 Found 24 hits with Last Name = 'dumelin' and Initial = 'ce'
Found 24 hits with Last Name = 'dumelin' and Initial = 'ce' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50298204
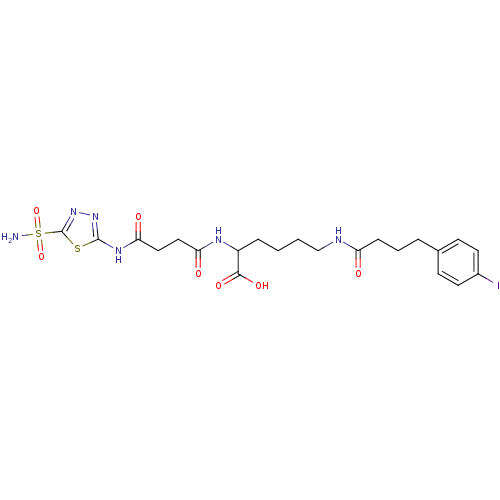
(6-(4-(4-iodophenyl)butanamido)-2-(4-oxo-4-(5-sulfa...)Show SMILES NS(=O)(=O)c1nnc(NC(=O)CCC(=O)NC(CCCCNC(=O)CCCc2ccc(I)cc2)C(O)=O)s1 Show InChI InChI=1S/C22H29IN6O7S2/c23-15-9-7-14(8-10-15)4-3-6-17(30)25-13-2-1-5-16(20(33)34)26-18(31)11-12-19(32)27-21-28-29-22(37-21)38(24,35)36/h7-10,16H,1-6,11-13H2,(H,25,30)(H,26,31)(H,33,34)(H2,24,35,36)(H,27,28,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human CA9 catalytic domain using 4-nitrophenylacetate substrate in presence of murine serum albumin by colorimetry |
Bioorg Med Chem Lett 19: 4851-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.022
BindingDB Entry DOI: 10.7270/Q298871J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50298204

(6-(4-(4-iodophenyl)butanamido)-2-(4-oxo-4-(5-sulfa...)Show SMILES NS(=O)(=O)c1nnc(NC(=O)CCC(=O)NC(CCCCNC(=O)CCCc2ccc(I)cc2)C(O)=O)s1 Show InChI InChI=1S/C22H29IN6O7S2/c23-15-9-7-14(8-10-15)4-3-6-17(30)25-13-2-1-5-16(20(33)34)26-18(31)11-12-19(32)27-21-28-29-22(37-21)38(24,35)36/h7-10,16H,1-6,11-13H2,(H,25,30)(H,26,31)(H,33,34)(H2,24,35,36)(H,27,28,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human CA9 catalytic domain using 4-nitrophenylacetate substrate by colorimetry |
Bioorg Med Chem Lett 19: 4851-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.022
BindingDB Entry DOI: 10.7270/Q298871J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50298205

(2-(6-hydroxy-3-oxo-3H-xanthen-9-yl)-5-(5-sulfamoyl...)Show SMILES NS(=O)(=O)c1nnc(NC(=O)c2ccc(c(c2)C(O)=O)-c2c3ccc(O)cc3oc3cc(=O)ccc23)s1 |(4.77,-16.65,;4.67,-15.1,;3.13,-15.21,;6.2,-15,;4.56,-13.56,;5.73,-12.57,;5.15,-11.14,;3.61,-11.26,;2.61,-10.08,;3.14,-8.63,;4.66,-8.37,;2.14,-7.45,;.62,-7.73,;-.37,-6.55,;.16,-5.11,;1.66,-4.83,;2.67,-6,;2.43,-3.49,;1.66,-2.15,;3.97,-3.49,;-1.38,-3.83,;-1.4,-2.3,;-.06,-1.52,;-.06,.03,;-1.4,.79,;-1.4,2.34,;-2.73,.03,;-2.73,-1.53,;-4.05,-2.3,;-4.05,-3.83,;-5.38,-4.6,;-5.37,-6.14,;-6.71,-6.91,;-4.03,-6.9,;-2.71,-6.13,;-2.72,-4.6,;3.24,-12.76,)| Show InChI InChI=1S/C23H14N4O8S2/c24-37(33,34)23-27-26-22(36-23)25-20(30)10-1-4-13(16(7-10)21(31)32)19-14-5-2-11(28)8-17(14)35-18-9-12(29)3-6-15(18)19/h1-9,28H,(H,31,32)(H2,24,33,34)(H,25,26,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human CA9 catalytic domain using 4-nitrophenylacetate substrate by colorimetry |
Bioorg Med Chem Lett 19: 4851-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.022
BindingDB Entry DOI: 10.7270/Q298871J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50298205

(2-(6-hydroxy-3-oxo-3H-xanthen-9-yl)-5-(5-sulfamoyl...)Show SMILES NS(=O)(=O)c1nnc(NC(=O)c2ccc(c(c2)C(O)=O)-c2c3ccc(O)cc3oc3cc(=O)ccc23)s1 |(4.77,-16.65,;4.67,-15.1,;3.13,-15.21,;6.2,-15,;4.56,-13.56,;5.73,-12.57,;5.15,-11.14,;3.61,-11.26,;2.61,-10.08,;3.14,-8.63,;4.66,-8.37,;2.14,-7.45,;.62,-7.73,;-.37,-6.55,;.16,-5.11,;1.66,-4.83,;2.67,-6,;2.43,-3.49,;1.66,-2.15,;3.97,-3.49,;-1.38,-3.83,;-1.4,-2.3,;-.06,-1.52,;-.06,.03,;-1.4,.79,;-1.4,2.34,;-2.73,.03,;-2.73,-1.53,;-4.05,-2.3,;-4.05,-3.83,;-5.38,-4.6,;-5.37,-6.14,;-6.71,-6.91,;-4.03,-6.9,;-2.71,-6.13,;-2.72,-4.6,;3.24,-12.76,)| Show InChI InChI=1S/C23H14N4O8S2/c24-37(33,34)23-27-26-22(36-23)25-20(30)10-1-4-13(16(7-10)21(31)32)19-14-5-2-11(28)8-17(14)35-18-9-12(29)3-6-15(18)19/h1-9,28H,(H,31,32)(H2,24,33,34)(H,25,26,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human CA9 catalytic domain using 4-nitrophenylacetate substrate in presence of murine serum albumin by colorimetry |
Bioorg Med Chem Lett 19: 4851-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.022
BindingDB Entry DOI: 10.7270/Q298871J |
More data for this
Ligand-Target Pair | |
Acyl-[acyl-carrier-protein]--UDP-N-acetylglucosamine O-acyltransferase
(Escherichia coli (Enterobacteria)) | BDBM50577526
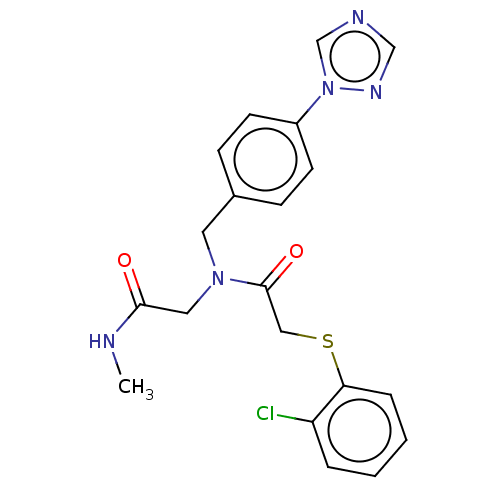
(CHEMBL4871671)Show SMILES CNC(=O)CN(Cc1ccc(cc1)-n1cncn1)C(=O)CSc1ccccc1Cl | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant Escherichia coli LpxA using UDP-GlcNAc as substrate incubated for 30 mins by spectrophotometric method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00888
BindingDB Entry DOI: 10.7270/Q2N301RT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50615229
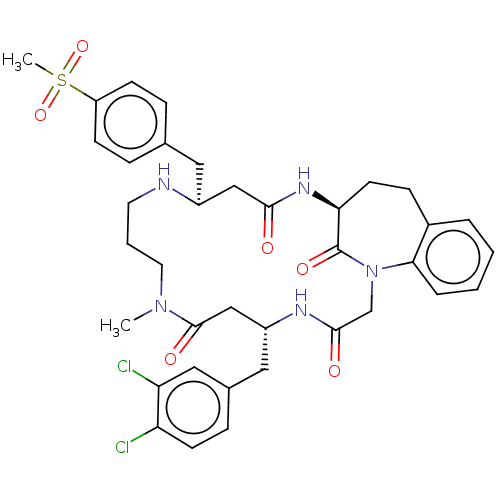
(CHEMBL5282122) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50508936
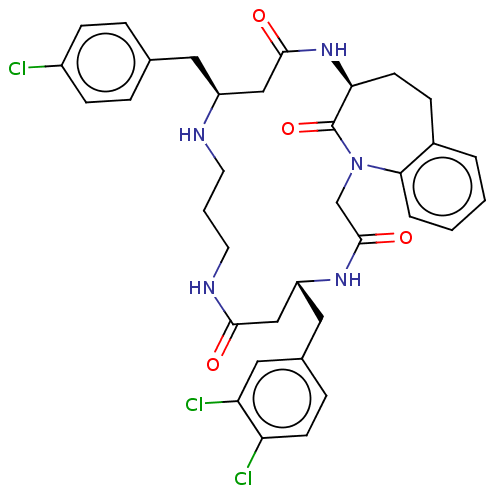
(CHEMBL4590345)Show SMILES Clc1ccc(C[C@H]2CC(=O)N[C@H]3CCc4ccccc4N(CC(=O)N[C@H](Cc4ccc(Cl)c(Cl)c4)CC(=O)NCCCN2)C3=O)cc1 |r| Show InChI InChI=1S/C35H38Cl3N5O4/c36-25-10-6-22(7-11-25)16-26-19-33(45)42-30-13-9-24-4-1-2-5-31(24)43(35(30)47)21-34(46)41-27(20-32(44)40-15-3-14-39-26)17-23-8-12-28(37)29(38)18-23/h1-2,4-8,10-12,18,26-27,30,39H,3,9,13-17,19-21H2,(H,40,44)(H,41,46)(H,42,45)/t26-,27+,30-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | 4.48E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50508947
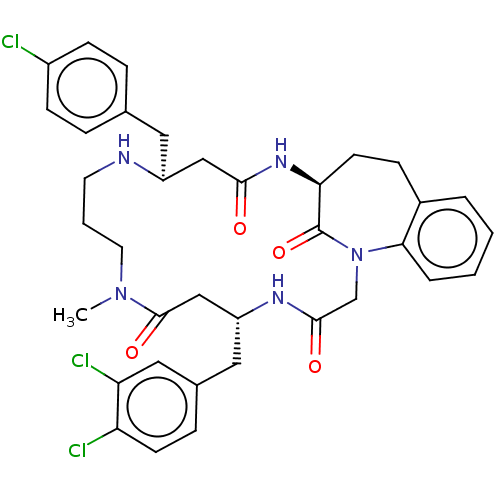
(CHEMBL4460550)Show SMILES CN1CCCN[C@@H](Cc2ccc(Cl)cc2)CC(=O)N[C@H]2CCc3ccccc3N(CC(=O)N[C@H](Cc3ccc(Cl)c(Cl)c3)CC1=O)C2=O |r| Show InChI InChI=1S/C36H40Cl3N5O4/c1-43-16-4-15-40-27(17-23-7-11-26(37)12-8-23)20-33(45)42-31-14-10-25-5-2-3-6-32(25)44(36(31)48)22-34(46)41-28(21-35(43)47)18-24-9-13-29(38)30(39)19-24/h2-3,5-9,11-13,19,27-28,31,40H,4,10,14-18,20-22H2,1H3,(H,41,46)(H,42,45)/t27-,28+,31-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50615230

(CHEMBL5268588) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50615231

(CHEMBL5266160) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50508940
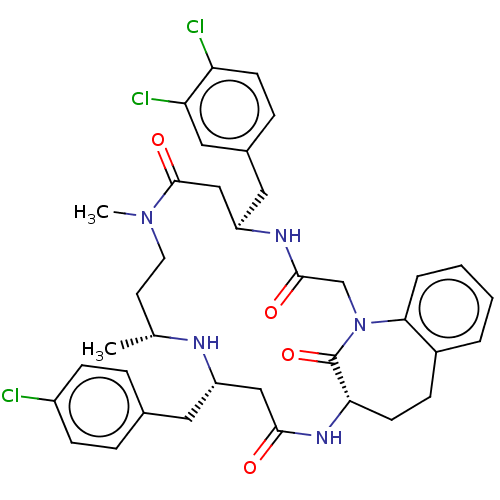
(CHEMBL4582512)Show SMILES C[C@@H]1CCN(C)C(=O)C[C@@H](Cc2ccc(Cl)c(Cl)c2)NC(=O)CN2c3ccccc3CC[C@H](NC(=O)C[C@H](Cc3ccc(Cl)cc3)N1)C2=O |r| Show InChI InChI=1S/C37H42Cl3N5O4/c1-23-15-16-44(2)36(48)21-29(18-25-9-13-30(39)31(40)19-25)42-35(47)22-45-33-6-4-3-5-26(33)10-14-32(37(45)49)43-34(46)20-28(41-23)17-24-7-11-27(38)12-8-24/h3-9,11-13,19,23,28-29,32,41H,10,14-18,20-22H2,1-2H3,(H,42,47)(H,43,46)/t23-,28+,29-,32+/m1/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | 3.54E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50508950

(CHEMBL4472439)Show SMILES C[C@H]1CCN(C)C(=O)C[C@@H](Cc2ccc(Cl)c(Cl)c2)NC(=O)CN2c3ccccc3CC[C@H](NC(=O)C[C@H](Cc3ccc(Cl)cc3)N1)C2=O |r| Show InChI InChI=1S/C37H42Cl3N5O4/c1-23-15-16-44(2)36(48)21-29(18-25-9-13-30(39)31(40)19-25)42-35(47)22-45-33-6-4-3-5-26(33)10-14-32(37(45)49)43-34(46)20-28(41-23)17-24-7-11-27(38)12-8-24/h3-9,11-13,19,23,28-29,32,41H,10,14-18,20-22H2,1-2H3,(H,42,47)(H,43,46)/t23-,28-,29+,32-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50508948
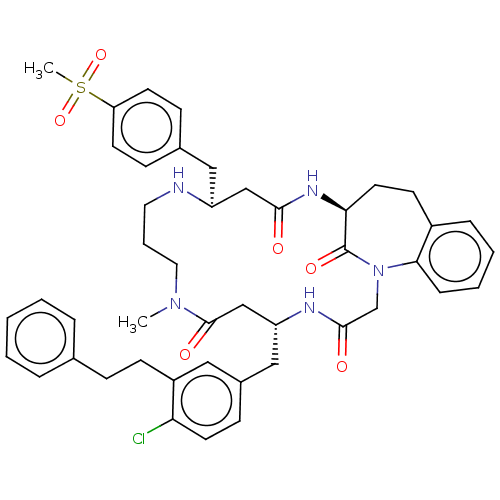
(CHEMBL4473598)Show SMILES CN1CCCN[C@@H](Cc2ccc(cc2)S(C)(=O)=O)CC(=O)N[C@H]2CCc3ccccc3N(CC(=O)N[C@H](Cc3ccc(Cl)c(CCc4ccccc4)c3)CC1=O)C2=O |r| Show InChI InChI=1S/C45H52ClN5O6S/c1-50-24-8-23-47-36(26-32-14-19-38(20-15-32)58(2,56)57)28-42(52)49-40-22-18-34-11-6-7-12-41(34)51(45(40)55)30-43(53)48-37(29-44(50)54)27-33-16-21-39(46)35(25-33)17-13-31-9-4-3-5-10-31/h3-7,9-12,14-16,19-21,25,36-37,40,47H,8,13,17-18,22-24,26-30H2,1-2H3,(H,48,53)(H,49,52)/t36-,37+,40-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50615232

(CHEMBL5280696) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50615233

(CHEMBL5280275) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50615234
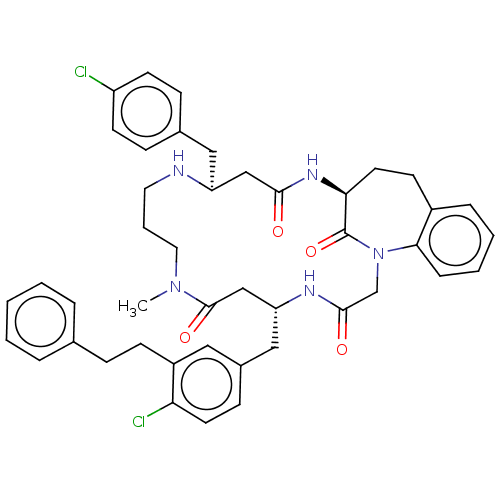
(CHEMBL5282328) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50615235
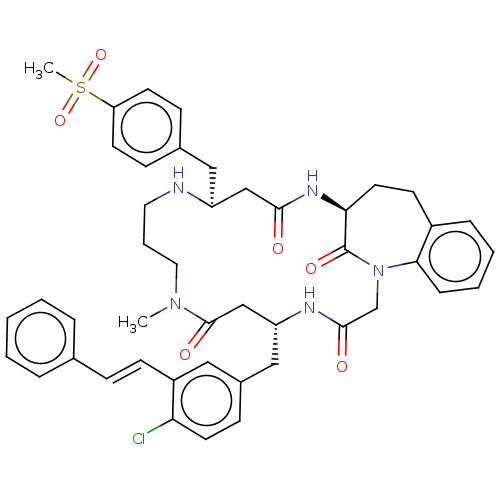
(CHEMBL5269798) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50615236
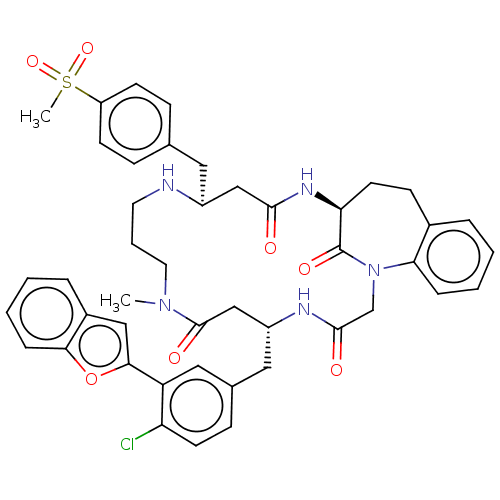
(CHEMBL5267198) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50615228
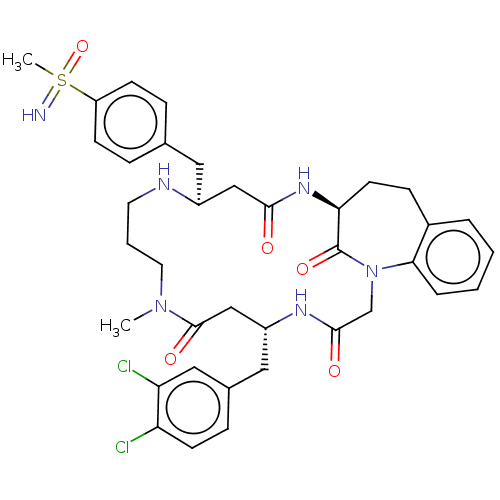
(CHEMBL5277654) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50615227
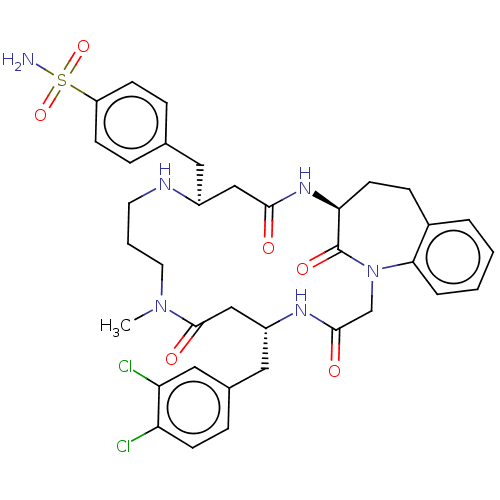
(CHEMBL5288551) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50615226

(CHEMBL5269652) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50615237
(CHEMBL5275336) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50298204

(6-(4-(4-iodophenyl)butanamido)-2-(4-oxo-4-(5-sulfa...)Show SMILES NS(=O)(=O)c1nnc(NC(=O)CCC(=O)NC(CCCCNC(=O)CCCc2ccc(I)cc2)C(O)=O)s1 Show InChI InChI=1S/C22H29IN6O7S2/c23-15-9-7-14(8-10-15)4-3-6-17(30)25-13-2-1-5-16(20(33)34)26-18(31)11-12-19(32)27-21-28-29-22(37-21)38(24,35)36/h7-10,16H,1-6,11-13H2,(H,25,30)(H,26,31)(H,33,34)(H2,24,35,36)(H,27,28,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Binding affinity to human CA9 catalytic domain by isothermal titration calorimetry |
Bioorg Med Chem Lett 19: 4851-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.022
BindingDB Entry DOI: 10.7270/Q298871J |
More data for this
Ligand-Target Pair | |
Albumin
(Homo sapiens (Human)) | BDBM50298204

(6-(4-(4-iodophenyl)butanamido)-2-(4-oxo-4-(5-sulfa...)Show SMILES NS(=O)(=O)c1nnc(NC(=O)CCC(=O)NC(CCCCNC(=O)CCCc2ccc(I)cc2)C(O)=O)s1 Show InChI InChI=1S/C22H29IN6O7S2/c23-15-9-7-14(8-10-15)4-3-6-17(30)25-13-2-1-5-16(20(33)34)26-18(31)11-12-19(32)27-21-28-29-22(37-21)38(24,35)36/h7-10,16H,1-6,11-13H2,(H,25,30)(H,26,31)(H,33,34)(H2,24,35,36)(H,27,28,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Binding affinity to HSA by isothermal titration calorimetry |
Bioorg Med Chem Lett 19: 4851-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.022
BindingDB Entry DOI: 10.7270/Q298871J |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data