6]-c2ccccc2)-[#6](=O)-[#7]-1 Show InChI InChI=1S/C29H29N3O5/c33-27-26(28(34)31-29(35)30-27)19-22-11-13-25(14-12-22)32(15-17-36-20-23-7-3-1-4-8-23)16-18-37-21-24-9-5-2-6-10-24/h1-14,19H,15-18,20-21H2,(H2,30,31,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged human PPAR-gamma receptor by FRET assay |
Eur J Med Chem 108: 423-35 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.030
BindingDB Entry DOI: 10.7270/Q2KP841X |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50137105
(CHEMBL3753871)Show SMILES [#6]-[#8]-c1ccc(-[#6]-[#8]-[#6]-[#6]-[#7](-[#6]-[#6]-[#8]-c2ccccn2)-c2ccc(\[#6]=[#6]-3/[#6](=O)-[#7]-[#6](=O)-[#7]-[#6]-3=O)cc2)cc1 Show InChI InChI=1S/C28H28N4O6/c1-36-23-11-7-21(8-12-23)19-37-16-14-32(15-17-38-25-4-2-3-13-29-25)22-9-5-20(6-10-22)18-24-26(33)30-28(35)31-27(24)34/h2-13,18H,14-17,19H2,1H3,(H2,30,31,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged human PPAR-gamma receptor by FRET assay |
Eur J Med Chem 108: 423-35 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.030
BindingDB Entry DOI: 10.7270/Q2KP841X |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50137096
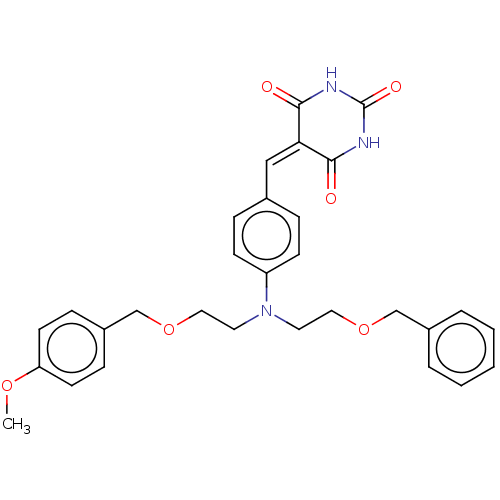
(CHEMBL3753308)Show SMILES [#6]-[#8]-c1ccc(-[#6]-[#8]-[#6]-[#6]-[#7](-[#6]-[#6]-[#8]-[#6]-c2ccccc2)-c2ccc(\[#6]=[#6]-3/[#6](=O)-[#7]-[#6](=O)-[#7]-[#6]-3=O)cc2)cc1 Show InChI InChI=1S/C30H31N3O6/c1-37-26-13-9-24(10-14-26)21-39-18-16-33(15-17-38-20-23-5-3-2-4-6-23)25-11-7-22(8-12-25)19-27-28(34)31-30(36)32-29(27)35/h2-14,19H,15-18,20-21H2,1H3,(H2,31,32,34,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.91E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged human PPAR-gamma receptor by FRET assay |
Eur J Med Chem 108: 423-35 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.030
BindingDB Entry DOI: 10.7270/Q2KP841X |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50137106
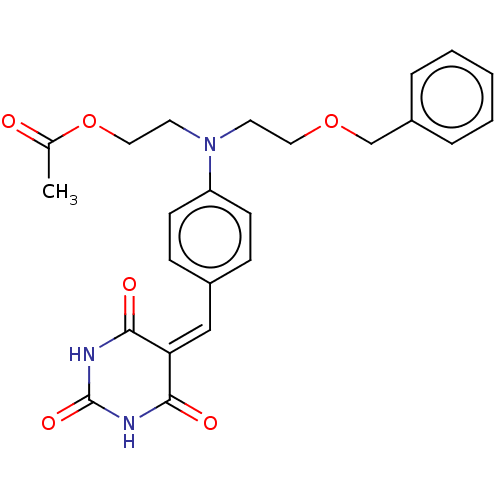
(CHEMBL3754373)Show SMILES [#6]-[#6](=O)-[#8]-[#6]-[#6]-[#7](-[#6]-[#6]-[#8]-[#6]-c1ccccc1)-c1ccc(\[#6]=[#6]-2/[#6](=O)-[#7]-[#6](=O)-[#7]-[#6]-2=O)cc1 Show InChI InChI=1S/C24H25N3O6/c1-17(28)33-14-12-27(11-13-32-16-19-5-3-2-4-6-19)20-9-7-18(8-10-20)15-21-22(29)25-24(31)26-23(21)30/h2-10,15H,11-14,16H2,1H3,(H2,25,26,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged human PPAR-gamma receptor by FRET assay |
Eur J Med Chem 108: 423-35 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.030
BindingDB Entry DOI: 10.7270/Q2KP841X |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data