

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sialidase-2 (Homo sapiens (Human)) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PF-IMSS-KEK-SBRC, Ibaraki 305-0801, Japan. Curated by ChEMBL | Assay Description Inhibition of human neuraminidase 2 | J Med Chem 53: 2998-3002 (2010) Article DOI: 10.1021/jm100078r BindingDB Entry DOI: 10.7270/Q2N58NBM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sialidase-2 (Homo sapiens (Human)) | BDBM4706 ((2R,3R,4S)-3-acetamido-4-hydroxy-2-[(1R,2R)-1,2,3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PF-IMSS-KEK-SBRC, Ibaraki 305-0801, Japan. Curated by ChEMBL | Assay Description Inhibition of human neuraminidase 2 | J Med Chem 53: 2998-3002 (2010) Article DOI: 10.1021/jm100078r BindingDB Entry DOI: 10.7270/Q2N58NBM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sialidase-2 (Homo sapiens (Human)) | BDBM5024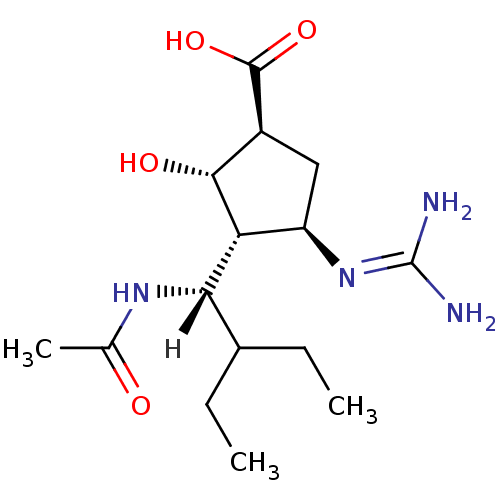 ((-)-(1S,2S,3R,4R)-3-[(1S)-1-(Acetylamino)-2-ethylb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 3.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PF-IMSS-KEK-SBRC, Ibaraki 305-0801, Japan. Curated by ChEMBL | Assay Description Inhibition of human neuraminidase 2 | J Med Chem 53: 2998-3002 (2010) Article DOI: 10.1021/jm100078r BindingDB Entry DOI: 10.7270/Q2N58NBM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sialidase-2 (Homo sapiens (Human)) | BDBM50314987 ((2S,3R,4R)-3-acetamido-4-hydroxy-2-(3-hydroxypropo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PF-IMSS-KEK-SBRC, Ibaraki 305-0801, Japan. Curated by ChEMBL | Assay Description Inhibition of human neuraminidase 2 | J Med Chem 53: 2998-3002 (2010) Article DOI: 10.1021/jm100078r BindingDB Entry DOI: 10.7270/Q2N58NBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase-2 (Homo sapiens (Human)) | BDBM50314988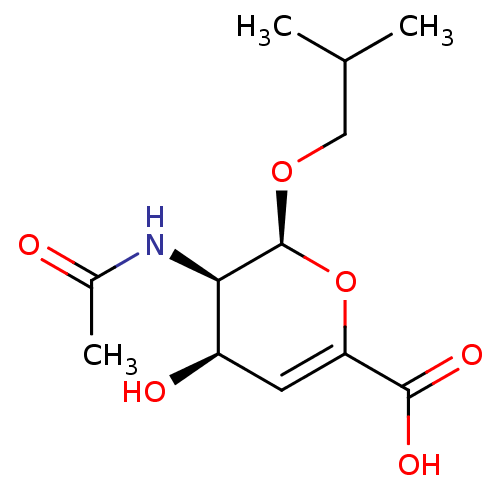 ((2S,3R,4R)-3-acetamido-4-hydroxy-2-isobutoxy-3,4-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PF-IMSS-KEK-SBRC, Ibaraki 305-0801, Japan. Curated by ChEMBL | Assay Description Inhibition of human neuraminidase 2 | J Med Chem 53: 2998-3002 (2010) Article DOI: 10.1021/jm100078r BindingDB Entry DOI: 10.7270/Q2N58NBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase-2 (Homo sapiens (Human)) | BDBM50314986 ((2S,3R,4R)-3-acetamido-2-(2,3-dihydroxypropoxy)-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PF-IMSS-KEK-SBRC, Ibaraki 305-0801, Japan. Curated by ChEMBL | Assay Description Inhibition of human neuraminidase 2 | J Med Chem 53: 2998-3002 (2010) Article DOI: 10.1021/jm100078r BindingDB Entry DOI: 10.7270/Q2N58NBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50497276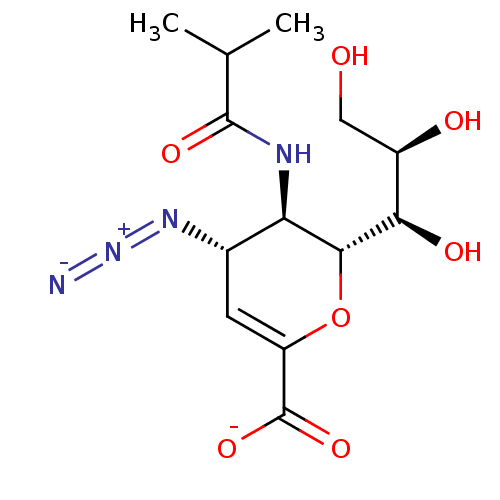 (CHEMBL3330708) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University Curated by ChEMBL | Assay Description Inhibition of neuraminidase activity of human parainfluenza virus 1 C35 hemagglutinin-neuraminidase pre-incubated for 20 mins before MUN substrate by... | J Med Chem 57: 7613-23 (2014) Article DOI: 10.1021/jm500759v BindingDB Entry DOI: 10.7270/Q2DB84VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50497281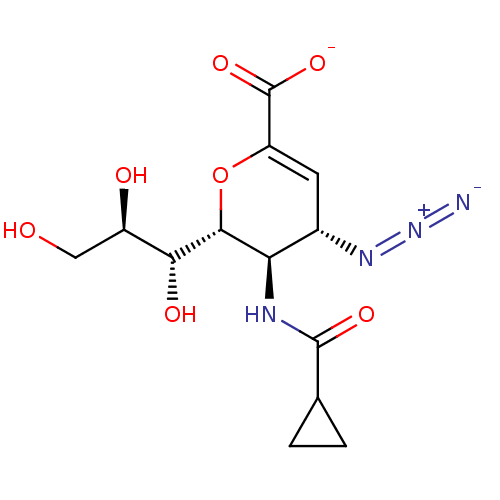 (CHEMBL3330844) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University Curated by ChEMBL | Assay Description Inhibition of neuraminidase activity of human parainfluenza virus 1 C35 hemagglutinin-neuraminidase pre-incubated for 20 mins before MUN substrate by... | J Med Chem 57: 7613-23 (2014) Article DOI: 10.1021/jm500759v BindingDB Entry DOI: 10.7270/Q2DB84VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50497279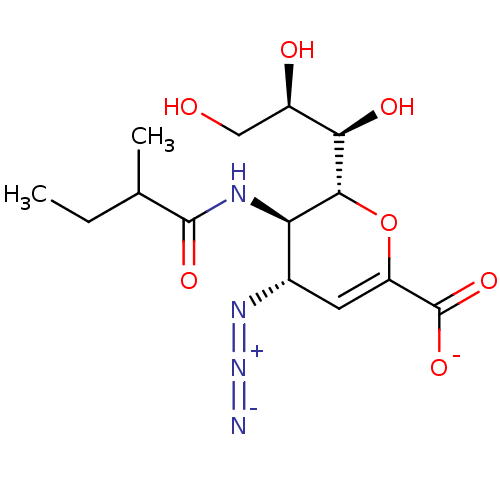 (CHEMBL3330846) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University Curated by ChEMBL | Assay Description Inhibition of neuraminidase activity of human parainfluenza virus 1 C35 hemagglutinin-neuraminidase pre-incubated for 20 mins before MUN substrate by... | J Med Chem 57: 7613-23 (2014) Article DOI: 10.1021/jm500759v BindingDB Entry DOI: 10.7270/Q2DB84VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50497280 (CHEMBL3330845) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University Curated by ChEMBL | Assay Description Inhibition of neuraminidase activity of human parainfluenza virus 1 C35 hemagglutinin-neuraminidase pre-incubated for 20 mins before MUN substrate by... | J Med Chem 57: 7613-23 (2014) Article DOI: 10.1021/jm500759v BindingDB Entry DOI: 10.7270/Q2DB84VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50497275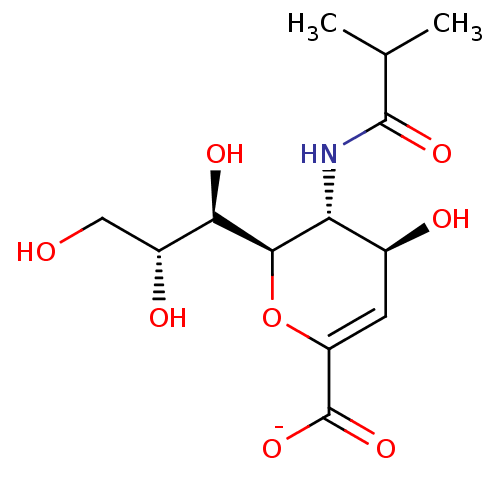 (CHEMBL3330709) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University Curated by ChEMBL | Assay Description Inhibition of neuraminidase activity of human parainfluenza virus 1 C35 hemagglutinin-neuraminidase pre-incubated for 20 mins before MUN substrate by... | J Med Chem 57: 7613-23 (2014) Article DOI: 10.1021/jm500759v BindingDB Entry DOI: 10.7270/Q2DB84VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50497285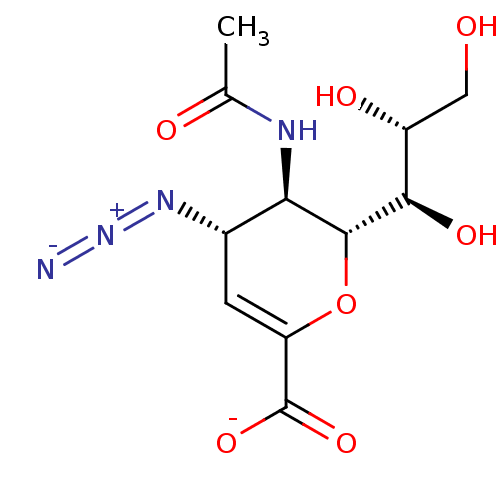 (CHEMBL3330704) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University Curated by ChEMBL | Assay Description Inhibition of neuraminidase activity of human parainfluenza virus 1 C35 hemagglutinin-neuraminidase pre-incubated for 20 mins before MUN substrate by... | J Med Chem 57: 7613-23 (2014) Article DOI: 10.1021/jm500759v BindingDB Entry DOI: 10.7270/Q2DB84VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50497278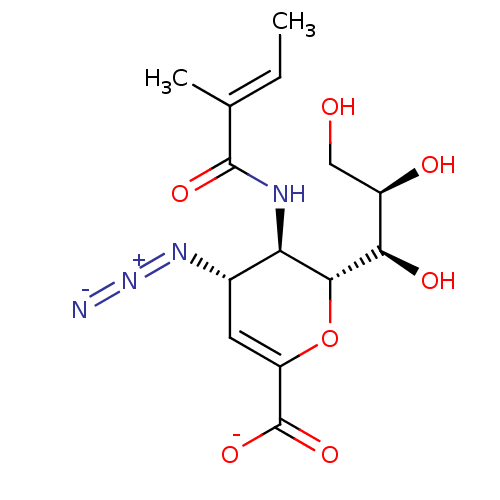 (CHEMBL3330847) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University Curated by ChEMBL | Assay Description Inhibition of neuraminidase activity of human parainfluenza virus 1 C35 hemagglutinin-neuraminidase pre-incubated for 20 mins before MUN substrate by... | J Med Chem 57: 7613-23 (2014) Article DOI: 10.1021/jm500759v BindingDB Entry DOI: 10.7270/Q2DB84VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50497277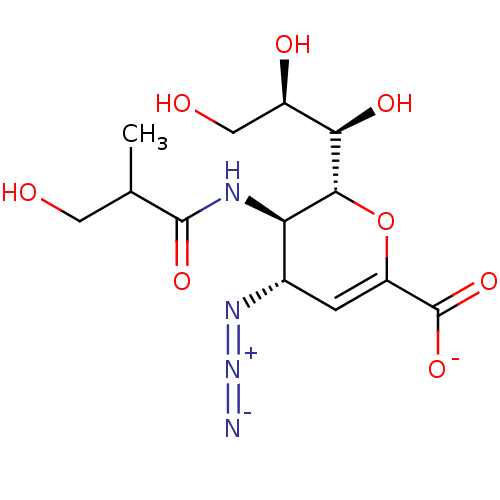 (CHEMBL3330849) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University Curated by ChEMBL | Assay Description Inhibition of neuraminidase activity of human parainfluenza virus 1 C35 hemagglutinin-neuraminidase pre-incubated for 20 mins before MUN substrate by... | J Med Chem 57: 7613-23 (2014) Article DOI: 10.1021/jm500759v BindingDB Entry DOI: 10.7270/Q2DB84VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50497283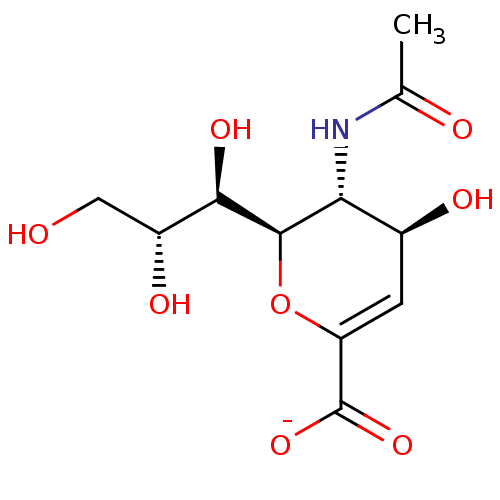 (CHEMBL3330705) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University Curated by ChEMBL | Assay Description Inhibition of neuraminidase activity of human parainfluenza virus 1 C35 hemagglutinin-neuraminidase pre-incubated for 20 mins before MUN substrate by... | J Med Chem 57: 7613-23 (2014) Article DOI: 10.1021/jm500759v BindingDB Entry DOI: 10.7270/Q2DB84VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50497282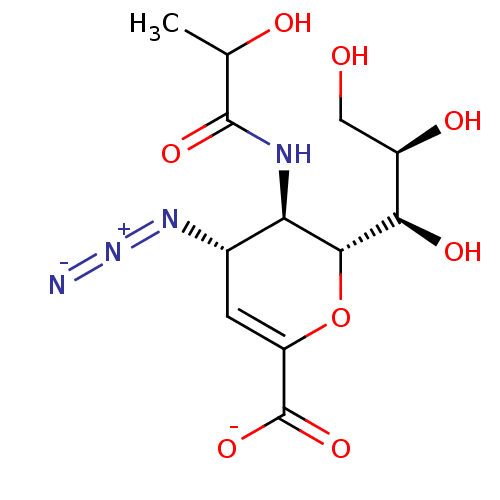 (CHEMBL3330848) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University Curated by ChEMBL | Assay Description Inhibition of neuraminidase activity of human parainfluenza virus 1 C35 hemagglutinin-neuraminidase pre-incubated for 20 mins before MUN substrate by... | J Med Chem 57: 7613-23 (2014) Article DOI: 10.1021/jm500759v BindingDB Entry DOI: 10.7270/Q2DB84VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50497286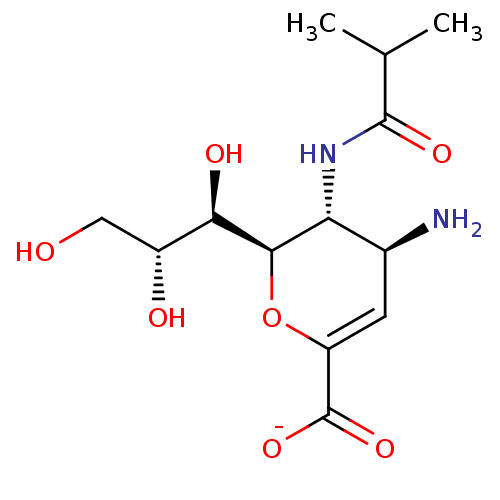 (CHEMBL3330710) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.61E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University Curated by ChEMBL | Assay Description Inhibition of neuraminidase activity of human parainfluenza virus 1 C35 hemagglutinin-neuraminidase pre-incubated for 20 mins before MUN substrate by... | J Med Chem 57: 7613-23 (2014) Article DOI: 10.1021/jm500759v BindingDB Entry DOI: 10.7270/Q2DB84VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50497284 (CHEMBL3330843) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University Curated by ChEMBL | Assay Description Inhibition of neuraminidase activity of human parainfluenza virus 1 C35 hemagglutinin-neuraminidase pre-incubated for 20 mins before MUN substrate by... | J Med Chem 57: 7613-23 (2014) Article DOI: 10.1021/jm500759v BindingDB Entry DOI: 10.7270/Q2DB84VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50497274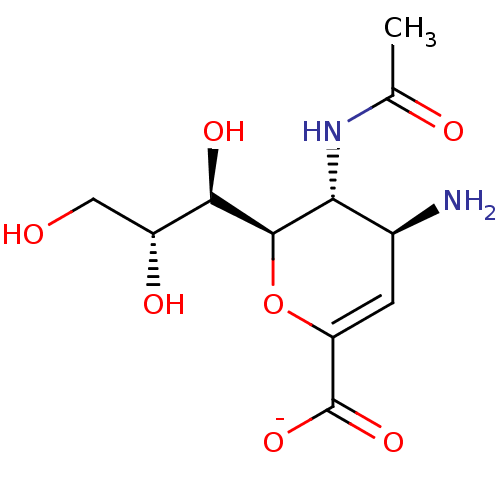 (CHEMBL3330706) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University Curated by ChEMBL | Assay Description Inhibition of neuraminidase activity of human parainfluenza virus 1 C35 hemagglutinin-neuraminidase pre-incubated for 20 mins before MUN substrate by... | J Med Chem 57: 7613-23 (2014) Article DOI: 10.1021/jm500759v BindingDB Entry DOI: 10.7270/Q2DB84VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50497287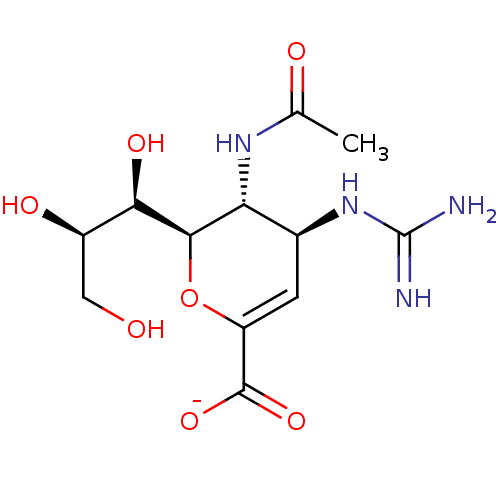 (CHEMBL3330707) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University Curated by ChEMBL | Assay Description Inhibition of neuraminidase activity of human parainfluenza virus 1 C35 hemagglutinin-neuraminidase pre-incubated for 20 mins before MUN substrate by... | J Med Chem 57: 7613-23 (2014) Article DOI: 10.1021/jm500759v BindingDB Entry DOI: 10.7270/Q2DB84VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||