 Found 28 hits with Last Name = 'engman' and Initial = 'l'
Found 28 hits with Last Name = 'engman' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Thioredoxin reductase
(Escherichia coli (strain K12)) | BDBM50385299
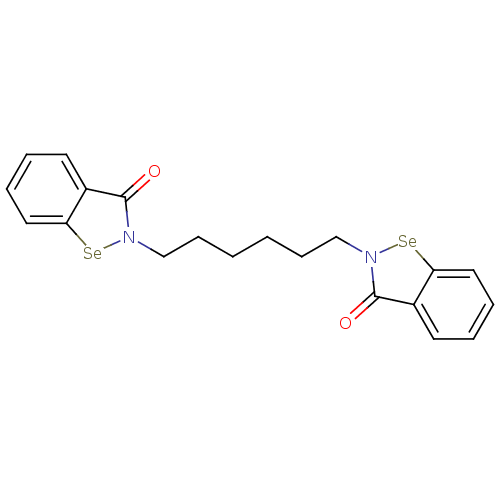
(CHEMBL2035464 | US8592468, EbSe16)Show SMILES O=c1n(CCCCCCn2[se]c3ccccc3c2=O)[se]c2ccccc12 Show InChI InChI=1S/C20H20N2O2Se2/c23-19-15-9-3-5-11-17(15)25-21(19)13-7-1-2-8-14-22-20(24)16-10-4-6-12-18(16)26-22/h3-6,9-12H,1-2,7-8,13-14H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 10 | n/a | 2.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Thioredoxin Systems AB
US Patent
| Assay Description
All the benzisoselenazol-3(2H)-one and bisbenzisoselenazol-3(2H)-one
derivatives were tested as potential E. coli TrxR inhibitors by standard
DTNB ... |
US Patent US8592468 (2013)
BindingDB Entry DOI: 10.7270/Q29P3081 |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase
(Escherichia coli (strain K12)) | BDBM50385302
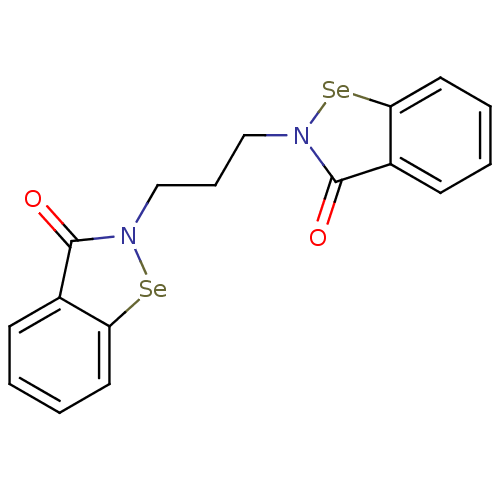
(CHEMBL2035461 | US8592468, EbSe15)Show InChI InChI=1S/C17H14N2O2Se2/c20-16-12-6-1-3-8-14(12)22-18(16)10-5-11-19-17(21)13-7-2-4-9-15(13)23-19/h1-4,6-9H,5,10-11H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 40 | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Thioredoxin Systems AB
US Patent
| Assay Description
All the benzisoselenazol-3(2H)-one and bisbenzisoselenazol-3(2H)-one
derivatives were tested as potential E. coli TrxR inhibitors by standard
DTNB ... |
US Patent US8592468 (2013)
BindingDB Entry DOI: 10.7270/Q29P3081 |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase
(Escherichia coli (strain K12)) | BDBM50385303
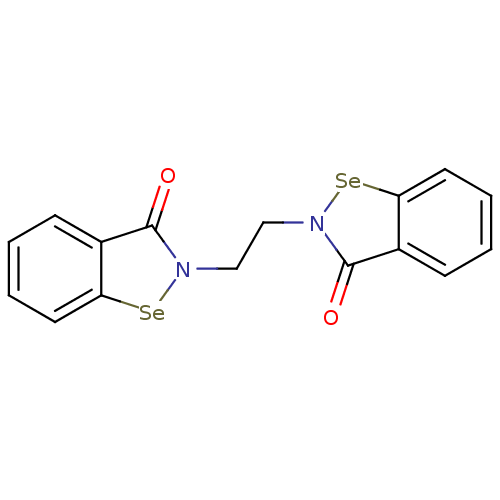
(CHEMBL2035460 | US8592468, EbSe14)Show InChI InChI=1S/C16H12N2O2Se2/c19-15-11-5-1-3-7-13(11)21-17(15)9-10-18-16(20)12-6-2-4-8-14(12)22-18/h1-8H,9-10H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| US Patent
| 50 | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Thioredoxin Systems AB
US Patent
| Assay Description
All the benzisoselenazol-3(2H)-one and bisbenzisoselenazol-3(2H)-one
derivatives were tested as potential E. coli TrxR inhibitors by standard
DTNB ... |
US Patent US8592468 (2013)
BindingDB Entry DOI: 10.7270/Q29P3081 |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase
(Escherichia coli (strain K12)) | BDBM106948

(US8592468, EbSe12)Show InChI InChI=1S/C12H7N3O3Se/c16-12-8-4-1-2-6-10(8)19-14(12)11-9(15(17)18)5-3-7-13-11/h1-7H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 250 | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Thioredoxin Systems AB
US Patent
| Assay Description
All the benzisoselenazol-3(2H)-one and bisbenzisoselenazol-3(2H)-one
derivatives were tested as potential E. coli TrxR inhibitors by standard
DTNB ... |
US Patent US8592468 (2013)
BindingDB Entry DOI: 10.7270/Q29P3081 |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase
(Escherichia coli (strain K12)) | BDBM106941
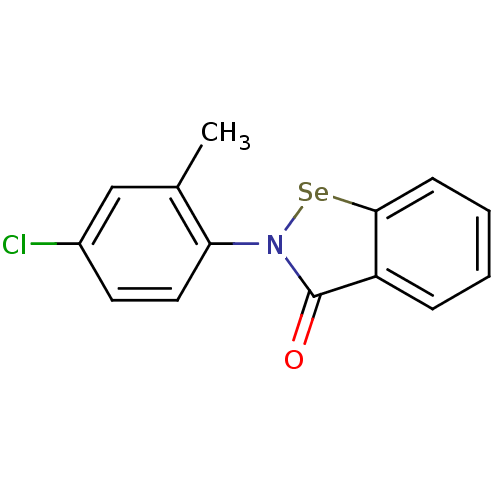
(US8592468, EbSe8)Show InChI InChI=1S/C14H10ClNOSe/c1-9-8-10(15)6-7-12(9)16-14(17)11-4-2-3-5-13(11)18-16/h2-8H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 250 | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Thioredoxin Systems AB
US Patent
| Assay Description
All the benzisoselenazol-3(2H)-one and bisbenzisoselenazol-3(2H)-one
derivatives were tested as potential E. coli TrxR inhibitors by standard
DTNB ... |
US Patent US8592468 (2013)
BindingDB Entry DOI: 10.7270/Q29P3081 |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase
(Escherichia coli (strain K12)) | BDBM34233

(2-Phenyl-benzo[d]isoselenazol-3-one | 2-Phenyl-ben...)Show InChI InChI=1S/C13H9NOSe/c15-13-11-8-4-5-9-12(11)16-14(13)10-6-2-1-3-7-10/h1-9H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| US Patent
| 300 | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Thioredoxin Systems AB
US Patent
| Assay Description
All the benzisoselenazol-3(2H)-one and bisbenzisoselenazol-3(2H)-one
derivatives were tested as potential E. coli TrxR inhibitors by standard
DTNB ... |
US Patent US8592468 (2013)
BindingDB Entry DOI: 10.7270/Q29P3081 |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase
(Escherichia coli (strain K12)) | BDBM106940
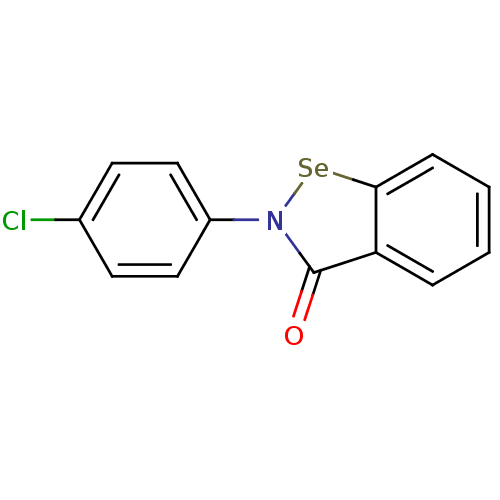
(US8592468, EbSe7)Show InChI InChI=1S/C13H8ClNOSe/c14-9-5-7-10(8-6-9)15-13(16)11-3-1-2-4-12(11)17-15/h1-8H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 550 | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Thioredoxin Systems AB
US Patent
| Assay Description
All the benzisoselenazol-3(2H)-one and bisbenzisoselenazol-3(2H)-one
derivatives were tested as potential E. coli TrxR inhibitors by standard
DTNB ... |
US Patent US8592468 (2013)
BindingDB Entry DOI: 10.7270/Q29P3081 |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase
(Escherichia coli (strain K12)) | BDBM106944
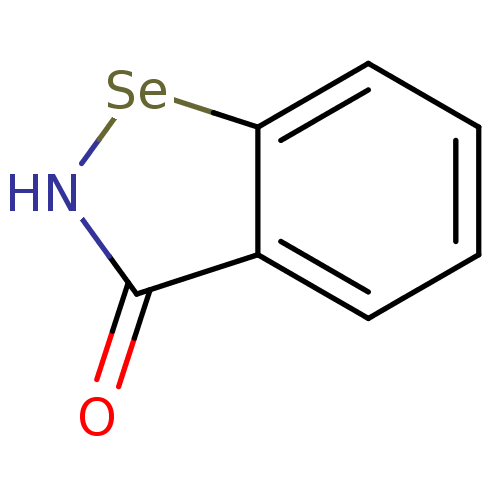
(US8592468, EbSe2)Show InChI InChI=1S/C7H5NOSe/c9-7-5-3-1-2-4-6(5)10-8-7/h1-4H,(H,8,9) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1.00E+3 | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Thioredoxin Systems AB
US Patent
| Assay Description
All the benzisoselenazol-3(2H)-one and bisbenzisoselenazol-3(2H)-one
derivatives were tested as potential E. coli TrxR inhibitors by standard
DTNB ... |
US Patent US8592468 (2013)
BindingDB Entry DOI: 10.7270/Q29P3081 |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase
(Escherichia coli (strain K12)) | BDBM106942
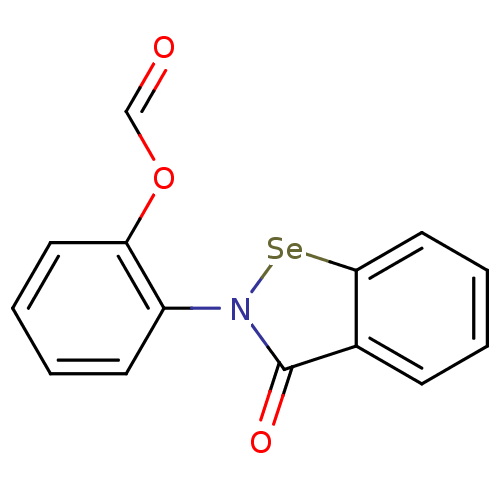
(US8592468, EbSe9)Show InChI InChI=1S/C14H9NO3Se/c16-9-18-12-7-3-2-6-11(12)15-14(17)10-5-1-4-8-13(10)19-15/h1-9H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1.20E+3 | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Thioredoxin Systems AB
US Patent
| Assay Description
All the benzisoselenazol-3(2H)-one and bisbenzisoselenazol-3(2H)-one
derivatives were tested as potential E. coli TrxR inhibitors by standard
DTNB ... |
US Patent US8592468 (2013)
BindingDB Entry DOI: 10.7270/Q29P3081 |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase
(Escherichia coli (strain K12)) | BDBM106949
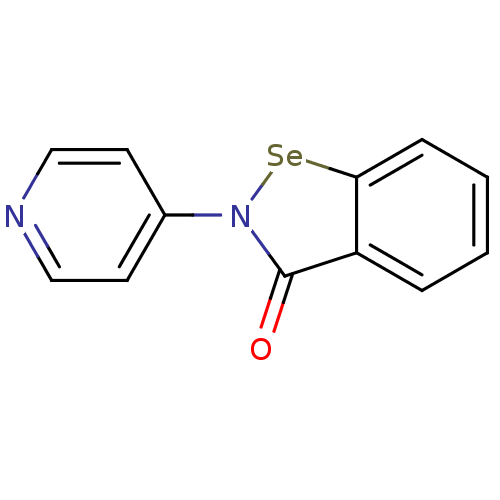
(US8592468, EbSe13)Show InChI InChI=1S/C12H8N2OSe/c15-12-10-3-1-2-4-11(10)16-14(12)9-5-7-13-8-6-9/h1-8H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.50E+3 | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Thioredoxin Systems AB
US Patent
| Assay Description
All the benzisoselenazol-3(2H)-one and bisbenzisoselenazol-3(2H)-one
derivatives were tested as potential E. coli TrxR inhibitors by standard
DTNB ... |
US Patent US8592468 (2013)
BindingDB Entry DOI: 10.7270/Q29P3081 |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 3
(Homo sapiens (Human)) | BDBM50474574
(CHEMBL217200)Show SMILES [H][C@]12[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]3([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]4([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]5([H])[#8]-[#6](-[#6][Te;v2]c6ccc(cc6)-[#7](-[#6])-[#6])[C@@]([H])([#8][C@@]6([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]7([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]8([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8]1)[#6](-[#8])-[#6]8-[#8])[#6](-[#8])-[#6]7-[#8])[#6](-[#8])-[#6]6-[#8])[#6](-[#8])-[#6]5-[#8])[#6](-[#8])-[#6]4-[#8])[#6](-[#8])-[#6]3-[#8])[#6](-[#8])-[#6]2-[#8] Show InChI InChI=1S/C50H79NO34Te/c1-51(2)14-3-5-15(6-4-14)86-13-22-43-29(64)36(71)50(78-22)84-42-21(12-57)76-48(34(69)27(42)62)82-40-19(10-55)74-46(32(67)25(40)60)80-38-17(8-53)72-44(30(65)23(38)58)79-37-16(7-52)73-45(31(66)24(37)59)81-39-18(9-54)75-47(33(68)26(39)61)83-41-20(11-56)77-49(85-43)35(70)28(41)63/h3-6,16-50,52-71H,7-13H2,1-2H3/t16?,17?,18?,19?,20?,21?,22?,23?,24?,25?,26?,27?,28?,29?,30?,31?,32?,33?,34?,35?,36?,37-,38-,39-,40-,41-,42-,43-,44+,45-,46-,47-,48-,49-,50-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of thioredoxin reductase |
J Med Chem 47: 233-9 (2004)
Article DOI: 10.1021/jm030916r
BindingDB Entry DOI: 10.7270/Q2542RBW |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 3
(Homo sapiens (Human)) | BDBM50474575
(CHEMBL412348)Show SMILES [H][C@@]12[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]3([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@]4([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]5([H])[#8]-[#6](-[#6][Te;v2][#6]-[#6]6-[#8][C@]7([H])[#8][C@]8([H])[#6](-[#6]-[#8])-[#8][C@]([H])([#8][C@]9([H])[#6](-[#6]-[#8])-[#8][C@]([H])([#8][C@]%10([H])[#6](-[#6]-[#8])-[#8][C@]([H])([#8][C@]%11([H])[#6](-[#6]-[#8])-[#8][C@]([H])([#8][C@]%12([H])[#6](-[#6]-[#8])-[#8][C@@]([H])([#8][C@]%13([H])[#6](-[#6]-[#8])-[#8][C@]([H])([#8][C@@]6([H])[#6](-[#8])-[#6]7-[#8])[#6](-[#8])-[#6]%13-[#8])[#6](-[#8])-[#6]%12-[#8])[#6](-[#8])-[#6]%11-[#8])[#6](-[#8])-[#6]%10-[#8])[#6](-[#8])-[#6]9-[#8])[#6](-[#8])-[#6]8-[#8])[C@@]([H])([#8][C@@]6([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]7([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]8([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8]1)[#6](-[#8])-[#6]8-[#8])[#6](-[#8])-[#6]7-[#8])[#6](-[#8])-[#6]6-[#8])[#6](-[#8])-[#6]5-[#8])[#6](-[#8])-[#6]4-[#8])[#6](-[#8])-[#6]3-[#8])[#6](-[#8])-[#6]2-[#8] Show InChI InChI=1S/C84H138O68Te/c85-1-15-57-31(99)45(113)73(127-15)143-61-19(5-89)131-77(49(117)35(61)103)147-65-23(9-93)135-81(53(121)39(65)107)151-69-27(137-83(55(123)41(69)109)149-67-25(11-95)133-79(51(119)37(67)105)145-63-21(7-91)129-75(47(115)33(63)101)141-59-17(3-87)125-71(139-57)43(111)29(59)97)13-153-14-28-70-42(110)56(124)84(138-28)150-68-26(12-96)134-80(52(120)38(68)106)146-64-22(8-92)130-76(48(116)34(64)102)142-60-18(4-88)126-72(44(112)30(60)98)140-58-16(2-86)128-74(46(114)32(58)100)144-62-20(6-90)132-78(50(118)36(62)104)148-66-24(10-94)136-82(152-70)54(122)40(66)108/h15-124H,1-14H2/t15?,16?,17?,18?,19?,20?,21?,22?,23?,24?,25?,26?,27?,28?,29?,30?,31?,32?,33?,34?,35?,36?,37?,38?,39?,40?,41?,42?,43?,44?,45?,46?,47?,48?,49?,50?,51?,52?,53?,54?,55?,56?,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70-,71-,72-,73-,74-,75-,76-,77-,78+,79+,80-,81-,82-,83-,84-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of thioredoxin reductase in the presence of thioredoxinand insulin |
J Med Chem 47: 233-9 (2004)
Article DOI: 10.1021/jm030916r
BindingDB Entry DOI: 10.7270/Q2542RBW |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 3
(Homo sapiens (Human)) | BDBM50474576

(CHEMBL413290)Show SMILES [H][C@]12[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]3([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]4([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]5([H])[#8]-[#6](-[#6][Te;v2][#6]-[#6]-[#6]-[#6])[C@@]([H])([#8][C@@]6([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]7([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]8([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8]1)[#6](-[#8])-[#6]8-[#8])[#6](-[#8])-[#6]7-[#8])[#6](-[#8])-[#6]6-[#8])[#6](-[#8])-[#6]5-[#8])[#6](-[#8])-[#6]4-[#8])[#6](-[#8])-[#6]3-[#8])[#6](-[#8])-[#6]2-[#8] Show InChI InChI=1S/C46H78O34Te/c1-2-3-4-81-11-18-39-25(59)32(66)46(73-18)79-38-17(10-52)71-44(30(64)23(38)57)77-36-15(8-50)69-42(28(62)21(36)55)75-34-13(6-48)67-40(26(60)19(34)53)74-33-12(5-47)68-41(27(61)20(33)54)76-35-14(7-49)70-43(29(63)22(35)56)78-37-16(9-51)72-45(80-39)31(65)24(37)58/h12-66H,2-11H2,1H3/t12?,13?,14?,15?,16?,17?,18?,19?,20?,21?,22?,23?,24?,25?,26?,27?,28?,29?,30?,31?,32?,33-,34-,35-,36-,37-,38-,39-,40+,41-,42-,43-,44-,45-,46-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of thioredoxin reductase in the presence of thioredoxinand insulin |
J Med Chem 47: 233-9 (2004)
Article DOI: 10.1021/jm030916r
BindingDB Entry DOI: 10.7270/Q2542RBW |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase
(Escherichia coli (strain K12)) | BDBM106943
(US8592468, EbSe10 | acs.jmedchem.1c00409_ST.159)Show InChI InChI=1S/C14H9NO3Se/c16-13-11-3-1-2-4-12(11)19-15(13)10-7-5-9(6-8-10)14(17)18/h1-8H,(H,17,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Thioredoxin Systems AB
US Patent
| Assay Description
All the benzisoselenazol-3(2H)-one and bisbenzisoselenazol-3(2H)-one
derivatives were tested as potential E. coli TrxR inhibitors by standard
DTNB ... |
US Patent US8592468 (2013)
BindingDB Entry DOI: 10.7270/Q29P3081 |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 3
(Homo sapiens (Human)) | BDBM50474573
(CHEMBL385299)Show SMILES [H][C@]12[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]3([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]4([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]5([H])[#8]-[#6](-[#6][Te;v2]c6ccc(-[#8]-[#6])cc6)[C@@]([H])([#8][C@@]6([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]7([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]8([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8]1)[#6](-[#8])-[#6]8-[#8])[#6](-[#8])-[#6]7-[#8])[#6](-[#8])-[#6]6-[#8])[#6](-[#8])-[#6]5-[#8])[#6](-[#8])-[#6]4-[#8])[#6](-[#8])-[#6]3-[#8])[#6](-[#8])-[#6]2-[#8] Show InChI InChI=1S/C49H76O35Te/c1-70-13-2-4-14(5-3-13)85-12-21-42-28(62)35(69)49(77-21)83-41-20(11-55)75-47(33(67)26(41)60)81-39-18(9-53)73-45(31(65)24(39)58)79-37-16(7-51)71-43(29(63)22(37)56)78-36-15(6-50)72-44(30(64)23(36)57)80-38-17(8-52)74-46(32(66)25(38)59)82-40-19(10-54)76-48(84-42)34(68)27(40)61/h2-5,15-69H,6-12H2,1H3/t15?,16?,17?,18?,19?,20?,21?,22?,23?,24?,25?,26?,27?,28?,29?,30?,31?,32?,33?,34?,35?,36-,37-,38-,39-,40-,41-,42-,43+,44-,45-,46-,47-,48-,49-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of thioredoxin reductase |
J Med Chem 47: 233-9 (2004)
Article DOI: 10.1021/jm030916r
BindingDB Entry DOI: 10.7270/Q2542RBW |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 3
(Homo sapiens (Human)) | BDBM50474579
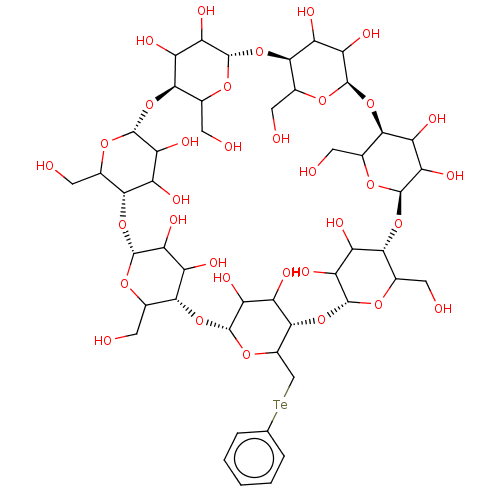
(CHEMBL440625)Show SMILES [H][C@]12[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]3([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]4([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]5([H])[#8]-[#6](-[#6][Te;v2]c6ccccc6)[C@@]([H])([#8][C@@]6([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]7([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]8([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8]1)[#6](-[#8])-[#6]8-[#8])[#6](-[#8])-[#6]7-[#8])[#6](-[#8])-[#6]6-[#8])[#6](-[#8])-[#6]5-[#8])[#6](-[#8])-[#6]4-[#8])[#6](-[#8])-[#6]3-[#8])[#6](-[#8])-[#6]2-[#8] Show InChI InChI=1S/C48H74O34Te/c49-6-14-35-22(56)29(63)43(70-14)78-37-16(8-51)72-45(31(65)24(37)58)80-39-18(10-53)74-47(33(67)26(39)60)82-41-20(12-83-13-4-2-1-3-5-13)75-48(34(68)27(41)61)81-40-19(11-54)73-46(32(66)25(40)59)79-38-17(9-52)71-44(30(64)23(38)57)77-36-15(7-50)69-42(76-35)28(62)21(36)55/h1-5,14-68H,6-12H2/t14?,15?,16?,17?,18?,19?,20?,21?,22?,23?,24?,25?,26?,27?,28?,29?,30?,31?,32?,33?,34?,35-,36-,37-,38-,39-,40-,41-,42+,43-,44-,45-,46-,47-,48-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of thioredoxin reductase |
J Med Chem 47: 233-9 (2004)
Article DOI: 10.1021/jm030916r
BindingDB Entry DOI: 10.7270/Q2542RBW |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 3
(Homo sapiens (Human)) | BDBM50474575

(CHEMBL412348)Show SMILES [H][C@@]12[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]3([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@]4([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]5([H])[#8]-[#6](-[#6][Te;v2][#6]-[#6]6-[#8][C@]7([H])[#8][C@]8([H])[#6](-[#6]-[#8])-[#8][C@]([H])([#8][C@]9([H])[#6](-[#6]-[#8])-[#8][C@]([H])([#8][C@]%10([H])[#6](-[#6]-[#8])-[#8][C@]([H])([#8][C@]%11([H])[#6](-[#6]-[#8])-[#8][C@]([H])([#8][C@]%12([H])[#6](-[#6]-[#8])-[#8][C@@]([H])([#8][C@]%13([H])[#6](-[#6]-[#8])-[#8][C@]([H])([#8][C@@]6([H])[#6](-[#8])-[#6]7-[#8])[#6](-[#8])-[#6]%13-[#8])[#6](-[#8])-[#6]%12-[#8])[#6](-[#8])-[#6]%11-[#8])[#6](-[#8])-[#6]%10-[#8])[#6](-[#8])-[#6]9-[#8])[#6](-[#8])-[#6]8-[#8])[C@@]([H])([#8][C@@]6([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]7([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]8([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8]1)[#6](-[#8])-[#6]8-[#8])[#6](-[#8])-[#6]7-[#8])[#6](-[#8])-[#6]6-[#8])[#6](-[#8])-[#6]5-[#8])[#6](-[#8])-[#6]4-[#8])[#6](-[#8])-[#6]3-[#8])[#6](-[#8])-[#6]2-[#8] Show InChI InChI=1S/C84H138O68Te/c85-1-15-57-31(99)45(113)73(127-15)143-61-19(5-89)131-77(49(117)35(61)103)147-65-23(9-93)135-81(53(121)39(65)107)151-69-27(137-83(55(123)41(69)109)149-67-25(11-95)133-79(51(119)37(67)105)145-63-21(7-91)129-75(47(115)33(63)101)141-59-17(3-87)125-71(139-57)43(111)29(59)97)13-153-14-28-70-42(110)56(124)84(138-28)150-68-26(12-96)134-80(52(120)38(68)106)146-64-22(8-92)130-76(48(116)34(64)102)142-60-18(4-88)126-72(44(112)30(60)98)140-58-16(2-86)128-74(46(114)32(58)100)144-62-20(6-90)132-78(50(118)36(62)104)148-66-24(10-94)136-82(152-70)54(122)40(66)108/h15-124H,1-14H2/t15?,16?,17?,18?,19?,20?,21?,22?,23?,24?,25?,26?,27?,28?,29?,30?,31?,32?,33?,34?,35?,36?,37?,38?,39?,40?,41?,42?,43?,44?,45?,46?,47?,48?,49?,50?,51?,52?,53?,54?,55?,56?,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70-,71-,72-,73-,74-,75-,76-,77-,78+,79+,80-,81-,82-,83-,84-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of thioredoxin reductase |
J Med Chem 47: 233-9 (2004)
Article DOI: 10.1021/jm030916r
BindingDB Entry DOI: 10.7270/Q2542RBW |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 3
(Homo sapiens (Human)) | BDBM50474574

(CHEMBL217200)Show SMILES [H][C@]12[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]3([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]4([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]5([H])[#8]-[#6](-[#6][Te;v2]c6ccc(cc6)-[#7](-[#6])-[#6])[C@@]([H])([#8][C@@]6([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]7([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]8([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8]1)[#6](-[#8])-[#6]8-[#8])[#6](-[#8])-[#6]7-[#8])[#6](-[#8])-[#6]6-[#8])[#6](-[#8])-[#6]5-[#8])[#6](-[#8])-[#6]4-[#8])[#6](-[#8])-[#6]3-[#8])[#6](-[#8])-[#6]2-[#8] Show InChI InChI=1S/C50H79NO34Te/c1-51(2)14-3-5-15(6-4-14)86-13-22-43-29(64)36(71)50(78-22)84-42-21(12-57)76-48(34(69)27(42)62)82-40-19(10-55)74-46(32(67)25(40)60)80-38-17(8-53)72-44(30(65)23(38)58)79-37-16(7-52)73-45(31(66)24(37)59)81-39-18(9-54)75-47(33(68)26(39)61)83-41-20(11-56)77-49(85-43)35(70)28(41)63/h3-6,16-50,52-71H,7-13H2,1-2H3/t16?,17?,18?,19?,20?,21?,22?,23?,24?,25?,26?,27?,28?,29?,30?,31?,32?,33?,34?,35?,36?,37-,38-,39-,40-,41-,42-,43-,44+,45-,46-,47-,48-,49-,50-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of thioredoxin reductase in the presence of thioredoxinand insulin |
J Med Chem 47: 233-9 (2004)
Article DOI: 10.1021/jm030916r
BindingDB Entry DOI: 10.7270/Q2542RBW |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 3
(Homo sapiens (Human)) | BDBM50474578
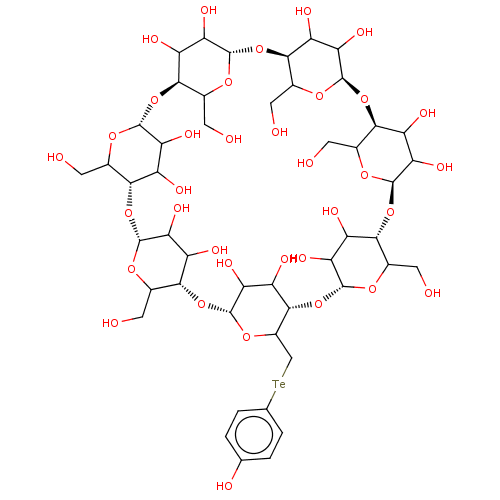
(CHEMBL437864)Show SMILES [H][C@]12[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]3([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]4([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]5([H])[#8]-[#6](-[#6][Te;v2]c6ccc(-[#8])cc6)[C@@]([H])([#8][C@@]6([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]7([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]8([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8]1)[#6](-[#8])-[#6]8-[#8])[#6](-[#8])-[#6]7-[#8])[#6](-[#8])-[#6]6-[#8])[#6](-[#8])-[#6]5-[#8])[#6](-[#8])-[#6]4-[#8])[#6](-[#8])-[#6]3-[#8])[#6](-[#8])-[#6]2-[#8] Show InChI InChI=1S/C48H74O35Te/c49-5-14-35-22(57)29(64)43(71-14)79-37-16(7-51)73-45(31(66)24(37)59)81-39-18(9-53)75-47(33(68)26(39)61)83-41-20(11-84-13-3-1-12(55)2-4-13)76-48(34(69)27(41)62)82-40-19(10-54)74-46(32(67)25(40)60)80-38-17(8-52)72-44(30(65)23(38)58)78-36-15(6-50)70-42(77-35)28(63)21(36)56/h1-4,14-69H,5-11H2/t14?,15?,16?,17?,18?,19?,20?,21?,22?,23?,24?,25?,26?,27?,28?,29?,30?,31?,32?,33?,34?,35-,36-,37-,38-,39-,40-,41-,42+,43-,44-,45-,46-,47-,48-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of thioredoxin reductase in the presence of thioredoxinand insulin |
J Med Chem 47: 233-9 (2004)
Article DOI: 10.1021/jm030916r
BindingDB Entry DOI: 10.7270/Q2542RBW |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 3
(Homo sapiens (Human)) | BDBM50474573

(CHEMBL385299)Show SMILES [H][C@]12[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]3([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]4([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]5([H])[#8]-[#6](-[#6][Te;v2]c6ccc(-[#8]-[#6])cc6)[C@@]([H])([#8][C@@]6([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]7([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]8([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8]1)[#6](-[#8])-[#6]8-[#8])[#6](-[#8])-[#6]7-[#8])[#6](-[#8])-[#6]6-[#8])[#6](-[#8])-[#6]5-[#8])[#6](-[#8])-[#6]4-[#8])[#6](-[#8])-[#6]3-[#8])[#6](-[#8])-[#6]2-[#8] Show InChI InChI=1S/C49H76O35Te/c1-70-13-2-4-14(5-3-13)85-12-21-42-28(62)35(69)49(77-21)83-41-20(11-55)75-47(33(67)26(41)60)81-39-18(9-53)73-45(31(65)24(39)58)79-37-16(7-51)71-43(29(63)22(37)56)78-36-15(6-50)72-44(30(64)23(36)57)80-38-17(8-52)74-46(32(66)25(38)59)82-40-19(10-54)76-48(84-42)34(68)27(40)61/h2-5,15-69H,6-12H2,1H3/t15?,16?,17?,18?,19?,20?,21?,22?,23?,24?,25?,26?,27?,28?,29?,30?,31?,32?,33?,34?,35?,36-,37-,38-,39-,40-,41-,42-,43+,44-,45-,46-,47-,48-,49-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of thioredoxin reductase in the presence of thioredoxinand insulin |
J Med Chem 47: 233-9 (2004)
Article DOI: 10.1021/jm030916r
BindingDB Entry DOI: 10.7270/Q2542RBW |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 3
(Homo sapiens (Human)) | BDBM50474579

(CHEMBL440625)Show SMILES [H][C@]12[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]3([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]4([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]5([H])[#8]-[#6](-[#6][Te;v2]c6ccccc6)[C@@]([H])([#8][C@@]6([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]7([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]8([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8]1)[#6](-[#8])-[#6]8-[#8])[#6](-[#8])-[#6]7-[#8])[#6](-[#8])-[#6]6-[#8])[#6](-[#8])-[#6]5-[#8])[#6](-[#8])-[#6]4-[#8])[#6](-[#8])-[#6]3-[#8])[#6](-[#8])-[#6]2-[#8] Show InChI InChI=1S/C48H74O34Te/c49-6-14-35-22(56)29(63)43(70-14)78-37-16(8-51)72-45(31(65)24(37)58)80-39-18(10-53)74-47(33(67)26(39)60)82-41-20(12-83-13-4-2-1-3-5-13)75-48(34(68)27(41)61)81-40-19(11-54)73-46(32(66)25(40)59)79-38-17(9-52)71-44(30(64)23(38)57)77-36-15(7-50)69-42(76-35)28(62)21(36)55/h1-5,14-68H,6-12H2/t14?,15?,16?,17?,18?,19?,20?,21?,22?,23?,24?,25?,26?,27?,28?,29?,30?,31?,32?,33?,34?,35-,36-,37-,38-,39-,40-,41-,42+,43-,44-,45-,46-,47-,48-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of thioredoxin reductase in the presence of thioredoxinand insulin |
J Med Chem 47: 233-9 (2004)
Article DOI: 10.1021/jm030916r
BindingDB Entry DOI: 10.7270/Q2542RBW |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase
(Escherichia coli (strain K12)) | BDBM106946
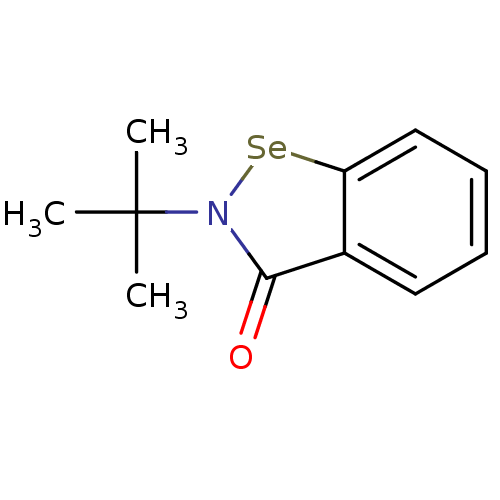
(US8592468, EbSe4)Show InChI InChI=1S/C11H13NOSe/c1-11(2,3)12-10(13)8-6-4-5-7-9(8)14-12/h4-7H,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Thioredoxin Systems AB
US Patent
| Assay Description
All the benzisoselenazol-3(2H)-one and bisbenzisoselenazol-3(2H)-one
derivatives were tested as potential E. coli TrxR inhibitors by standard
DTNB ... |
US Patent US8592468 (2013)
BindingDB Entry DOI: 10.7270/Q29P3081 |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase
(Escherichia coli (strain K12)) | BDBM106945

(US8592468, EbSe3)Show InChI InChI=1S/C8H7NOSe/c1-9-8(10)6-4-2-3-5-7(6)11-9/h2-5H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Thioredoxin Systems AB
US Patent
| Assay Description
All the benzisoselenazol-3(2H)-one and bisbenzisoselenazol-3(2H)-one
derivatives were tested as potential E. coli TrxR inhibitors by standard
DTNB ... |
US Patent US8592468 (2013)
BindingDB Entry DOI: 10.7270/Q29P3081 |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 3
(Homo sapiens (Human)) | BDBM50474577

(CHEMBL411553)Show SMILES [H][C@]12[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]3([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]4([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]5([H])[#8]-[#6](-[#6][Se;v2]c6ccccc6)[C@@]([H])([#8][C@@]6([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]7([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]8([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8]1)[#6](-[#8])-[#6]8-[#8])[#6](-[#8])-[#6]7-[#8])[#6](-[#8])-[#6]6-[#8])[#6](-[#8])-[#6]5-[#8])[#6](-[#8])-[#6]4-[#8])[#6](-[#8])-[#6]3-[#8])[#6](-[#8])-[#6]2-[#8] Show InChI InChI=1S/C48H74O34Se/c49-6-14-35-22(56)29(63)43(70-14)78-37-16(8-51)72-45(31(65)24(37)58)80-39-18(10-53)74-47(33(67)26(39)60)82-41-20(12-83-13-4-2-1-3-5-13)75-48(34(68)27(41)61)81-40-19(11-54)73-46(32(66)25(40)59)79-38-17(9-52)71-44(30(64)23(38)57)77-36-15(7-50)69-42(76-35)28(62)21(36)55/h1-5,14-68H,6-12H2/t14?,15?,16?,17?,18?,19?,20?,21?,22?,23?,24?,25?,26?,27?,28?,29?,30?,31?,32?,33?,34?,35-,36-,37-,38-,39-,40-,41-,42+,43-,44-,45-,46-,47-,48-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of thioredoxin reductase |
J Med Chem 47: 233-9 (2004)
Article DOI: 10.1021/jm030916r
BindingDB Entry DOI: 10.7270/Q2542RBW |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 3
(Homo sapiens (Human)) | BDBM50474576

(CHEMBL413290)Show SMILES [H][C@]12[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]3([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]4([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]5([H])[#8]-[#6](-[#6][Te;v2][#6]-[#6]-[#6]-[#6])[C@@]([H])([#8][C@@]6([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]7([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]8([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8]1)[#6](-[#8])-[#6]8-[#8])[#6](-[#8])-[#6]7-[#8])[#6](-[#8])-[#6]6-[#8])[#6](-[#8])-[#6]5-[#8])[#6](-[#8])-[#6]4-[#8])[#6](-[#8])-[#6]3-[#8])[#6](-[#8])-[#6]2-[#8] Show InChI InChI=1S/C46H78O34Te/c1-2-3-4-81-11-18-39-25(59)32(66)46(73-18)79-38-17(10-52)71-44(30(64)23(38)57)77-36-15(8-50)69-42(28(62)21(36)55)75-34-13(6-48)67-40(26(60)19(34)53)74-33-12(5-47)68-41(27(61)20(33)54)76-35-14(7-49)70-43(29(63)22(35)56)78-37-16(9-51)72-45(80-39)31(65)24(37)58/h12-66H,2-11H2,1H3/t12?,13?,14?,15?,16?,17?,18?,19?,20?,21?,22?,23?,24?,25?,26?,27?,28?,29?,30?,31?,32?,33-,34-,35-,36-,37-,38-,39-,40+,41-,42-,43-,44-,45-,46-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of thioredoxin reductase |
J Med Chem 47: 233-9 (2004)
Article DOI: 10.1021/jm030916r
BindingDB Entry DOI: 10.7270/Q2542RBW |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 3
(Homo sapiens (Human)) | BDBM50474578

(CHEMBL437864)Show SMILES [H][C@]12[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]3([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]4([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]5([H])[#8]-[#6](-[#6][Te;v2]c6ccc(-[#8])cc6)[C@@]([H])([#8][C@@]6([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]7([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]8([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8]1)[#6](-[#8])-[#6]8-[#8])[#6](-[#8])-[#6]7-[#8])[#6](-[#8])-[#6]6-[#8])[#6](-[#8])-[#6]5-[#8])[#6](-[#8])-[#6]4-[#8])[#6](-[#8])-[#6]3-[#8])[#6](-[#8])-[#6]2-[#8] Show InChI InChI=1S/C48H74O35Te/c49-5-14-35-22(57)29(64)43(71-14)79-37-16(7-51)73-45(31(66)24(37)59)81-39-18(9-53)75-47(33(68)26(39)61)83-41-20(11-84-13-3-1-12(55)2-4-13)76-48(34(69)27(41)62)82-40-19(10-54)74-46(32(67)25(40)60)80-38-17(8-52)72-44(30(65)23(38)58)78-36-15(6-50)70-42(77-35)28(63)21(36)56/h1-4,14-69H,5-11H2/t14?,15?,16?,17?,18?,19?,20?,21?,22?,23?,24?,25?,26?,27?,28?,29?,30?,31?,32?,33?,34?,35-,36-,37-,38-,39-,40-,41-,42+,43-,44-,45-,46-,47-,48-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of thioredoxin reductase |
J Med Chem 47: 233-9 (2004)
Article DOI: 10.1021/jm030916r
BindingDB Entry DOI: 10.7270/Q2542RBW |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 3
(Homo sapiens (Human)) | BDBM50474577

(CHEMBL411553)Show SMILES [H][C@]12[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]3([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]4([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]5([H])[#8]-[#6](-[#6][Se;v2]c6ccccc6)[C@@]([H])([#8][C@@]6([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]7([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8][C@@]8([H])[#8]-[#6](-[#6]-[#8])[C@@]([H])([#8]1)[#6](-[#8])-[#6]8-[#8])[#6](-[#8])-[#6]7-[#8])[#6](-[#8])-[#6]6-[#8])[#6](-[#8])-[#6]5-[#8])[#6](-[#8])-[#6]4-[#8])[#6](-[#8])-[#6]3-[#8])[#6](-[#8])-[#6]2-[#8] Show InChI InChI=1S/C48H74O34Se/c49-6-14-35-22(56)29(63)43(70-14)78-37-16(8-51)72-45(31(65)24(37)58)80-39-18(10-53)74-47(33(67)26(39)60)82-41-20(12-83-13-4-2-1-3-5-13)75-48(34(68)27(41)61)81-40-19(11-54)73-46(32(66)25(40)59)79-38-17(9-52)71-44(30(64)23(38)57)77-36-15(7-50)69-42(76-35)28(62)21(36)55/h1-5,14-68H,6-12H2/t14?,15?,16?,17?,18?,19?,20?,21?,22?,23?,24?,25?,26?,27?,28?,29?,30?,31?,32?,33?,34?,35-,36-,37-,38-,39-,40-,41-,42+,43-,44-,45-,46-,47-,48-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of thioredoxin reductase in the presence of thioredoxinand insulin |
J Med Chem 47: 233-9 (2004)
Article DOI: 10.1021/jm030916r
BindingDB Entry DOI: 10.7270/Q2542RBW |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase
(Escherichia coli (strain K12)) | BDBM106947
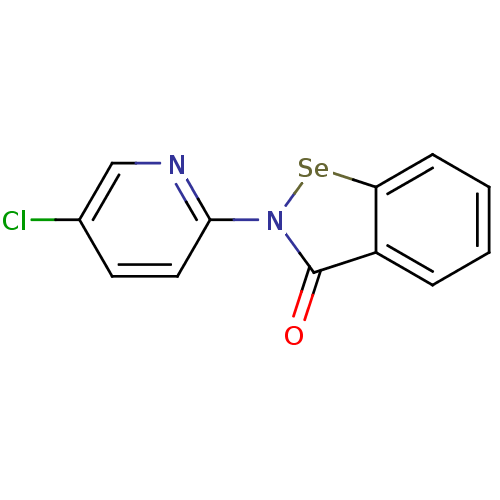
(US8592468, EbSe11)Show InChI InChI=1S/C12H7ClN2OSe/c13-8-5-6-11(14-7-8)15-12(16)9-3-1-2-4-10(9)17-15/h1-7H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Thioredoxin Systems AB
US Patent
| Assay Description
All the benzisoselenazol-3(2H)-one and bisbenzisoselenazol-3(2H)-one
derivatives were tested as potential E. coli TrxR inhibitors by standard
DTNB ... |
US Patent US8592468 (2013)
BindingDB Entry DOI: 10.7270/Q29P3081 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data



























