 Found 189 hits with Last Name = 'dullea' and Initial = 'r'
Found 189 hits with Last Name = 'dullea' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50275437
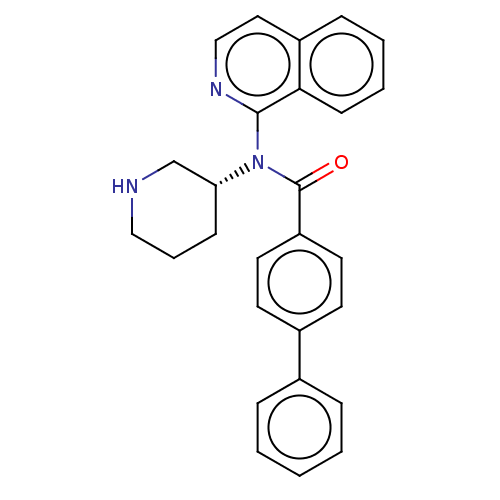
(CHEMBL4129620)Show SMILES O=C(N([C@@H]1CCCNC1)c1nccc2ccccc12)c1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C27H25N3O/c31-27(23-14-12-21(13-15-23)20-7-2-1-3-8-20)30(24-10-6-17-28-19-24)26-25-11-5-4-9-22(25)16-18-29-26/h1-5,7-9,11-16,18,24,28H,6,10,17,19H2/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibition of dofetilide binding to human ERG by fluorescence polarization assay |
J Med Chem 61: 5704-5718 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00650
BindingDB Entry DOI: 10.7270/Q28C9ZRR |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM200318
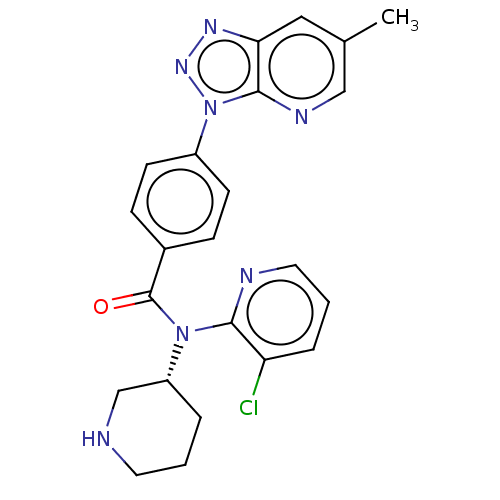
(US9227956, 3)Show SMILES Cc1cnc2n(nnc2c1)-c1ccc(cc1)C(=O)N([C@@H]1CCCNC1)c1ncccc1Cl |r| Show InChI InChI=1S/C23H22ClN7O/c1-15-12-20-22(27-13-15)31(29-28-20)17-8-6-16(7-9-17)23(32)30(18-4-2-10-25-14-18)21-19(24)5-3-11-26-21/h3,5-9,11-13,18,25H,2,4,10,14H2,1H3/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibition of dofetilide binding to human ERG by fluorescence polarization assay |
J Med Chem 61: 5704-5718 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00650
BindingDB Entry DOI: 10.7270/Q28C9ZRR |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50275430

(CHEMBL4130157)Show SMILES O=C(N([C@@H]1CCCNC1)c1nccc2ccccc12)c1ccc(cc1)-c1cnn2cccnc12 |r| Show InChI InChI=1S/C27H24N6O/c34-27(21-10-8-20(9-11-21)24-18-31-32-16-4-14-29-25(24)32)33(22-6-3-13-28-17-22)26-23-7-2-1-5-19(23)12-15-30-26/h1-2,4-5,7-12,14-16,18,22,28H,3,6,13,17H2/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibition of dofetilide binding to human ERG by fluorescence polarization assay |
J Med Chem 61: 5704-5718 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00650
BindingDB Entry DOI: 10.7270/Q28C9ZRR |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM200324
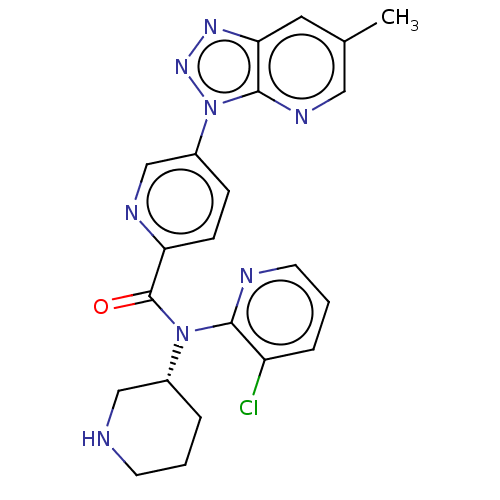
(US9227956, 9)Show SMILES Cc1cnc2n(nnc2c1)-c1ccc(nc1)C(=O)N([C@@H]1CCCNC1)c1ncccc1Cl |r| Show InChI InChI=1S/C22H21ClN8O/c1-14-10-19-21(27-11-14)31(29-28-19)16-6-7-18(26-13-16)22(32)30(15-4-2-8-24-12-15)20-17(23)5-3-9-25-20/h3,5-7,9-11,13,15,24H,2,4,8,12H2,1H3/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibition of dofetilide binding to human ERG by fluorescence polarization assay |
J Med Chem 61: 5704-5718 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00650
BindingDB Entry DOI: 10.7270/Q28C9ZRR |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50275486
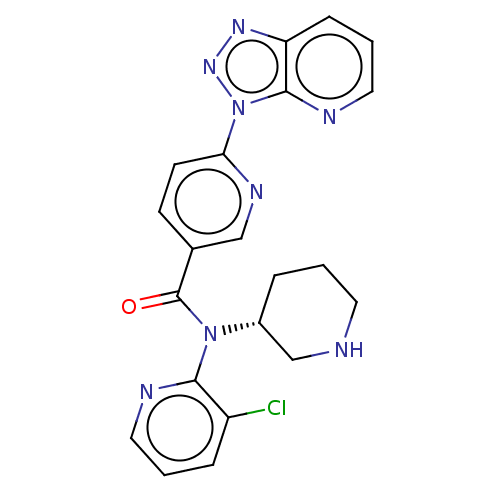
(CHEMBL4128560)Show SMILES Clc1cccnc1N([C@@H]1CCCNC1)C(=O)c1ccc(nc1)-n1nnc2cccnc12 |r| Show InChI InChI=1S/C21H19ClN8O/c22-16-5-2-10-24-19(16)29(15-4-1-9-23-13-15)21(31)14-7-8-18(26-12-14)30-20-17(27-28-30)6-3-11-25-20/h2-3,5-8,10-12,15,23H,1,4,9,13H2/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibition of dofetilide binding to human ERG by fluorescence polarization assay |
J Med Chem 61: 5704-5718 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00650
BindingDB Entry DOI: 10.7270/Q28C9ZRR |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50275436

(CHEMBL4128250)Show SMILES COc1ccc(CCC(=O)N([C@@H]2CCCNC2)c2nccc3ccccc23)cc1 |r| Show InChI InChI=1S/C24H27N3O2/c1-29-21-11-8-18(9-12-21)10-13-23(28)27(20-6-4-15-25-17-20)24-22-7-3-2-5-19(22)14-16-26-24/h2-3,5,7-9,11-12,14,16,20,25H,4,6,10,13,15,17H2,1H3/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibition of dofetilide binding to human ERG by fluorescence polarization assay |
J Med Chem 61: 5704-5718 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00650
BindingDB Entry DOI: 10.7270/Q28C9ZRR |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50275433
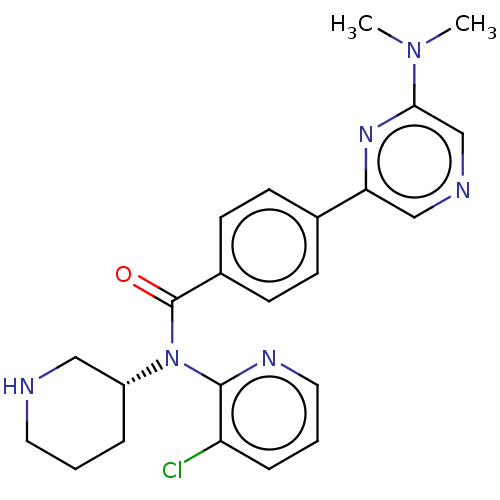
(CHEMBL4127311)Show SMILES CN(C)c1cncc(n1)-c1ccc(cc1)C(=O)N([C@@H]1CCCNC1)c1ncccc1Cl |r| Show InChI InChI=1S/C23H25ClN6O/c1-29(2)21-15-26-14-20(28-21)16-7-9-17(10-8-16)23(31)30(18-5-3-11-25-13-18)22-19(24)6-4-12-27-22/h4,6-10,12,14-15,18,25H,3,5,11,13H2,1-2H3/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibition of dofetilide binding to human ERG by fluorescence polarization assay |
J Med Chem 61: 5704-5718 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00650
BindingDB Entry DOI: 10.7270/Q28C9ZRR |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50275485
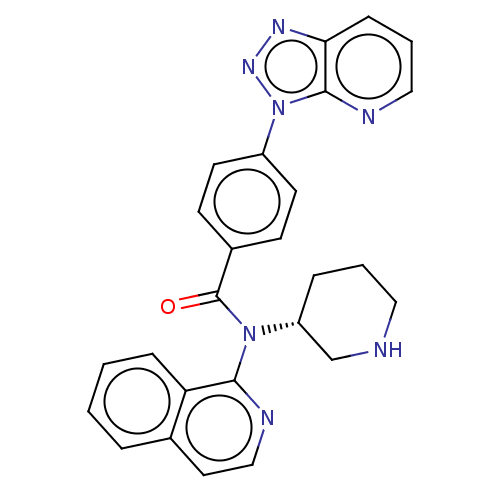
(CHEMBL4126894)Show SMILES O=C(N([C@@H]1CCCNC1)c1nccc2ccccc12)c1ccc(cc1)-n1nnc2cccnc12 |r| Show InChI InChI=1S/C26H23N7O/c34-26(19-9-11-20(12-10-19)33-25-23(30-31-33)8-4-15-28-25)32(21-6-3-14-27-17-21)24-22-7-2-1-5-18(22)13-16-29-24/h1-2,4-5,7-13,15-16,21,27H,3,6,14,17H2/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibition of dofetilide binding to human ERG by fluorescence polarization assay |
J Med Chem 61: 5704-5718 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00650
BindingDB Entry DOI: 10.7270/Q28C9ZRR |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50275484
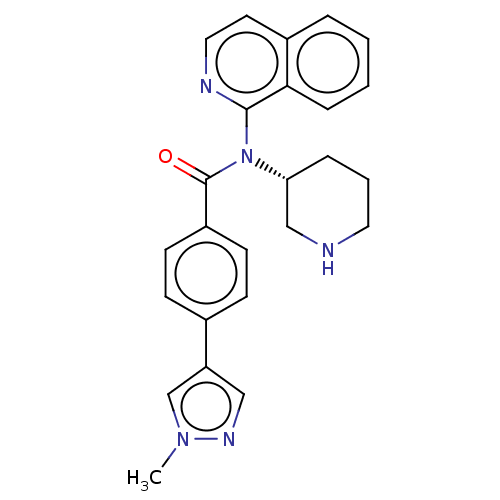
(CHEMBL4128388)Show SMILES Cn1cc(cn1)-c1ccc(cc1)C(=O)N([C@@H]1CCCNC1)c1nccc2ccccc12 |r| Show InChI InChI=1S/C25H25N5O/c1-29-17-21(15-28-29)18-8-10-20(11-9-18)25(31)30(22-6-4-13-26-16-22)24-23-7-3-2-5-19(23)12-14-27-24/h2-3,5,7-12,14-15,17,22,26H,4,6,13,16H2,1H3/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibition of dofetilide binding to human ERG by fluorescence polarization assay |
J Med Chem 61: 5704-5718 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00650
BindingDB Entry DOI: 10.7270/Q28C9ZRR |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM200332
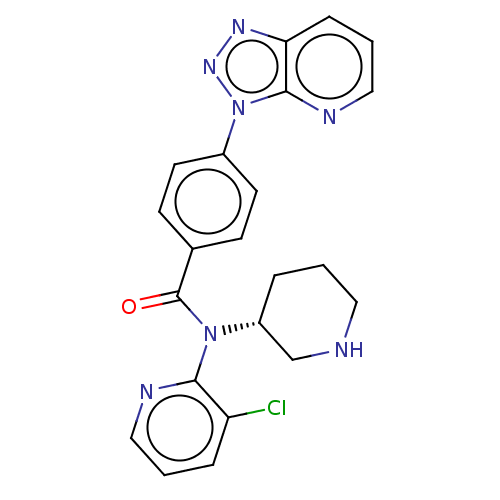
(US9227956, 16)Show SMILES Clc1cccnc1N([C@@H]1CCCNC1)C(=O)c1ccc(cc1)-n1nnc2cccnc12 |r| Show InChI InChI=1S/C22H20ClN7O/c23-18-5-2-12-25-20(18)29(17-4-1-11-24-14-17)22(31)15-7-9-16(10-8-15)30-21-19(27-28-30)6-3-13-26-21/h2-3,5-10,12-13,17,24H,1,4,11,14H2/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibition of dofetilide binding to human ERG by fluorescence polarization assay |
J Med Chem 61: 5704-5718 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00650
BindingDB Entry DOI: 10.7270/Q28C9ZRR |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM200320
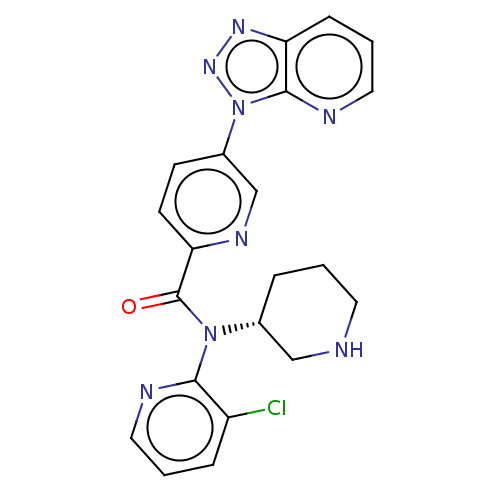
(US9227956, 5)Show SMILES Clc1cccnc1N([C@@H]1CCCNC1)C(=O)c1ccc(cn1)-n1nnc2cccnc12 |r| Show InChI InChI=1S/C21H19ClN8O/c22-16-5-2-10-24-19(16)29(14-4-1-9-23-12-14)21(31)18-8-7-15(13-26-18)30-20-17(27-28-30)6-3-11-25-20/h2-3,5-8,10-11,13-14,23H,1,4,9,12H2/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibition of dofetilide binding to human ERG by fluorescence polarization assay |
J Med Chem 61: 5704-5718 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00650
BindingDB Entry DOI: 10.7270/Q28C9ZRR |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50275487
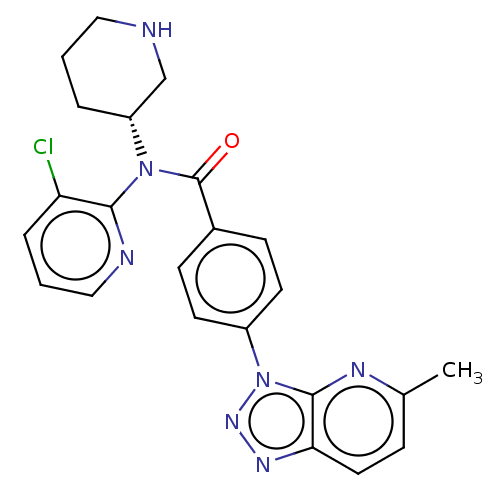
(CHEMBL4126496)Show SMILES Cc1ccc2nnn(-c3ccc(cc3)C(=O)N([C@@H]3CCCNC3)c3ncccc3Cl)c2n1 |r| Show InChI InChI=1S/C23H22ClN7O/c1-15-6-11-20-22(27-15)31(29-28-20)17-9-7-16(8-10-17)23(32)30(18-4-2-12-25-14-18)21-19(24)5-3-13-26-21/h3,5-11,13,18,25H,2,4,12,14H2,1H3/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibition of dofetilide binding to human ERG by fluorescence polarization assay |
J Med Chem 61: 5704-5718 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00650
BindingDB Entry DOI: 10.7270/Q28C9ZRR |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50275434
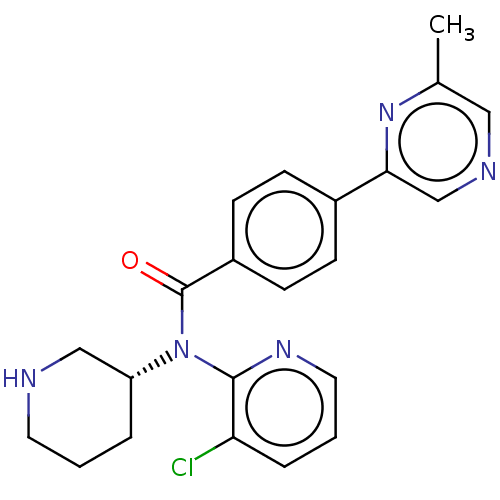
(CHEMBL4127458)Show SMILES Cc1cncc(n1)-c1ccc(cc1)C(=O)N([C@@H]1CCCNC1)c1ncccc1Cl |r| Show InChI InChI=1S/C22H22ClN5O/c1-15-12-25-14-20(27-15)16-6-8-17(9-7-16)22(29)28(18-4-2-10-24-13-18)21-19(23)5-3-11-26-21/h3,5-9,11-12,14,18,24H,2,4,10,13H2,1H3/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibition of dofetilide binding to human ERG by fluorescence polarization assay |
J Med Chem 61: 5704-5718 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00650
BindingDB Entry DOI: 10.7270/Q28C9ZRR |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50275435
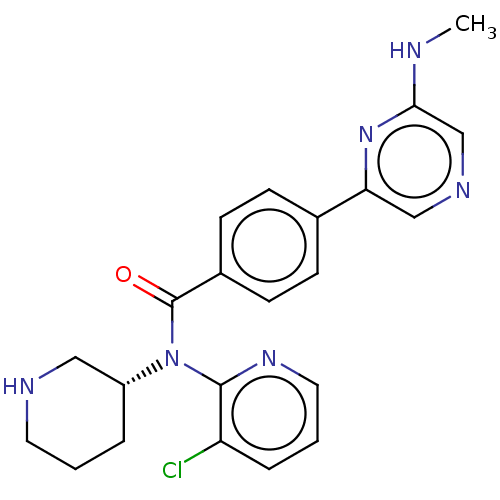
(CHEMBL4126072)Show SMILES CNc1cncc(n1)-c1ccc(cc1)C(=O)N([C@@H]1CCCNC1)c1ncccc1Cl |r| Show InChI InChI=1S/C22H23ClN6O/c1-24-20-14-26-13-19(28-20)15-6-8-16(9-7-15)22(30)29(17-4-2-10-25-12-17)21-18(23)5-3-11-27-21/h3,5-9,11,13-14,17,25H,2,4,10,12H2,1H3,(H,24,28)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibition of dofetilide binding to human ERG by fluorescence polarization assay |
J Med Chem 61: 5704-5718 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00650
BindingDB Entry DOI: 10.7270/Q28C9ZRR |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50275431
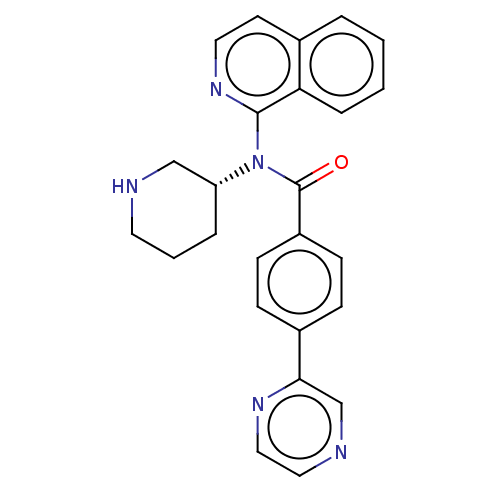
(CHEMBL4127016)Show SMILES O=C(N([C@@H]1CCCNC1)c1nccc2ccccc12)c1ccc(cc1)-c1cnccn1 |r| Show InChI InChI=1S/C25H23N5O/c31-25(20-9-7-19(8-10-20)23-17-27-14-15-28-23)30(21-5-3-12-26-16-21)24-22-6-2-1-4-18(22)11-13-29-24/h1-2,4,6-11,13-15,17,21,26H,3,5,12,16H2/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibition of dofetilide binding to human ERG by fluorescence polarization assay |
J Med Chem 61: 5704-5718 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00650
BindingDB Entry DOI: 10.7270/Q28C9ZRR |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM504495
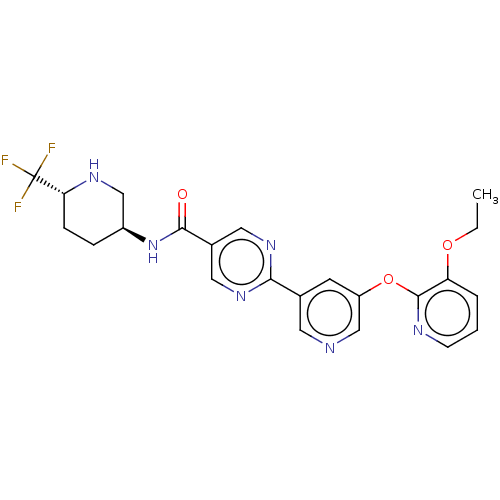
(First-eluting isomer (see footnote 4 in Table 1); ...)Show SMILES CCOc1cccnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)N[C@H]1CC[C@@H](NC1)C(F)(F)F |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM504419
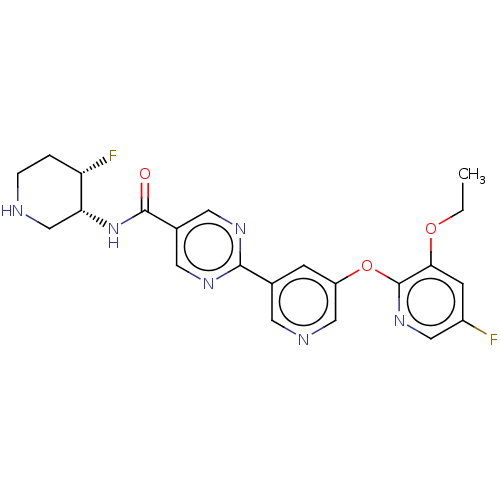
(2-{5-[(3-Ethoxy-5-fluoropyridin-2-yl)oxy]pyridin-3...)Show SMILES CCOc1cc(F)cnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)N[C@@H]1CNCC[C@@H]1F |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM276748
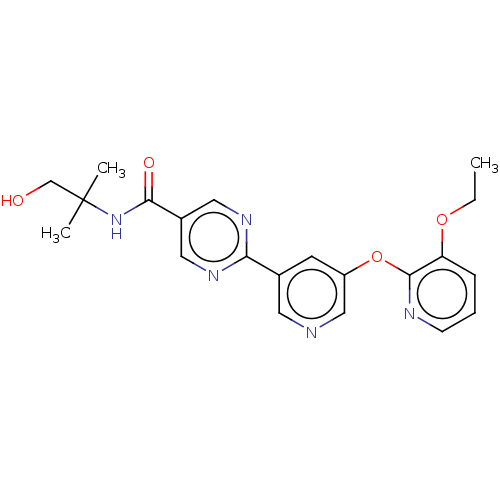
(US10071992, Example 3.5 | US10071992, Example 5 | ...)Show SMILES CCOc1cccnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)NC(C)(C)CO Show InChI InChI=1S/C21H23N5O4/c1-4-29-17-6-5-7-23-20(17)30-16-8-14(9-22-12-16)18-24-10-15(11-25-18)19(28)26-21(2,3)13-27/h5-12,27H,4,13H2,1-3H3,(H,26,28) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01200
BindingDB Entry DOI: 10.7270/Q23N27CP |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM276749
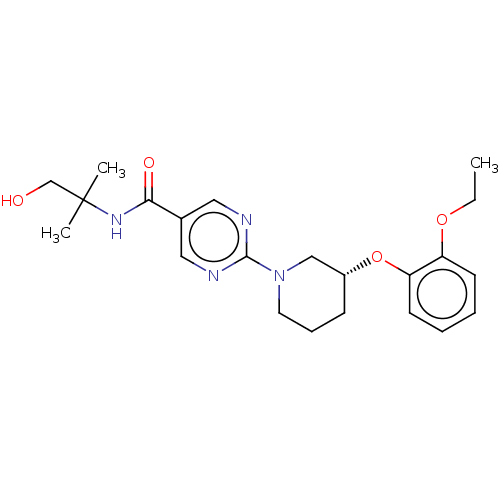
(US10188653, Example 19.21 | US11034678, WO20151406...)Show SMILES CCOc1ccccc1O[C@@H]1CCCN(C1)c1ncc(cn1)C(=O)NC(C)(C)CO |r| Show InChI InChI=1S/C22H30N4O4/c1-4-29-18-9-5-6-10-19(18)30-17-8-7-11-26(14-17)21-23-12-16(13-24-21)20(28)25-22(2,3)15-27/h5-6,9-10,12-13,17,27H,4,7-8,11,14-15H2,1-3H3,(H,25,28)/t17-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01200
BindingDB Entry DOI: 10.7270/Q23N27CP |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM504424
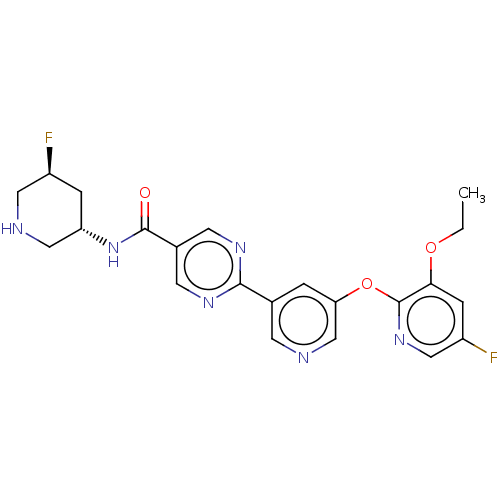
(2-{5-[(3-Ethoxy-5-fluoropyridin-2-yl)oxy]pyridin-3...)Show SMILES CCOc1cc(F)cnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)N[C@@H]1CNC[C@@H](F)C1 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM504484

(2-{5-[(3-Ethoxypyridin-2-yl)oxy]pyridin-3-yl}-N-[(...)Show SMILES CCOc1cccnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)N[C@@H]1CNC[C@@H](F)C1 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50588577

(CHEMBL5176366)Show SMILES CCOc1cccnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)NC1CCCOC1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01200
BindingDB Entry DOI: 10.7270/Q23N27CP |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM276750
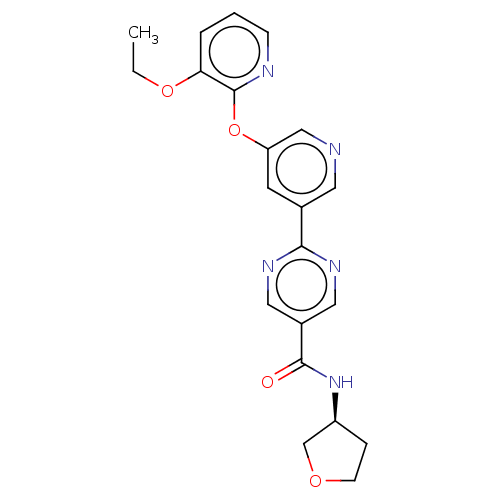
((S)-2-(5-((3-ethoxypyridin-2-yl)oxy)pyridin-3-yl)-...)Show SMILES CCOc1cccnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)N[C@H]1CCOC1 |r| Show InChI InChI=1S/C21H21N5O4/c1-2-29-18-4-3-6-23-21(18)30-17-8-14(9-22-12-17)19-24-10-15(11-25-19)20(27)26-16-5-7-28-13-16/h3-4,6,8-12,16H,2,5,7,13H2,1H3,(H,26,27)/t16-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01200
BindingDB Entry DOI: 10.7270/Q23N27CP |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM642329
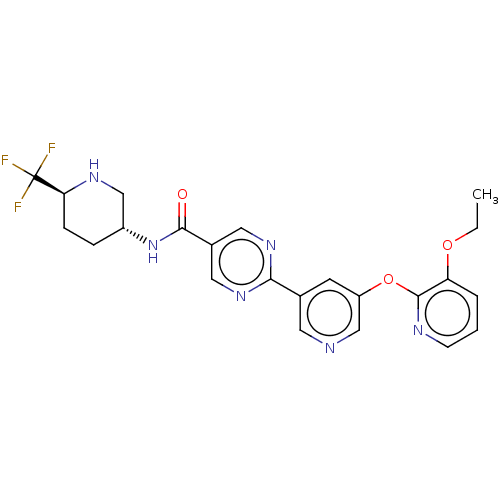
(US20230405002, Example 10 | US20230405002, Example...)Show SMILES CCOc1cccnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)N[C@@H]1CC[C@H](NC1)C(F)(F)F |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM276760
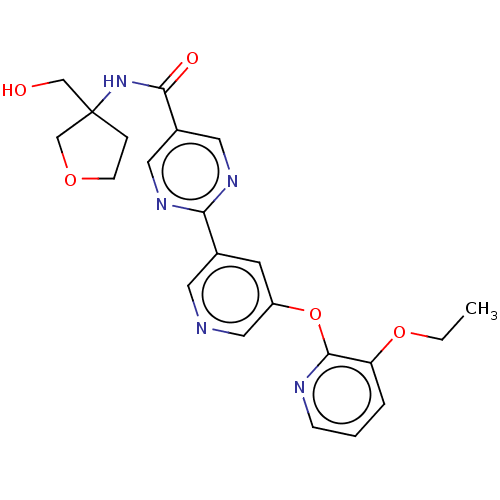
(US10071992, Example 6a)Show SMILES CCOc1cccnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)NC1(CO)CCOC1 Show InChI InChI=1S/C22H23N5O5/c1-2-31-18-4-3-6-24-21(18)32-17-8-15(9-23-12-17)19-25-10-16(11-26-19)20(29)27-22(13-28)5-7-30-14-22/h3-4,6,8-12,28H,2,5,7,13-14H2,1H3,(H,27,29) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 29.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01200
BindingDB Entry DOI: 10.7270/Q23N27CP |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM276760

(US10071992, Example 6a)Show SMILES CCOc1cccnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)NC1(CO)CCOC1 Show InChI InChI=1S/C22H23N5O5/c1-2-31-18-4-3-6-24-21(18)32-17-8-15(9-23-12-17)19-25-10-16(11-26-19)20(29)27-22(13-28)5-7-30-14-22/h3-4,6,8-12,28H,2,5,7,13-14H2,1H3,(H,27,29) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01200
BindingDB Entry DOI: 10.7270/Q23N27CP |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50588576
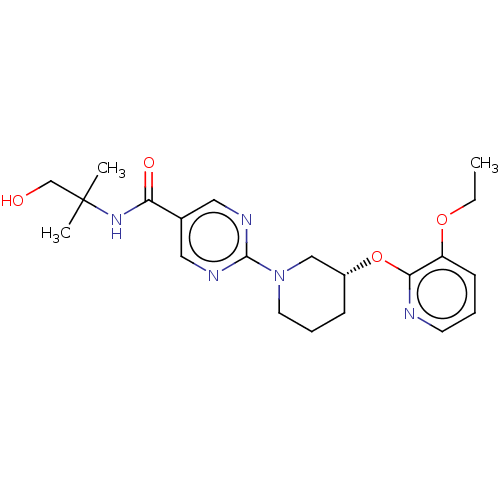
(CHEMBL5207410)Show SMILES CCOc1cccnc1O[C@@H]1CCCN(C1)c1ncc(cn1)C(=O)NC(C)(C)CO |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01200
BindingDB Entry DOI: 10.7270/Q23N27CP |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM503720
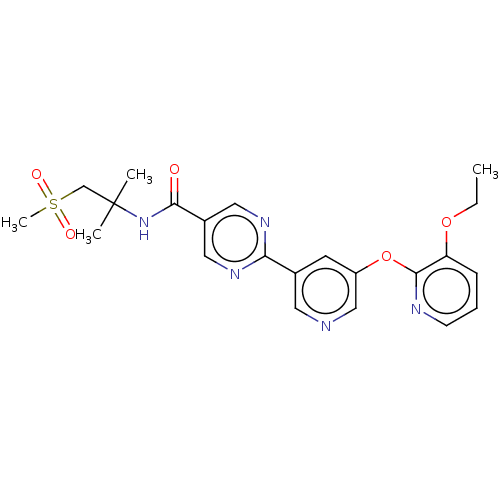
(US11034678, Example 3.6)Show SMILES CCOc1cccnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)NC(C)(C)CS(C)(=O)=O | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01200
BindingDB Entry DOI: 10.7270/Q23N27CP |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM642329

(US20230405002, Example 10 | US20230405002, Example...)Show SMILES CCOc1cccnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)N[C@@H]1CC[C@H](NC1)C(F)(F)F |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM504482
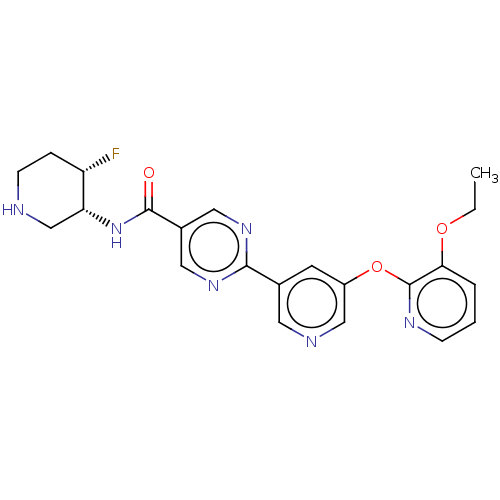
(2-{5-[(3-Ethoxypyridin-2-yl)oxy]pyridin-3-yl}-N-[(...)Show SMILES CCOc1cccnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)N[C@@H]1CNCC[C@@H]1F |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM504494
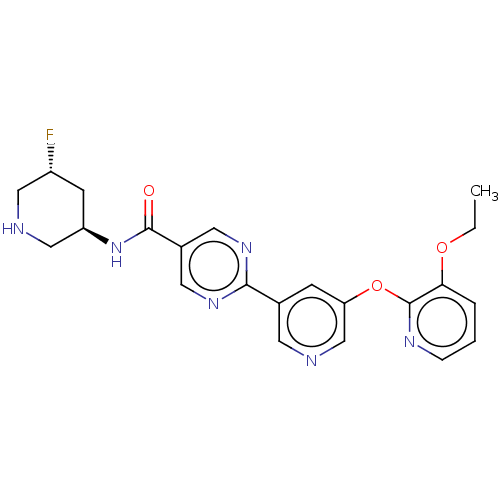
(2-{5-[(3-ethoxypyridin-2-yl)oxy]pyridin- 3-yl}-N-[...)Show SMILES CCOc1cccnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)N[C@H]1CNC[C@H](F)C1 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM504500
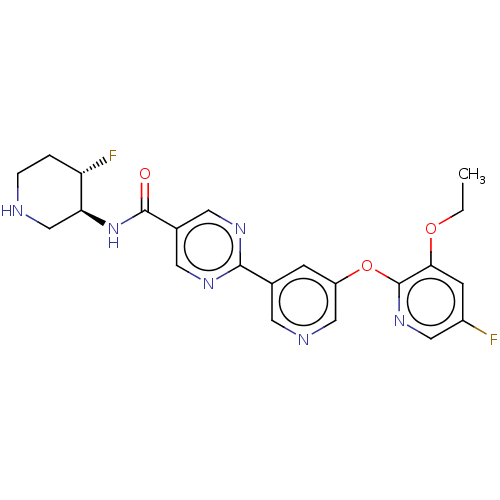
(2-{5-[(3-ethoxy-5-fluoropyridin-2- yl)oxy]pyridin-...)Show SMILES CCOc1cc(F)cnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)N[C@H]1CNCC[C@@H]1F |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM504493
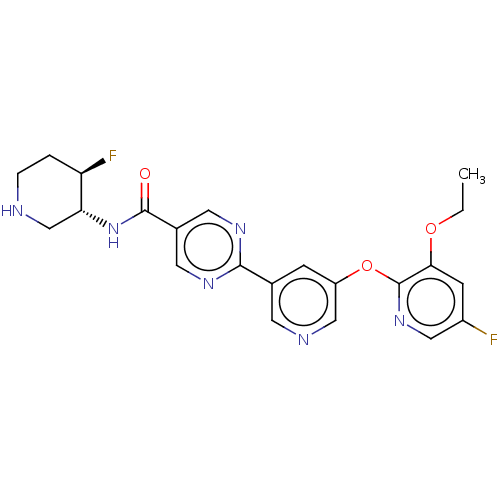
(2-{5-[(3-Ethoxy-5-fluoropyridin-2-yl)oxy]pyridin-3...)Show SMILES CCOc1cc(F)cnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)N[C@@H]1CNCC[C@H]1F |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50588580
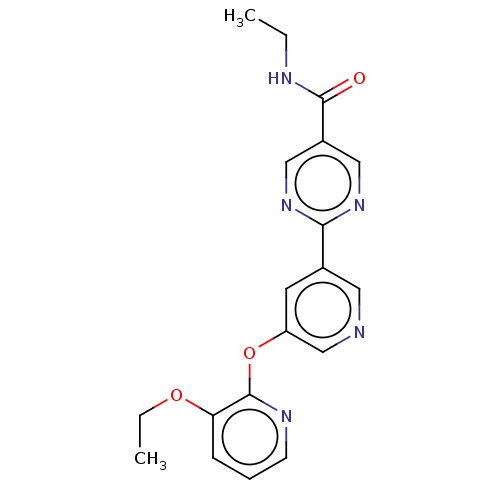
(CHEMBL5176043) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01200
BindingDB Entry DOI: 10.7270/Q23N27CP |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM323589
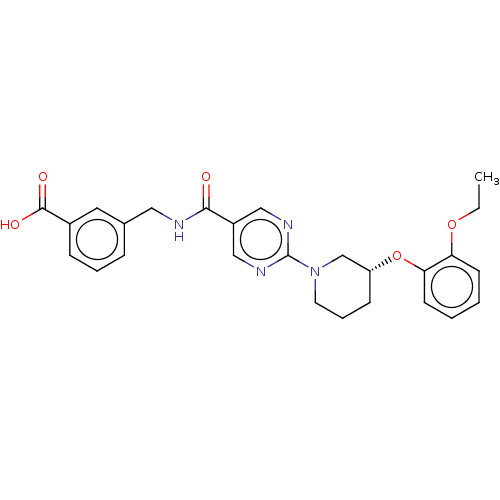
(US10188653, Example 1 | US9789110, 1)Show SMILES CCOc1ccccc1O[C@@H]1CCCN(C1)c1ncc(cn1)C(=O)NCc1cccc(c1)C(O)=O |r| Show InChI InChI=1S/C26H28N4O5/c1-2-34-22-10-3-4-11-23(22)35-21-9-6-12-30(17-21)26-28-15-20(16-29-26)24(31)27-14-18-7-5-8-19(13-18)25(32)33/h3-5,7-8,10-11,13,15-16,21H,2,6,9,12,14,17H2,1H3,(H,27,31)(H,32,33)/t21-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01200
BindingDB Entry DOI: 10.7270/Q23N27CP |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM504492
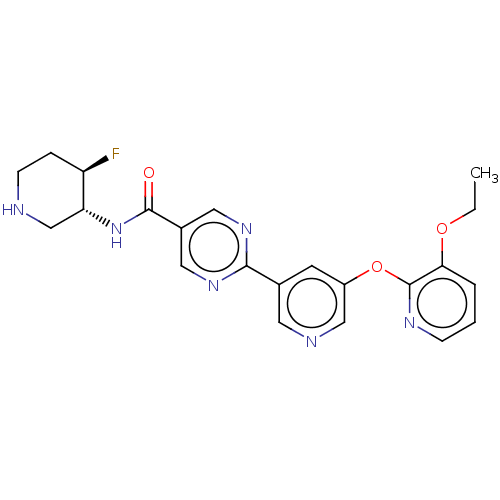
(2-{5-[(3-Ethoxypyridin-2-yl)oxy]pyridin-3-yl}-N-[(...)Show SMILES CCOc1cccnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)N[C@@H]1CNCC[C@H]1F |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM504505

(First-eluting enantiomer (see footnote 8 in Table ...)Show SMILES CCOc1cccnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)N[C@@H]1CNCC(F)(F)C1 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM276754
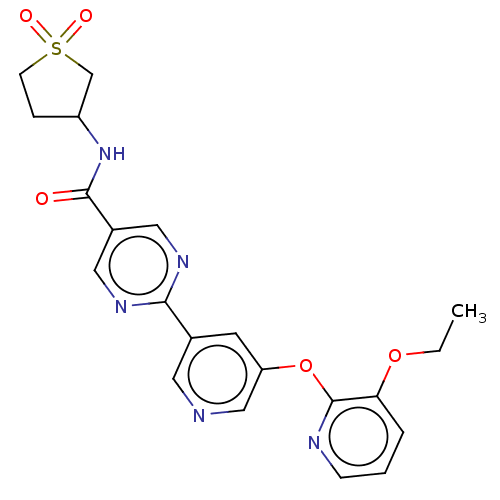
(N-(1,1-dioxidotetrahydro- thiophen-3-yl)-2-(5-((3-...)Show SMILES CCOc1cccnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)NC1CCS(=O)(=O)C1 Show InChI InChI=1S/C21H21N5O5S/c1-2-30-18-4-3-6-23-21(18)31-17-8-14(9-22-12-17)19-24-10-15(11-25-19)20(27)26-16-5-7-32(28,29)13-16/h3-4,6,8-12,16H,2,5,7,13H2,1H3,(H,26,27) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 196 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01200
BindingDB Entry DOI: 10.7270/Q23N27CP |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM642345
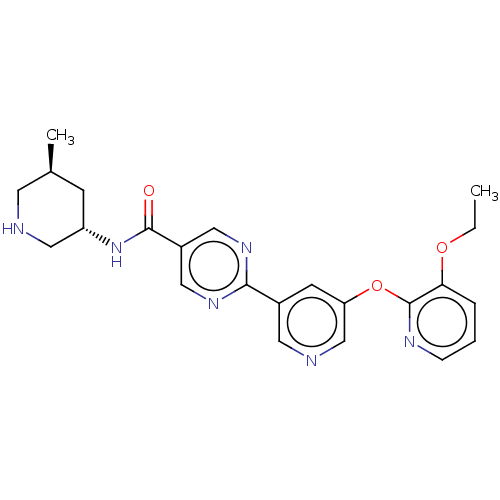
(US20230405002, Example 25 | ac-2-{5-[(3-ethoxypyri...)Show SMILES CCOc1cccnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)N[C@@H]1CNC[C@@H](C)C1 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM276751
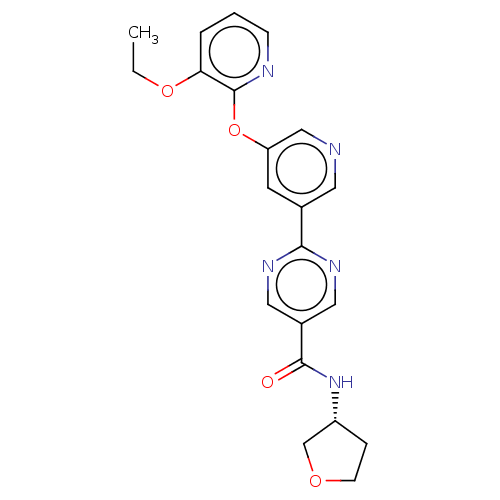
((R)-2-(5-((3-Ethoxypyridin-2-yl)oxy)pyridin-3-yl)-...)Show SMILES CCOc1cccnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)N[C@@H]1CCOC1 |r| Show InChI InChI=1S/C21H21N5O4/c1-2-29-18-4-3-6-23-21(18)30-17-8-14(9-22-12-17)19-24-10-15(11-25-19)20(27)26-16-5-7-28-13-16/h3-4,6,8-12,16H,2,5,7,13H2,1H3,(H,26,27)/t16-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01200
BindingDB Entry DOI: 10.7270/Q23N27CP |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM504504
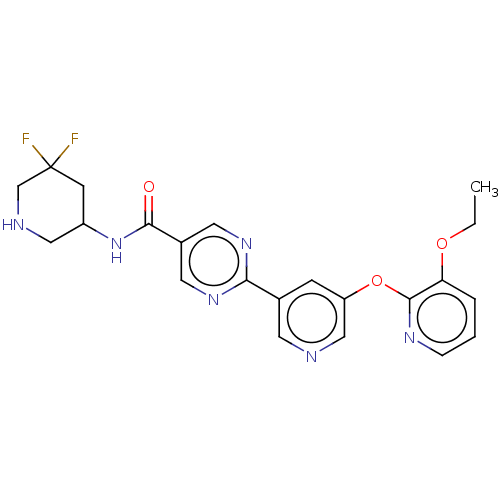
(N-(5,5-difluoropiperidin-3-yl)-2-{5-[(3- ethoxypyr...)Show SMILES CCOc1cccnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)NC1CNCC(F)(F)C1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM642339
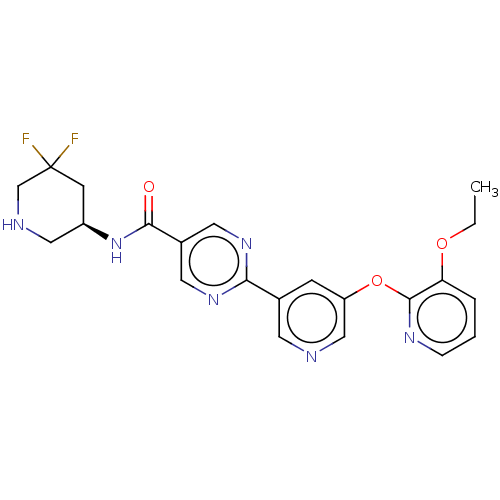
(N-[(3S)-5,5-difluoropiperidin-3-yl]-2-{5-[(3- etho...)Show SMILES CCOc1cccnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)N[C@H]1CNCC(F)(F)C1 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50588578
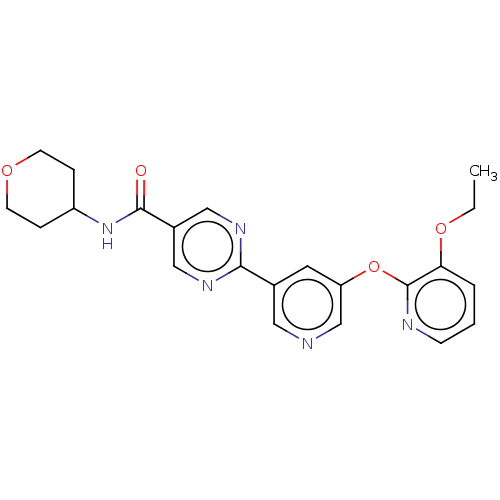
(CHEMBL5171697)Show SMILES CCOc1cccnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)NC1CCOCC1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 353 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01200
BindingDB Entry DOI: 10.7270/Q23N27CP |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM200316
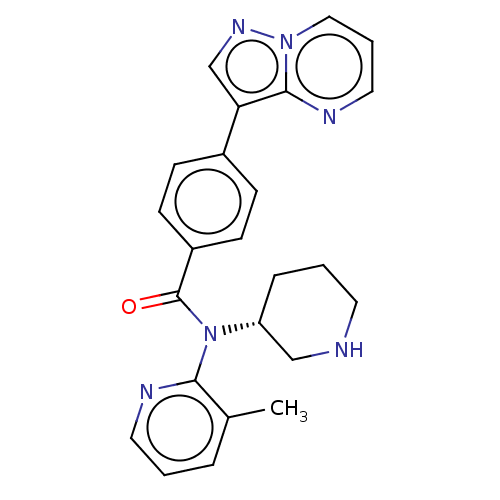
(US9227956, 1)Show SMILES Cc1cccnc1N([C@@H]1CCCNC1)C(=O)c1ccc(cc1)-c1cnn2cccnc12 |r| Show InChI InChI=1S/C24H24N6O/c1-17-5-2-12-26-22(17)30(20-6-3-11-25-15-20)24(31)19-9-7-18(8-10-19)21-16-28-29-14-4-13-27-23(21)29/h2,4-5,7-10,12-14,16,20,25H,3,6,11,15H2,1H3/t20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
In order to eliminate the permeability barrier inherent to the WT7 and SCHH cell-based assays a cell-free system was also established to access compo... |
US Patent US9227956 (2016)
BindingDB Entry DOI: 10.7270/Q20R9N7M |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM576482
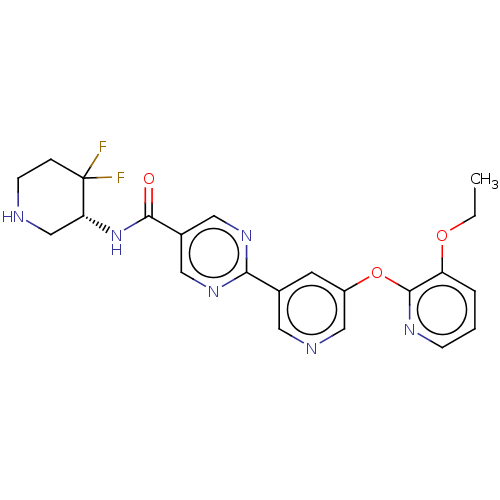
(US11471458, Example 16 | US20230405002, Example 16)Show SMILES CCOc1cccnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)N[C@@H]1CNCCC1(F)F |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50588579

(CHEMBL5173066)Show SMILES CCOc1cccnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)NC1CN(C)C(=O)C1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 501 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01200
BindingDB Entry DOI: 10.7270/Q23N27CP |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM200332

(US9227956, 16)Show SMILES Clc1cccnc1N([C@@H]1CCCNC1)C(=O)c1ccc(cc1)-n1nnc2cccnc12 |r| Show InChI InChI=1S/C22H20ClN7O/c23-18-5-2-12-25-20(18)29(17-4-1-11-24-14-17)22(31)15-7-9-16(10-8-15)30-21-19(27-28-30)6-3-13-26-21/h2-3,5-10,12-13,17,24H,1,4,11,14H2/t17-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| US Patent
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
In order to eliminate the permeability barrier inherent to the WT7 and SCHH cell-based assays a cell-free system was also established to access compo... |
US Patent US9227956 (2016)
BindingDB Entry DOI: 10.7270/Q20R9N7M |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM504499
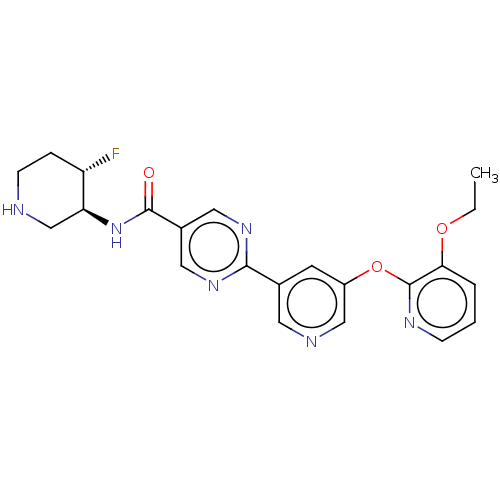
(2-{5-[(3-ethoxypyridin-2-yl)oxy]pyridin- 3-yl}-N-[...)Show SMILES CCOc1cccnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)N[C@H]1CNCC[C@@H]1F |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM200354

(US9227956, 38 | US9227956, 39)Show SMILES CC(C)OC(=O)OC(C)n1nnc(n1)-c1c(cnn1C)-c1ccc(cc1)C(=O)N([C@@H]1CCCNC1)c1ncccc1C |r| Show InChI InChI=1S/C29H35N9O4/c1-18(2)41-29(40)42-20(4)38-34-26(33-35-38)25-24(17-32-36(25)5)21-10-12-22(13-11-21)28(39)37(23-9-7-14-30-16-23)27-19(3)8-6-15-31-27/h6,8,10-13,15,17-18,20,23,30H,7,9,14,16H2,1-5H3/t20?,23-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 592 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
In order to eliminate the permeability barrier inherent to the WT7 and SCHH cell-based assays a cell-free system was also established to access compo... |
US Patent US9227956 (2016)
BindingDB Entry DOI: 10.7270/Q20R9N7M |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM200325
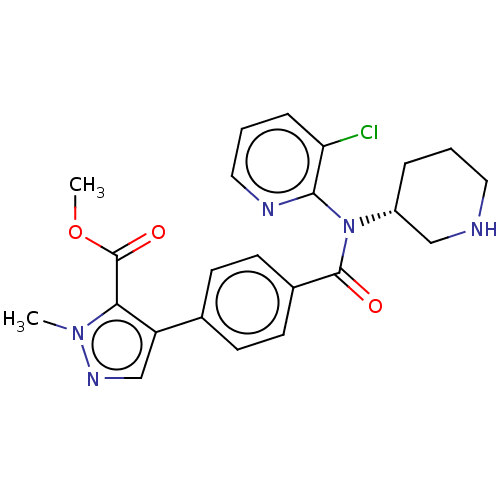
(US9227956, 10)Show SMILES COC(=O)c1c(cnn1C)-c1ccc(cc1)C(=O)N([C@@H]1CCCNC1)c1ncccc1Cl |r| Show InChI InChI=1S/C23H24ClN5O3/c1-28-20(23(31)32-2)18(14-27-28)15-7-9-16(10-8-15)22(30)29(17-5-3-11-25-13-17)21-19(24)6-4-12-26-21/h4,6-10,12,14,17,25H,3,5,11,13H2,1-2H3/t17-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
In order to eliminate the permeability barrier inherent to the WT7 and SCHH cell-based assays a cell-free system was also established to access compo... |
US Patent US9227956 (2016)
BindingDB Entry DOI: 10.7270/Q20R9N7M |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data