 Found 151 hits with Last Name = 'erdmann' and Initial = 'd'
Found 151 hits with Last Name = 'erdmann' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50343731

((R)-N-alpha-(2,2-Diphenylacetyl)-N-(4-ureidomethyl...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6](-c1ccccc1)-c1ccccc1)-[#6](=O)-[#7]-[#6]-c1ccc(-[#6]-[#7]-[#6](-[#7])=O)cc1 |r| Show InChI InChI=1S/C29H35N7O3/c30-28(31)33-17-7-12-24(26(37)34-18-20-13-15-21(16-14-20)19-35-29(32)39)36-27(38)25(22-8-3-1-4-9-22)23-10-5-2-6-11-23/h1-6,8-11,13-16,24-25H,7,12,17-19H2,(H,34,37)(H,36,38)(H4,30,31,33)(H3,32,35,39)/t24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-UR-MK114 from Y1R in human SK-N-MC cells |
Bioorg Med Chem 19: 2859-78 (2011)
Article DOI: 10.1016/j.bmc.2011.03.045
BindingDB Entry DOI: 10.7270/Q2F47PG7 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50060728

((R)-2-(2,2-diphenylacetamido)-5-guanidino-N-(4-hyd...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6](-c1ccccc1)-c1ccccc1)-[#6](=O)-[#7]-[#6]-c1ccc(-[#8])cc1 |r| Show InChI InChI=1S/C27H31N5O3/c28-27(29)30-17-7-12-23(25(34)31-18-19-13-15-22(33)16-14-19)32-26(35)24(20-8-3-1-4-9-20)21-10-5-2-6-11-21/h1-6,8-11,13-16,23-24,33H,7,12,17-18H2,(H,31,34)(H,32,35)(H4,28,29,30)/t23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-UR-MK114 from Y1R in human SK-N-MC cells |
Bioorg Med Chem 19: 2859-78 (2011)
Article DOI: 10.1016/j.bmc.2011.03.045
BindingDB Entry DOI: 10.7270/Q2F47PG7 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50343733
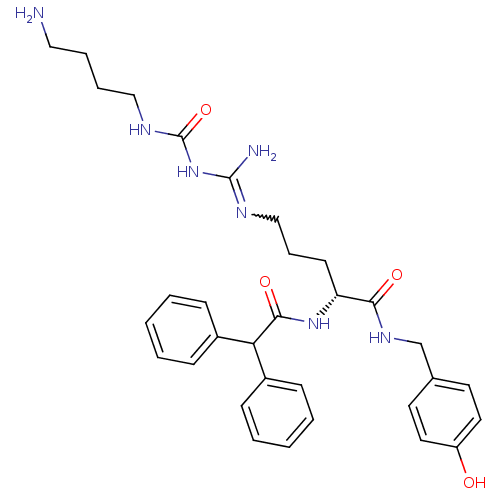
((R)-N-omega-[(4-Aminobutyl)aminocarbonyl]-Na-(2,2-...)Show SMILES NCCCCNC(=O)NC(N)=NCCC[C@@H](NC(=O)C(c1ccccc1)c1ccccc1)C(=O)NCc1ccc(O)cc1 |r,w:11.11| Show InChI InChI=1S/C32H41N7O4/c33-19-7-8-20-36-32(43)39-31(34)35-21-9-14-27(29(41)37-22-23-15-17-26(40)18-16-23)38-30(42)28(24-10-3-1-4-11-24)25-12-5-2-6-13-25/h1-6,10-13,15-18,27-28,40H,7-9,14,19-22,33H2,(H,37,41)(H,38,42)(H4,34,35,36,39,43)/t27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-UR-MK114 from Y1R in human SK-N-MC cells |
Bioorg Med Chem 19: 2859-78 (2011)
Article DOI: 10.1016/j.bmc.2011.03.045
BindingDB Entry DOI: 10.7270/Q2F47PG7 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50060728

((R)-2-(2,2-diphenylacetamido)-5-guanidino-N-(4-hyd...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6](-c1ccccc1)-c1ccccc1)-[#6](=O)-[#7]-[#6]-c1ccc(-[#8])cc1 |r| Show InChI InChI=1S/C27H31N5O3/c28-27(29)30-17-7-12-23(25(34)31-18-19-13-15-22(33)16-14-19)32-26(35)24(20-8-3-1-4-9-20)21-10-5-2-6-11-21/h1-6,8-11,13-16,23-24,33H,7,12,17-18H2,(H,31,34)(H,32,35)(H4,28,29,30)/t23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Displacement of radiolabled NPY from human Y1R expressed in human SK-N-MC cells |
Bioorg Med Chem 19: 2859-78 (2011)
Article DOI: 10.1016/j.bmc.2011.03.045
BindingDB Entry DOI: 10.7270/Q2F47PG7 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50343738
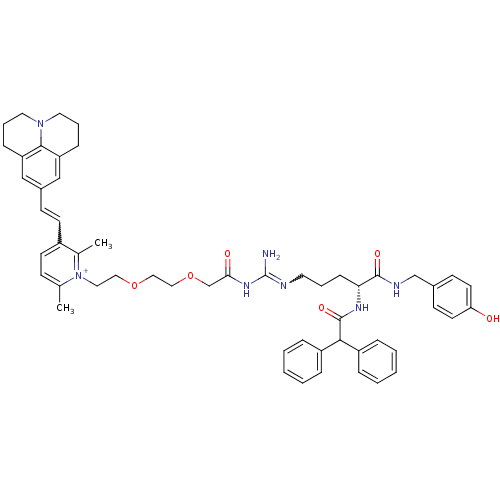
(1-((R)-9-Amino-4-(2,2-diphenylacetamido)-1-(4-hydr...)Show SMILES Cc1ccc(C=Cc2cc3CCCN4CCCc(c2)c34)c(C)[n+]1CCOCCOCC(=O)NC(N)=NCCC[C@@H](NC(=O)C(c1ccccc1)c1ccccc1)C(=O)NCc1ccc(O)cc1 |r,w:35.39,5.4| Show InChI InChI=1S/C54H63N7O6/c1-38-19-23-42(24-20-41-34-45-16-10-28-60-29-11-17-46(35-41)51(45)60)39(2)61(38)30-31-66-32-33-67-37-49(63)59-54(55)56-27-9-18-48(52(64)57-36-40-21-25-47(62)26-22-40)58-53(65)50(43-12-5-3-6-13-43)44-14-7-4-8-15-44/h3-8,12-15,19-26,34-35,48,50H,9-11,16-18,27-33,36-37H2,1-2H3,(H5-,55,56,57,58,59,62,63,64,65)/p+1/t48-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-UR-MK114 from Y1R in human SK-N-MC cells |
Bioorg Med Chem 19: 2859-78 (2011)
Article DOI: 10.1016/j.bmc.2011.03.045
BindingDB Entry DOI: 10.7270/Q2F47PG7 |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50543277

(CHEMBL4642548)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.NCCCCCCNc1c(NCCCOc2cccc(CN3CCCCC3)c2)c(=O)c1=O Show InChI InChI=1S/C25H38N4O3/c26-12-4-1-2-5-13-27-22-23(25(31)24(22)30)28-14-9-17-32-21-11-8-10-20(18-21)19-29-15-6-3-7-16-29/h8,10-11,18,27-28H,1-7,9,12-17,19,26H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]UR-DE257 from Gsalphas-coupled human H2R expressed in baculovirus infected Sf9 cells incubated for 60 mins by scintillation count... |
ACS Med Chem Lett 11: 1521-1528 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00033
BindingDB Entry DOI: 10.7270/Q2W380WD |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50543278

(CHEMBL4644794)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.NCCCCCCCNc1c(NCCCOc2cccc(CN3CCCCC3)c2)c(=O)c1=O Show InChI InChI=1S/C26H40N4O3/c27-13-5-2-1-3-6-14-28-23-24(26(32)25(23)31)29-15-10-18-33-22-12-9-11-21(19-22)20-30-16-7-4-8-17-30/h9,11-12,19,28-29H,1-8,10,13-18,20,27H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]UR-DE257 from Gsalphas-coupled human H2R expressed in baculovirus infected Sf9 cells incubated for 60 mins by scintillation count... |
ACS Med Chem Lett 11: 1521-1528 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00033
BindingDB Entry DOI: 10.7270/Q2W380WD |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50543289
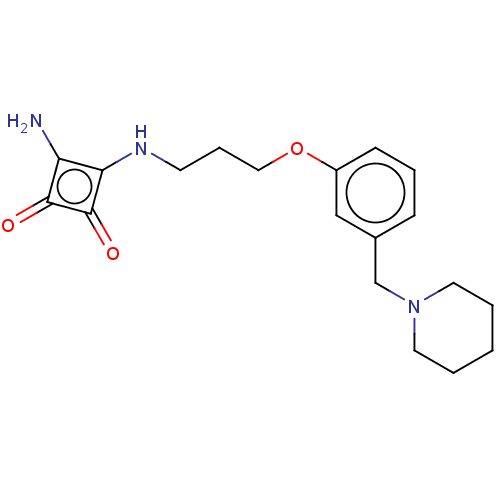
(CHEMBL4643501)Show InChI InChI=1S/C19H25N3O3/c20-16-17(19(24)18(16)23)21-8-5-11-25-15-7-4-6-14(12-15)13-22-9-2-1-3-10-22/h4,6-7,12,21H,1-3,5,8-11,13,20H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Binding affinity to human H2R expressing Sf9 cells incubated for 60 mins by microbeta scintillation counter method |
ACS Med Chem Lett 11: 1521-1528 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00033
BindingDB Entry DOI: 10.7270/Q2W380WD |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50543281

(CHEMBL4644467)Show SMILES OC(=O)C(F)(F)F.[O-]C(=O)C(F)(F)F.CN(C)c1ccc(\C=C\C=C\c2cc(C)[n+](CCCCCCCNc3c(NCCCOc4cccc(CN5CCCCC5)c4)c(=O)c3=O)c(C)c2)cc1 Show InChI InChI=1S/C45H59N5O3/c1-35-31-38(18-10-9-17-37-21-23-40(24-22-37)48(3)4)32-36(2)50(35)29-14-7-5-6-11-25-46-42-43(45(52)44(42)51)47-26-16-30-53-41-20-15-19-39(33-41)34-49-27-12-8-13-28-49/h9-10,15,17-24,31-33H,5-8,11-14,16,25-30,34H2,1-4H3,(H-,46,47,51,52)/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]UR-DE257 from Gsalphas-coupled human H2R expressed in baculovirus infected Sf9 cells incubated for 60 mins by scintillation count... |
ACS Med Chem Lett 11: 1521-1528 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00033
BindingDB Entry DOI: 10.7270/Q2W380WD |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50543279
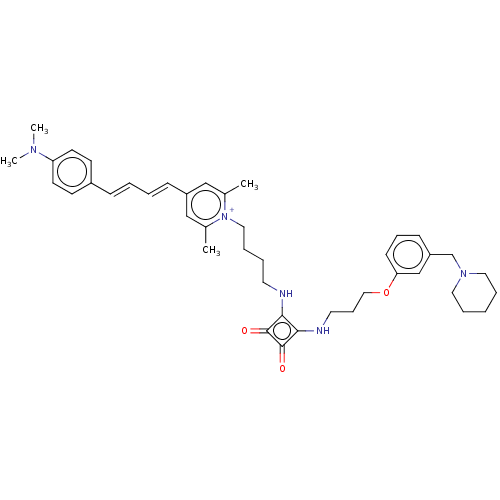
(CHEMBL4641916)Show SMILES OC(=O)C(F)(F)F.[O-]C(=O)C(F)(F)F.CN(C)c1ccc(\C=C\C=C\c2cc(C)[n+](CCCCNc3c(NCCCOc4cccc(CN5CCCCC5)c4)c(=O)c3=O)c(C)c2)cc1 Show InChI InChI=1S/C42H53N5O3/c1-32-28-35(15-7-6-14-34-18-20-37(21-19-34)45(3)4)29-33(2)47(32)26-11-8-22-43-39-40(42(49)41(39)48)44-23-13-27-50-38-17-12-16-36(30-38)31-46-24-9-5-10-25-46/h6-7,12,14-21,28-30H,5,8-11,13,22-27,31H2,1-4H3,(H-,43,44,48,49)/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]UR-DE257 from Gsalphas-coupled human H2R expressed in baculovirus infected Sf9 cells incubated for 60 mins by scintillation count... |
ACS Med Chem Lett 11: 1521-1528 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00033
BindingDB Entry DOI: 10.7270/Q2W380WD |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50343732
((R)-N-omega-(6-Aminohexanoyl)-Na-(2,2-diphenylacet...)Show SMILES NCCCCCC(=O)NC(N)=NCCC[C@@H](NC(=O)C(c1ccccc1)c1ccccc1)C(=O)NCc1ccc(O)cc1 |r,w:11.11| Show InChI InChI=1S/C33H42N6O4/c34-21-9-3-8-16-29(41)39-33(35)36-22-10-15-28(31(42)37-23-24-17-19-27(40)20-18-24)38-32(43)30(25-11-4-1-5-12-25)26-13-6-2-7-14-26/h1-2,4-7,11-14,17-20,28,30,40H,3,8-10,15-16,21-23,34H2,(H,37,42)(H,38,43)(H3,35,36,39,41)/t28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-UR-MK114 from Y1R in human SK-N-MC cells |
Bioorg Med Chem 19: 2859-78 (2011)
Article DOI: 10.1016/j.bmc.2011.03.045
BindingDB Entry DOI: 10.7270/Q2F47PG7 |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50543280
(CHEMBL4637893)Show SMILES OC(=O)C(F)(F)F.[O-]C(=O)C(F)(F)F.CN(C)c1ccc(\C=C\C=C\c2cc(C)[n+](CCCCCCNc3c(NCCCOc4cccc(CN5CCCCC5)c4)c(=O)c3=O)c(C)c2)cc1 Show InChI InChI=1S/C44H57N5O3/c1-34-30-37(17-9-8-16-36-20-22-39(23-21-36)47(3)4)31-35(2)49(34)28-13-6-5-10-24-45-41-42(44(51)43(41)50)46-25-15-29-52-40-19-14-18-38(32-40)33-48-26-11-7-12-27-48/h8-9,14,16-23,30-32H,5-7,10-13,15,24-29,33H2,1-4H3,(H-,45,46,50,51)/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]UR-DE257 from Gsalphas-coupled human H2R expressed in baculovirus infected Sf9 cells incubated for 60 mins by scintillation count... |
ACS Med Chem Lett 11: 1521-1528 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00033
BindingDB Entry DOI: 10.7270/Q2W380WD |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50543283
(CHEMBL4647775)Show SMILES OC(=O)C(F)(F)F.[O-]C(=O)C(F)(F)F.C[N+]1=C(\C=C\C=C\C=C2/N(CCCCCC(=O)NCCCCCCNc3c(NCCCOc4cccc(CN5CCCCC5)c4)c(=O)c3=O)c3ccccc3C2(C)C)C(C)(C)c2ccccc12 |c:13| Show InChI InChI=1S/C57H74N6O4/c1-56(2)45-27-14-16-29-47(45)61(5)49(56)31-11-8-12-32-50-57(3,4)46-28-15-17-30-48(46)63(50)39-22-9-13-33-51(64)58-34-18-6-7-19-35-59-52-53(55(66)54(52)65)60-36-24-40-67-44-26-23-25-43(41-44)42-62-37-20-10-21-38-62/h8,11-12,14-17,23,25-32,41H,6-7,9-10,13,18-22,24,33-40,42H2,1-5H3,(H2-,58,59,60,64,65,66)/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]UR-DE257 from Gsalphas-coupled human H2R expressed in baculovirus infected Sf9 cells incubated for 60 mins by scintillation count... |
ACS Med Chem Lett 11: 1521-1528 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00033
BindingDB Entry DOI: 10.7270/Q2W380WD |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50343729
(1-((R)-9-Amino-4-(2,2-diphenylacetamido)-1-(4-hydr...)Show SMILES Cc1cc(C=Cc2cc3CCCN4CCCc(c2)c34)cc(C)[n+]1CCNC(=O)CCCC(=O)NC(N)=NCCC[C@@H](NC(=O)C(c1ccccc1)c1ccccc1)C(=O)NCc1ccc(O)cc1 |r,w:4.3,36.40| Show InChI InChI=1S/C55H64N8O5/c1-38-33-41(22-23-42-35-45-17-11-30-62-31-12-18-46(36-42)52(45)62)34-39(2)63(38)32-29-57-49(65)20-9-21-50(66)61-55(56)58-28-10-19-48(53(67)59-37-40-24-26-47(64)27-25-40)60-54(68)51(43-13-5-3-6-14-43)44-15-7-4-8-16-44/h3-8,13-16,22-27,33-36,48,51H,9-12,17-21,28-32,37H2,1-2H3,(H6-,56,57,58,59,60,61,64,65,66,67,68)/p+1/t48-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-UR-MK114 from Y1R in human SK-N-MC cells |
Bioorg Med Chem 19: 2859-78 (2011)
Article DOI: 10.1016/j.bmc.2011.03.045
BindingDB Entry DOI: 10.7270/Q2F47PG7 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50343729

(1-((R)-9-Amino-4-(2,2-diphenylacetamido)-1-(4-hydr...)Show SMILES Cc1cc(C=Cc2cc3CCCN4CCCc(c2)c34)cc(C)[n+]1CCNC(=O)CCCC(=O)NC(N)=NCCC[C@@H](NC(=O)C(c1ccccc1)c1ccccc1)C(=O)NCc1ccc(O)cc1 |r,w:4.3,36.40| Show InChI InChI=1S/C55H64N8O5/c1-38-33-41(22-23-42-35-45-17-11-30-62-31-12-18-46(36-42)52(45)62)34-39(2)63(38)32-29-57-49(65)20-9-21-50(66)61-55(56)58-28-10-19-48(53(67)59-37-40-24-26-47(64)27-25-40)60-54(68)51(43-13-5-3-6-14-43)44-15-7-4-8-16-44/h3-8,13-16,22-27,33-36,48,51H,9-12,17-21,28-32,37H2,1-2H3,(H6-,56,57,58,59,60,61,64,65,66,67,68)/p+1/t48-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-UR-MK114 from Y1R in human SK-N-MC cells by flowcytometry |
Bioorg Med Chem 19: 2859-78 (2011)
Article DOI: 10.1016/j.bmc.2011.03.045
BindingDB Entry DOI: 10.7270/Q2F47PG7 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50343735
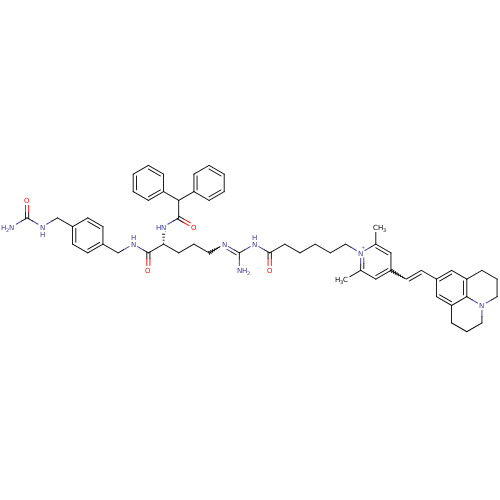
(1-(6-(Amino((R)-4-(2,2-diphenylacetamido)-5-oxo-5-...)Show SMILES Cc1cc(C=Cc2cc3CCCN4CCCc(c2)c34)cc(C)[n+]1CCCCCC(=O)NC(N)=NCCC[C@@H](NC(=O)C(c1ccccc1)c1ccccc1)C(=O)NCc1ccc(CNC(N)=O)cc1 |r,w:33.37,4.3| Show InChI InChI=1S/C56H67N9O4/c1-39-33-43(27-28-44-35-47-19-13-30-64-31-14-20-48(36-44)52(47)64)34-40(2)65(39)32-11-5-10-22-50(66)63-55(57)59-29-12-21-49(53(67)60-37-41-23-25-42(26-24-41)38-61-56(58)69)62-54(68)51(45-15-6-3-7-16-45)46-17-8-4-9-18-46/h3-4,6-9,15-18,23-28,33-36,49,51H,5,10-14,19-22,29-32,37-38H2,1-2H3,(H7-,57,58,59,60,61,62,63,66,67,68,69)/p+1/t49-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-UR-MK114 from Y1R in human SK-N-MC cells |
Bioorg Med Chem 19: 2859-78 (2011)
Article DOI: 10.1016/j.bmc.2011.03.045
BindingDB Entry DOI: 10.7270/Q2F47PG7 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50343741
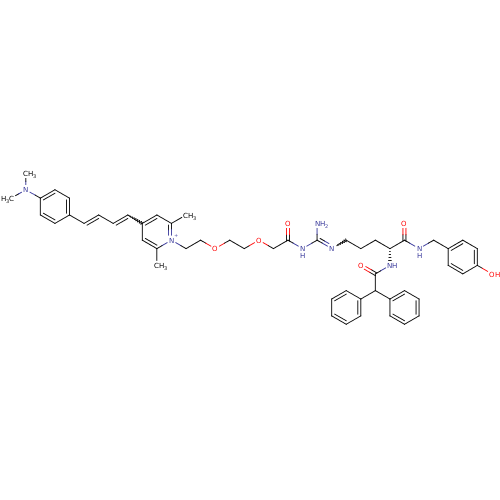
(1-((R)-9-Amino-4-(2,2-diphenylacetamido)-1-(4-hydr...)Show SMILES CN(C)c1ccc(\C=C\C=Cc2cc(C)[n+](CCOCCOCC(=O)NC(N)=NCCC[C@@H](NC(=O)C(c3ccccc3)c3ccccc3)C(=O)NCc3ccc(O)cc3)c(C)c2)cc1 |r,w:10.10,28.28| Show InChI InChI=1S/C52H61N7O6/c1-38-34-42(15-12-11-14-40-21-25-45(26-22-40)58(3)4)35-39(2)59(38)30-31-64-32-33-65-37-48(61)57-52(53)54-29-13-20-47(50(62)55-36-41-23-27-46(60)28-24-41)56-51(63)49(43-16-7-5-8-17-43)44-18-9-6-10-19-44/h5-12,14-19,21-28,34-35,47,49H,13,20,29-33,36-37H2,1-4H3,(H5-,53,54,55,56,57,60,61,62,63)/p+1/t47-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-UR-MK114 from Y1R in human SK-N-MC cells |
Bioorg Med Chem 19: 2859-78 (2011)
Article DOI: 10.1016/j.bmc.2011.03.045
BindingDB Entry DOI: 10.7270/Q2F47PG7 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50343728

(3-((R)-9-Amino-4-(2,2-diphenylacetamido)-1-(4-hydr...)Show SMILES CN(C)c1ccc(C=CC=Cc2sc3ccccc3[n+]2CCCC(=O)NCCCCNC(=O)NC(N)=NCCC[C@@H](NC(=O)C(c2ccccc2)c2ccccc2)C(=O)NCc2ccc(O)cc2)cc1 |r,w:8.8,36.38,10.10| Show InChI InChI=1S/C55H63N9O5S/c1-63(2)44-31-27-40(28-32-44)17-9-12-26-50-64(47-23-10-11-24-48(47)70-50)38-16-25-49(66)57-35-13-14-36-59-55(69)62-54(56)58-37-15-22-46(52(67)60-39-41-29-33-45(65)34-30-41)61-53(68)51(42-18-5-3-6-19-42)43-20-7-4-8-21-43/h3-12,17-21,23-24,26-34,46,51H,13-16,22,25,35-39H2,1-2H3,(H7-,56,57,58,59,60,61,62,65,66,67,68,69)/p+1/t46-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-UR-MK114 from Y1R in human SK-N-MC cells |
Bioorg Med Chem 19: 2859-78 (2011)
Article DOI: 10.1016/j.bmc.2011.03.045
BindingDB Entry DOI: 10.7270/Q2F47PG7 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50343728

(3-((R)-9-Amino-4-(2,2-diphenylacetamido)-1-(4-hydr...)Show SMILES CN(C)c1ccc(C=CC=Cc2sc3ccccc3[n+]2CCCC(=O)NCCCCNC(=O)NC(N)=NCCC[C@@H](NC(=O)C(c2ccccc2)c2ccccc2)C(=O)NCc2ccc(O)cc2)cc1 |r,w:8.8,36.38,10.10| Show InChI InChI=1S/C55H63N9O5S/c1-63(2)44-31-27-40(28-32-44)17-9-12-26-50-64(47-23-10-11-24-48(47)70-50)38-16-25-49(66)57-35-13-14-36-59-55(69)62-54(56)58-37-15-22-46(52(67)60-39-41-29-33-45(65)34-30-41)61-53(68)51(42-18-5-3-6-19-42)43-20-7-4-8-21-43/h3-12,17-21,23-24,26-34,46,51H,13-16,22,25,35-39H2,1-2H3,(H7-,56,57,58,59,60,61,62,65,66,67,68,69)/p+1/t46-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-UR-MK114 from Y1R in human SK-N-MC cells by flowcytometry |
Bioorg Med Chem 19: 2859-78 (2011)
Article DOI: 10.1016/j.bmc.2011.03.045
BindingDB Entry DOI: 10.7270/Q2F47PG7 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50343730
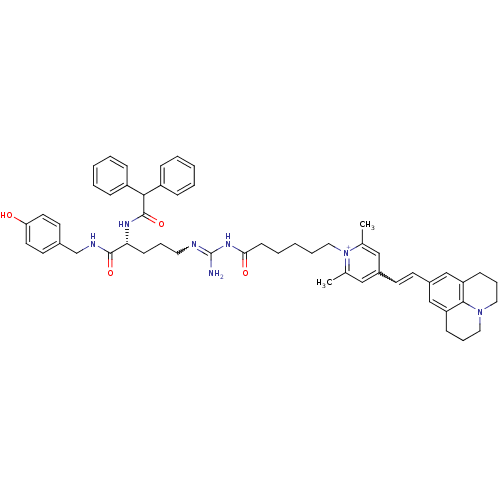
(1-(6-((Z)-amino((R)-4-(2,2-diphenylacetamido)-5-(4...)Show SMILES Cc1cc(C=Cc2cc3CCCN4CCCc(c2)c34)cc(C)[n+]1CCCCCC(=O)NC(N)=NCCC[C@@H](NC(=O)C(c1ccccc1)c1ccccc1)C(=O)NCc1ccc(O)cc1 |r,w:4.3,33.37| Show InChI InChI=1S/C54H63N7O4/c1-38-33-41(23-24-42-35-45-19-13-30-60-31-14-20-46(36-42)51(45)60)34-39(2)61(38)32-11-5-10-22-49(63)59-54(55)56-29-12-21-48(52(64)57-37-40-25-27-47(62)28-26-40)58-53(65)50(43-15-6-3-7-16-43)44-17-8-4-9-18-44/h3-4,6-9,15-18,23-28,33-36,48,50H,5,10-14,19-22,29-32,37H2,1-2H3,(H5-,55,56,57,58,59,62,63,64,65)/p+1/t48-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-UR-MK114 from Y1R in human SK-N-MC cells |
Bioorg Med Chem 19: 2859-78 (2011)
Article DOI: 10.1016/j.bmc.2011.03.045
BindingDB Entry DOI: 10.7270/Q2F47PG7 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50343736
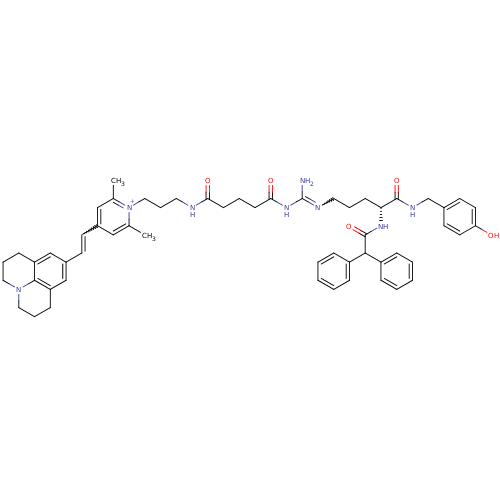
(1-((R)-9-Amino-4-(2,2-diphenylacetamido)-1-(4-hydr...)Show SMILES Cc1cc(C=Cc2cc3CCCN4CCCc(c2)c34)cc(C)[n+]1CCCNC(=O)CCCC(=O)NC(N)=NCCC[C@@H](NC(=O)C(c1ccccc1)c1ccccc1)C(=O)NCc1ccc(O)cc1 |r,w:37.41,4.3| Show InChI InChI=1S/C56H66N8O5/c1-39-34-42(23-24-43-36-46-18-11-31-63-32-12-19-47(37-43)53(46)63)35-40(2)64(39)33-13-30-58-50(66)21-9-22-51(67)62-56(57)59-29-10-20-49(54(68)60-38-41-25-27-48(65)28-26-41)61-55(69)52(44-14-5-3-6-15-44)45-16-7-4-8-17-45/h3-8,14-17,23-28,34-37,49,52H,9-13,18-22,29-33,38H2,1-2H3,(H6-,57,58,59,60,61,62,65,66,67,68,69)/p+1/t49-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-UR-MK114 from Y1R in human SK-N-MC cells |
Bioorg Med Chem 19: 2859-78 (2011)
Article DOI: 10.1016/j.bmc.2011.03.045
BindingDB Entry DOI: 10.7270/Q2F47PG7 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50343734
((R)-5-(3-(4-aminobutyl)-1-carbamimidoylureido)-2-(...)Show SMILES NCCCCNC(=O)N(CCC[C@@H](NC(=O)C(c1ccccc1)c1ccccc1)C(=O)NCc1ccc(O)cc1)C(N)=N |r| Show InChI InChI=1S/C32H41N7O4/c33-19-7-8-20-36-32(43)39(31(34)35)21-9-14-27(29(41)37-22-23-15-17-26(40)18-16-23)38-30(42)28(24-10-3-1-4-11-24)25-12-5-2-6-13-25/h1-6,10-13,15-18,27-28,40H,7-9,14,19-22,33H2,(H3,34,35)(H,36,43)(H,37,41)(H,38,42)/t27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-UR-MK114 from Y1R in human SK-N-MC cells |
Bioorg Med Chem 19: 2859-78 (2011)
Article DOI: 10.1016/j.bmc.2011.03.045
BindingDB Entry DOI: 10.7270/Q2F47PG7 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50343746
(3-((R)-9-Amino-4-(2,2-diphenylacetamido)-1-(4-hydr...)Show SMILES CN(C)c1ccc(C=CC=Cc2sc3ccccc3[n+]2CCCC(=O)NCCNC(=O)CCCC(=O)NC(N)=NCCC[C@@H](NC(=O)C(c2ccccc2)c2ccccc2)C(=O)NCc2ccc(O)cc2)cc1 |r,w:8.8,39.41,10.10| Show InChI InChI=1S/C57H65N9O6S/c1-65(2)45-32-28-41(29-33-45)16-9-12-27-53-66(48-22-10-11-23-49(48)73-53)39-15-26-51(69)60-38-37-59-50(68)24-13-25-52(70)64-57(58)61-36-14-21-47(55(71)62-40-42-30-34-46(67)35-31-42)63-56(72)54(43-17-5-3-6-18-43)44-19-7-4-8-20-44/h3-12,16-20,22-23,27-35,47,54H,13-15,21,24-26,36-40H2,1-2H3,(H7-,58,59,60,61,62,63,64,67,68,69,70,71,72)/p+1/t47-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-UR-MK114 from Y1R in human SK-N-MC cells |
Bioorg Med Chem 19: 2859-78 (2011)
Article DOI: 10.1016/j.bmc.2011.03.045
BindingDB Entry DOI: 10.7270/Q2F47PG7 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50343740
(1-(6-(Amino((R)-4-(2,2-diphenylacetamido)-5-(4-hyd...)Show SMILES CN(C)c1ccc(\C=C\C=Cc2cc(C)[n+](CCCCCC(=O)NC(N)=NCCC[C@@H](NC(=O)C(c3ccccc3)c3ccccc3)C(=O)NCc3ccc(O)cc3)c(C)c2)cc1 |r,w:26.26,10.10| Show InChI InChI=1S/C52H61N7O4/c1-38-35-42(18-14-13-17-40-25-29-45(30-26-40)58(3)4)36-39(2)59(38)34-15-7-12-24-48(61)57-52(53)54-33-16-23-47(50(62)55-37-41-27-31-46(60)32-28-41)56-51(63)49(43-19-8-5-9-20-43)44-21-10-6-11-22-44/h5-6,8-11,13-14,17-22,25-32,35-36,47,49H,7,12,15-16,23-24,33-34,37H2,1-4H3,(H5-,53,54,55,56,57,60,61,62,63)/p+1/t47-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-UR-MK114 from Y1R in human SK-N-MC cells |
Bioorg Med Chem 19: 2859-78 (2011)
Article DOI: 10.1016/j.bmc.2011.03.045
BindingDB Entry DOI: 10.7270/Q2F47PG7 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50343749
(1-((R)-9-Amino-24-((R)-9-amino-4-(2,2-diphenylacet...)Show SMILES Cc1cc(C=Cc2cc3CCCN4CCCc(c2)c34)cc(C)[n+]1CCN(CCNC(=O)CCCNC(=O)CCCC(=O)NC(=N)NCCC[C@@H](NC(=O)C(c1ccccc1)c1ccccc1)C(=O)NCc1ccc(O)cc1)CCNC(=O)CCCNC(=O)CCCC(=O)NC(N)=NCCC[C@@H](NC(=O)C(c1ccccc1)c1ccccc1)C(=O)NCc1ccc(O)cc1 |r,w:96.103,4.3| Show InChI InChI=1S/C99H123N17O12/c1-69-63-73(41-42-74-65-79-31-21-57-115-58-22-32-80(66-74)93(79)115)64-70(2)116(69)62-61-114(59-55-104-87(121)39-19-51-102-85(119)35-15-37-89(123)112-98(100)106-53-17-33-83(94(125)108-67-71-43-47-81(117)48-44-71)110-96(127)91(75-23-7-3-8-24-75)76-25-9-4-10-26-76)60-56-105-88(122)40-20-52-103-86(120)36-16-38-90(124)113-99(101)107-54-18-34-84(95(126)109-68-72-45-49-82(118)50-46-72)111-97(128)92(77-27-11-5-12-28-77)78-29-13-6-14-30-78/h3-14,23-30,41-50,63-66,83-84,91-92H,15-22,31-40,51-62,67-68H2,1-2H3,(H15-,100,101,102,103,104,105,106,107,108,109,110,111,112,113,117,118,119,120,121,122,123,124,125,126,127,128)/p+1/t83-,84-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-UR-MK114 from Y1R in human SK-N-MC cells |
Bioorg Med Chem 19: 2859-78 (2011)
Article DOI: 10.1016/j.bmc.2011.03.045
BindingDB Entry DOI: 10.7270/Q2F47PG7 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50343747
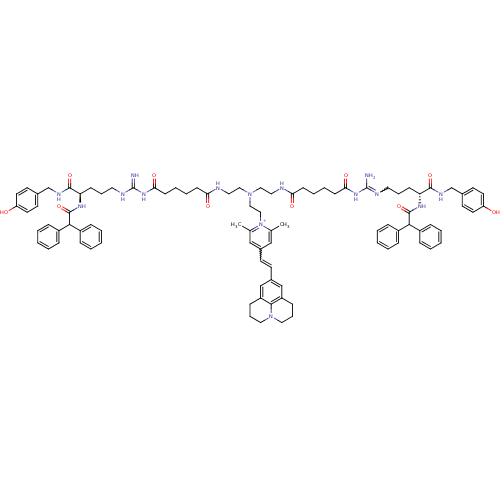
(1-((R)-9-Amino-20-((R)-9-amino-4-(2,2-diphenylacet...)Show SMILES Cc1cc(C=Cc2cc3CCCN4CCCc(c2)c34)cc(C)[n+]1CCN(CCNC(=O)CCCCC(=O)NC(=N)NCCC[C@@H](NC(=O)C(c1ccccc1)c1ccccc1)C(=O)NCc1ccc(O)cc1)CCNC(=O)CCCCC(=O)NC(N)=NCCC[C@@H](NC(=O)C(c1ccccc1)c1ccccc1)C(=O)NCc1ccc(O)cc1 |r,w:86.93,4.3| Show InChI InChI=1S/C93H113N15O10/c1-65-59-69(39-40-70-61-75-31-21-53-107-54-22-32-76(62-70)87(75)107)60-66(2)108(65)58-57-106(55-51-96-81(111)35-15-17-37-83(113)104-92(94)98-49-19-33-79(88(115)100-63-67-41-45-77(109)46-42-67)102-90(117)85(71-23-7-3-8-24-71)72-25-9-4-10-26-72)56-52-97-82(112)36-16-18-38-84(114)105-93(95)99-50-20-34-80(89(116)101-64-68-43-47-78(110)48-44-68)103-91(118)86(73-27-11-5-12-28-73)74-29-13-6-14-30-74/h3-14,23-30,39-48,59-62,79-80,85-86H,15-22,31-38,49-58,63-64H2,1-2H3,(H13-,94,95,96,97,98,99,100,101,102,103,104,105,109,110,111,112,113,114,115,116,117,118)/p+1/t79-,80-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-UR-MK114 from Y1R in human SK-N-MC cells |
Bioorg Med Chem 19: 2859-78 (2011)
Article DOI: 10.1016/j.bmc.2011.03.045
BindingDB Entry DOI: 10.7270/Q2F47PG7 |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50543285

(CHEMBL4637356)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.CC1(C)\C(=C\C=C\C=C\C2=[N+](CCCCS([O-])(=O)=O)c3ccccc3C2(C)C)N(CCCCCC(=O)NCCCCCCNc2c(NCCCOc3cccc(CN4CCCCC4)c3)c(=O)c2=O)c2ccccc12 |c:21| Show InChI InChI=1S/C60H80N6O7S/c1-59(2)48-28-13-15-30-50(48)65(52(59)32-10-7-11-33-53-60(3,4)49-29-14-16-31-51(49)66(53)41-22-23-43-74(70,71)72)40-21-8-12-34-54(67)61-35-17-5-6-18-36-62-55-56(58(69)57(55)68)63-37-25-42-73-47-27-24-26-46(44-47)45-64-38-19-9-20-39-64/h7,10-11,13-16,24,26-33,44H,5-6,8-9,12,17-23,25,34-43,45H2,1-4H3,(H3-,61,62,63,67,68,69,70,71,72) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]UR-DE257 from Gsalphas-coupled human H2R expressed in baculovirus infected Sf9 cells incubated for 60 mins by scintillation count... |
ACS Med Chem Lett 11: 1521-1528 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00033
BindingDB Entry DOI: 10.7270/Q2W380WD |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50343743

(1-((R)-9-Amino-4-(2,2-diphenylacetamido)-1-(4-hydr...)Show SMILES CN1\C(=C/C=Cc2cc(C)[n+](CCOCCOCCNC(=O)CCCC(=O)NC(N)=NCCC[C@@H](NC(=O)C(c3ccccc3)c3ccccc3)C(=O)NCc3ccc(O)cc3)c(C)c2)C(C)(C)c2c1ccc1ccccc21 |r,w:5.5,30.30| Show InChI InChI=1S/C63H74N8O7/c1-44-41-47(17-14-25-55-63(3,4)59-52-23-13-12-18-48(52)30-33-54(59)70(55)5)42-45(2)71(44)36-38-78-40-39-77-37-35-65-56(73)26-15-27-57(74)69-62(64)66-34-16-24-53(60(75)67-43-46-28-31-51(72)32-29-46)68-61(76)58(49-19-8-6-9-20-49)50-21-10-7-11-22-50/h6-14,17-23,25,28-33,41-42,53,58H,15-16,24,26-27,34-40,43H2,1-5H3,(H6-,64,65,66,67,68,69,72,73,74,75,76)/p+1/t53-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-UR-MK114 from Y1R in human SK-N-MC cells |
Bioorg Med Chem 19: 2859-78 (2011)
Article DOI: 10.1016/j.bmc.2011.03.045
BindingDB Entry DOI: 10.7270/Q2F47PG7 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50343737
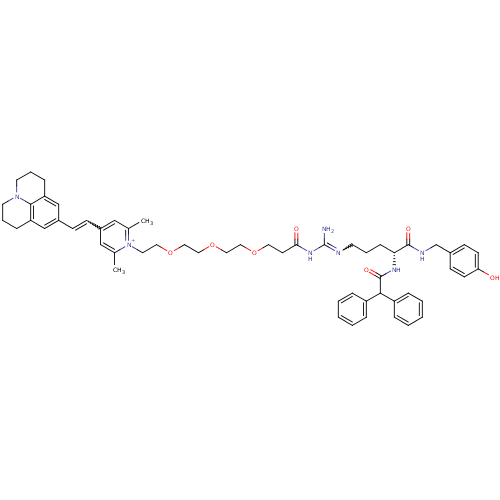
(1-((R)-9-Amino-4-(2,2-diphenylacetamido)-1-(4-hydr...)Show SMILES Cc1cc(C=Cc2cc3CCCN4CCCc(c2)c34)cc(C)[n+]1CCOCCOCCOCCC(=O)NC(N)=NCCC[C@@H](NC(=O)C(c1ccccc1)c1ccccc1)C(=O)NCc1ccc(O)cc1 |r,w:39.43,4.3| Show InChI InChI=1S/C57H69N7O7/c1-41-36-44(19-20-45-38-48-16-10-27-63-28-11-17-49(39-45)54(48)63)37-42(2)64(41)29-31-70-33-35-71-34-32-69-30-25-52(66)62-57(58)59-26-9-18-51(55(67)60-40-43-21-23-50(65)24-22-43)61-56(68)53(46-12-5-3-6-13-46)47-14-7-4-8-15-47/h3-8,12-15,19-24,36-39,51,53H,9-11,16-18,25-35,40H2,1-2H3,(H5-,58,59,60,61,62,65,66,67,68)/p+1/t51-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-UR-MK114 from Y1R in human SK-N-MC cells |
Bioorg Med Chem 19: 2859-78 (2011)
Article DOI: 10.1016/j.bmc.2011.03.045
BindingDB Entry DOI: 10.7270/Q2F47PG7 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50343748
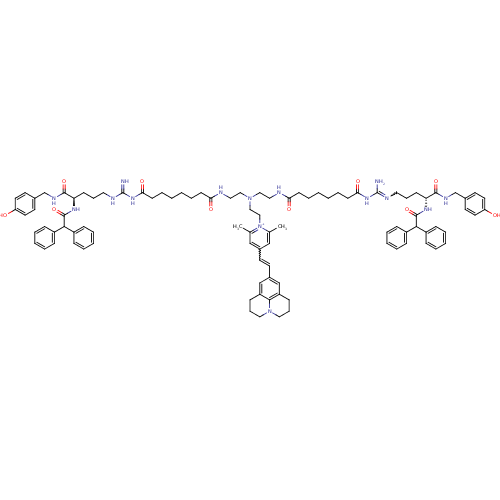
(1-((R)-9-Amino-22-((R)-9-amino-4-(2,2-diphenylacet...)Show SMILES Cc1cc(C=Cc2cc3CCCN4CCCc(c2)c34)cc(C)[n+]1CCN(CCNC(=O)CCCCCCC(=O)NC(=N)NCCC[C@@H](NC(=O)C(c1ccccc1)c1ccccc1)C(=O)NCc1ccc(O)cc1)CCNC(=O)CCCCCCC(=O)NC(N)=NCCC[C@@H](NC(=O)C(c1ccccc1)c1ccccc1)C(=O)NCc1ccc(O)cc1 |r,w:4.3,90.97| Show InChI InChI=1S/C97H121N15O10/c1-69-63-73(43-44-74-65-79-35-25-57-111-58-26-36-80(66-74)91(79)111)64-70(2)112(69)62-61-110(59-55-100-85(115)39-19-3-5-21-41-87(117)108-96(98)102-53-23-37-83(92(119)104-67-71-45-49-81(113)50-46-71)106-94(121)89(75-27-11-7-12-28-75)76-29-13-8-14-30-76)60-56-101-86(116)40-20-4-6-22-42-88(118)109-97(99)103-54-24-38-84(93(120)105-68-72-47-51-82(114)52-48-72)107-95(122)90(77-31-15-9-16-32-77)78-33-17-10-18-34-78/h7-18,27-34,43-52,63-66,83-84,89-90H,3-6,19-26,35-42,53-62,67-68H2,1-2H3,(H13-,98,99,100,101,102,103,104,105,106,107,108,109,113,114,115,116,117,118,119,120,121,122)/p+1/t83-,84-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-UR-MK114 from Y1R in human SK-N-MC cells |
Bioorg Med Chem 19: 2859-78 (2011)
Article DOI: 10.1016/j.bmc.2011.03.045
BindingDB Entry DOI: 10.7270/Q2F47PG7 |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(RAT) | BDBM50404029
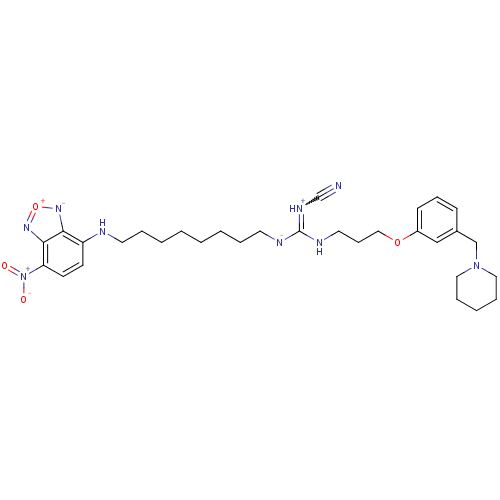
(CHEMBL45728)Show SMILES [O-][N+](=O)c1ccc(NCCCCCCCC[N-]C(NCCCOc2cccc(CN3CCCCC3)c2)=[NH+]C#N)c2[n-][o+]nc12 |w:36.38| Show InChI InChI=1S/C31H42N9O4/c32-24-36-31(35-18-11-21-43-26-13-10-12-25(22-26)23-39-19-8-5-9-20-39)34-17-7-4-2-1-3-6-16-33-27-14-15-28(40(41)42)30-29(27)37-44-38-30/h10,12-15,22,33H,1-9,11,16-21,23H2,(H-,34,35,36)/q-1/p+1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [125I]-aminopotentidine from rat H2R expressed in human COS7 cells incubated for 90 mins gamma counting method |
ACS Med Chem Lett 11: 1521-1528 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00033
BindingDB Entry DOI: 10.7270/Q2W380WD |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(RAT) | BDBM50543288
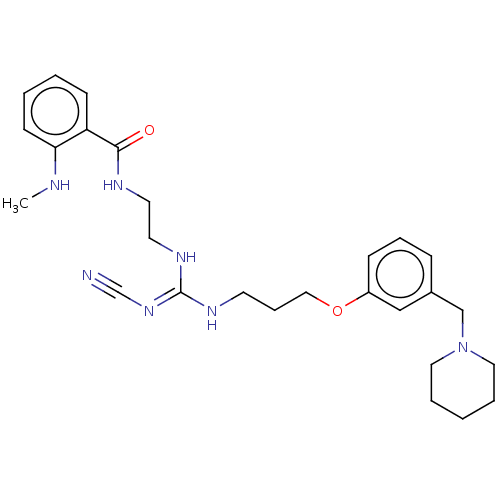
(CHEMBL4638742)Show SMILES CNc1ccccc1C(=O)NCCN\C(NCCCOc1cccc(CN2CCCCC2)c1)=N/C#N Show InChI InChI=1S/C27H37N7O2/c1-29-25-12-4-3-11-24(25)26(35)30-14-15-32-27(33-21-28)31-13-8-18-36-23-10-7-9-22(19-23)20-34-16-5-2-6-17-34/h3-4,7,9-12,19,29H,2,5-6,8,13-18,20H2,1H3,(H,30,35)(H2,31,32,33) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [125I]-aminopotentidine from rat H2R expressed in human COS7 cells incubated for 90 mins gamma counting method |
ACS Med Chem Lett 11: 1521-1528 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00033
BindingDB Entry DOI: 10.7270/Q2W380WD |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50343745
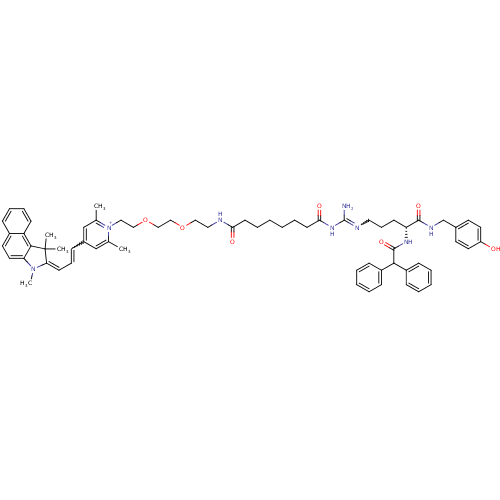
(1-((R)-9-Amino-4-(2,2-diphenylacetamido)-1-(4-hydr...)Show SMILES CN1\C(=C\C=Cc2cc(C)[n+](CCOCCOCCNC(=O)CCCCCCC(=O)NC(N)=NCCC[C@@H](NC(=O)C(c3ccccc3)c3ccccc3)C(=O)NCc3ccc(O)cc3)c(C)c2)C(C)(C)c2c1ccc1ccccc21 |r,w:33.33,5.5| Show InChI InChI=1S/C66H80N8O7/c1-47-44-50(20-18-28-58-66(3,4)62-55-26-17-16-21-51(55)33-36-57(62)73(58)5)45-48(2)74(47)39-41-81-43-42-80-40-38-68-59(76)29-14-6-7-15-30-60(77)72-65(67)69-37-19-27-56(63(78)70-46-49-31-34-54(75)35-32-49)71-64(79)61(52-22-10-8-11-23-52)53-24-12-9-13-25-53/h8-13,16-18,20-26,28,31-36,44-45,56,61H,6-7,14-15,19,27,29-30,37-43,46H2,1-5H3,(H6-,67,68,69,70,71,72,75,76,77,78,79)/p+1/t56-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-UR-MK114 from Y1R in human SK-N-MC cells |
Bioorg Med Chem 19: 2859-78 (2011)
Article DOI: 10.1016/j.bmc.2011.03.045
BindingDB Entry DOI: 10.7270/Q2F47PG7 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50343744
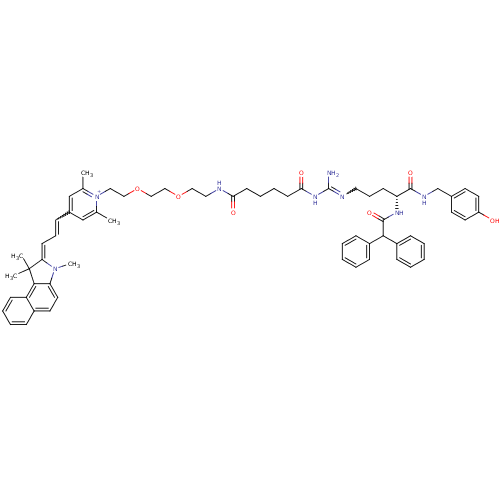
(1-((R)-9-Amino-4-(2,2-diphenylacetamido)-1-(4-hydr...)Show SMILES CN1\C(=C/C=Cc2cc(C)[n+](CCOCCOCCNC(=O)CCCCC(=O)NC(N)=NCCC[C@@H](NC(=O)C(c3ccccc3)c3ccccc3)C(=O)NCc3ccc(O)cc3)c(C)c2)C(C)(C)c2c1ccc1ccccc21 |r,w:31.31,5.5| Show InChI InChI=1S/C64H76N8O7/c1-45-42-48(18-16-26-56-64(3,4)60-53-24-13-12-19-49(53)31-34-55(60)71(56)5)43-46(2)72(45)37-39-79-41-40-78-38-36-66-57(74)27-14-15-28-58(75)70-63(65)67-35-17-25-54(61(76)68-44-47-29-32-52(73)33-30-47)69-62(77)59(50-20-8-6-9-21-50)51-22-10-7-11-23-51/h6-13,16,18-24,26,29-34,42-43,54,59H,14-15,17,25,27-28,35-41,44H2,1-5H3,(H6-,65,66,67,68,69,70,73,74,75,76,77)/p+1/t54-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-UR-MK114 from Y1R in human SK-N-MC cells |
Bioorg Med Chem 19: 2859-78 (2011)
Article DOI: 10.1016/j.bmc.2011.03.045
BindingDB Entry DOI: 10.7270/Q2F47PG7 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50343739
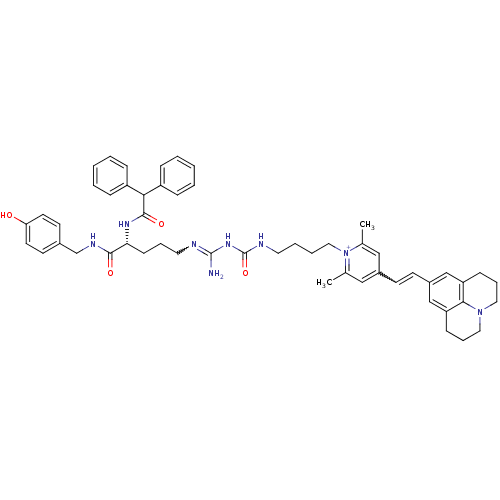
(1-((R)-9-Amino-4-(2,2-diphenylacetamido)-1-(4-hydr...)Show SMILES Cc1cc(C=Cc2cc3CCCN4CCCc(c2)c34)cc(C)[n+]1CCCCNC(=O)NC(N)=NCCC[C@@H](NC(=O)C(c1ccccc1)c1ccccc1)C(=O)NCc1ccc(O)cc1 |r,w:33.37,4.3| Show InChI InChI=1S/C53H62N8O4/c1-37-32-40(21-22-41-34-44-18-12-29-60-30-13-19-45(35-41)49(44)60)33-38(2)61(37)31-10-9-27-56-53(65)59-52(54)55-28-11-20-47(50(63)57-36-39-23-25-46(62)26-24-39)58-51(64)48(42-14-5-3-6-15-42)43-16-7-4-8-17-43/h3-8,14-17,21-26,32-35,47-48H,9-13,18-20,27-31,36H2,1-2H3,(H6-,54,55,56,57,58,59,62,63,64,65)/p+1/t47-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-UR-MK114 from Y1R in human SK-N-MC cells |
Bioorg Med Chem 19: 2859-78 (2011)
Article DOI: 10.1016/j.bmc.2011.03.045
BindingDB Entry DOI: 10.7270/Q2F47PG7 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50343742
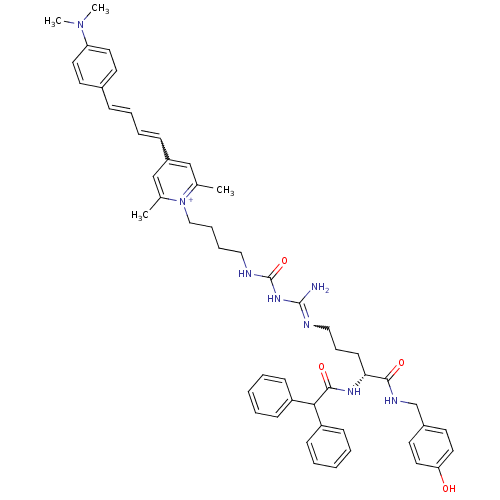
(1-((R)-9-Amino-4-(2,2-diphenylacetamido)-1-(4-hydr...)Show SMILES CN(C)c1ccc(\C=C\C=Cc2cc(C)[n+](CCCCNC(=O)NC(N)=NCCC[C@@H](NC(=O)C(c3ccccc3)c3ccccc3)C(=O)NCc3ccc(O)cc3)c(C)c2)cc1 |r,w:26.26,10.10| Show InChI InChI=1S/C51H60N8O4/c1-37-34-41(17-12-11-16-39-23-27-44(28-24-39)58(3)4)35-38(2)59(37)33-14-13-31-54-51(63)57-50(52)53-32-15-22-46(48(61)55-36-40-25-29-45(60)30-26-40)56-49(62)47(42-18-7-5-8-19-42)43-20-9-6-10-21-43/h5-12,16-21,23-30,34-35,46-47H,13-15,22,31-33,36H2,1-4H3,(H6-,52,53,54,55,56,57,60,61,62,63)/p+1/t46-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-UR-MK114 from Y1R in human SK-N-MC cells |
Bioorg Med Chem 19: 2859-78 (2011)
Article DOI: 10.1016/j.bmc.2011.03.045
BindingDB Entry DOI: 10.7270/Q2F47PG7 |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50543282
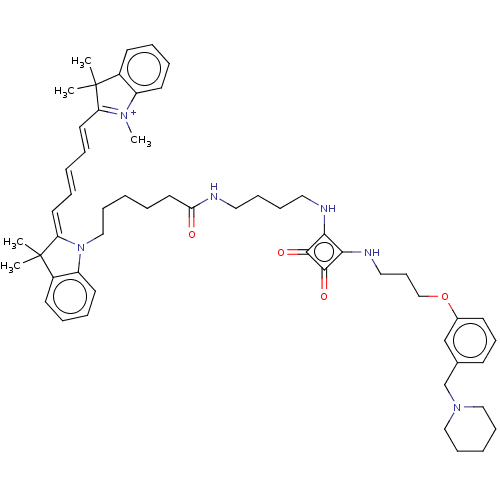
(CHEMBL4645867)Show SMILES OC(=O)C(F)(F)F.[O-]C(=O)C(F)(F)F.C[N+]1=C(\C=C\C=C\C=C2/N(CCCCCC(=O)NCCCCNc3c(NCCCOc4cccc(CN5CCCCC5)c4)c(=O)c3=O)c3ccccc3C2(C)C)C(C)(C)c2ccccc12 |c:13| Show InChI InChI=1S/C55H70N6O4/c1-54(2)43-25-12-14-27-45(43)59(5)47(54)29-9-6-10-30-48-55(3,4)44-26-13-15-28-46(44)61(48)37-20-7-11-31-49(62)56-32-16-17-33-57-50-51(53(64)52(50)63)58-34-22-38-65-42-24-21-23-41(39-42)40-60-35-18-8-19-36-60/h6,9-10,12-15,21,23-30,39H,7-8,11,16-20,22,31-38,40H2,1-5H3,(H2-,56,57,58,62,63,64)/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 269 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]UR-DE257 from Gsalphas-coupled human H2R expressed in baculovirus infected Sf9 cells incubated for 60 mins by scintillation count... |
ACS Med Chem Lett 11: 1521-1528 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00033
BindingDB Entry DOI: 10.7270/Q2W380WD |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50121205

(CHEBI:18295 | Histamine)Show InChI InChI=1S/C5H9N3/c6-2-1-5-3-7-4-8-5/h3-4H,1-2,6H2,(H,7,8) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 295 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]UR-DE257 from Gsalphas-coupled human H2R expressed in baculovirus infected Sf9 cells incubated for 60 mins by scintillation count... |
ACS Med Chem Lett 11: 1521-1528 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00033
BindingDB Entry DOI: 10.7270/Q2W380WD |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50543276
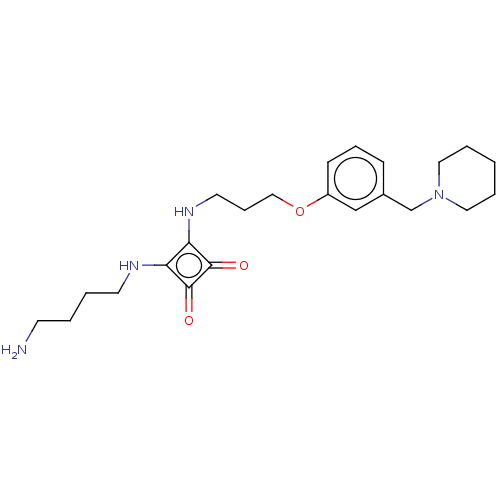
(CHEMBL4632998)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.NCCCCNc1c(NCCCOc2cccc(CN3CCCCC3)c2)c(=O)c1=O Show InChI InChI=1S/C23H34N4O3/c24-10-2-3-11-25-20-21(23(29)22(20)28)26-12-7-15-30-19-9-6-8-18(16-19)17-27-13-4-1-5-14-27/h6,8-9,16,25-26H,1-5,7,10-15,17,24H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 302 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]UR-DE257 from Gsalphas-coupled human H2R expressed in baculovirus infected Sf9 cells incubated for 60 mins by scintillation count... |
ACS Med Chem Lett 11: 1521-1528 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00033
BindingDB Entry DOI: 10.7270/Q2W380WD |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50543284
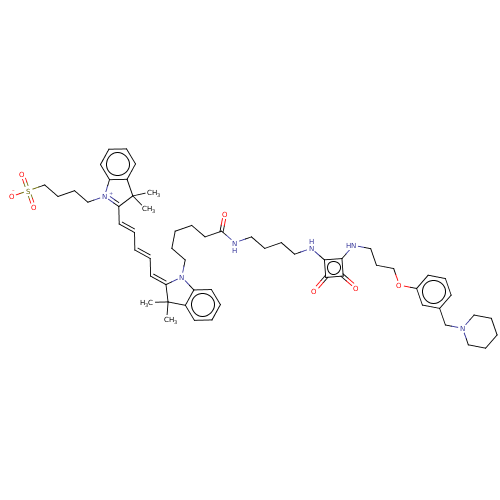
(CHEMBL4645213)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.CC1(C)\C(=C\C=C\C=C\C2=[N+](CCCCS([O-])(=O)=O)c3ccccc3C2(C)C)N(CCCCCC(=O)NCCCCNc2c(NCCCOc3cccc(CN4CCCCC4)c3)c(=O)c2=O)c2ccccc12 |c:21| Show InChI InChI=1S/C58H76N6O7S/c1-57(2)46-26-11-13-28-48(46)63(50(57)30-8-5-9-31-51-58(3,4)47-27-12-14-29-49(47)64(51)39-20-21-41-72(68,69)70)38-19-6-10-32-52(65)59-33-15-16-34-60-53-54(56(67)55(53)66)61-35-23-40-71-45-25-22-24-44(42-45)43-62-36-17-7-18-37-62/h5,8-9,11-14,22,24-31,42H,6-7,10,15-21,23,32-41,43H2,1-4H3,(H3-,59,60,61,65,66,67,68,69,70) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 324 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]UR-DE257 from Gsalphas-coupled human H2R expressed in baculovirus infected Sf9 cells incubated for 60 mins by scintillation count... |
ACS Med Chem Lett 11: 1521-1528 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00033
BindingDB Entry DOI: 10.7270/Q2W380WD |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50343728

(3-((R)-9-Amino-4-(2,2-diphenylacetamido)-1-(4-hydr...)Show SMILES CN(C)c1ccc(C=CC=Cc2sc3ccccc3[n+]2CCCC(=O)NCCCCNC(=O)NC(N)=NCCC[C@@H](NC(=O)C(c2ccccc2)c2ccccc2)C(=O)NCc2ccc(O)cc2)cc1 |r,w:8.8,36.38,10.10| Show InChI InChI=1S/C55H63N9O5S/c1-63(2)44-31-27-40(28-32-44)17-9-12-26-50-64(47-23-10-11-24-48(47)70-50)38-16-25-49(66)57-35-13-14-36-59-55(69)62-54(56)58-37-15-22-46(52(67)60-39-41-29-33-45(65)34-30-41)61-53(68)51(42-18-5-3-6-19-42)43-20-7-4-8-21-43/h3-12,17-21,23-24,26-34,46,51H,13-16,22,25,35-39H2,1-2H3,(H7-,56,57,58,59,60,61,62,65,66,67,68,69)/p+1/t46-/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Displacement of Dy-635-pNPY from human Y5R expressed in CHO cells by flowcytometry |
Bioorg Med Chem 19: 2859-78 (2011)
Article DOI: 10.1016/j.bmc.2011.03.045
BindingDB Entry DOI: 10.7270/Q2F47PG7 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 4
(Homo sapiens (Human)) | BDBM50343728

(3-((R)-9-Amino-4-(2,2-diphenylacetamido)-1-(4-hydr...)Show SMILES CN(C)c1ccc(C=CC=Cc2sc3ccccc3[n+]2CCCC(=O)NCCCCNC(=O)NC(N)=NCCC[C@@H](NC(=O)C(c2ccccc2)c2ccccc2)C(=O)NCc2ccc(O)cc2)cc1 |r,w:8.8,36.38,10.10| Show InChI InChI=1S/C55H63N9O5S/c1-63(2)44-31-27-40(28-32-44)17-9-12-26-50-64(47-23-10-11-24-48(47)70-50)38-16-25-49(66)57-35-13-14-36-59-55(69)62-54(56)58-37-15-22-46(52(67)60-39-41-29-33-45(65)34-30-41)61-53(68)51(42-18-5-3-6-19-42)43-20-7-4-8-21-43/h3-12,17-21,23-24,26-34,46,51H,13-16,22,25,35-39H2,1-2H3,(H7-,56,57,58,59,60,61,62,65,66,67,68,69)/p+1/t46-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Displacement of Cy5-[K4]-hpp from human Y4R expressed in CHO cells by flowcytometry |
Bioorg Med Chem 19: 2859-78 (2011)
Article DOI: 10.1016/j.bmc.2011.03.045
BindingDB Entry DOI: 10.7270/Q2F47PG7 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 2
(Homo sapiens (Human)) | BDBM50343728

(3-((R)-9-Amino-4-(2,2-diphenylacetamido)-1-(4-hydr...)Show SMILES CN(C)c1ccc(C=CC=Cc2sc3ccccc3[n+]2CCCC(=O)NCCCCNC(=O)NC(N)=NCCC[C@@H](NC(=O)C(c2ccccc2)c2ccccc2)C(=O)NCc2ccc(O)cc2)cc1 |r,w:8.8,36.38,10.10| Show InChI InChI=1S/C55H63N9O5S/c1-63(2)44-31-27-40(28-32-44)17-9-12-26-50-64(47-23-10-11-24-48(47)70-50)38-16-25-49(66)57-35-13-14-36-59-55(69)62-54(56)58-37-15-22-46(52(67)60-39-41-29-33-45(65)34-30-41)61-53(68)51(42-18-5-3-6-19-42)43-20-7-4-8-21-43/h3-12,17-21,23-24,26-34,46,51H,13-16,22,25,35-39H2,1-2H3,(H7-,56,57,58,59,60,61,62,65,66,67,68,69)/p+1/t46-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Displacement of Dy-635-pNPY from human Y2R expressed in CHO cells by flowcytometry |
Bioorg Med Chem 19: 2859-78 (2011)
Article DOI: 10.1016/j.bmc.2011.03.045
BindingDB Entry DOI: 10.7270/Q2F47PG7 |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50543287
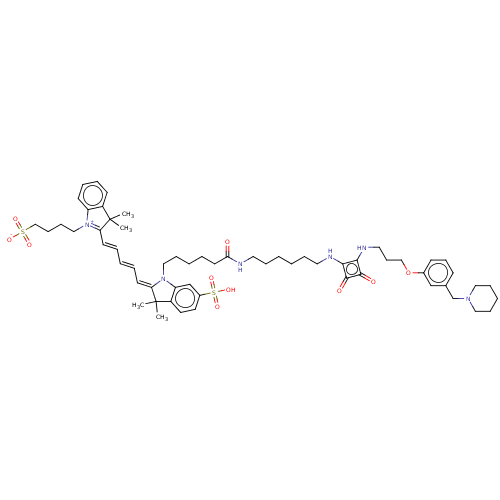
(CHEMBL4641747)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.CC1(C)\C(=C\C=C\C=C\C2=[N+](CCCCS([O-])(=O)=O)c3ccccc3C2(C)C)N(CCCCCC(=O)NCCCCCCNc2c(NCCCOc3cccc(CN4CCCCC4)c3)c(=O)c2=O)c2cc(ccc12)S(O)(=O)=O |c:21| Show InChI InChI=1S/C60H80N6O10S2/c1-59(2)48-26-13-14-27-50(48)65(39-20-21-41-77(70,71)72)52(59)28-10-7-11-29-53-60(3,4)49-32-31-47(78(73,74)75)43-51(49)66(53)38-19-8-12-30-54(67)61-33-15-5-6-16-34-62-55-56(58(69)57(55)68)63-35-23-40-76-46-25-22-24-45(42-46)44-64-36-17-9-18-37-64/h7,10-11,13-14,22,24-29,31-32,42-43H,5-6,8-9,12,15-21,23,30,33-41,44H2,1-4H3,(H4-,61,62,63,67,68,69,70,71,72,73,74,75) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]UR-DE257 from Gsalphas-coupled human H2R expressed in baculovirus infected Sf9 cells incubated for 60 mins by scintillation count... |
ACS Med Chem Lett 11: 1521-1528 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00033
BindingDB Entry DOI: 10.7270/Q2W380WD |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50543286
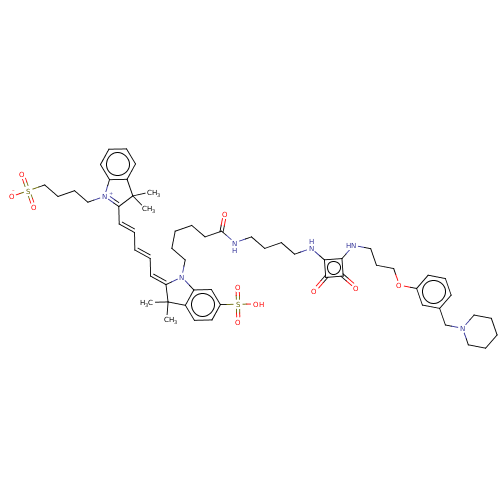
(CHEMBL4648203)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.CC1(C)\C(=C\C=C\C=C\C2=[N+](CCCCS([O-])(=O)=O)c3ccccc3C2(C)C)N(CCCCCC(=O)NCCCCNc2c(NCCCOc3cccc(CN4CCCCC4)c3)c(=O)c2=O)c2cc(ccc12)S(O)(=O)=O |c:21| Show InChI InChI=1S/C58H76N6O10S2/c1-57(2)46-24-11-12-25-48(46)63(37-18-19-39-75(68,69)70)50(57)26-8-5-9-27-51-58(3,4)47-30-29-45(76(71,72)73)41-49(47)64(51)36-17-6-10-28-52(65)59-31-13-14-32-60-53-54(56(67)55(53)66)61-33-21-38-74-44-23-20-22-43(40-44)42-62-34-15-7-16-35-62/h5,8-9,11-12,20,22-27,29-30,40-41H,6-7,10,13-19,21,28,31-39,42H2,1-4H3,(H4-,59,60,61,65,66,67,68,69,70,71,72,73) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]UR-DE257 from Gsalphas-coupled human H2R expressed in baculovirus infected Sf9 cells incubated for 60 mins by scintillation count... |
ACS Med Chem Lett 11: 1521-1528 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00033
BindingDB Entry DOI: 10.7270/Q2W380WD |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 2
(Homo sapiens (Human)) | BDBM50343729

(1-((R)-9-Amino-4-(2,2-diphenylacetamido)-1-(4-hydr...)Show SMILES Cc1cc(C=Cc2cc3CCCN4CCCc(c2)c34)cc(C)[n+]1CCNC(=O)CCCC(=O)NC(N)=NCCC[C@@H](NC(=O)C(c1ccccc1)c1ccccc1)C(=O)NCc1ccc(O)cc1 |r,w:4.3,36.40| Show InChI InChI=1S/C55H64N8O5/c1-38-33-41(22-23-42-35-45-17-11-30-62-31-12-18-46(36-42)52(45)62)34-39(2)63(38)32-29-57-49(65)20-9-21-50(66)61-55(56)58-28-10-19-48(53(67)59-37-40-24-26-47(64)27-25-40)60-54(68)51(43-13-5-3-6-14-43)44-15-7-4-8-16-44/h3-8,13-16,22-27,33-36,48,51H,9-12,17-21,28-32,37H2,1-2H3,(H6-,56,57,58,59,60,61,64,65,66,67,68)/p+1/t48-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Displacement of Dy-635-pNPY from human Y2R expressed in CHO cells by flowcytometry |
Bioorg Med Chem 19: 2859-78 (2011)
Article DOI: 10.1016/j.bmc.2011.03.045
BindingDB Entry DOI: 10.7270/Q2F47PG7 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 4
(Homo sapiens (Human)) | BDBM50343729

(1-((R)-9-Amino-4-(2,2-diphenylacetamido)-1-(4-hydr...)Show SMILES Cc1cc(C=Cc2cc3CCCN4CCCc(c2)c34)cc(C)[n+]1CCNC(=O)CCCC(=O)NC(N)=NCCC[C@@H](NC(=O)C(c1ccccc1)c1ccccc1)C(=O)NCc1ccc(O)cc1 |r,w:4.3,36.40| Show InChI InChI=1S/C55H64N8O5/c1-38-33-41(22-23-42-35-45-17-11-30-62-31-12-18-46(36-42)52(45)62)34-39(2)63(38)32-29-57-49(65)20-9-21-50(66)61-55(56)58-28-10-19-48(53(67)59-37-40-24-26-47(64)27-25-40)60-54(68)51(43-13-5-3-6-14-43)44-15-7-4-8-16-44/h3-8,13-16,22-27,33-36,48,51H,9-12,17-21,28-32,37H2,1-2H3,(H6-,56,57,58,59,60,61,64,65,66,67,68)/p+1/t48-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Displacement of Cy5-[K4]-hpp from human Y4R expressed in CHO cells by flowcytometry |
Bioorg Med Chem 19: 2859-78 (2011)
Article DOI: 10.1016/j.bmc.2011.03.045
BindingDB Entry DOI: 10.7270/Q2F47PG7 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50343729

(1-((R)-9-Amino-4-(2,2-diphenylacetamido)-1-(4-hydr...)Show SMILES Cc1cc(C=Cc2cc3CCCN4CCCc(c2)c34)cc(C)[n+]1CCNC(=O)CCCC(=O)NC(N)=NCCC[C@@H](NC(=O)C(c1ccccc1)c1ccccc1)C(=O)NCc1ccc(O)cc1 |r,w:4.3,36.40| Show InChI InChI=1S/C55H64N8O5/c1-38-33-41(22-23-42-35-45-17-11-30-62-31-12-18-46(36-42)52(45)62)34-39(2)63(38)32-29-57-49(65)20-9-21-50(66)61-55(56)58-28-10-19-48(53(67)59-37-40-24-26-47(64)27-25-40)60-54(68)51(43-13-5-3-6-14-43)44-15-7-4-8-16-44/h3-8,13-16,22-27,33-36,48,51H,9-12,17-21,28-32,37H2,1-2H3,(H6-,56,57,58,59,60,61,64,65,66,67,68)/p+1/t48-/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Displacement of Dy-635-pNPY from human Y5R expressed in CHO cells by flowcytometry |
Bioorg Med Chem 19: 2859-78 (2011)
Article DOI: 10.1016/j.bmc.2011.03.045
BindingDB Entry DOI: 10.7270/Q2F47PG7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50312987
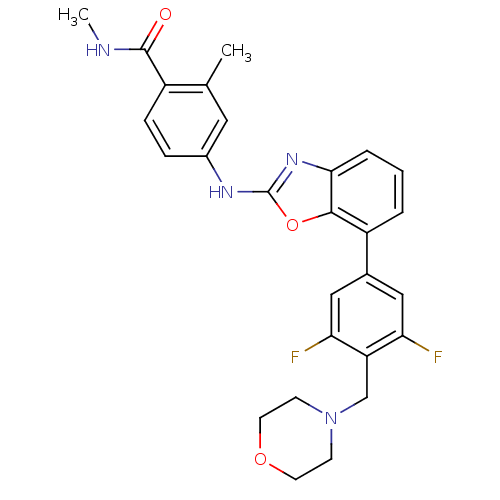
(4-(7-(3,5-difluoro-4-(morpholinomethyl)phenyl)benz...)Show SMILES CNC(=O)c1ccc(Nc2nc3cccc(-c4cc(F)c(CN5CCOCC5)c(F)c4)c3o2)cc1C Show InChI InChI=1S/C27H26F2N4O3/c1-16-12-18(6-7-19(16)26(34)30-2)31-27-32-24-5-3-4-20(25(24)36-27)17-13-22(28)21(23(29)14-17)15-33-8-10-35-11-9-33/h3-7,12-14H,8-11,15H2,1-2H3,(H,30,34)(H,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged JAK2 assessed as inhibition of biotinylated JAK3tide peptide phosphorylation after 60 mins by caliper mobility shift assay |
Bioorg Med Chem Lett 20: 1724-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.069
BindingDB Entry DOI: 10.7270/Q2M32VW0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50312989
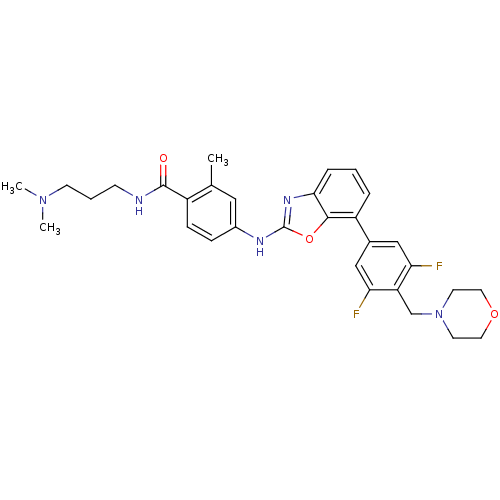
(4-(7-(3,5-difluoro-4-(morpholinomethyl)phenyl)benz...)Show SMILES CN(C)CCCNC(=O)c1ccc(Nc2nc3cccc(-c4cc(F)c(CN5CCOCC5)c(F)c4)c3o2)cc1C Show InChI InChI=1S/C31H35F2N5O3/c1-20-16-22(8-9-23(20)30(39)34-10-5-11-37(2)3)35-31-36-28-7-4-6-24(29(28)41-31)21-17-26(32)25(27(33)18-21)19-38-12-14-40-15-13-38/h4,6-9,16-18H,5,10-15,19H2,1-3H3,(H,34,39)(H,35,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged JAK2 assessed as inhibition of biotinylated JAK3tide peptide phosphorylation after 60 mins by caliper mobility shift assay |
Bioorg Med Chem Lett 20: 1724-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.069
BindingDB Entry DOI: 10.7270/Q2M32VW0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data