 Found 67 hits with Last Name = 'dong' and Initial = 'wl'
Found 67 hits with Last Name = 'dong' and Initial = 'wl' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Ketol-acid reductoisomerase (NADP(+))
(Escherichia coli) | BDBM82145
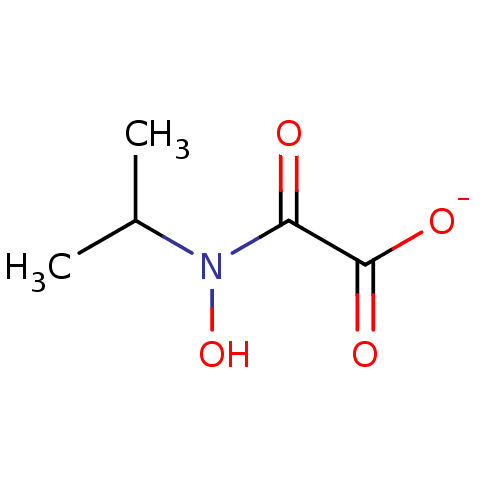
(N-hydroxy-N-isopropyloxamate, IpOHA)Show InChI InChI=1S/C5H9NO4/c1-3(2)6(10)4(7)5(8)9/h3,10H,1-2H3,(H,8,9)/p-1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 2.75E+3 | -32.3 | n/a | n/a | n/a | n/a | n/a | 8.0 | 30 |
Nankai University
| Assay Description
Inhibition of E. coli KARI (ketol-acid reductoisomerase) is time dependent, and activity was measured by the decrease in A340 at 30 C in solutions co... |
Chem Biol Drug Des 75: 228-32 (2010)
Article DOI: 10.1111/j.1747-0285.2009.00924.x
BindingDB Entry DOI: 10.7270/Q2BV7F45 |
More data for this
Ligand-Target Pair | |
Ketol-acid reductoisomerase (NADP(+))
(Escherichia coli) | BDBM82144
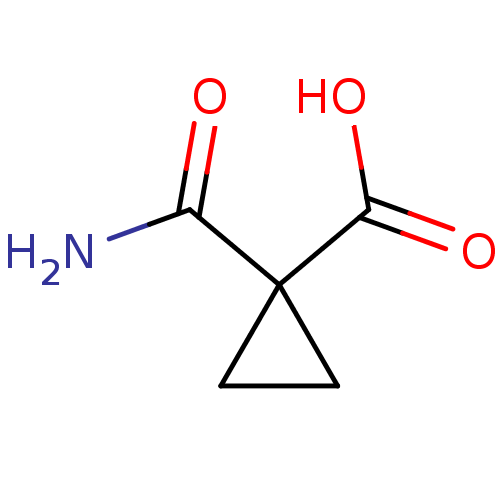
(Cyclopropane, 5)Show InChI InChI=1S/C5H7NO3/c6-3(7)5(1-2-5)4(8)9/h1-2H2,(H2,6,7)(H,8,9) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 3.12E+4 | -26.2 | n/a | n/a | n/a | n/a | n/a | 8.0 | 30 |
Nankai University
| Assay Description
Inhibition of E. coli KARI (ketol-acid reductoisomerase) is time dependent, and activity was measured by the decrease in A340 at 30 C in solutions co... |
Chem Biol Drug Des 75: 228-32 (2010)
Article DOI: 10.1111/j.1747-0285.2009.00924.x
BindingDB Entry DOI: 10.7270/Q2BV7F45 |
More data for this
Ligand-Target Pair | |
Ketol-acid reductoisomerase (NADP(+))
(Escherichia coli) | BDBM82142
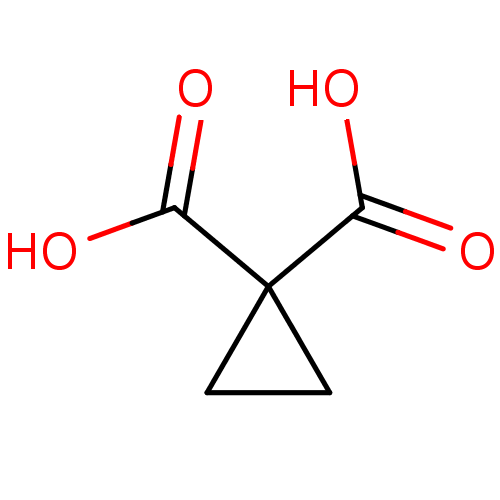
(Cyclopropane, 3)Show InChI InChI=1S/C5H6O4/c6-3(7)5(1-2-5)4(8)9/h1-2H2,(H,6,7)(H,8,9) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 7.66E+4 | -23.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 30 |
Nankai University
| Assay Description
Inhibition of E. coli KARI (ketol-acid reductoisomerase) is time dependent, and activity was measured by the decrease in A340 at 30 C in solutions co... |
Chem Biol Drug Des 75: 228-32 (2010)
Article DOI: 10.1111/j.1747-0285.2009.00924.x
BindingDB Entry DOI: 10.7270/Q2BV7F45 |
More data for this
Ligand-Target Pair | |
Ketol-acid reductoisomerase (NADP(+))
(Escherichia coli) | BDBM82143

(Cyclopropane, 4)Show InChI InChI=1S/C5H5NO2/c6-3-5(1-2-5)4(7)8/h1-2H2,(H,7,8) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 9.53E+4 | -23.3 | n/a | n/a | n/a | n/a | n/a | 8.0 | 30 |
Nankai University
| Assay Description
Inhibition of E. coli KARI (ketol-acid reductoisomerase) is time dependent, and activity was measured by the decrease in A340 at 30 C in solutions co... |
Chem Biol Drug Des 75: 228-32 (2010)
Article DOI: 10.1111/j.1747-0285.2009.00924.x
BindingDB Entry DOI: 10.7270/Q2BV7F45 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50127122
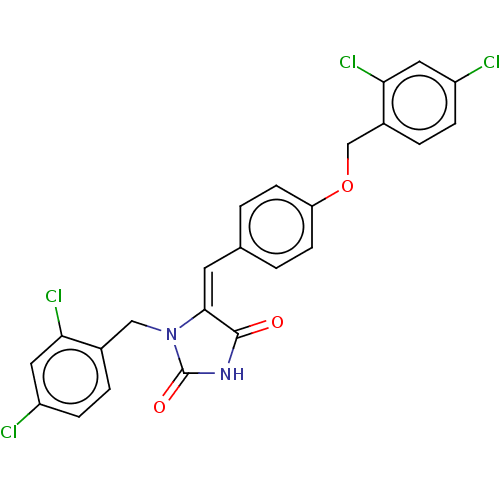
(CHEMBL3628469)Show SMILES Clc1ccc(COc2ccc(\C=C3\N(Cc4ccc(Cl)cc4Cl)C(=O)NC3=O)cc2)c(Cl)c1 Show InChI InChI=1S/C24H16Cl4N2O3/c25-17-5-3-15(20(27)10-17)12-30-22(23(31)29-24(30)32)9-14-1-7-19(8-2-14)33-13-16-4-6-18(26)11-21(16)28/h1-11H,12-13H2,(H,29,31,32)/b22-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Tianjin Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli pre-incubated for 15 mins before pNPP substrate addition by spectrophotometry |
Eur J Med Chem 103: 91-104 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.037
BindingDB Entry DOI: 10.7270/Q2PK0HZF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50127132
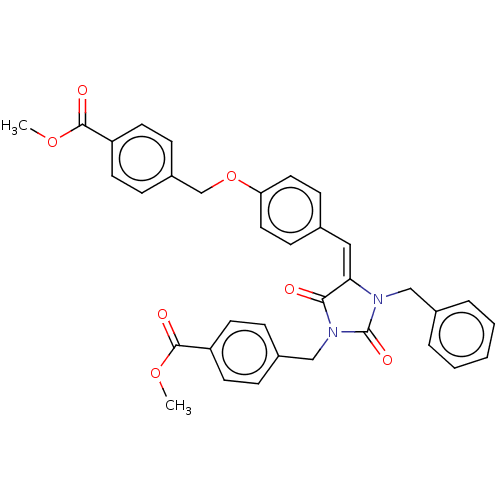
(CHEMBL3628484)Show SMILES COC(=O)c1ccc(COc2ccc(\C=C3\N(Cc4ccccc4)C(=O)N(Cc4ccc(cc4)C(=O)OC)C3=O)cc2)cc1 Show InChI InChI=1S/C35H30N2O7/c1-42-33(39)28-14-8-26(9-15-28)22-37-32(38)31(36(35(37)41)21-25-6-4-3-5-7-25)20-24-12-18-30(19-13-24)44-23-27-10-16-29(17-11-27)34(40)43-2/h3-20H,21-23H2,1-2H3/b31-20+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 960 | n/a | n/a | n/a | n/a | n/a | n/a |
Tianjin Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli pre-incubated for 15 mins before pNPP substrate addition by spectrophotometry |
Eur J Med Chem 103: 91-104 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.037
BindingDB Entry DOI: 10.7270/Q2PK0HZF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50127100
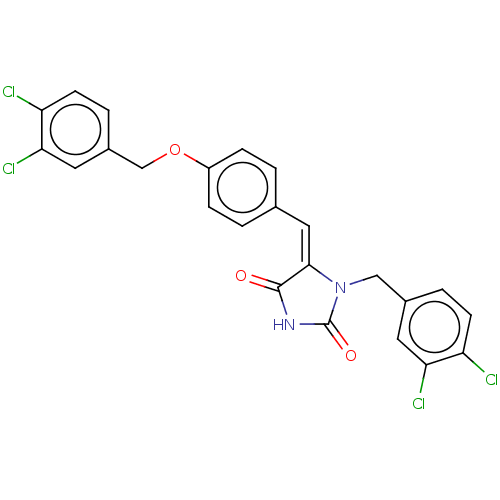
(CHEMBL3628468)Show SMILES Clc1ccc(COc2ccc(\C=C3\N(Cc4ccc(Cl)c(Cl)c4)C(=O)NC3=O)cc2)cc1Cl Show InChI InChI=1S/C24H16Cl4N2O3/c25-18-7-3-15(9-20(18)27)12-30-22(23(31)29-24(30)32)11-14-1-5-17(6-2-14)33-13-16-4-8-19(26)21(28)10-16/h1-11H,12-13H2,(H,29,31,32)/b22-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tianjin Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli pre-incubated for 15 mins before pNPP substrate addition by spectrophotometry |
Eur J Med Chem 103: 91-104 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.037
BindingDB Entry DOI: 10.7270/Q2PK0HZF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50127125
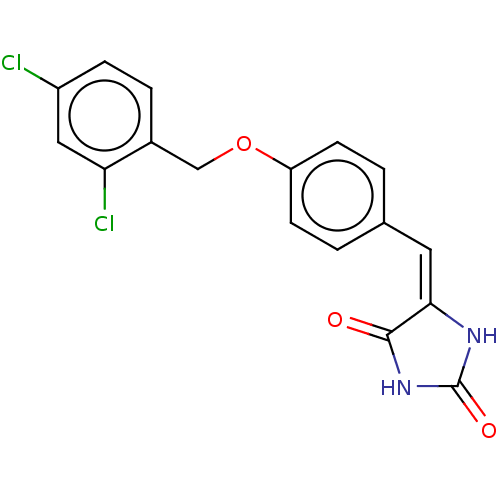
(CHEMBL3628475)Show SMILES Clc1ccc(COc2ccc(\C=C3\NC(=O)NC3=O)cc2)c(Cl)c1 Show InChI InChI=1S/C17H12Cl2N2O3/c18-12-4-3-11(14(19)8-12)9-24-13-5-1-10(2-6-13)7-15-16(22)21-17(23)20-15/h1-8H,9H2,(H2,20,21,22,23)/b15-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tianjin Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli pre-incubated for 15 mins before pNPP substrate addition by spectrophotometry |
Eur J Med Chem 103: 91-104 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.037
BindingDB Entry DOI: 10.7270/Q2PK0HZF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM106797
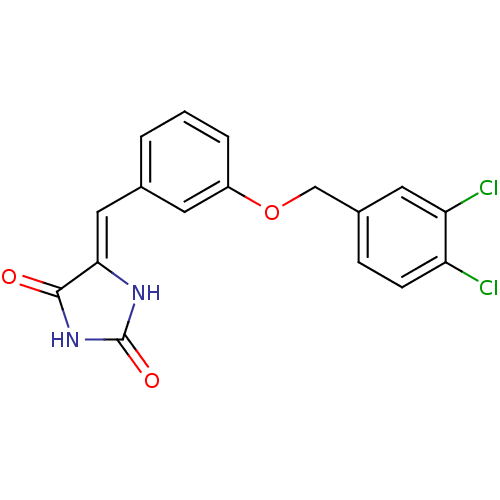
((5Z)‐5‐({3‐[(3,4‐ dichloro...)Show SMILES Clc1ccc(COc2cccc(\C=C3/NC(=O)NC3=O)c2)cc1Cl Show InChI InChI=1S/C17H12Cl2N2O3/c18-13-5-4-11(7-14(13)19)9-24-12-3-1-2-10(6-12)8-15-16(22)21-17(23)20-15/h1-8H,9H2,(H2,20,21,22,23)/b15-8- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Tianjin Medical University
| Assay Description
The enzyme activity was measured using p-nitrophenyl phosphate (pNPP) as substrate in a 96-well plate and by detecting the absorbance at 405 nm for t... |
Chem Biol Drug Des 82: 595-602 (2013)
Article DOI: 10.1111/cbdd.12189
BindingDB Entry DOI: 10.7270/Q2K64GQP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50127121
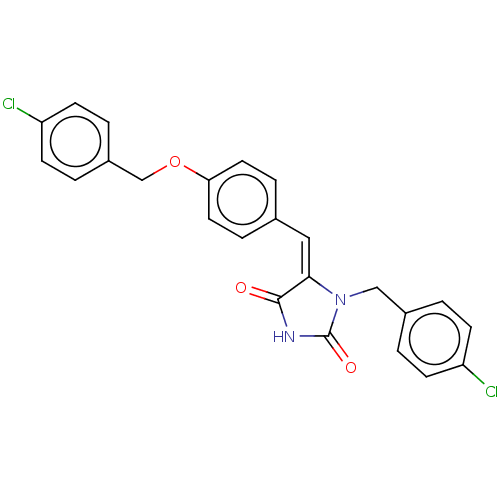
(CHEMBL3628467)Show SMILES Clc1ccc(COc2ccc(\C=C3\N(Cc4ccc(Cl)cc4)C(=O)NC3=O)cc2)cc1 Show InChI InChI=1S/C24H18Cl2N2O3/c25-19-7-1-17(2-8-19)14-28-22(23(29)27-24(28)30)13-16-5-11-21(12-6-16)31-15-18-3-9-20(26)10-4-18/h1-13H,14-15H2,(H,27,29,30)/b22-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tianjin Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli pre-incubated for 15 mins before pNPP substrate addition by spectrophotometry |
Eur J Med Chem 103: 91-104 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.037
BindingDB Entry DOI: 10.7270/Q2PK0HZF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM106800

((5Z)‐5‐({4‐[(4‐ methoxyphe...)Show InChI InChI=1S/C18H16N2O4/c1-23-14-6-4-13(5-7-14)11-24-15-8-2-12(3-9-15)10-16-17(21)20-18(22)19-16/h2-10H,11H2,1H3,(H2,19,20,21,22)/b16-10- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Tianjin Medical University
| Assay Description
The enzyme activity was measured using p-nitrophenyl phosphate (pNPP) as substrate in a 96-well plate and by detecting the absorbance at 405 nm for t... |
Chem Biol Drug Des 82: 595-602 (2013)
Article DOI: 10.1111/cbdd.12189
BindingDB Entry DOI: 10.7270/Q2K64GQP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50127101
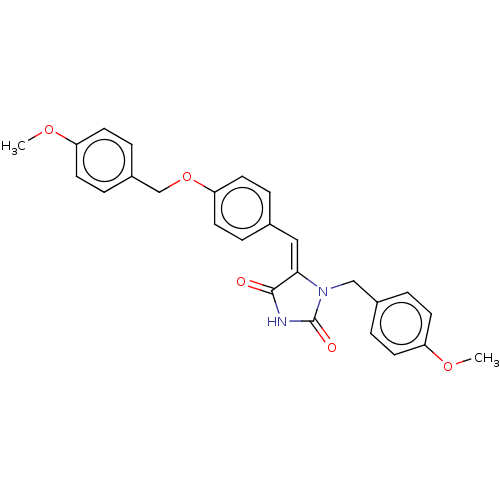
(CHEMBL3628470)Show SMILES COc1ccc(COc2ccc(\C=C3\N(Cc4ccc(OC)cc4)C(=O)NC3=O)cc2)cc1 Show InChI InChI=1S/C26H24N2O5/c1-31-21-9-5-19(6-10-21)16-28-24(25(29)27-26(28)30)15-18-3-13-23(14-4-18)33-17-20-7-11-22(32-2)12-8-20/h3-15H,16-17H2,1-2H3,(H,27,29,30)/b24-15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tianjin Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli pre-incubated for 15 mins before pNPP substrate addition by spectrophotometry |
Eur J Med Chem 103: 91-104 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.037
BindingDB Entry DOI: 10.7270/Q2PK0HZF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50127096
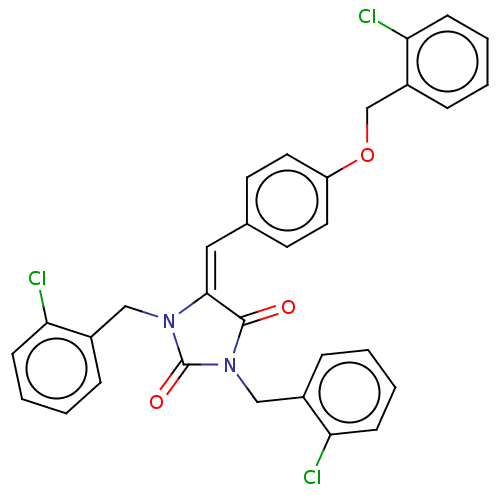
(CHEMBL3628384)Show SMILES Clc1ccccc1COc1ccc(\C=C2\N(Cc3ccccc3Cl)C(=O)N(Cc3ccccc3Cl)C2=O)cc1 Show InChI InChI=1S/C31H23Cl3N2O3/c32-26-10-4-1-7-22(26)18-35-29(30(37)36(31(35)38)19-23-8-2-5-11-27(23)33)17-21-13-15-25(16-14-21)39-20-24-9-3-6-12-28(24)34/h1-17H,18-20H2/b29-17+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tianjin Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli pre-incubated for 15 mins before pNPP substrate addition by spectrophotometry |
Eur J Med Chem 103: 91-104 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.037
BindingDB Entry DOI: 10.7270/Q2PK0HZF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50127098
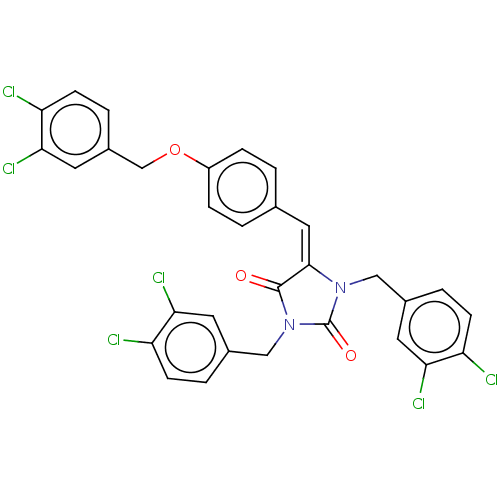
(CHEMBL3628464)Show SMILES Clc1ccc(COc2ccc(\C=C3\N(Cc4ccc(Cl)c(Cl)c4)C(=O)N(Cc4ccc(Cl)c(Cl)c4)C3=O)cc2)cc1Cl Show InChI InChI=1S/C31H20Cl6N2O3/c32-23-8-3-19(11-26(23)35)15-38-29(30(40)39(31(38)41)16-20-4-9-24(33)27(36)12-20)14-18-1-6-22(7-2-18)42-17-21-5-10-25(34)28(37)13-21/h1-14H,15-17H2/b29-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tianjin Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli pre-incubated for 15 mins before pNPP substrate addition by spectrophotometry |
Eur J Med Chem 103: 91-104 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.037
BindingDB Entry DOI: 10.7270/Q2PK0HZF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM106791
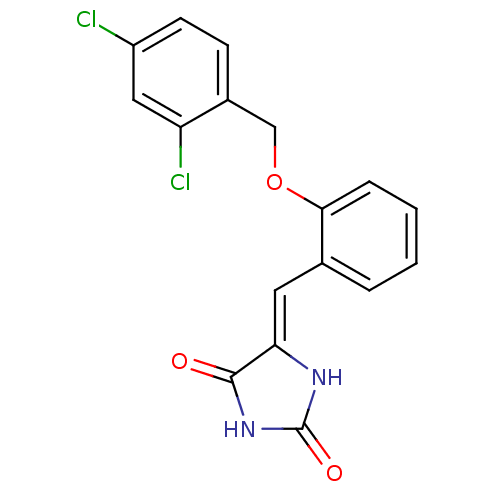
((5Z)‐5‐({2‐[(2,4‐ dichloro...)Show SMILES Clc1ccc(COc2ccccc2\C=C2/NC(=O)NC2=O)c(Cl)c1 Show InChI InChI=1S/C17H12Cl2N2O3/c18-12-6-5-11(13(19)8-12)9-24-15-4-2-1-3-10(15)7-14-16(22)21-17(23)20-14/h1-8H,9H2,(H2,20,21,22,23)/b14-7- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Tianjin Medical University
| Assay Description
The enzyme activity was measured using p-nitrophenyl phosphate (pNPP) as substrate in a 96-well plate and by detecting the absorbance at 405 nm for t... |
Chem Biol Drug Des 82: 595-602 (2013)
Article DOI: 10.1111/cbdd.12189
BindingDB Entry DOI: 10.7270/Q2K64GQP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM106796

((5Z)‐5‐({3‐[(2,4‐ dichloro...)Show SMILES Clc1ccc(COc2cccc(\C=C3/NC(=O)NC3=O)c2)c(Cl)c1 Show InChI InChI=1S/C17H12Cl2N2O3/c18-12-5-4-11(14(19)8-12)9-24-13-3-1-2-10(6-13)7-15-16(22)21-17(23)20-15/h1-8H,9H2,(H2,20,21,22,23)/b15-7- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Tianjin Medical University
| Assay Description
The enzyme activity was measured using p-nitrophenyl phosphate (pNPP) as substrate in a 96-well plate and by detecting the absorbance at 405 nm for t... |
Chem Biol Drug Des 82: 595-602 (2013)
Article DOI: 10.1111/cbdd.12189
BindingDB Entry DOI: 10.7270/Q2K64GQP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM106790
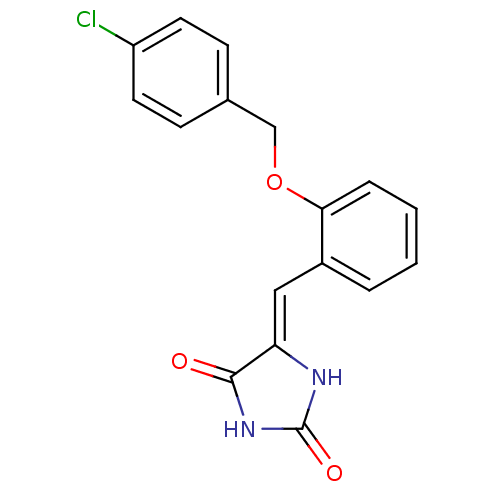
((5Z)‐5‐({2‐[(4‐ chlorophen...)Show InChI InChI=1S/C17H13ClN2O3/c18-13-7-5-11(6-8-13)10-23-15-4-2-1-3-12(15)9-14-16(21)20-17(22)19-14/h1-9H,10H2,(H2,19,20,21,22)/b14-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Tianjin Medical University
| Assay Description
The enzyme activity was measured using p-nitrophenyl phosphate (pNPP) as substrate in a 96-well plate and by detecting the absorbance at 405 nm for t... |
Chem Biol Drug Des 82: 595-602 (2013)
Article DOI: 10.1111/cbdd.12189
BindingDB Entry DOI: 10.7270/Q2K64GQP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM106798
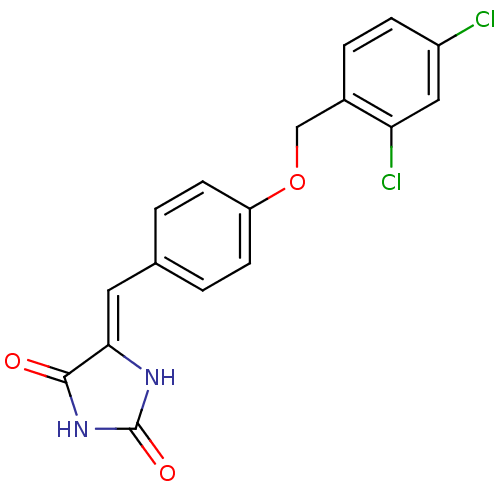
((5Z)‐5‐({4‐[(2,4‐ dichloro...)Show SMILES Clc1ccc(COc2ccc(\C=C3/NC(=O)NC3=O)cc2)c(Cl)c1 Show InChI InChI=1S/C17H12Cl2N2O3/c18-12-4-3-11(14(19)8-12)9-24-13-5-1-10(2-6-13)7-15-16(22)21-17(23)20-15/h1-8H,9H2,(H2,20,21,22,23)/b15-7- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Tianjin Medical University
| Assay Description
The enzyme activity was measured using p-nitrophenyl phosphate (pNPP) as substrate in a 96-well plate and by detecting the absorbance at 405 nm for t... |
Chem Biol Drug Des 82: 595-602 (2013)
Article DOI: 10.1111/cbdd.12189
BindingDB Entry DOI: 10.7270/Q2K64GQP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50127131
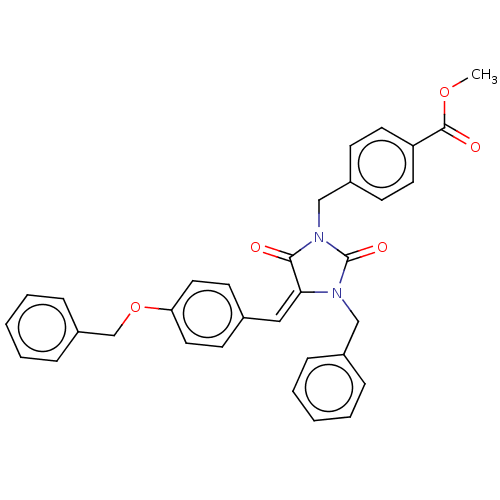
(CHEMBL3628539)Show SMILES COC(=O)c1ccc(CN2C(=O)N(Cc3ccccc3)\C(=C\c3ccc(OCc4ccccc4)cc3)C2=O)cc1 Show InChI InChI=1S/C33H28N2O5/c1-39-32(37)28-16-12-26(13-17-28)22-35-31(36)30(34(33(35)38)21-25-8-4-2-5-9-25)20-24-14-18-29(19-15-24)40-23-27-10-6-3-7-11-27/h2-20H,21-23H2,1H3/b30-20+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tianjin Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli pre-incubated for 15 mins before pNPP substrate addition by spectrophotometry |
Eur J Med Chem 103: 91-104 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.037
BindingDB Entry DOI: 10.7270/Q2PK0HZF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50127126
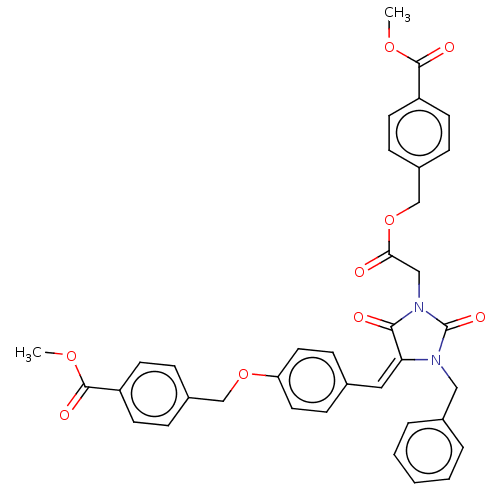
(CHEMBL3628548)Show SMILES COC(=O)c1ccc(COC(=O)CN2C(=O)N(Cc3ccccc3)\C(=C\c3ccc(OCc4ccc(cc4)C(=O)OC)cc3)C2=O)cc1 Show InChI InChI=1S/C37H32N2O9/c1-45-35(42)29-14-8-27(9-15-29)23-47-31-18-12-25(13-19-31)20-32-34(41)39(37(44)38(32)21-26-6-4-3-5-7-26)22-33(40)48-24-28-10-16-30(17-11-28)36(43)46-2/h3-20H,21-24H2,1-2H3/b32-20+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tianjin Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli pre-incubated for 15 mins before pNPP substrate addition by spectrophotometry |
Eur J Med Chem 103: 91-104 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.037
BindingDB Entry DOI: 10.7270/Q2PK0HZF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50127119
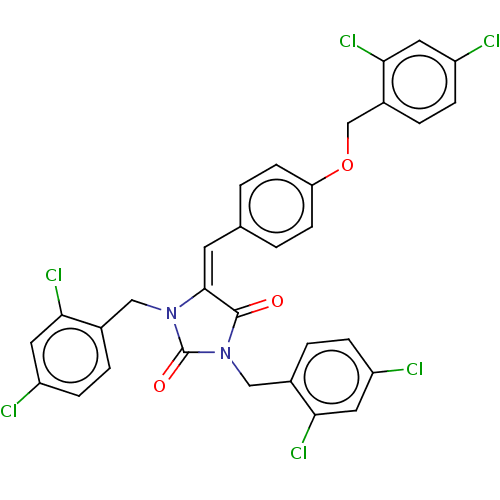
(CHEMBL3628463)Show SMILES Clc1ccc(COc2ccc(\C=C3\N(Cc4ccc(Cl)cc4Cl)C(=O)N(Cc4ccc(Cl)cc4Cl)C3=O)cc2)c(Cl)c1 Show InChI InChI=1S/C31H20Cl6N2O3/c32-22-6-3-19(26(35)12-22)15-38-29(30(40)39(31(38)41)16-20-4-7-23(33)13-27(20)36)11-18-1-9-25(10-2-18)42-17-21-5-8-24(34)14-28(21)37/h1-14H,15-17H2/b29-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tianjin Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli pre-incubated for 15 mins before pNPP substrate addition by spectrophotometry |
Eur J Med Chem 103: 91-104 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.037
BindingDB Entry DOI: 10.7270/Q2PK0HZF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50127116
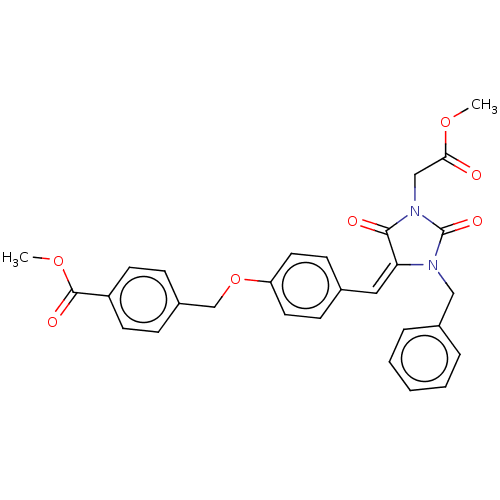
(CHEMBL3628481)Show SMILES COC(=O)CN1C(=O)N(Cc2ccccc2)\C(=C\c2ccc(OCc3ccc(cc3)C(=O)OC)cc2)C1=O Show InChI InChI=1S/C29H26N2O7/c1-36-26(32)18-31-27(33)25(30(29(31)35)17-21-6-4-3-5-7-21)16-20-10-14-24(15-11-20)38-19-22-8-12-23(13-9-22)28(34)37-2/h3-16H,17-19H2,1-2H3/b25-16+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tianjin Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli pre-incubated for 15 mins before pNPP substrate addition by spectrophotometry |
Eur J Med Chem 103: 91-104 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.037
BindingDB Entry DOI: 10.7270/Q2PK0HZF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50127097
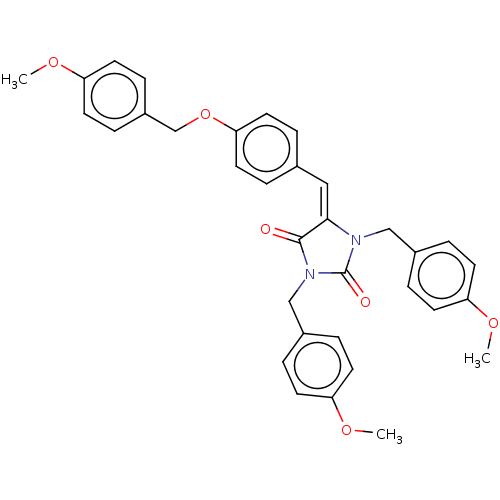
(CHEMBL3628462)Show SMILES COc1ccc(COc2ccc(\C=C3\N(Cc4ccc(OC)cc4)C(=O)N(Cc4ccc(OC)cc4)C3=O)cc2)cc1 Show InChI InChI=1S/C34H32N2O6/c1-39-28-12-6-25(7-13-28)21-35-32(33(37)36(34(35)38)22-26-8-14-29(40-2)15-9-26)20-24-4-18-31(19-5-24)42-23-27-10-16-30(41-3)17-11-27/h4-20H,21-23H2,1-3H3/b32-20+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tianjin Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli pre-incubated for 15 mins before pNPP substrate addition by spectrophotometry |
Eur J Med Chem 103: 91-104 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.037
BindingDB Entry DOI: 10.7270/Q2PK0HZF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50127115
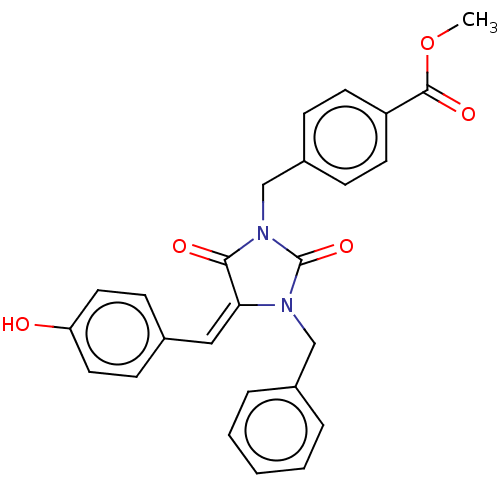
(CHEMBL3628480)Show SMILES COC(=O)c1ccc(CN2C(=O)N(Cc3ccccc3)\C(=C\c3ccc(O)cc3)C2=O)cc1 Show InChI InChI=1S/C26H22N2O5/c1-33-25(31)21-11-7-20(8-12-21)17-28-24(30)23(15-18-9-13-22(29)14-10-18)27(26(28)32)16-19-5-3-2-4-6-19/h2-15,29H,16-17H2,1H3/b23-15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tianjin Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli pre-incubated for 15 mins before pNPP substrate addition by spectrophotometry |
Eur J Med Chem 103: 91-104 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.037
BindingDB Entry DOI: 10.7270/Q2PK0HZF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50127110
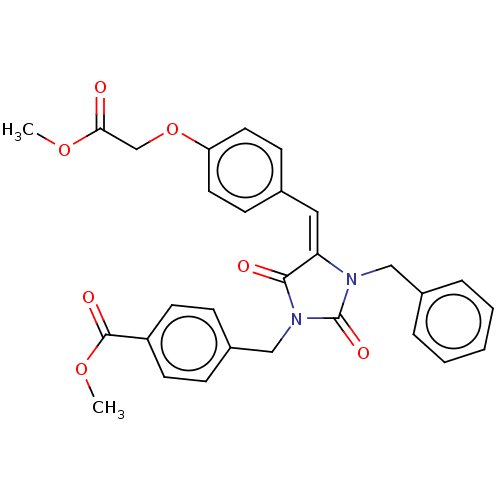
(CHEMBL3628538)Show SMILES COC(=O)COc1ccc(\C=C2\N(Cc3ccccc3)C(=O)N(Cc3ccc(cc3)C(=O)OC)C2=O)cc1 Show InChI InChI=1S/C29H26N2O7/c1-36-26(32)19-38-24-14-10-20(11-15-24)16-25-27(33)31(18-22-8-12-23(13-9-22)28(34)37-2)29(35)30(25)17-21-6-4-3-5-7-21/h3-16H,17-19H2,1-2H3/b25-16+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tianjin Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli pre-incubated for 15 mins before pNPP substrate addition by spectrophotometry |
Eur J Med Chem 103: 91-104 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.037
BindingDB Entry DOI: 10.7270/Q2PK0HZF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50127120
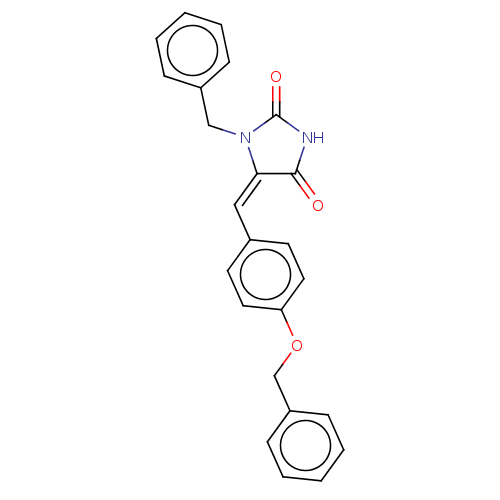
(CHEMBL3628465)Show SMILES O=C1NC(=O)\C(=C/c2ccc(OCc3ccccc3)cc2)N1Cc1ccccc1 Show InChI InChI=1S/C24H20N2O3/c27-23-22(26(24(28)25-23)16-19-7-3-1-4-8-19)15-18-11-13-21(14-12-18)29-17-20-9-5-2-6-10-20/h1-15H,16-17H2,(H,25,27,28)/b22-15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tianjin Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli pre-incubated for 15 mins before pNPP substrate addition by spectrophotometry |
Eur J Med Chem 103: 91-104 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.037
BindingDB Entry DOI: 10.7270/Q2PK0HZF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50127109
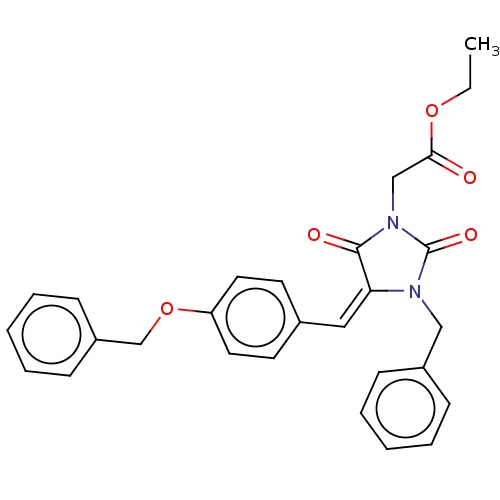
(CHEMBL3628540)Show SMILES CCOC(=O)CN1C(=O)N(Cc2ccccc2)\C(=C\c2ccc(OCc3ccccc3)cc2)C1=O Show InChI InChI=1S/C28H26N2O5/c1-2-34-26(31)19-30-27(32)25(29(28(30)33)18-22-9-5-3-6-10-22)17-21-13-15-24(16-14-21)35-20-23-11-7-4-8-12-23/h3-17H,2,18-20H2,1H3/b25-17+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tianjin Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli pre-incubated for 15 mins before pNPP substrate addition by spectrophotometry |
Eur J Med Chem 103: 91-104 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.037
BindingDB Entry DOI: 10.7270/Q2PK0HZF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50127113
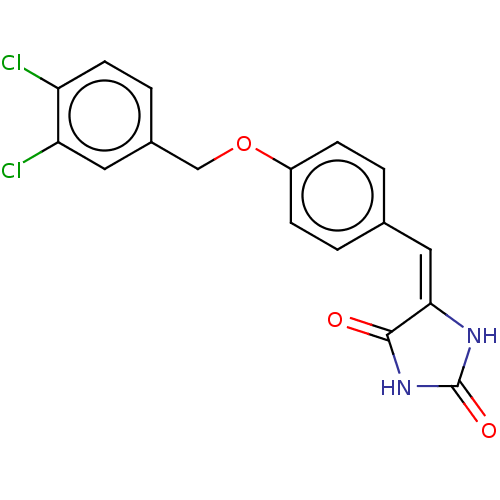
(CHEMBL3628478)Show SMILES Clc1ccc(COc2ccc(\C=C3\NC(=O)NC3=O)cc2)cc1Cl Show InChI InChI=1S/C17H12Cl2N2O3/c18-13-6-3-11(7-14(13)19)9-24-12-4-1-10(2-5-12)8-15-16(22)21-17(23)20-15/h1-8H,9H2,(H2,20,21,22,23)/b15-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tianjin Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli pre-incubated for 15 mins before pNPP substrate addition by spectrophotometry |
Eur J Med Chem 103: 91-104 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.037
BindingDB Entry DOI: 10.7270/Q2PK0HZF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM106799

((5Z)‐5‐({4‐[(3,4‐ dichloro...)Show SMILES Clc1ccc(COc2ccc(\C=C3/NC(=O)NC3=O)cc2)cc1Cl Show InChI InChI=1S/C17H12Cl2N2O3/c18-13-6-3-11(7-14(13)19)9-24-12-4-1-10(2-5-12)8-15-16(22)21-17(23)20-15/h1-8H,9H2,(H2,20,21,22,23)/b15-8- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Tianjin Medical University
| Assay Description
The enzyme activity was measured using p-nitrophenyl phosphate (pNPP) as substrate in a 96-well plate and by detecting the absorbance at 405 nm for t... |
Chem Biol Drug Des 82: 595-602 (2013)
Article DOI: 10.1111/cbdd.12189
BindingDB Entry DOI: 10.7270/Q2K64GQP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50127118
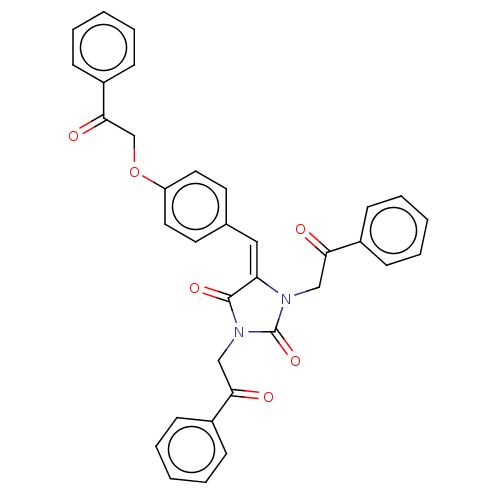
(CHEMBL3628461)Show SMILES O=C(COc1ccc(\C=C2\N(CC(=O)c3ccccc3)C(=O)N(CC(=O)c3ccccc3)C2=O)cc1)c1ccccc1 Show InChI InChI=1S/C34H26N2O6/c37-30(25-10-4-1-5-11-25)21-35-29(33(40)36(34(35)41)22-31(38)26-12-6-2-7-13-26)20-24-16-18-28(19-17-24)42-23-32(39)27-14-8-3-9-15-27/h1-20H,21-23H2/b29-20+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tianjin Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli pre-incubated for 15 mins before pNPP substrate addition by spectrophotometry |
Eur J Med Chem 103: 91-104 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.037
BindingDB Entry DOI: 10.7270/Q2PK0HZF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM106793

((5Z)‐5‐{[3‐(2‐ phenylethox...)Show InChI InChI=1S/C18H16N2O3/c21-17-16(19-18(22)20-17)12-14-7-4-8-15(11-14)23-10-9-13-5-2-1-3-6-13/h1-8,11-12H,9-10H2,(H2,19,20,21,22)/b16-12- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.14E+4 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Tianjin Medical University
| Assay Description
The enzyme activity was measured using p-nitrophenyl phosphate (pNPP) as substrate in a 96-well plate and by detecting the absorbance at 405 nm for t... |
Chem Biol Drug Des 82: 595-602 (2013)
Article DOI: 10.1111/cbdd.12189
BindingDB Entry DOI: 10.7270/Q2K64GQP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM106798

((5Z)‐5‐({4‐[(2,4‐ dichloro...)Show SMILES Clc1ccc(COc2ccc(\C=C3/NC(=O)NC3=O)cc2)c(Cl)c1 Show InChI InChI=1S/C17H12Cl2N2O3/c18-12-4-3-11(14(19)8-12)9-24-13-5-1-10(2-6-13)7-15-16(22)21-17(23)20-15/h1-8H,9H2,(H2,20,21,22,23)/b15-7- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.16E+4 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Tianjin Medical University
| Assay Description
The enzyme activity was measured using p-nitrophenyl phosphate (pNPP) as substrate in a 96-well plate and by detecting the absorbance at 405 nm for t... |
Chem Biol Drug Des 82: 595-602 (2013)
Article DOI: 10.1111/cbdd.12189
BindingDB Entry DOI: 10.7270/Q2K64GQP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50127112
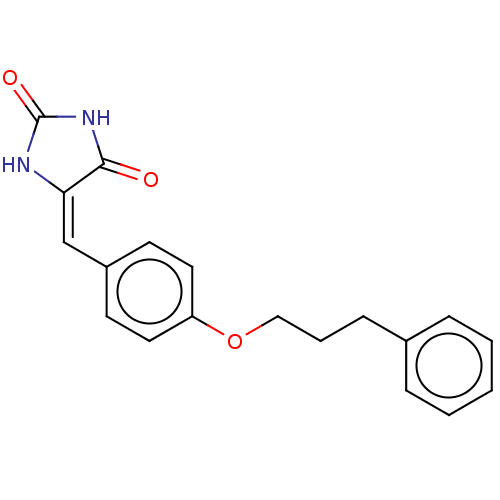
(CHEMBL3628477)Show InChI InChI=1S/C19H18N2O3/c22-18-17(20-19(23)21-18)13-15-8-10-16(11-9-15)24-12-4-7-14-5-2-1-3-6-14/h1-3,5-6,8-11,13H,4,7,12H2,(H2,20,21,22,23)/b17-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tianjin Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli pre-incubated for 15 mins before pNPP substrate addition by spectrophotometry |
Eur J Med Chem 103: 91-104 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.037
BindingDB Entry DOI: 10.7270/Q2PK0HZF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50127103
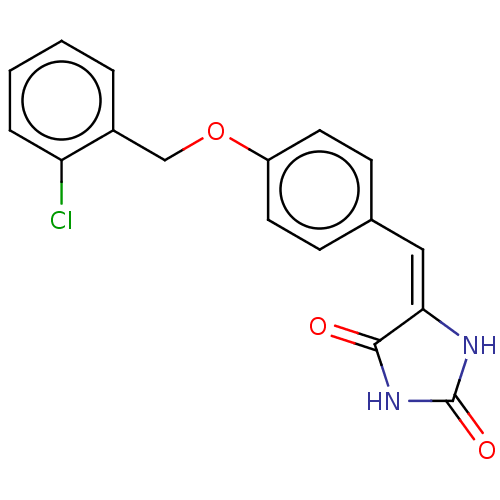
(CHEMBL3628474)Show InChI InChI=1S/C17H13ClN2O3/c18-14-4-2-1-3-12(14)10-23-13-7-5-11(6-8-13)9-15-16(21)20-17(22)19-15/h1-9H,10H2,(H2,19,20,21,22)/b15-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tianjin Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli pre-incubated for 15 mins before pNPP substrate addition by spectrophotometry |
Eur J Med Chem 103: 91-104 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.037
BindingDB Entry DOI: 10.7270/Q2PK0HZF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM106791

((5Z)‐5‐({2‐[(2,4‐ dichloro...)Show SMILES Clc1ccc(COc2ccccc2\C=C2/NC(=O)NC2=O)c(Cl)c1 Show InChI InChI=1S/C17H12Cl2N2O3/c18-12-6-5-11(13(19)8-12)9-24-15-4-2-1-3-10(15)7-14-16(22)21-17(23)20-14/h1-8H,9H2,(H2,20,21,22,23)/b14-7- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.87E+4 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Tianjin Medical University
| Assay Description
The enzyme activity was measured using p-nitrophenyl phosphate (pNPP) as substrate in a 96-well plate and by detecting the absorbance at 405 nm for t... |
Chem Biol Drug Des 82: 595-602 (2013)
Article DOI: 10.1111/cbdd.12189
BindingDB Entry DOI: 10.7270/Q2K64GQP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50127117
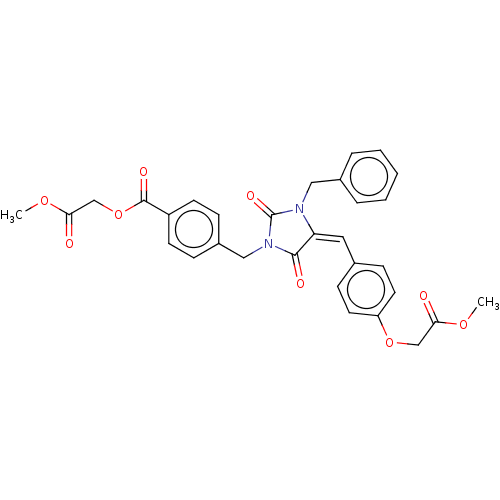
(CHEMBL3628550)Show SMILES COC(=O)COC(=O)c1ccc(CN2C(=O)N(Cc3ccccc3)\C(=C\c3ccc(OCC(=O)OC)cc3)C2=O)cc1 Show InChI InChI=1S/C31H28N2O9/c1-39-27(34)19-41-25-14-10-21(11-15-25)16-26-29(36)33(31(38)32(26)17-22-6-4-3-5-7-22)18-23-8-12-24(13-9-23)30(37)42-20-28(35)40-2/h3-16H,17-20H2,1-2H3/b26-16+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tianjin Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli pre-incubated for 15 mins before pNPP substrate addition by spectrophotometry |
Eur J Med Chem 103: 91-104 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.037
BindingDB Entry DOI: 10.7270/Q2PK0HZF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM106794
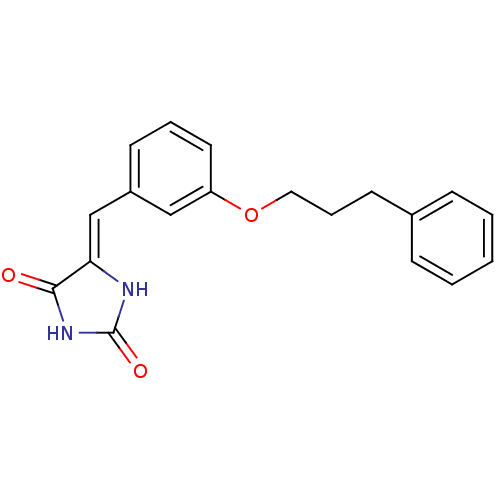
((5Z)‐5‐{[3‐(3‐ phenylpropo...)Show InChI InChI=1S/C19H18N2O3/c22-18-17(20-19(23)21-18)13-15-8-4-10-16(12-15)24-11-5-9-14-6-2-1-3-7-14/h1-4,6-8,10,12-13H,5,9,11H2,(H2,20,21,22,23)/b17-13- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.23E+4 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Tianjin Medical University
| Assay Description
The enzyme activity was measured using p-nitrophenyl phosphate (pNPP) as substrate in a 96-well plate and by detecting the absorbance at 405 nm for t... |
Chem Biol Drug Des 82: 595-602 (2013)
Article DOI: 10.1111/cbdd.12189
BindingDB Entry DOI: 10.7270/Q2K64GQP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM106800

((5Z)‐5‐({4‐[(4‐ methoxyphe...)Show InChI InChI=1S/C18H16N2O4/c1-23-14-6-4-13(5-7-14)11-24-15-8-2-12(3-9-15)10-16-17(21)20-18(22)19-16/h2-10H,11H2,1H3,(H2,19,20,21,22)/b16-10- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.14E+4 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Tianjin Medical University
| Assay Description
The enzyme activity was measured using p-nitrophenyl phosphate (pNPP) as substrate in a 96-well plate and by detecting the absorbance at 405 nm for t... |
Chem Biol Drug Des 82: 595-602 (2013)
Article DOI: 10.1111/cbdd.12189
BindingDB Entry DOI: 10.7270/Q2K64GQP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM106797

((5Z)‐5‐({3‐[(3,4‐ dichloro...)Show SMILES Clc1ccc(COc2cccc(\C=C3/NC(=O)NC3=O)c2)cc1Cl Show InChI InChI=1S/C17H12Cl2N2O3/c18-13-5-4-11(7-14(13)19)9-24-12-3-1-2-10(6-12)8-15-16(22)21-17(23)20-15/h1-8H,9H2,(H2,20,21,22,23)/b15-8- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.36E+4 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Tianjin Medical University
| Assay Description
The enzyme activity was measured using p-nitrophenyl phosphate (pNPP) as substrate in a 96-well plate and by detecting the absorbance at 405 nm for t... |
Chem Biol Drug Des 82: 595-602 (2013)
Article DOI: 10.1111/cbdd.12189
BindingDB Entry DOI: 10.7270/Q2K64GQP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM106799

((5Z)‐5‐({4‐[(3,4‐ dichloro...)Show SMILES Clc1ccc(COc2ccc(\C=C3/NC(=O)NC3=O)cc2)cc1Cl Show InChI InChI=1S/C17H12Cl2N2O3/c18-13-6-3-11(7-14(13)19)9-24-12-4-1-10(2-5-12)8-15-16(22)21-17(23)20-15/h1-8H,9H2,(H2,20,21,22,23)/b15-8- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.39E+4 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Tianjin Medical University
| Assay Description
The enzyme activity was measured using p-nitrophenyl phosphate (pNPP) as substrate in a 96-well plate and by detecting the absorbance at 405 nm for t... |
Chem Biol Drug Des 82: 595-602 (2013)
Article DOI: 10.1111/cbdd.12189
BindingDB Entry DOI: 10.7270/Q2K64GQP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM106790

((5Z)‐5‐({2‐[(4‐ chlorophen...)Show InChI InChI=1S/C17H13ClN2O3/c18-13-7-5-11(6-8-13)10-23-15-4-2-1-3-12(15)9-14-16(21)20-17(22)19-14/h1-9H,10H2,(H2,19,20,21,22)/b14-9- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.59E+4 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Tianjin Medical University
| Assay Description
The enzyme activity was measured using p-nitrophenyl phosphate (pNPP) as substrate in a 96-well plate and by detecting the absorbance at 405 nm for t... |
Chem Biol Drug Des 82: 595-602 (2013)
Article DOI: 10.1111/cbdd.12189
BindingDB Entry DOI: 10.7270/Q2K64GQP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM106789
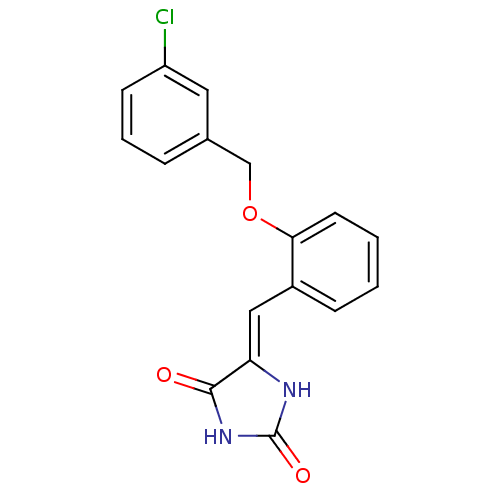
((5Z)‐5‐({2‐[(3‐ chlorophen...)Show InChI InChI=1S/C17H13ClN2O3/c18-13-6-3-4-11(8-13)10-23-15-7-2-1-5-12(15)9-14-16(21)20-17(22)19-14/h1-9H,10H2,(H2,19,20,21,22)/b14-9- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.68E+4 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Tianjin Medical University
| Assay Description
The enzyme activity was measured using p-nitrophenyl phosphate (pNPP) as substrate in a 96-well plate and by detecting the absorbance at 405 nm for t... |
Chem Biol Drug Des 82: 595-602 (2013)
Article DOI: 10.1111/cbdd.12189
BindingDB Entry DOI: 10.7270/Q2K64GQP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM106789

((5Z)‐5‐({2‐[(3‐ chlorophen...)Show InChI InChI=1S/C17H13ClN2O3/c18-13-6-3-4-11(8-13)10-23-15-7-2-1-5-12(15)9-14-16(21)20-17(22)19-14/h1-9H,10H2,(H2,19,20,21,22)/b14-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.74E+4 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Tianjin Medical University
| Assay Description
The enzyme activity was measured using p-nitrophenyl phosphate (pNPP) as substrate in a 96-well plate and by detecting the absorbance at 405 nm for t... |
Chem Biol Drug Des 82: 595-602 (2013)
Article DOI: 10.1111/cbdd.12189
BindingDB Entry DOI: 10.7270/Q2K64GQP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM106795
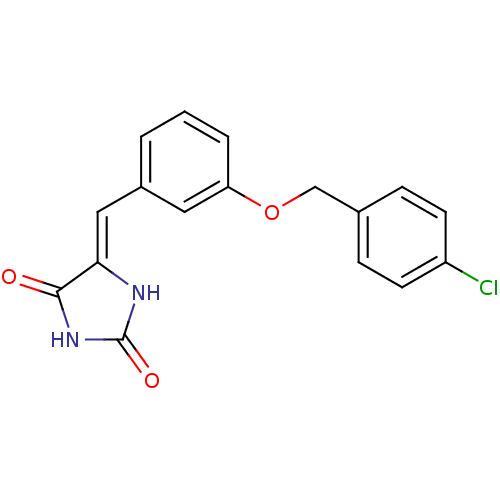
((5Z)‐5‐({3‐[(4‐ chlorophen...)Show InChI InChI=1S/C17H13ClN2O3/c18-13-6-4-11(5-7-13)10-23-14-3-1-2-12(8-14)9-15-16(21)20-17(22)19-15/h1-9H,10H2,(H2,19,20,21,22)/b15-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.08E+4 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Tianjin Medical University
| Assay Description
The enzyme activity was measured using p-nitrophenyl phosphate (pNPP) as substrate in a 96-well plate and by detecting the absorbance at 405 nm for t... |
Chem Biol Drug Des 82: 595-602 (2013)
Article DOI: 10.1111/cbdd.12189
BindingDB Entry DOI: 10.7270/Q2K64GQP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM106794

((5Z)‐5‐{[3‐(3‐ phenylpropo...)Show InChI InChI=1S/C19H18N2O3/c22-18-17(20-19(23)21-18)13-15-8-4-10-16(12-15)24-11-5-9-14-6-2-1-3-7-14/h1-4,6-8,10,12-13H,5,9,11H2,(H2,20,21,22,23)/b17-13- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.19E+4 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Tianjin Medical University
| Assay Description
The enzyme activity was measured using p-nitrophenyl phosphate (pNPP) as substrate in a 96-well plate and by detecting the absorbance at 405 nm for t... |
Chem Biol Drug Des 82: 595-602 (2013)
Article DOI: 10.1111/cbdd.12189
BindingDB Entry DOI: 10.7270/Q2K64GQP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM106793

((5Z)‐5‐{[3‐(2‐ phenylethox...)Show InChI InChI=1S/C18H16N2O3/c21-17-16(19-18(22)20-17)12-14-7-4-8-15(11-14)23-10-9-13-5-2-1-3-6-13/h1-8,11-12H,9-10H2,(H2,19,20,21,22)/b16-12- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.41E+4 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Tianjin Medical University
| Assay Description
The enzyme activity was measured using p-nitrophenyl phosphate (pNPP) as substrate in a 96-well plate and by detecting the absorbance at 405 nm for t... |
Chem Biol Drug Des 82: 595-602 (2013)
Article DOI: 10.1111/cbdd.12189
BindingDB Entry DOI: 10.7270/Q2K64GQP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50127107
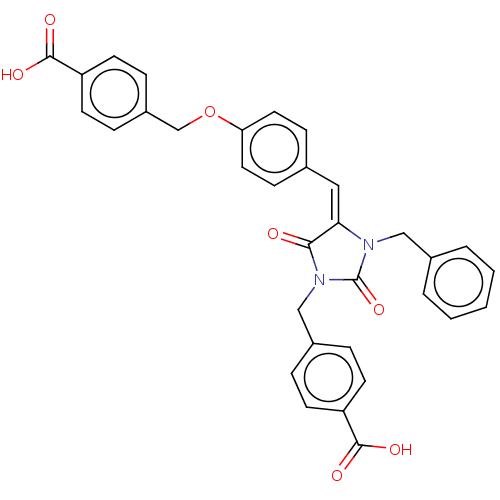
(CHEMBL3628544)Show SMILES OC(=O)c1ccc(COc2ccc(\C=C3\N(Cc4ccccc4)C(=O)N(Cc4ccc(cc4)C(O)=O)C3=O)cc2)cc1 Show InChI InChI=1S/C33H26N2O7/c36-30-29(18-22-10-16-28(17-11-22)42-21-25-8-14-27(15-9-25)32(39)40)34(19-23-4-2-1-3-5-23)33(41)35(30)20-24-6-12-26(13-7-24)31(37)38/h1-18H,19-21H2,(H,37,38)(H,39,40)/b29-18+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tianjin Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli pre-incubated for 15 mins before pNPP substrate addition by spectrophotometry |
Eur J Med Chem 103: 91-104 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.037
BindingDB Entry DOI: 10.7270/Q2PK0HZF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50127106
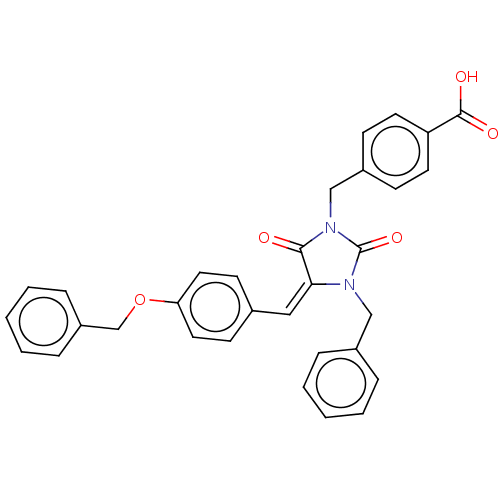
(CHEMBL3627729)Show SMILES OC(=O)c1ccc(CN2C(=O)N(Cc3ccccc3)\C(=C\c3ccc(OCc4ccccc4)cc3)C2=O)cc1 Show InChI InChI=1S/C32H26N2O5/c35-30-29(19-23-13-17-28(18-14-23)39-22-26-9-5-2-6-10-26)33(20-24-7-3-1-4-8-24)32(38)34(30)21-25-11-15-27(16-12-25)31(36)37/h1-19H,20-22H2,(H,36,37)/b29-19+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tianjin Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli pre-incubated for 15 mins before pNPP substrate addition by spectrophotometry |
Eur J Med Chem 103: 91-104 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.037
BindingDB Entry DOI: 10.7270/Q2PK0HZF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50127104
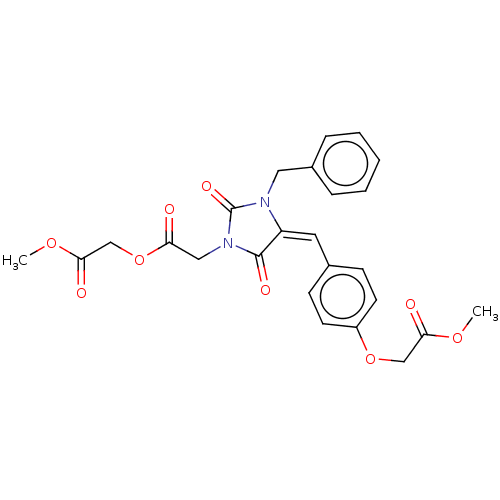
(CHEMBL3628549)Show SMILES COC(=O)COC(=O)CN1C(=O)N(Cc2ccccc2)\C(=C\c2ccc(OCC(=O)OC)cc2)C1=O Show InChI InChI=1S/C25H24N2O9/c1-33-22(29)15-35-19-10-8-17(9-11-19)12-20-24(31)27(14-21(28)36-16-23(30)34-2)25(32)26(20)13-18-6-4-3-5-7-18/h3-12H,13-16H2,1-2H3/b20-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.01E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tianjin Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli pre-incubated for 15 mins before pNPP substrate addition by spectrophotometry |
Eur J Med Chem 103: 91-104 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.037
BindingDB Entry DOI: 10.7270/Q2PK0HZF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50127130
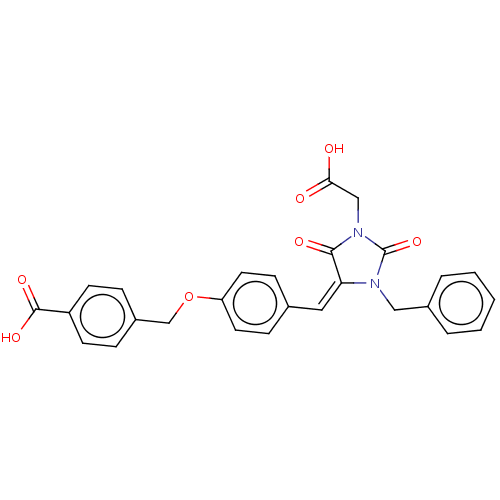
(CHEMBL3628541)Show SMILES OC(=O)CN1C(=O)N(Cc2ccccc2)\C(=C\c2ccc(OCc3ccc(cc3)C(O)=O)cc2)C1=O Show InChI InChI=1S/C27H22N2O7/c30-24(31)16-29-25(32)23(28(27(29)35)15-19-4-2-1-3-5-19)14-18-8-12-22(13-9-18)36-17-20-6-10-21(11-7-20)26(33)34/h1-14H,15-17H2,(H,30,31)(H,33,34)/b23-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.03E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tianjin Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli pre-incubated for 15 mins before pNPP substrate addition by spectrophotometry |
Eur J Med Chem 103: 91-104 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.037
BindingDB Entry DOI: 10.7270/Q2PK0HZF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data