

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50568920 (CHEMBL4846823) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50568920 (CHEMBL4846823) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.741 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50362863 (CHEMBL1940418) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50237599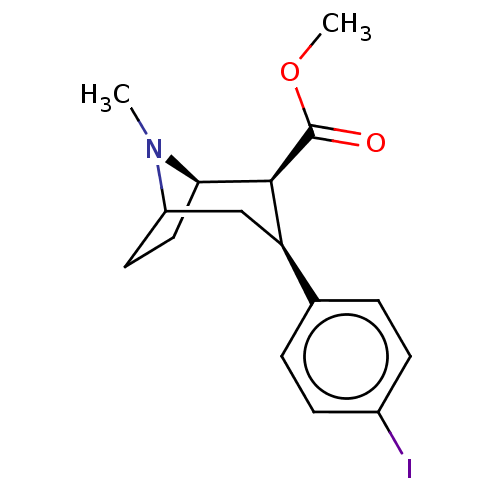 (CHEMBL4066427) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of [3H]mazindol binding to recombinant human SERT expressed in HEK293 cell membranes preincubated for 10 mins followed by radioligand addi... | J Med Chem 60: 3109-3123 (2017) Article DOI: 10.1021/acs.jmedchem.7b00141 BindingDB Entry DOI: 10.7270/Q2Z60RBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane domain-containing protein TMIGD3 (Homo sapiens (Human)) | BDBM50236785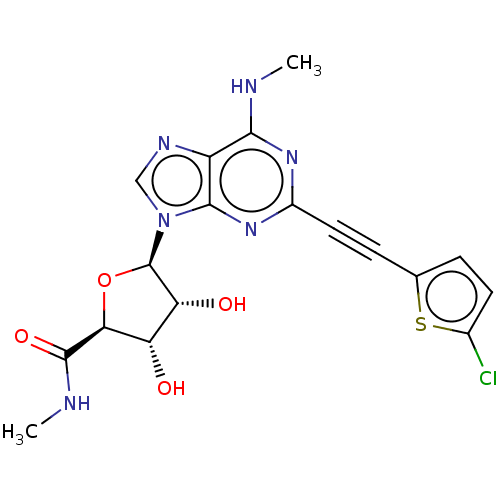 (CHEMBL4090355) | UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]N6-(4-amino-3-iodobenzyl)adenosine-5'-N-methyluronamide from human A3AR expressed in HEK293T cells pre-incubated for 10 mins be... | J Med Chem 60: 3109-3123 (2017) Article DOI: 10.1021/acs.jmedchem.7b00141 BindingDB Entry DOI: 10.7270/Q2Z60RBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane domain-containing protein TMIGD3 (Homo sapiens (Human)) | BDBM50116878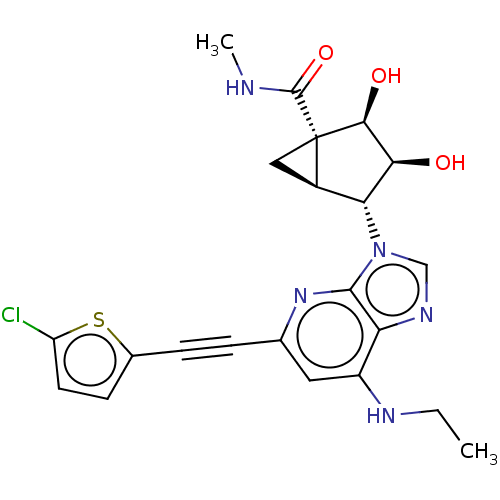 (CHEMBL3612940) | UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]N6-(4-amino-3-iodobenzyl)adenosine-5'-N-methyluronamide from human A3AR expressed in CHO cells | J Med Chem 60: 3109-3123 (2017) Article DOI: 10.1021/acs.jmedchem.7b00141 BindingDB Entry DOI: 10.7270/Q2Z60RBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50568924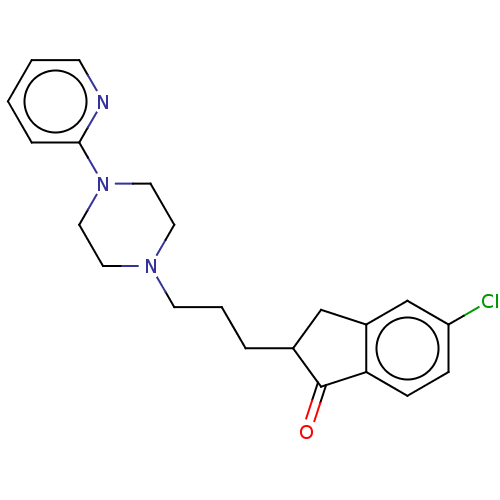 (CHEMBL4862180) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50568924 (CHEMBL4862180) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50568921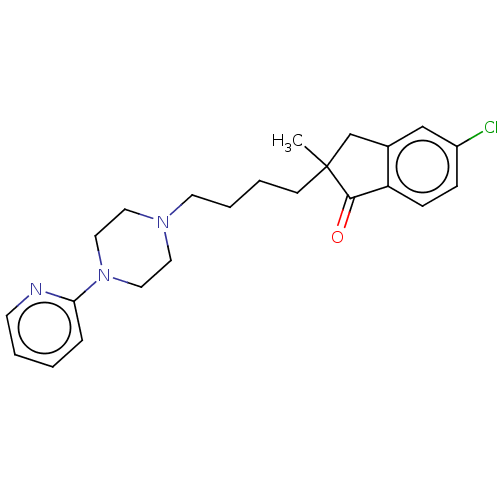 (CHEMBL4871868) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50568921 (CHEMBL4871868) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50130293 (7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]N-methylspiperone from human dopamine D2 receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50362863 (CHEMBL1940418) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]N-methylspiperone from human dopamine D4 receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50568911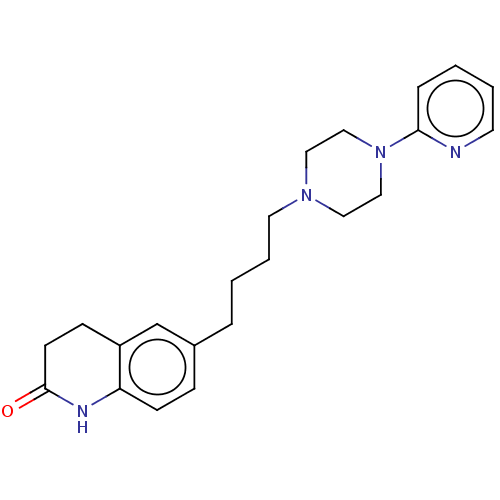 (CHEMBL4857597) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50568919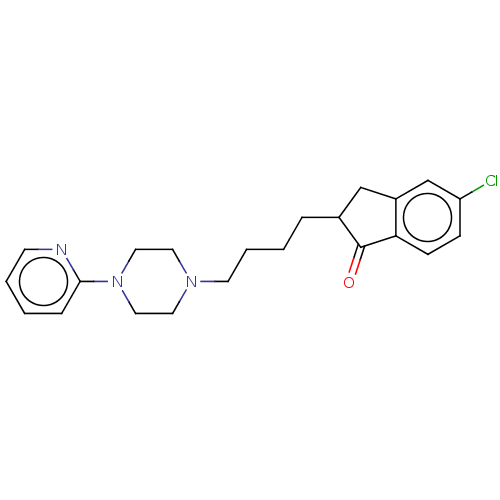 (CHEMBL4857665) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50568919 (CHEMBL4857665) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50568911 (CHEMBL4857597) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50568910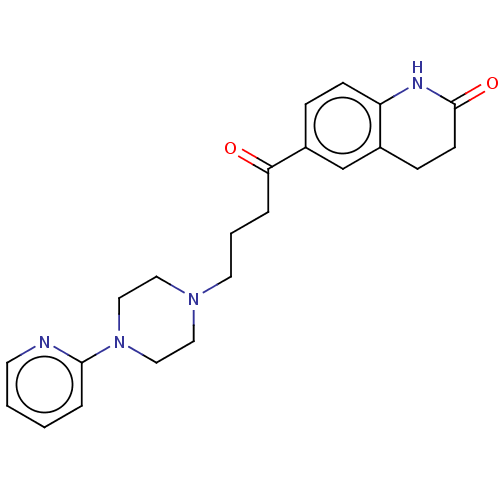 (CHEMBL4845947) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5-HT7 receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50568910 (CHEMBL4845947) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5-HT7 receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50568917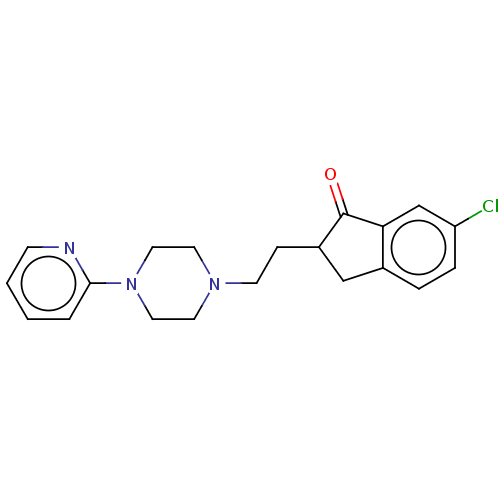 (CHEMBL4876297) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50568917 (CHEMBL4876297) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50568914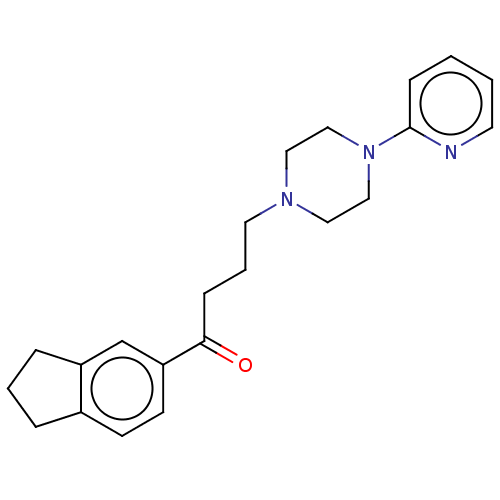 (CHEMBL4874069) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50568914 (CHEMBL4874069) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Synaptic vesicular amine transporter (Homo sapiens (Human)) | BDBM50017712 ((-)-reserpine | (3beta,16beta,17alpha,18beta,20alp...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organix Inc Curated by ChEMBL | Assay Description Displacement of [3H]reserpine from human VMAT2 expressed in HEK293 cell membranes incubated for 60 mins by scintillation counting method | J Med Chem 61: 9121-9131 (2018) Article DOI: 10.1021/acs.jmedchem.8b00542 BindingDB Entry DOI: 10.7270/Q2KH0QZ9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Transmembrane domain-containing protein TMIGD3 (Homo sapiens (Human)) | BDBM50236804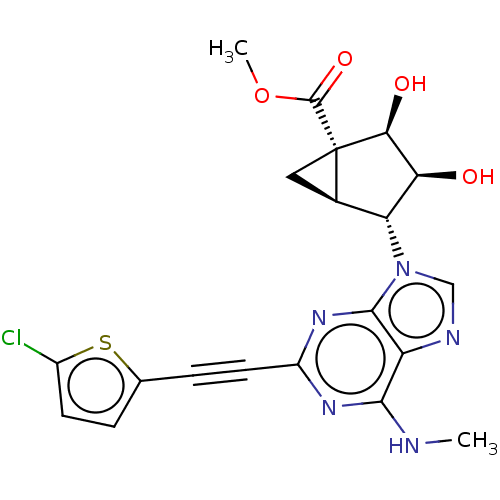 (CHEMBL4066973) | UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of binding of [3H]nisoxetine to (NET) norepinephrine transporter in rat striatum | J Med Chem 60: 3109-3123 (2017) Article DOI: 10.1021/acs.jmedchem.7b00141 BindingDB Entry DOI: 10.7270/Q2Z60RBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50130293 (7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Transmembrane domain-containing protein TMIGD3 (Homo sapiens (Human)) | BDBM50236774 (CHEMBL4105127) | UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of [125I]RANTES binding to CCR5 receptor. | J Med Chem 60: 3109-3123 (2017) Article DOI: 10.1021/acs.jmedchem.7b00141 BindingDB Entry DOI: 10.7270/Q2Z60RBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50568916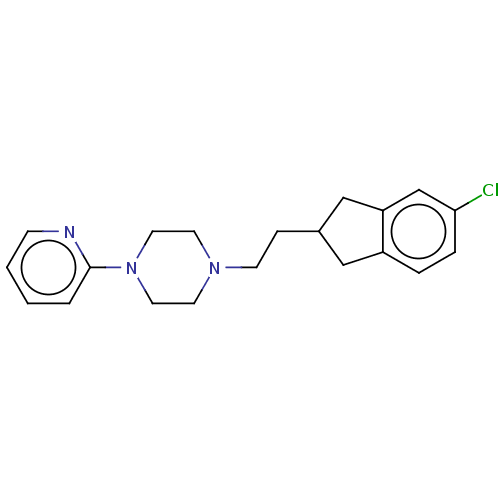 (CHEMBL4866058) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50568916 (CHEMBL4866058) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane domain-containing protein TMIGD3 (Homo sapiens (Human)) | BDBM50236779 (CHEMBL4086593) | UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]N6-(4-amino-3-iodobenzyl)adenosine-5'-N-methyluronamide from human A3AR expressed in HEK293T cells pre-incubated for 10 mins be... | J Med Chem 60: 3109-3123 (2017) Article DOI: 10.1021/acs.jmedchem.7b00141 BindingDB Entry DOI: 10.7270/Q2Z60RBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50036733 (1-(4-Fluoro-phenyl)-4-(4-pyridin-2-yl-piperazin-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]N-methylspiperone from human dopamine D4 receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane domain-containing protein TMIGD3 (Homo sapiens (Human)) | BDBM50236714 (CHEMBL4097426) | UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]N6-(4-amino-3-iodobenzyl)adenosine-5'-N-methyluronamide from human A3AR expressed in HEK293T cells pre-incubated for 10 mins be... | J Med Chem 60: 3109-3123 (2017) Article DOI: 10.1021/acs.jmedchem.7b00141 BindingDB Entry DOI: 10.7270/Q2Z60RBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50459619 (CHEMBL4206824) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organix Inc Curated by ChEMBL | Assay Description Displacement of [125I]DOI from human 5HT2A receptor expressed in HEK293 cell membranes | J Med Chem 61: 9121-9131 (2018) Article DOI: 10.1021/acs.jmedchem.8b00542 BindingDB Entry DOI: 10.7270/Q2KH0QZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50036733 (1-(4-Fluoro-phenyl)-4-(4-pyridin-2-yl-piperazin-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]N-methylspiperone from human dopamine D4 receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane domain-containing protein TMIGD3 (Homo sapiens (Human)) | BDBM50236765 (CHEMBL4089071) | UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description mu-1 receptor binding affinity in rat brain by 3H [d-Ala2, d-Leu5] enkephalin displacement. | J Med Chem 60: 3109-3123 (2017) Article DOI: 10.1021/acs.jmedchem.7b00141 BindingDB Entry DOI: 10.7270/Q2Z60RBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50036733 (1-(4-Fluoro-phenyl)-4-(4-pyridin-2-yl-piperazin-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50568915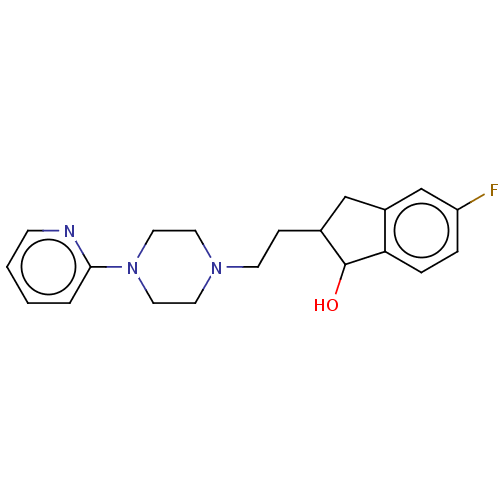 (CHEMBL4866053) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]N-methylspiperone from human dopamine D4 receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50568915 (CHEMBL4866053) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50036733 (1-(4-Fluoro-phenyl)-4-(4-pyridin-2-yl-piperazin-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50568920 (CHEMBL4846823) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5-HT7 receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50568920 (CHEMBL4846823) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5-HT7 receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane domain-containing protein TMIGD3 (Homo sapiens (Human)) | BDBM50236762 (CHEMBL4071830) | UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]N6-(4-amino-3-iodobenzyl)adenosine-5'-N-methyluronamide from human A3AR expressed in HEK293T cells pre-incubated for 10 mins be... | J Med Chem 60: 3109-3123 (2017) Article DOI: 10.1021/acs.jmedchem.7b00141 BindingDB Entry DOI: 10.7270/Q2Z60RBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50130293 (7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-Ketanserin from human 5-HT2A receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane domain-containing protein TMIGD3 (Homo sapiens (Human)) | BDBM50237600 (CHEMBL4071867) | UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]N6-(4-amino-3-iodobenzyl)adenosine-5'-N-methyluronamide from human A3AR expressed in HEK293T cells pre-incubated for 10 mins be... | J Med Chem 60: 3109-3123 (2017) Article DOI: 10.1021/acs.jmedchem.7b00141 BindingDB Entry DOI: 10.7270/Q2Z60RBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM50130293 (7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]N-methylspiperone from human dopamine D3 receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50130293 (7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5-HT7 receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50568917 (CHEMBL4876297) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]N-methylspiperone from human dopamine D4 receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50568917 (CHEMBL4876297) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]N-methylspiperone from human dopamine D4 receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50568923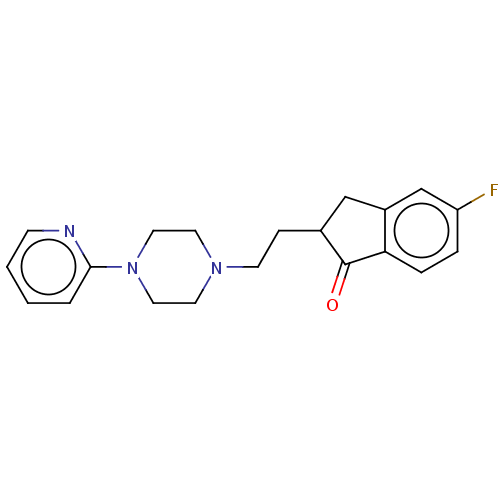 (CHEMBL4871850) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM21395 (3-(2-(4-(4-Fluorobenzoyl)piperidinol)ethyl)-2,4(1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organix Inc Curated by ChEMBL | Assay Description Displacement of [125I]DOI from human 5HT2A receptor expressed in HEK293 cell membranes | J Med Chem 61: 9121-9131 (2018) Article DOI: 10.1021/acs.jmedchem.8b00542 BindingDB Entry DOI: 10.7270/Q2KH0QZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50237599 (CHEMBL4066427) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Compound tested in vitro for inhibition of translation using highly active Escherichia coli S30 and a plasmid containing a gene expressing truncated ... | J Med Chem 60: 3109-3123 (2017) Article DOI: 10.1021/acs.jmedchem.7b00141 BindingDB Entry DOI: 10.7270/Q2Z60RBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 659 total ) | Next | Last >> |