

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50296131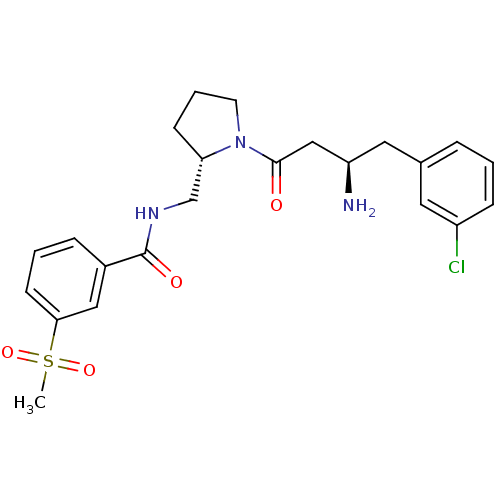 (CHEMBL564249 | N-(((S)-1-((R)-3-amino-4-(3-chlorop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents | MMDB PDB Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Santhera Pharmaceuticals (Switzerland) Ltd Curated by ChEMBL | Assay Description Inhibition of DPP4 by continuous fluorimetric assay | Bioorg Med Chem Lett 19: 4201-3 (2009) Article DOI: 10.1016/j.bmcl.2009.05.109 BindingDB Entry DOI: 10.7270/Q24749XM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50302961 ((R)-3-amino-1-((S)-2-(3-(1-(methylsulfonyl)azetidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Santhera Pharmaceuticals (Switzerland) Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP4 transfected in Pichia methanolica expression system after 10 mins by continuous fluorimetric assay | Bioorg Med Chem Lett 19: 6340-5 (2009) Article DOI: 10.1016/j.bmcl.2009.09.078 BindingDB Entry DOI: 10.7270/Q2WS8TB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50296761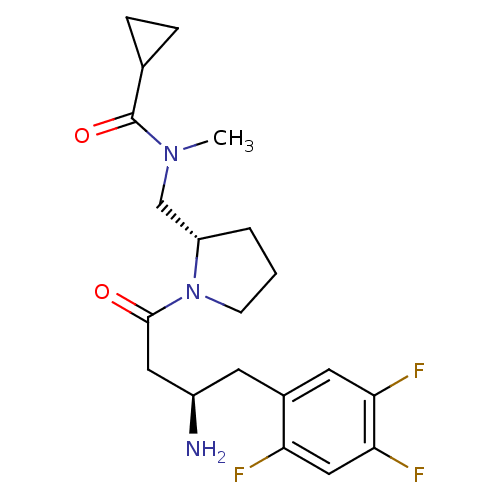 (CHEMBL564105 | N-(((S)-1-((R)-3-amino-4-(2,4,5-tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Santhera Pharmaceuticals (Switzerland) Ltd Curated by ChEMBL | Assay Description Inhibition of DPP4 | Bioorg Med Chem Lett 19: 4818-23 (2009) Article DOI: 10.1016/j.bmcl.2009.06.036 BindingDB Entry DOI: 10.7270/Q20865B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50296768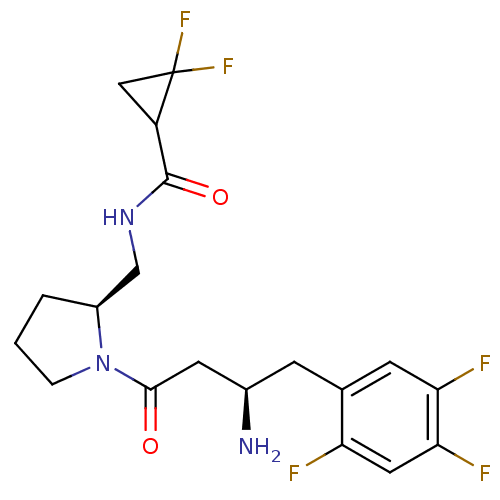 (CHEMBL554908 | N-(((S)-1-((R)-3-amino-4-(2,4,5-tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Santhera Pharmaceuticals (Switzerland) Ltd Curated by ChEMBL | Assay Description Inhibition of DPP4 | Bioorg Med Chem Lett 19: 4818-23 (2009) Article DOI: 10.1016/j.bmcl.2009.06.036 BindingDB Entry DOI: 10.7270/Q20865B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50296755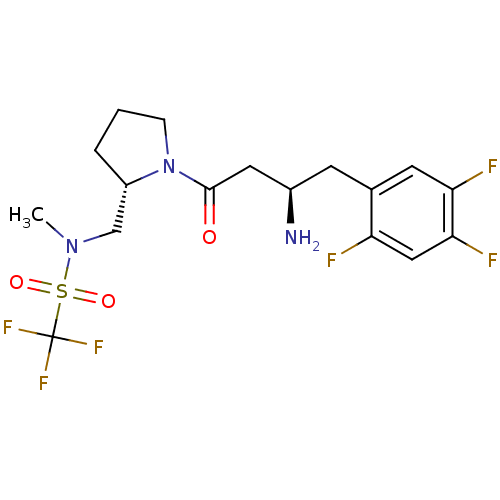 (CHEMBL558452 | N-(((S)-1-((R)-3-amino-4-(2,4,5-tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Santhera Pharmaceuticals (Switzerland) Ltd Curated by ChEMBL | Assay Description Inhibition of DPP4 | Bioorg Med Chem Lett 19: 4818-23 (2009) Article DOI: 10.1016/j.bmcl.2009.06.036 BindingDB Entry DOI: 10.7270/Q20865B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50302972 ((R)-3-amino-1-((S)-2-(5-(3-methyloxetan-3-yl)-1,2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Santhera Pharmaceuticals (Switzerland) Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP4 transfected in Pichia methanolica expression system after 10 mins by continuous fluorimetric assay | Bioorg Med Chem Lett 19: 6340-5 (2009) Article DOI: 10.1016/j.bmcl.2009.09.078 BindingDB Entry DOI: 10.7270/Q2WS8TB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50302958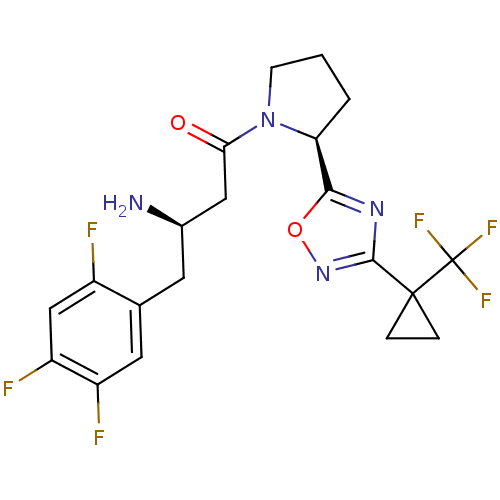 ((R)-3-amino-1-((S)-2-(3-(1-(trifluoromethyl)cyclop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Santhera Pharmaceuticals (Switzerland) Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP4 transfected in Pichia methanolica expression system after 10 mins by continuous fluorimetric assay | Bioorg Med Chem Lett 19: 6340-5 (2009) Article DOI: 10.1016/j.bmcl.2009.09.078 BindingDB Entry DOI: 10.7270/Q2WS8TB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50302964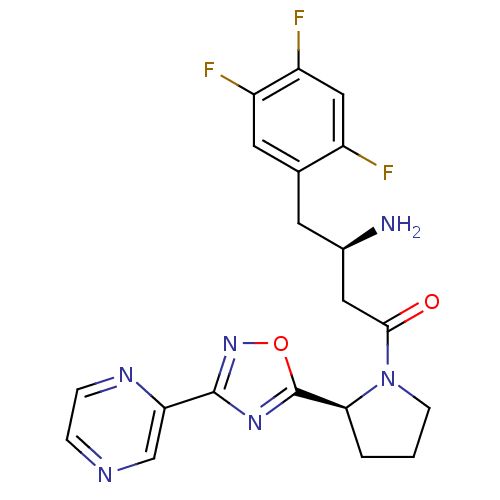 ((R)-3-amino-1-((S)-2-(3-(pyrazin-2-yl)-1,2,4-oxadi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Santhera Pharmaceuticals (Switzerland) Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP4 transfected in Pichia methanolica expression system after 10 mins by continuous fluorimetric assay | Bioorg Med Chem Lett 19: 6340-5 (2009) Article DOI: 10.1016/j.bmcl.2009.09.078 BindingDB Entry DOI: 10.7270/Q2WS8TB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50302956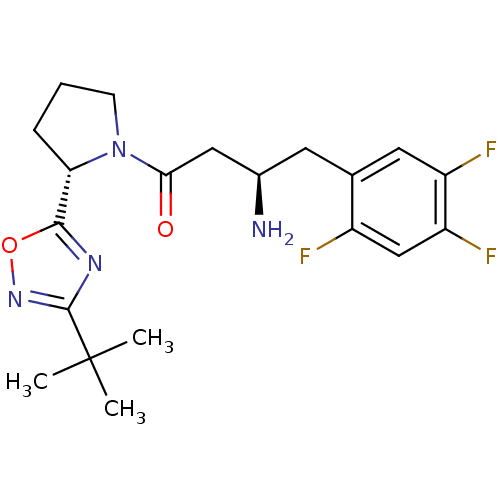 ((R)-3-amino-1-((S)-2-(3-tert-butyl-1,2,4-oxadiazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Santhera Pharmaceuticals (Switzerland) Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP4 transfected in Pichia methanolica expression system after 10 mins by continuous fluorimetric assay | Bioorg Med Chem Lett 19: 6340-5 (2009) Article DOI: 10.1016/j.bmcl.2009.09.078 BindingDB Entry DOI: 10.7270/Q2WS8TB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50296773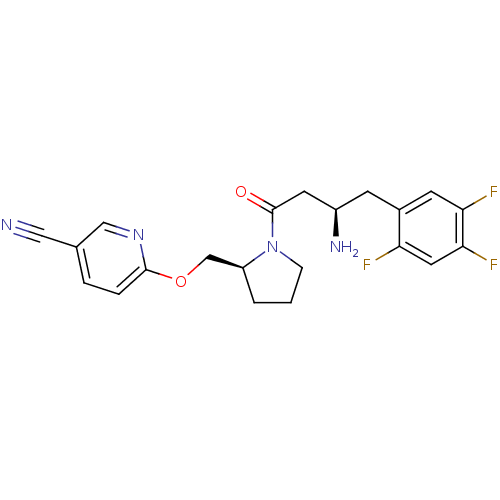 (6-(((S)-1-((R)-3-amino-4-(2,4,5-trifluorophenyl)bu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Santhera Pharmaceuticals (Switzerland) Ltd Curated by ChEMBL | Assay Description Inhibition of DPP4 | Bioorg Med Chem Lett 19: 4818-23 (2009) Article DOI: 10.1016/j.bmcl.2009.06.036 BindingDB Entry DOI: 10.7270/Q20865B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50296767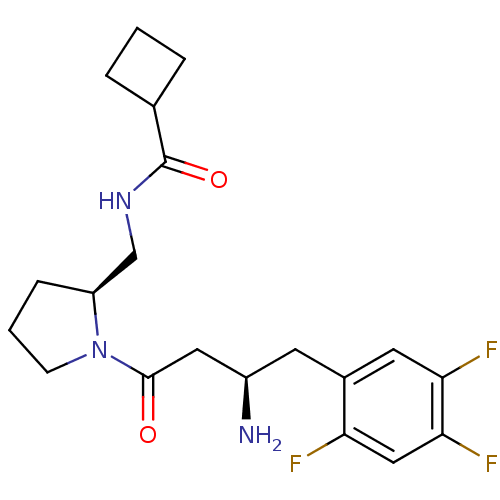 (CHEMBL563901 | N-(((S)-1-((R)-3-amino-4-(2,4,5-tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Santhera Pharmaceuticals (Switzerland) Ltd Curated by ChEMBL | Assay Description Inhibition of DPP4 | Bioorg Med Chem Lett 19: 4818-23 (2009) Article DOI: 10.1016/j.bmcl.2009.06.036 BindingDB Entry DOI: 10.7270/Q20865B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50296772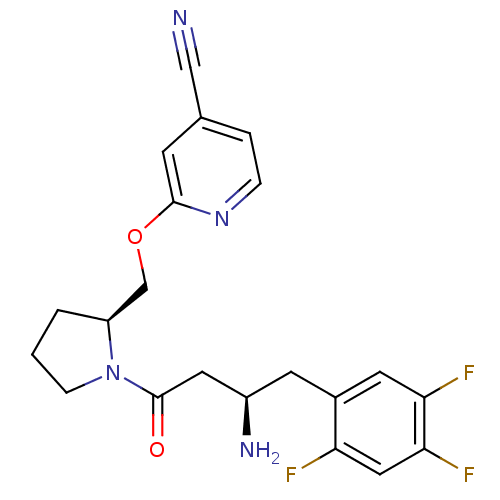 (2-(((S)-1-((R)-3-amino-4-(2,4,5-trifluorophenyl)bu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Santhera Pharmaceuticals (Switzerland) Ltd Curated by ChEMBL | Assay Description Inhibition of DPP4 | Bioorg Med Chem Lett 19: 4818-23 (2009) Article DOI: 10.1016/j.bmcl.2009.06.036 BindingDB Entry DOI: 10.7270/Q20865B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492164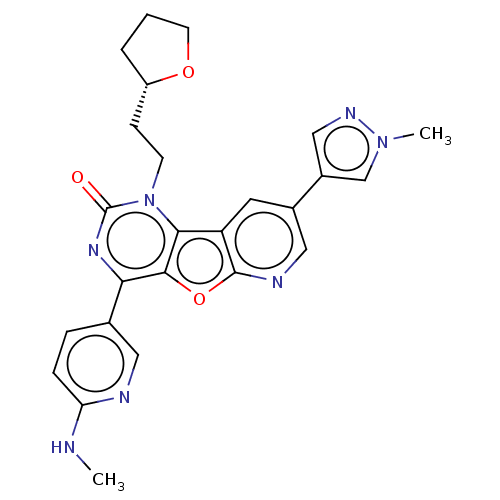 (CHEMBL2381561) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492156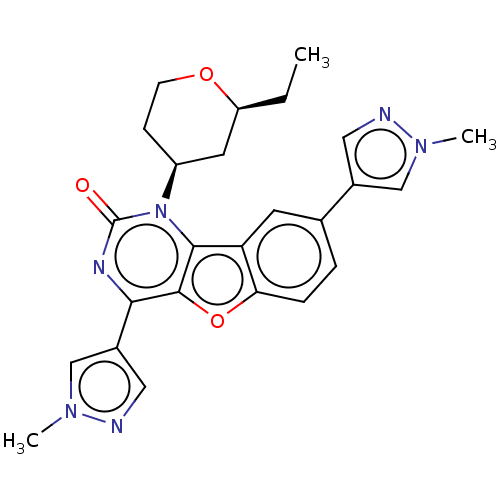 (CHEMBL2397560) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50296754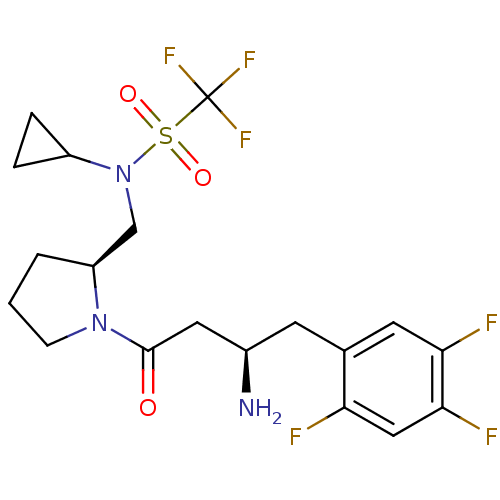 (CHEMBL558647 | N-(((S)-1-((R)-3-amino-4-(2,4,5-tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Santhera Pharmaceuticals (Switzerland) Ltd Curated by ChEMBL | Assay Description Inhibition of DPP4 | Bioorg Med Chem Lett 19: 4818-23 (2009) Article DOI: 10.1016/j.bmcl.2009.06.036 BindingDB Entry DOI: 10.7270/Q20865B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50296769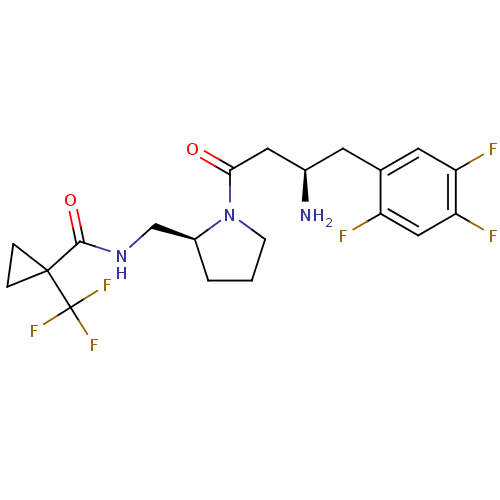 (CHEMBL552069 | N-(((S)-1-((R)-3-amino-4-(2,4,5-tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Santhera Pharmaceuticals (Switzerland) Ltd Curated by ChEMBL | Assay Description Inhibition of DPP4 | Bioorg Med Chem Lett 19: 4818-23 (2009) Article DOI: 10.1016/j.bmcl.2009.06.036 BindingDB Entry DOI: 10.7270/Q20865B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492155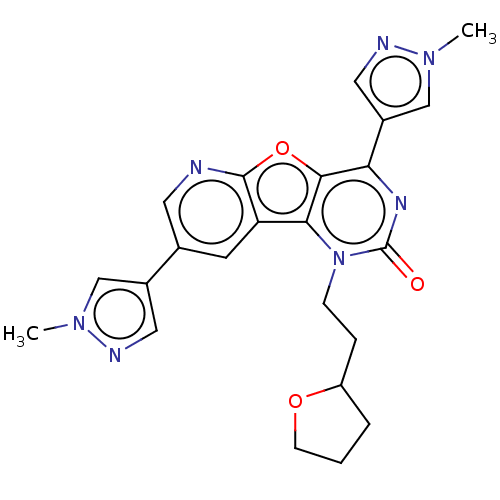 (CHEMBL2397554) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50302966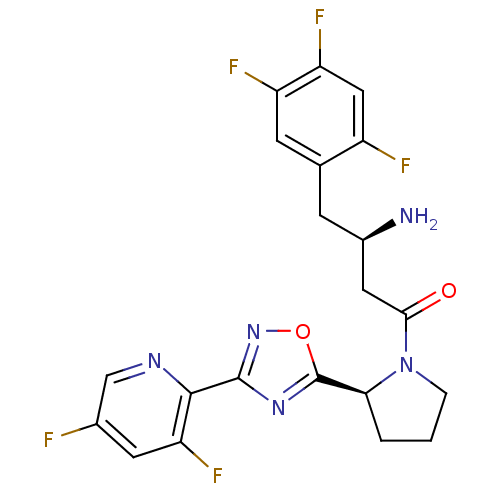 ((R)-3-amino-1-((S)-2-(3-(3,5-difluoropyridin-2-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Santhera Pharmaceuticals (Switzerland) Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP4 transfected in Pichia methanolica expression system after 10 mins by continuous fluorimetric assay | Bioorg Med Chem Lett 19: 6340-5 (2009) Article DOI: 10.1016/j.bmcl.2009.09.078 BindingDB Entry DOI: 10.7270/Q2WS8TB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492176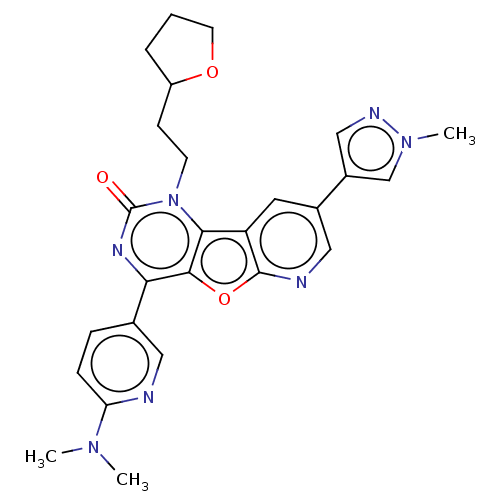 (CHEMBL2397567) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50302957 ((R)-3-amino-1-((S)-2-(3-(cyclopropylmethyl)-1,2,4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Santhera Pharmaceuticals (Switzerland) Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP4 transfected in Pichia methanolica expression system after 10 mins by continuous fluorimetric assay | Bioorg Med Chem Lett 19: 6340-5 (2009) Article DOI: 10.1016/j.bmcl.2009.09.078 BindingDB Entry DOI: 10.7270/Q2WS8TB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492175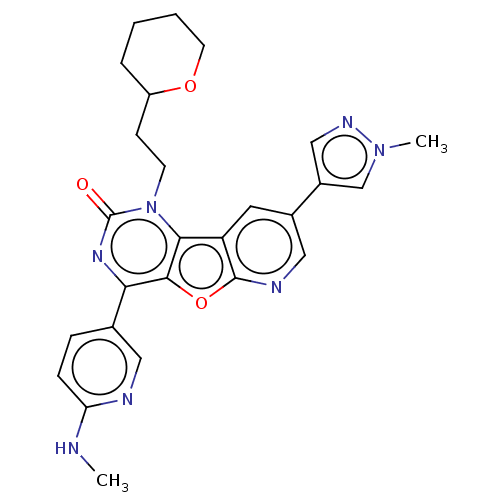 (CHEMBL2397583) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50296779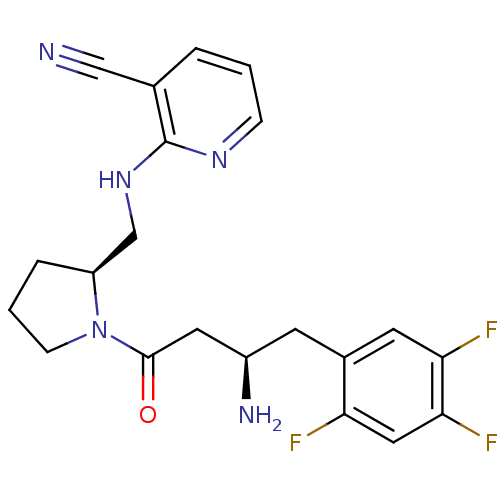 (2-(((S)-1-((R)-3-amino-4-(2,4,5-trifluorophenyl)bu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Santhera Pharmaceuticals (Switzerland) Ltd Curated by ChEMBL | Assay Description Inhibition of DPP4 | Bioorg Med Chem Lett 19: 4818-23 (2009) Article DOI: 10.1016/j.bmcl.2009.06.036 BindingDB Entry DOI: 10.7270/Q20865B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492163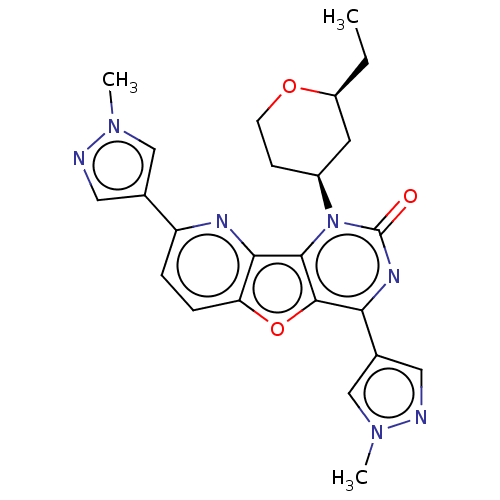 (CHEMBL2397557) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50296762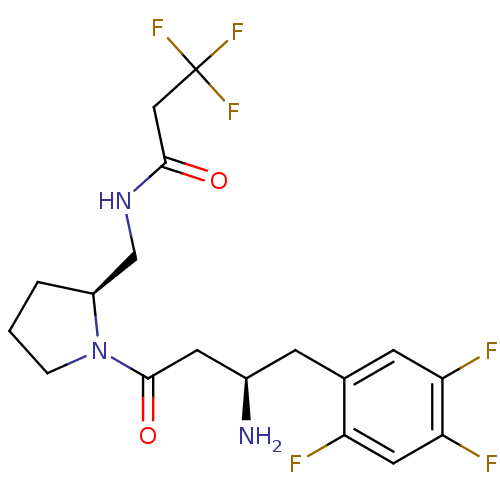 (CHEMBL550114 | N-(((S)-1-((R)-3-amino-4-(2,4,5-tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Santhera Pharmaceuticals (Switzerland) Ltd Curated by ChEMBL | Assay Description Inhibition of DPP4 | Bioorg Med Chem Lett 19: 4818-23 (2009) Article DOI: 10.1016/j.bmcl.2009.06.036 BindingDB Entry DOI: 10.7270/Q20865B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492154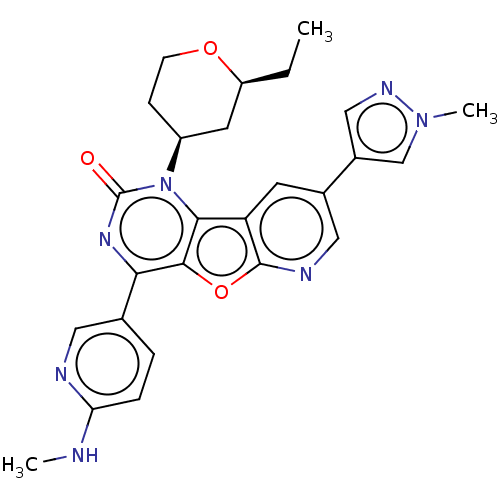 (CHEMBL2397572) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50296781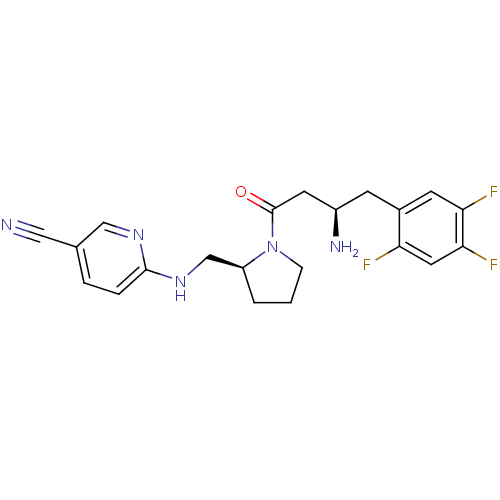 (6-(((S)-1-((R)-3-amino-4-(2,4,5-trifluorophenyl)bu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Santhera Pharmaceuticals (Switzerland) Ltd Curated by ChEMBL | Assay Description Inhibition of DPP4 | Bioorg Med Chem Lett 19: 4818-23 (2009) Article DOI: 10.1016/j.bmcl.2009.06.036 BindingDB Entry DOI: 10.7270/Q20865B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50296765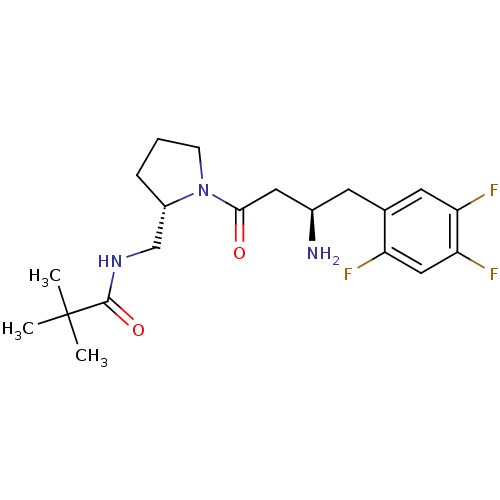 (CHEMBL558322 | N-(((S)-1-((R)-3-amino-4-(2,4,5-tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Santhera Pharmaceuticals (Switzerland) Ltd Curated by ChEMBL | Assay Description Inhibition of DPP4 | Bioorg Med Chem Lett 19: 4818-23 (2009) Article DOI: 10.1016/j.bmcl.2009.06.036 BindingDB Entry DOI: 10.7270/Q20865B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50296774 ((R)-3-amino-1-((S)-2-((4-(trifluoromethyl)pyrimidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Santhera Pharmaceuticals (Switzerland) Ltd Curated by ChEMBL | Assay Description Inhibition of DPP4 | Bioorg Med Chem Lett 19: 4818-23 (2009) Article DOI: 10.1016/j.bmcl.2009.06.036 BindingDB Entry DOI: 10.7270/Q20865B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492168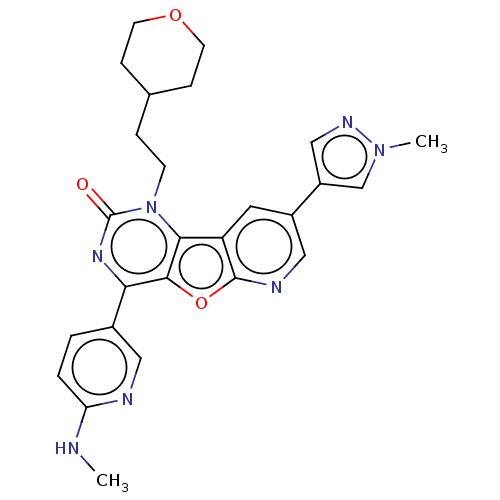 (CHEMBL2397584) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50302959 ((R)-3-amino-1-((S)-2-(3-morpholino-1,2,4-oxadiazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Santhera Pharmaceuticals (Switzerland) Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP4 transfected in Pichia methanolica expression system after 10 mins by continuous fluorimetric assay | Bioorg Med Chem Lett 19: 6340-5 (2009) Article DOI: 10.1016/j.bmcl.2009.09.078 BindingDB Entry DOI: 10.7270/Q2WS8TB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50302960 ((R)-3-amino-1-((S)-2-(3-(pyrrolidin-1-yl)-1,2,4-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Santhera Pharmaceuticals (Switzerland) Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP4 transfected in Pichia methanolica expression system after 10 mins by continuous fluorimetric assay | Bioorg Med Chem Lett 19: 6340-5 (2009) Article DOI: 10.1016/j.bmcl.2009.09.078 BindingDB Entry DOI: 10.7270/Q2WS8TB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50302965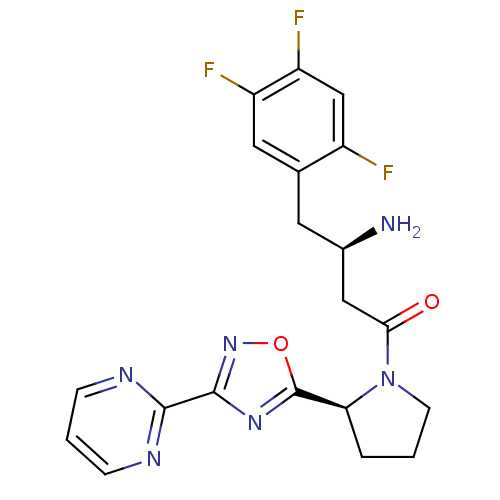 ((R)-3-amino-1-((S)-2-(3-(pyrimidin-2-yl)-1,2,4-oxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Santhera Pharmaceuticals (Switzerland) Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP4 transfected in Pichia methanolica expression system after 10 mins by continuous fluorimetric assay | Bioorg Med Chem Lett 19: 6340-5 (2009) Article DOI: 10.1016/j.bmcl.2009.09.078 BindingDB Entry DOI: 10.7270/Q2WS8TB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50296780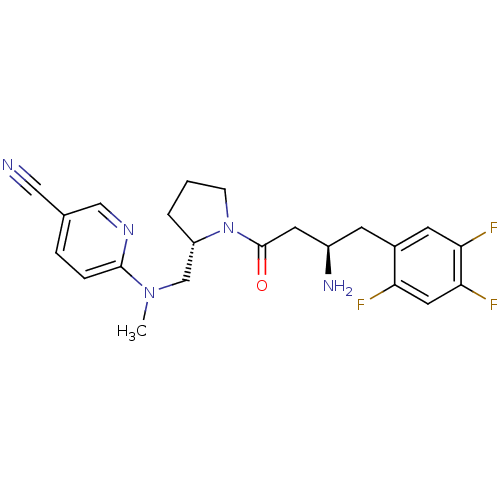 (6-((((S)-1-((R)-3-amino-4-(2,4,5-trifluorophenyl)b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Santhera Pharmaceuticals (Switzerland) Ltd Curated by ChEMBL | Assay Description Inhibition of DPP4 | Bioorg Med Chem Lett 19: 4818-23 (2009) Article DOI: 10.1016/j.bmcl.2009.06.036 BindingDB Entry DOI: 10.7270/Q20865B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492153 (CHEMBL2397563) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50296766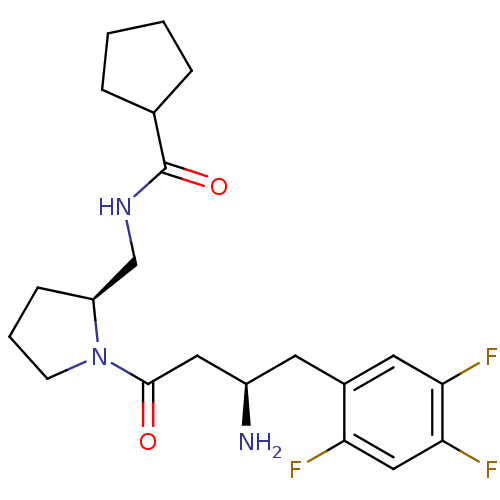 (CHEMBL557723 | N-(((S)-1-((R)-3-amino-4-(2,4,5-tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Santhera Pharmaceuticals (Switzerland) Ltd Curated by ChEMBL | Assay Description Inhibition of DPP4 | Bioorg Med Chem Lett 19: 4818-23 (2009) Article DOI: 10.1016/j.bmcl.2009.06.036 BindingDB Entry DOI: 10.7270/Q20865B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492174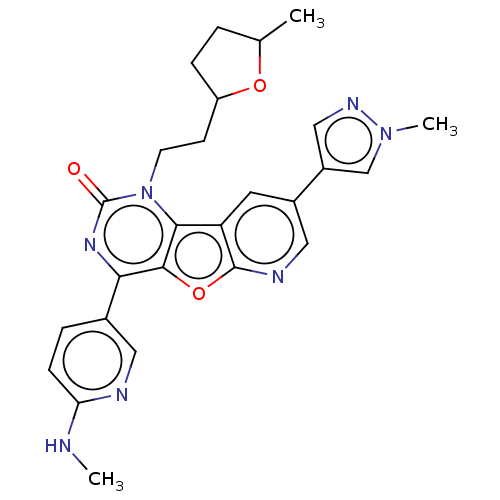 (CHEMBL2397586) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50302962 ((R)-1-((S)-2-(3-(1-acetylazetidin-3-yl)-1,2,4-oxad...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Santhera Pharmaceuticals (Switzerland) Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP4 transfected in Pichia methanolica expression system after 10 mins by continuous fluorimetric assay | Bioorg Med Chem Lett 19: 6340-5 (2009) Article DOI: 10.1016/j.bmcl.2009.09.078 BindingDB Entry DOI: 10.7270/Q2WS8TB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492169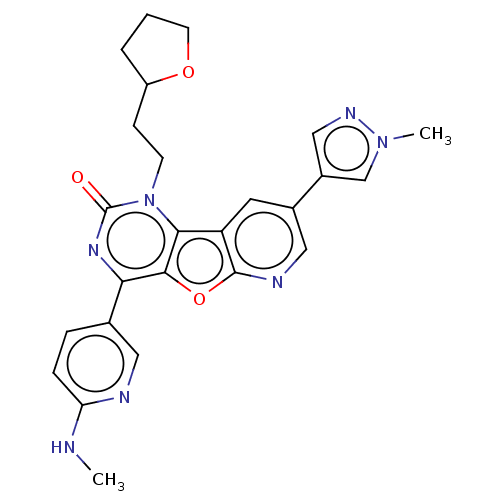 (CHEMBL2397582) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492179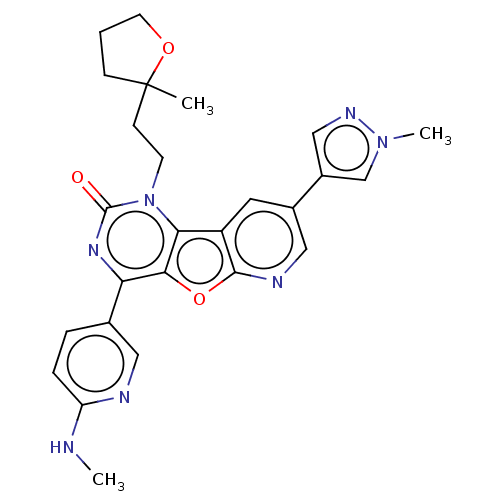 (CHEMBL2397587) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492152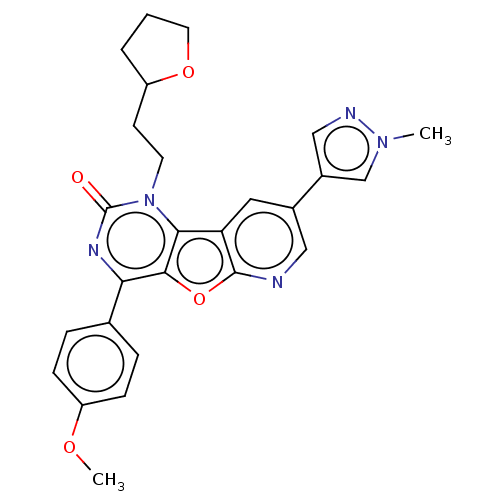 (CHEMBL2397565) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50296776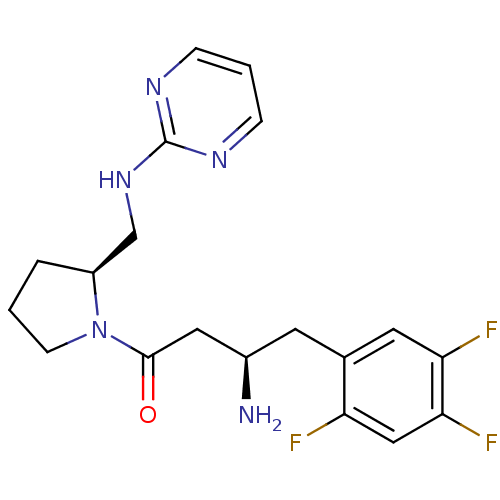 ((R)-3-amino-1-((S)-2-((pyrimidin-2-ylamino)methyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Santhera Pharmaceuticals (Switzerland) Ltd Curated by ChEMBL | Assay Description Inhibition of DPP4 | Bioorg Med Chem Lett 19: 4818-23 (2009) Article DOI: 10.1016/j.bmcl.2009.06.036 BindingDB Entry DOI: 10.7270/Q20865B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492151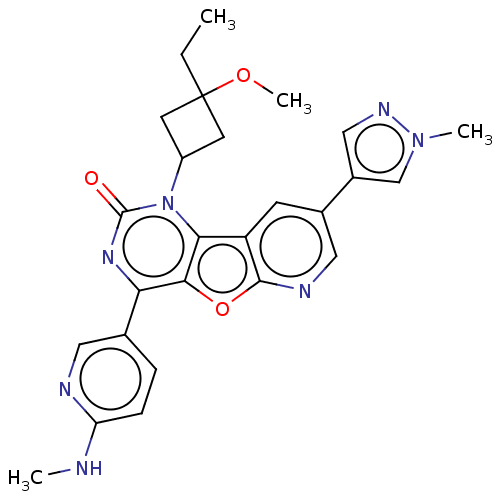 (CHEMBL2397588) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50296756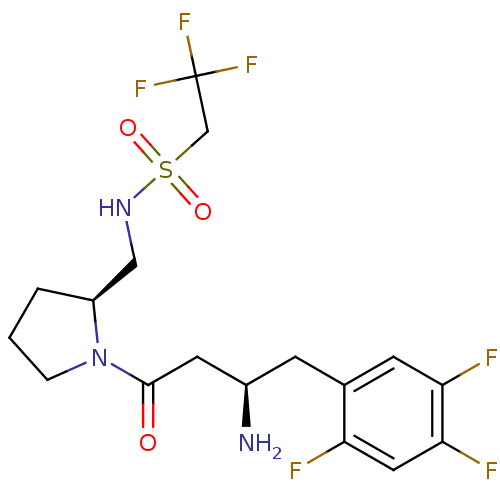 (CHEMBL559602 | N-(((S)-1-((R)-3-amino-4-(2,4,5-tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Santhera Pharmaceuticals (Switzerland) Ltd Curated by ChEMBL | Assay Description Inhibition of DPP4 | Bioorg Med Chem Lett 19: 4818-23 (2009) Article DOI: 10.1016/j.bmcl.2009.06.036 BindingDB Entry DOI: 10.7270/Q20865B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492162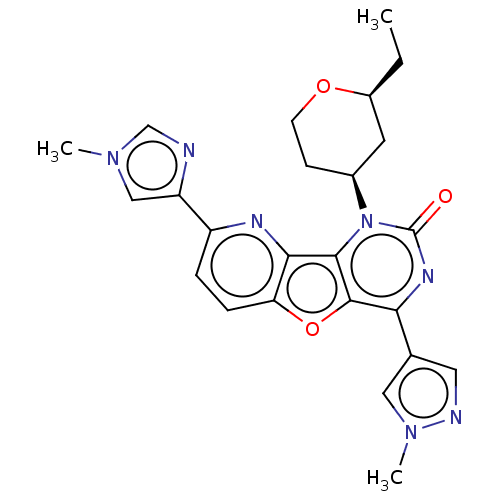 (CHEMBL2397549) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492158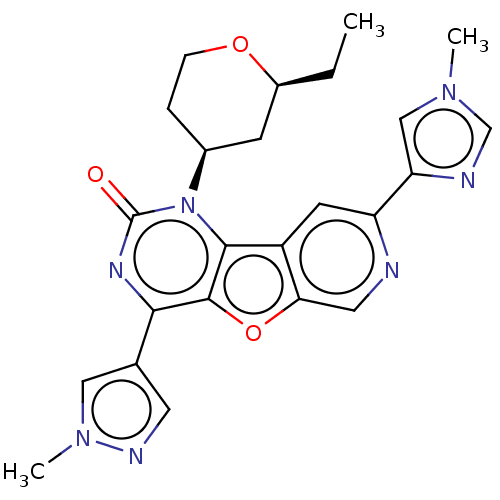 (CHEMBL2397548) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492173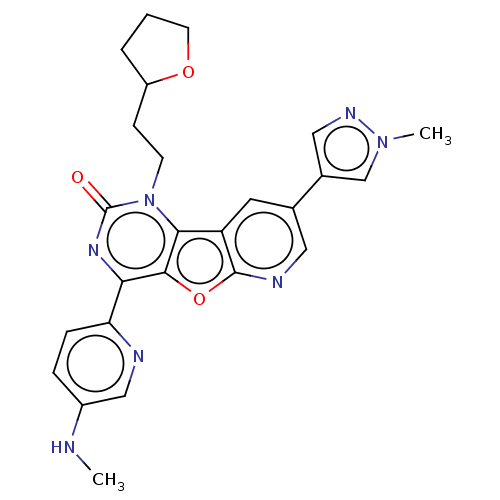 (CHEMBL2397573) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50296763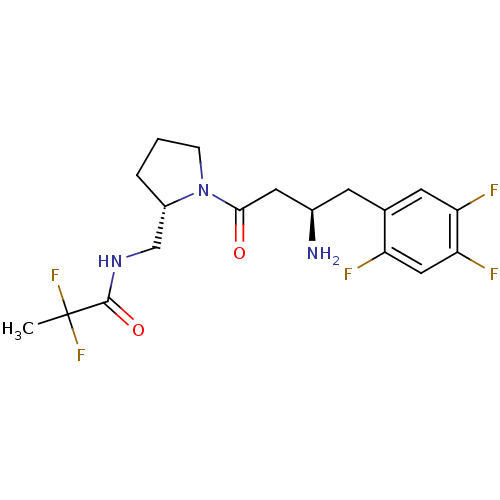 (CHEMBL557860 | N-(((S)-1-((R)-3-amino-4-(2,4,5-tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Santhera Pharmaceuticals (Switzerland) Ltd Curated by ChEMBL | Assay Description Inhibition of DPP4 | Bioorg Med Chem Lett 19: 4818-23 (2009) Article DOI: 10.1016/j.bmcl.2009.06.036 BindingDB Entry DOI: 10.7270/Q20865B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492150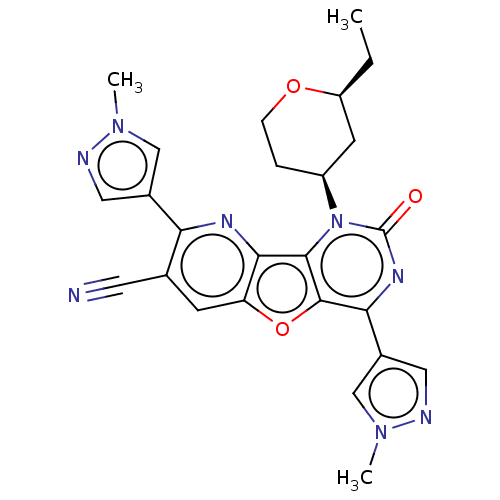 (CHEMBL2397559) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50296771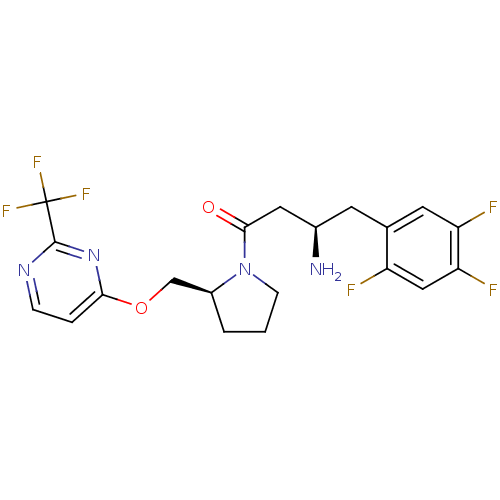 ((R)-3-amino-1-((S)-2-((2-(trifluoromethyl)pyrimidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Santhera Pharmaceuticals (Switzerland) Ltd Curated by ChEMBL | Assay Description Inhibition of DPP4 | Bioorg Med Chem Lett 19: 4818-23 (2009) Article DOI: 10.1016/j.bmcl.2009.06.036 BindingDB Entry DOI: 10.7270/Q20865B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50296778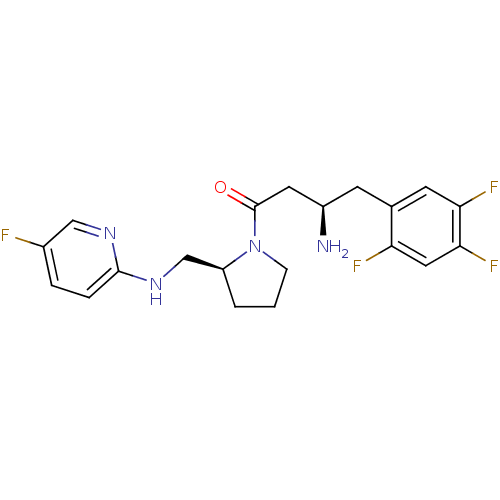 ((R)-3-amino-1-((S)-2-((5-fluoropyridin-2-ylamino)m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Santhera Pharmaceuticals (Switzerland) Ltd Curated by ChEMBL | Assay Description Inhibition of DPP4 | Bioorg Med Chem Lett 19: 4818-23 (2009) Article DOI: 10.1016/j.bmcl.2009.06.036 BindingDB Entry DOI: 10.7270/Q20865B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 384 total ) | Next | Last >> |