

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM22988 ((5Z,8Z,11Z,14Z)-N-(2-hydroxyethyl)icosa-5,8,11,14-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montpellier I Curated by ChEMBL | Assay Description Displacement of [3H]CP55-940 from human recombinant CB1 receptor expressed in COS cells | J Med Chem 52: 1005-17 (2009) Article DOI: 10.1021/jm8011382 BindingDB Entry DOI: 10.7270/Q2GF0TH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM22988 ((5Z,8Z,11Z,14Z)-N-(2-hydroxyethyl)icosa-5,8,11,14-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Montpellier 1 Curated by ChEMBL | Assay Description Displacement of [3H]CP55-940 from human recombinant CB1 receptor in COS cells | Bioorg Med Chem Lett 16: 3765-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.059 BindingDB Entry DOI: 10.7270/Q2M61JVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM22988 ((5Z,8Z,11Z,14Z)-N-(2-hydroxyethyl)icosa-5,8,11,14-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR CNRS 5074 Curated by ChEMBL | Assay Description Binding affinity of the compound towards Cannabinoid receptor 1 in rat forebrain membranes using [3H]-CP-55,940 as radioligand | Bioorg Med Chem Lett 13: 1977-80 (2003) BindingDB Entry DOI: 10.7270/Q23X861T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM22988 ((5Z,8Z,11Z,14Z)-N-(2-hydroxyethyl)icosa-5,8,11,14-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montpellier I Curated by ChEMBL | Assay Description Displacement of [3H]CP55-940 from human recombinant CB2 receptor expressed in COS cells | J Med Chem 52: 1005-17 (2009) Article DOI: 10.1021/jm8011382 BindingDB Entry DOI: 10.7270/Q2GF0TH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM22988 ((5Z,8Z,11Z,14Z)-N-(2-hydroxyethyl)icosa-5,8,11,14-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Montpellier 1 Curated by ChEMBL | Assay Description Displacement of [3H]CP55-940 from human recombinant CB2 receptor in COS cells | Bioorg Med Chem Lett 16: 3765-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.059 BindingDB Entry DOI: 10.7270/Q2M61JVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50186533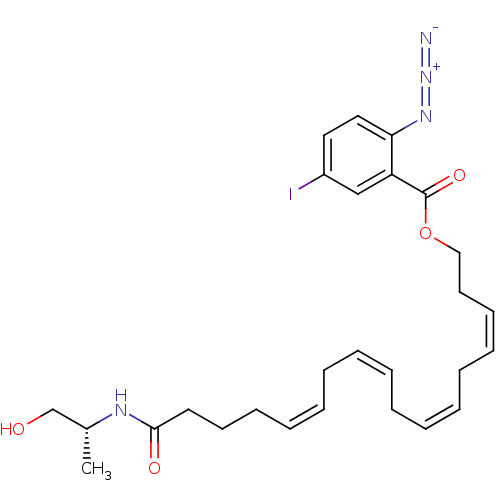 ((3Z,6Z,9Z,12Z)-17-((R)-1-hydroxypropan-2-ylamino)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Montpellier 1 Curated by ChEMBL | Assay Description Displacement of [3H]CP55-940 from human recombinant CB2 receptor in COS cells | Bioorg Med Chem Lett 16: 3765-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.059 BindingDB Entry DOI: 10.7270/Q2M61JVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50128915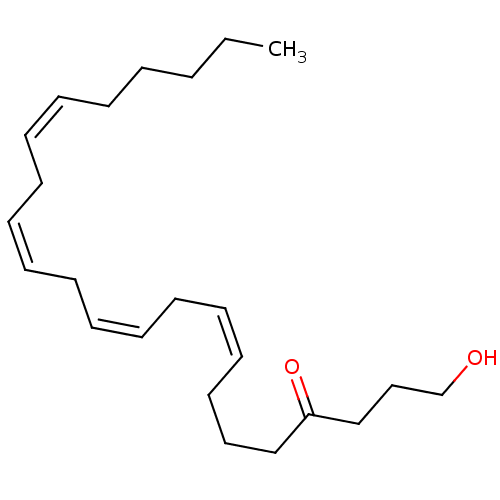 ((8Z,11Z,14Z)-1-Hydroxy-tricosa-8,11,14,17-tetraen-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR CNRS 5074 Curated by ChEMBL | Assay Description Binding affinity of the compound towards Cannabinoid receptor 1 in rat forebrain membranes using [3H]-CP-55,940 as radioligand | Bioorg Med Chem Lett 13: 1977-80 (2003) BindingDB Entry DOI: 10.7270/Q23X861T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM20462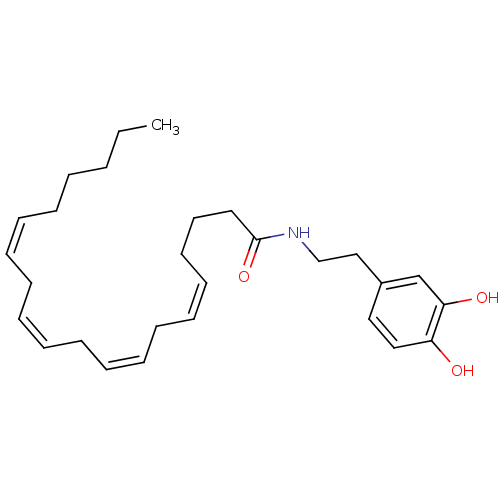 ((5Z,8Z,11Z,14Z)-N-[2-(3,4-dihydroxyphenyl)ethyl]ic...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Bioorganic Chemistry RAS Curated by ChEMBL | Assay Description Concentration required to displace 0.4 nM [3H]-SR-141,716A from CB1 receptor in rat brain preparations in the presence of 0.1 mM phenylmethyl sulphon... | Bioorg Med Chem Lett 11: 447-9 (2001) BindingDB Entry DOI: 10.7270/Q2VT1RBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50186533 ((3Z,6Z,9Z,12Z)-17-((R)-1-hydroxypropan-2-ylamino)-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Montpellier 1 Curated by ChEMBL | Assay Description Displacement of [3H]CP55-940 from human recombinant CB1 receptor in COS cells | Bioorg Med Chem Lett 16: 3765-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.059 BindingDB Entry DOI: 10.7270/Q2M61JVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50096883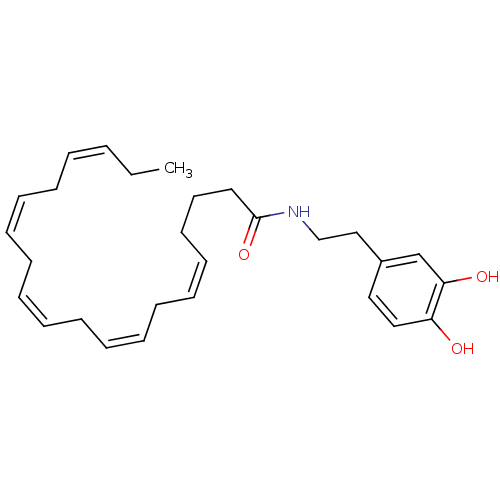 ((5Z,8Z,11Z,14Z,17Z)-Icosa-5,8,11,14,17-pentaenoic ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Bioorganic Chemistry RAS Curated by ChEMBL | Assay Description Concentration required to displace 0.4 nM [3H]-SR-141,716A from CB1 receptor in rat brain preparations in the presence of 0.1 mM phenylmethyl sulphon... | Bioorg Med Chem Lett 11: 447-9 (2001) BindingDB Entry DOI: 10.7270/Q2VT1RBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM22988 ((5Z,8Z,11Z,14Z)-N-(2-hydroxyethyl)icosa-5,8,11,14-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Bioorganic Chemistry RAS Curated by ChEMBL | Assay Description Concentration required to displace 0.4 nM [3H]-SR-141,716A from CB1 receptor in rat brain preparations in the presence of 0.1 mM phenylmethyl sulphon... | Bioorg Med Chem Lett 11: 447-9 (2001) BindingDB Entry DOI: 10.7270/Q2VT1RBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50292882 ((3Z,6Z,9Z,12Z)-17-((R)-1-hydroxypropan-2-ylamino)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montpellier I Curated by ChEMBL | Assay Description Displacement of [3H]CP55-940 from human recombinant CB2 receptor expressed in COS cells | J Med Chem 52: 1005-17 (2009) Article DOI: 10.1021/jm8011382 BindingDB Entry DOI: 10.7270/Q2GF0TH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50096881 ((10Z,13Z,16Z,19Z)-Docosa-7,10,13,16,19-pentaenoic ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Bioorganic Chemistry RAS Curated by ChEMBL | Assay Description Concentration required to displace 0.4 nM [3H]-SR-141,716A from CB1 receptor in rat brain preparations in the presence of 0.1 mM phenylmethyl sulphon... | Bioorg Med Chem Lett 11: 447-9 (2001) BindingDB Entry DOI: 10.7270/Q2VT1RBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50096882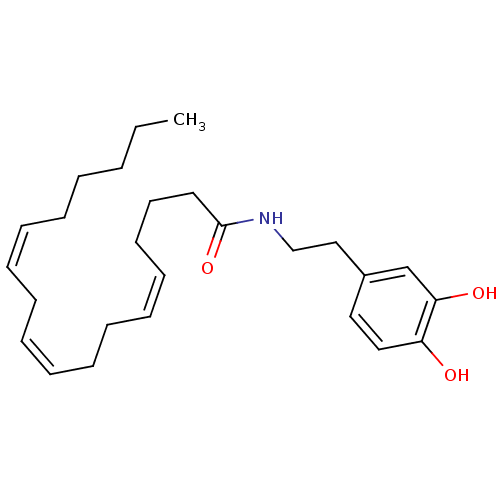 ((5Z,9Z,12Z)-Octadeca-5,9,12-trienoic acid [2-(3,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Bioorganic Chemistry RAS Curated by ChEMBL | Assay Description Concentration required to displace 0.4 nM [3H]-SR-141,716A from CB1 receptor in rat brain preparations in the presence of 0.1 mM phenylmethyl sulphon... | Bioorg Med Chem Lett 11: 447-9 (2001) BindingDB Entry DOI: 10.7270/Q2VT1RBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50128914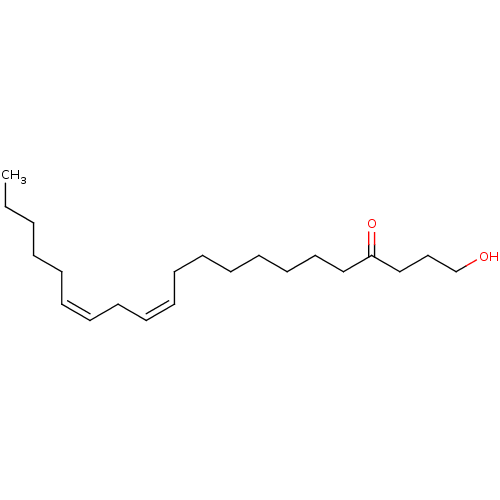 ((12Z,15Z)-1-Hydroxy-henicosa-12,15-dien-4-one | CH...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR CNRS 5074 Curated by ChEMBL | Assay Description Binding affinity of the compound towards Cannabinoid receptor 1 in rat forebrain membranes using [3H]-CP-55,940 as radioligand | Bioorg Med Chem Lett 13: 1977-80 (2003) BindingDB Entry DOI: 10.7270/Q23X861T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50128912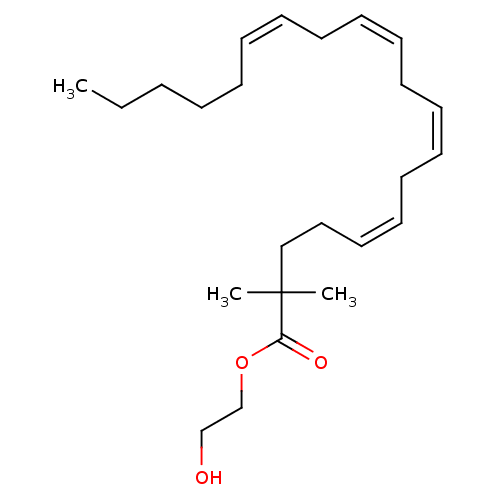 ((5Z,8Z,11Z)-2,2-Dimethyl-icosa-5,8,11,14-tetraenoi...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR CNRS 5074 Curated by ChEMBL | Assay Description Binding affinity of the compound towards Fatty-acid amide hydrolase (FAAH) in rat forebrain membranes | Bioorg Med Chem Lett 13: 1977-80 (2003) BindingDB Entry DOI: 10.7270/Q23X861T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50096885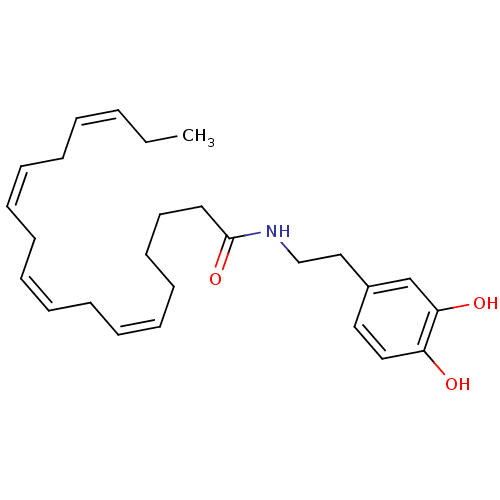 ((6Z,9Z,12Z,15Z)-Octadeca-6,9,12,15-tetraenoic acid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Bioorganic Chemistry RAS Curated by ChEMBL | Assay Description Concentration required to displace 0.4 nM [3H]-SR-141,716A from CB1 receptor in rat brain preparations in the presence of 0.1 mM phenylmethyl sulphon... | Bioorg Med Chem Lett 11: 447-9 (2001) BindingDB Entry DOI: 10.7270/Q2VT1RBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50292882 ((3Z,6Z,9Z,12Z)-17-((R)-1-hydroxypropan-2-ylamino)-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montpellier I Curated by ChEMBL | Assay Description Displacement of [3H]CP55-940 from human recombinant CB1 receptor expressed in COS cells | J Med Chem 52: 1005-17 (2009) Article DOI: 10.1021/jm8011382 BindingDB Entry DOI: 10.7270/Q2GF0TH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM22988 ((5Z,8Z,11Z,14Z)-N-(2-hydroxyethyl)icosa-5,8,11,14-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR CNRS 5074 Curated by ChEMBL | Assay Description Binding affinity of the compound towards Fatty-acid amide hydrolase (FAAH) in rat forebrain membranes | Bioorg Med Chem Lett 13: 1977-80 (2003) BindingDB Entry DOI: 10.7270/Q23X861T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50096884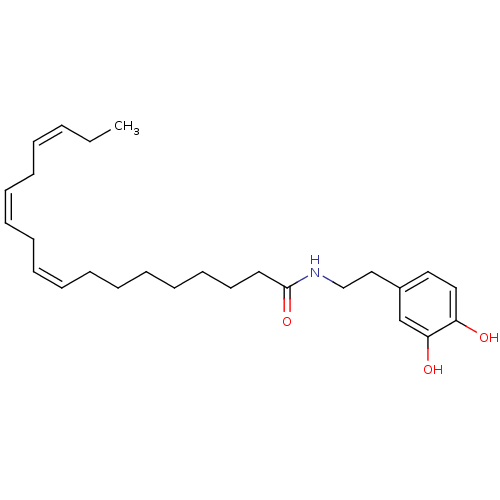 ((9Z,12Z,15Z)-Octadeca-9,12,15-trienoic acid [2-(3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Bioorganic Chemistry RAS Curated by ChEMBL | Assay Description Concentration required to displace 0.4 nM [3H]-SR-141,716A from CB1 receptor in rat brain preparations in the presence of 0.1 mM phenylmethyl sulphon... | Bioorg Med Chem Lett 11: 447-9 (2001) BindingDB Entry DOI: 10.7270/Q2VT1RBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50128912 ((5Z,8Z,11Z)-2,2-Dimethyl-icosa-5,8,11,14-tetraenoi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR CNRS 5074 Curated by ChEMBL | Assay Description Binding affinity of the compound towards Cannabinoid receptor 1 in rat forebrain membranes using [3H]-CP-55,940 as radioligand | Bioorg Med Chem Lett 13: 1977-80 (2003) BindingDB Entry DOI: 10.7270/Q23X861T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50128913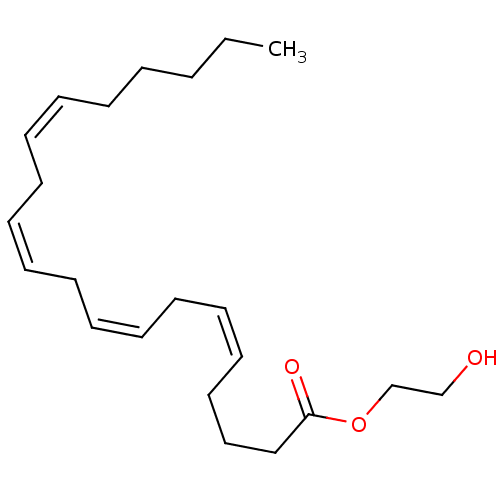 ((5Z,8Z,11Z)-Icosa-5,8,11,14-tetraenoic acid 2-hydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR CNRS 5074 Curated by ChEMBL | Assay Description Binding affinity of the compound towards Cannabinoid receptor 1 in rat forebrain membranes using [3H]-CP-55,940 as radioligand | Bioorg Med Chem Lett 13: 1977-80 (2003) BindingDB Entry DOI: 10.7270/Q23X861T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM467000 (2-(((2S,5R)-2-carbamoyl-4-methyl-7-oxo-1,6-diazabi...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50200120 (CHEMBL260091 | CHIR-090 | US10875832, Compound ChI...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of fluorescent ligand from Pseudomonas aeruginosa LpxC measured after 30 mins by fluorescence anisotrophy assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115826 BindingDB Entry DOI: 10.7270/Q2C2513C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-lactamase (Klebsiella pneumoniae) | BDBM467002 ((2R)-2-(((2S,5R)-2-cyano-4-methyl-7-oxo-1,6-diazab...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM467003 (US10800778, Comparator 98) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM466965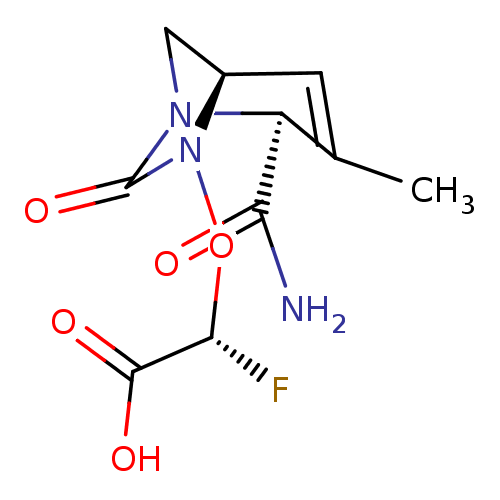 ((2R)-{[(2S,5R)-2-carbamoyl-3-methyl-7-oxo-1,6-diaz...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.35 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM466967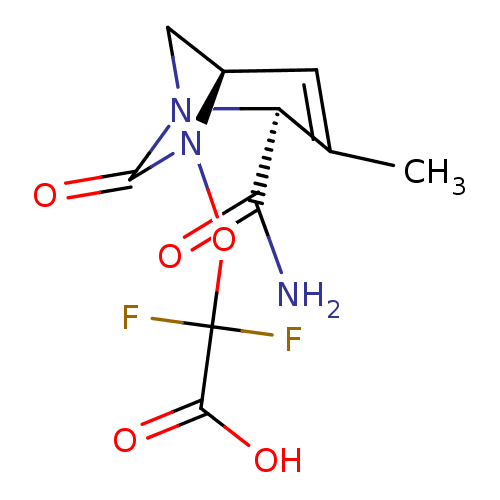 (US10800778, Example 8 | {[(2S,5R)-2-carbamoyl-3-me...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM466971 (2-fluoro-2-(((2S,5R)-3-methyl-7-oxo-2-((pyrazin-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM466985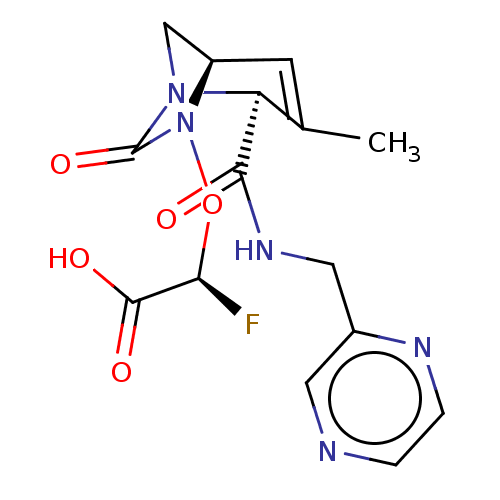 ((2S)-2-fluoro-2-[[(2S,5R)-3-methyl-7-oxo-2-(pyrazi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM466999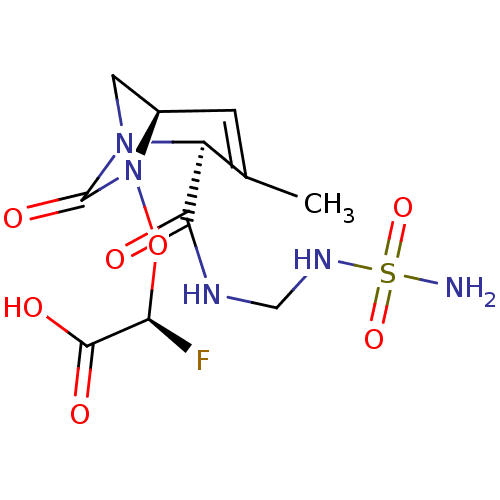 ((2S)-2-fluoro-2-[[(2S,5R)-3-methyl-7-oxo-2-[(sulfa...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM466998 ((2S)-2-fluoro-2-[[(2S,5R)-2-(acetamidomethylcarbam...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM466972 ((2R)-2-(((2S,5R)-2-carbamoyl-4-methyl-7-oxo-1,6-di...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM466974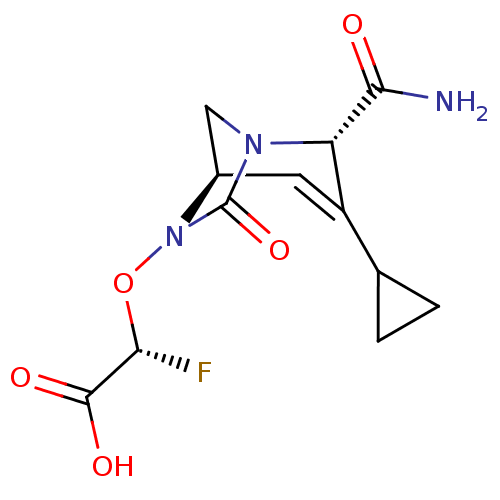 ((2R)-2-(((2S,5R)-2-carbamoyl-3-cyclopropyl-7-oxo-1...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM466979 ((2S)-2-fluoro-2-[[(2S,5R)-3-methyl-7-oxo-2-(2-sulf...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM466987 ((2S)-2-[[(2S,5R)-2-[(3-amino-3-oxo-propyl)carbamoy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM466966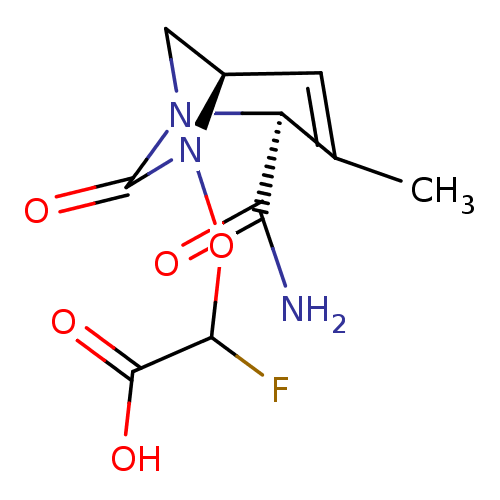 (US10800778, Example 5 | {[(2S,5R)-2-carbamoyl-3-me...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Klebsiella pneumoniae) | BDBM466975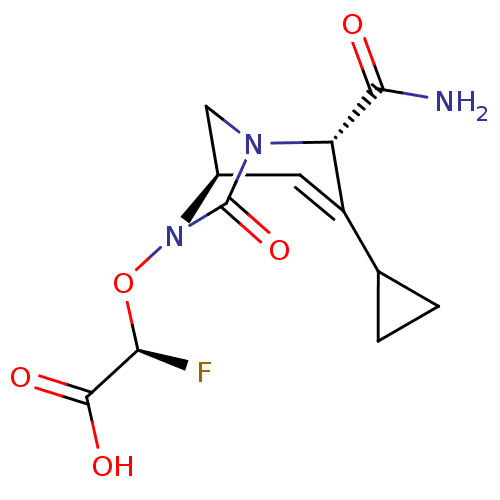 ((2R)-2-(((2S,5R)-2-carbamoyl-3-cyclopropyl-7-oxo-1...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Klebsiella pneumoniae) | BDBM466974 ((2R)-2-(((2S,5R)-2-carbamoyl-3-cyclopropyl-7-oxo-1...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Klebsiella pneumoniae) | BDBM466977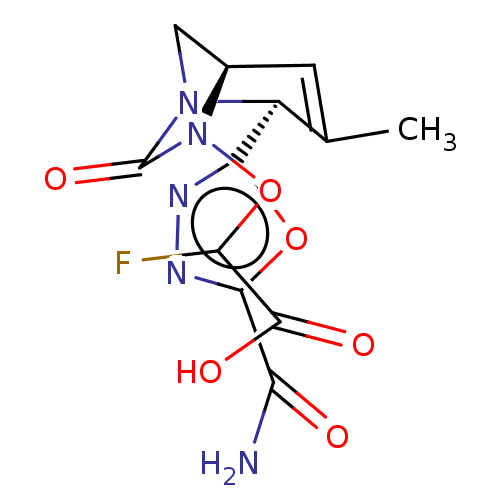 (2-[[(2S,5R)-2-(5-carbamoyl-1,3,4-oxadiazol-2-yl)-3...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM466983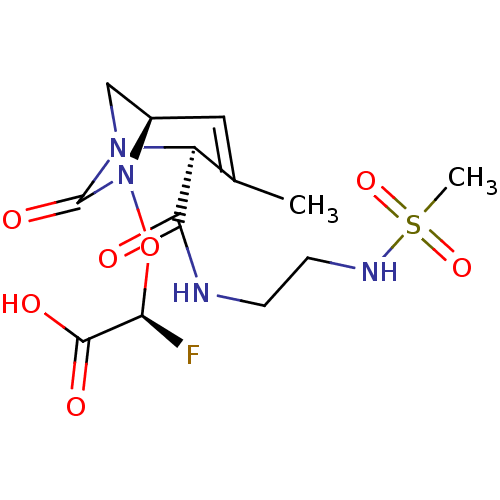 ((2S)-2-fluoro-2-[[(2S,5R)-2-[2-(methanesulfonamido...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM466970 (2-(((2S,5R)-2-(2-acetylhydrazinecarbonyl)-3-methyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM466984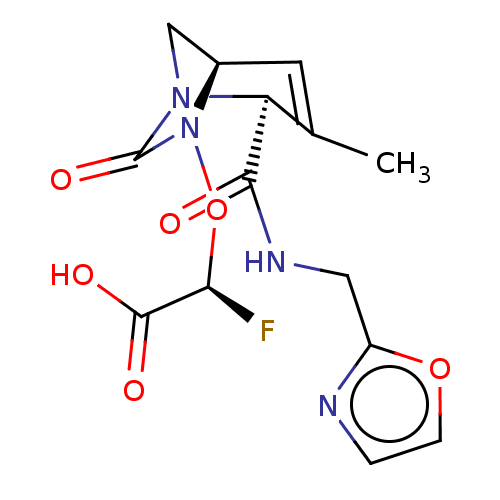 ((2S)-2-fluoro-2-[[(2S,5R)-3-methyl-2-(oxazol-2-ylm...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM466989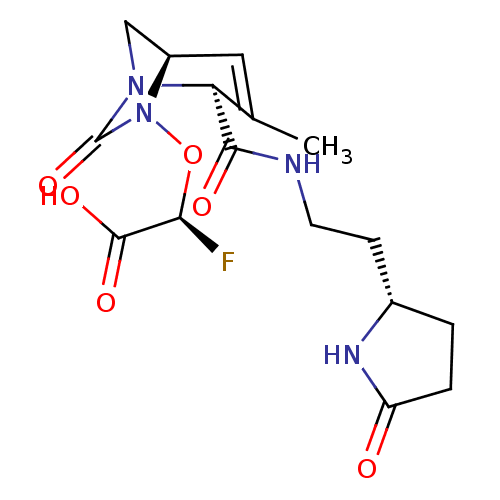 ((2S)-2-fluoro-2-[[(2S,5R)-3-methyl-7-oxo-2-[2-(5-o...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Klebsiella pneumoniae) | BDBM466997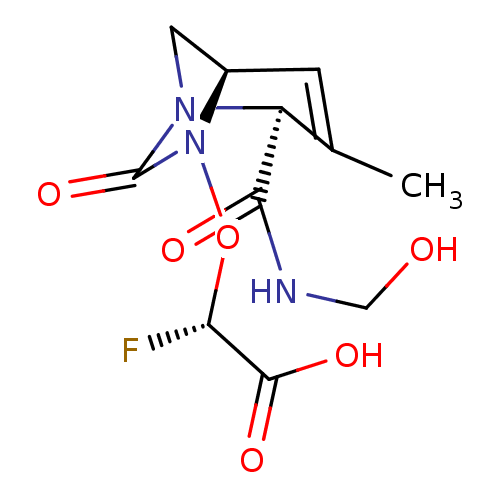 ((2S)-2-fluoro-2-[[(2S,5R)-2-(hydroxymethylcarbamoy...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM466965 ((2R)-{[(2S,5R)-2-carbamoyl-3-methyl-7-oxo-1,6-diaz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM466967 (US10800778, Example 8 | {[(2S,5R)-2-carbamoyl-3-me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Klebsiella pneumoniae) | BDBM466982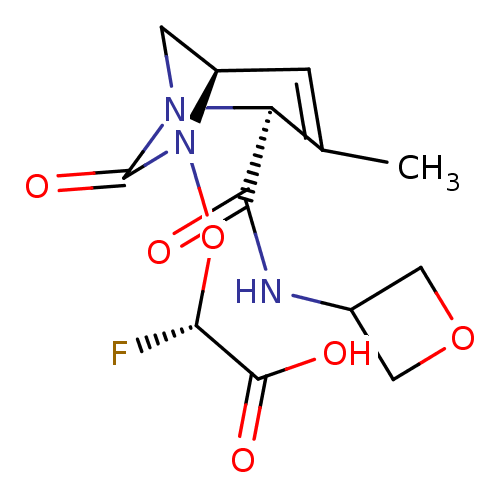 ((2S)-2-fluoro-2-[[(2S,5R)-3-methyl-2-(oxetan-3-ylc...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM466991 ((2S)-2-fluoro-2-[[(2S,5R)-3-methyl-7-oxo-2-[2-(sul...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Klebsiella pneumoniae) | BDBM466967 (US10800778, Example 8 | {[(2S,5R)-2-carbamoyl-3-me...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Entasis Therapeutics Limited US Patent | Assay Description A buffer consisting of 0.1 M sodium phosphate (pH 7.0), 10 mM NaHCO3, and 0.005% Triton X-100 was used for all enzymes. The chromogenic substrate nit... | US Patent US10800778 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 190 total ) | Next | Last >> |