 Found 86 hits with Last Name = 'evanno' and Initial = 'y'
Found 86 hits with Last Name = 'evanno' and Initial = 'y' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gamma-aminobutyric acid receptor subunit alpha-2
(Rattus norvegicus (Rat)) | BDBM50000766

(CHEMBL12 | DIAZEPAM | US9271961, Diazepam)Show InChI InChI=1S/C16H13ClN2O/c1-19-14-8-7-12(17)9-13(14)16(18-10-15(19)20)11-5-3-2-4-6-11/h2-9H,10H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Synthelabo
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 753-68 (2001)
BindingDB Entry DOI: 10.7270/Q2KS6Q34 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gamma-aminobutyric acid receptor subunit alpha-1
(Rattus norvegicus (Rat)) | BDBM85806

(6-FLUOR-2-FENYL-9-METHYL-4-(PYRROLIDINE-1-CARBONYL...)Show SMILES Cn1c2ccc(F)cc2c2c(cn(-c3ccccc3)c(=O)c12)C(=O)N1CCCC1 Show InChI InChI=1S/C23H20FN3O2/c1-25-19-10-9-15(24)13-17(19)20-18(22(28)26-11-5-6-12-26)14-27(23(29)21(20)25)16-7-3-2-4-8-16/h2-4,7-10,13-14H,5-6,11-12H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Synthelabo
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 753-68 (2001)
BindingDB Entry DOI: 10.7270/Q2KS6Q34 |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-2
(Rattus norvegicus (Rat)) | BDBM50000766

(CHEMBL12 | DIAZEPAM | US9271961, Diazepam)Show InChI InChI=1S/C16H13ClN2O/c1-19-14-8-7-12(17)9-13(14)16(18-10-15(19)20)11-5-3-2-4-6-11/h2-9H,10H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Synthelabo
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 753-68 (2001)
BindingDB Entry DOI: 10.7270/Q2KS6Q34 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gamma-aminobutyric acid receptor subunit alpha-5
(RAT) | BDBM50000766

(CHEMBL12 | DIAZEPAM | US9271961, Diazepam)Show InChI InChI=1S/C16H13ClN2O/c1-19-14-8-7-12(17)9-13(14)16(18-10-15(19)20)11-5-3-2-4-6-11/h2-9H,10H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Synthelabo
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 753-68 (2001)
BindingDB Entry DOI: 10.7270/Q2KS6Q34 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gamma-aminobutyric acid receptor subunit alpha-2
(Rattus norvegicus (Rat)) | BDBM85806

(6-FLUOR-2-FENYL-9-METHYL-4-(PYRROLIDINE-1-CARBONYL...)Show SMILES Cn1c2ccc(F)cc2c2c(cn(-c3ccccc3)c(=O)c12)C(=O)N1CCCC1 Show InChI InChI=1S/C23H20FN3O2/c1-25-19-10-9-15(24)13-17(19)20-18(22(28)26-11-5-6-12-26)14-27(23(29)21(20)25)16-7-3-2-4-8-16/h2-4,7-10,13-14H,5-6,11-12H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| 12.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Synthelabo
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 753-68 (2001)
BindingDB Entry DOI: 10.7270/Q2KS6Q34 |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5
(RAT) | BDBM50000766

(CHEMBL12 | DIAZEPAM | US9271961, Diazepam)Show InChI InChI=1S/C16H13ClN2O/c1-19-14-8-7-12(17)9-13(14)16(18-10-15(19)20)11-5-3-2-4-6-11/h2-9H,10H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 13.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Synthelabo
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 753-68 (2001)
BindingDB Entry DOI: 10.7270/Q2KS6Q34 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gamma-aminobutyric acid receptor subunit alpha-3
(RAT) | BDBM50000766

(CHEMBL12 | DIAZEPAM | US9271961, Diazepam)Show InChI InChI=1S/C16H13ClN2O/c1-19-14-8-7-12(17)9-13(14)16(18-10-15(19)20)11-5-3-2-4-6-11/h2-9H,10H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 13.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Synthelabo
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 753-68 (2001)
BindingDB Entry DOI: 10.7270/Q2KS6Q34 |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1
(Rattus norvegicus (Rat)) | BDBM50000766

(CHEMBL12 | DIAZEPAM | US9271961, Diazepam)Show InChI InChI=1S/C16H13ClN2O/c1-19-14-8-7-12(17)9-13(14)16(18-10-15(19)20)11-5-3-2-4-6-11/h2-9H,10H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Synthelabo
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 753-68 (2001)
BindingDB Entry DOI: 10.7270/Q2KS6Q34 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gamma-aminobutyric acid receptor subunit alpha-1
(Rattus norvegicus (Rat)) | BDBM85806

(6-FLUOR-2-FENYL-9-METHYL-4-(PYRROLIDINE-1-CARBONYL...)Show SMILES Cn1c2ccc(F)cc2c2c(cn(-c3ccccc3)c(=O)c12)C(=O)N1CCCC1 Show InChI InChI=1S/C23H20FN3O2/c1-25-19-10-9-15(24)13-17(19)20-18(22(28)26-11-5-6-12-26)14-27(23(29)21(20)25)16-7-3-2-4-8-16/h2-4,7-10,13-14H,5-6,11-12H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Synthelabo
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 753-68 (2001)
BindingDB Entry DOI: 10.7270/Q2KS6Q34 |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-2
(Rattus norvegicus (Rat)) | BDBM85806

(6-FLUOR-2-FENYL-9-METHYL-4-(PYRROLIDINE-1-CARBONYL...)Show SMILES Cn1c2ccc(F)cc2c2c(cn(-c3ccccc3)c(=O)c12)C(=O)N1CCCC1 Show InChI InChI=1S/C23H20FN3O2/c1-25-19-10-9-15(24)13-17(19)20-18(22(28)26-11-5-6-12-26)14-27(23(29)21(20)25)16-7-3-2-4-8-16/h2-4,7-10,13-14H,5-6,11-12H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| 73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Synthelabo
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 753-68 (2001)
BindingDB Entry DOI: 10.7270/Q2KS6Q34 |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-3
(RAT) | BDBM85806

(6-FLUOR-2-FENYL-9-METHYL-4-(PYRROLIDINE-1-CARBONYL...)Show SMILES Cn1c2ccc(F)cc2c2c(cn(-c3ccccc3)c(=O)c12)C(=O)N1CCCC1 Show InChI InChI=1S/C23H20FN3O2/c1-25-19-10-9-15(24)13-17(19)20-18(22(28)26-11-5-6-12-26)14-27(23(29)21(20)25)16-7-3-2-4-8-16/h2-4,7-10,13-14H,5-6,11-12H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| 80.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Synthelabo
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 753-68 (2001)
BindingDB Entry DOI: 10.7270/Q2KS6Q34 |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5
(RAT) | BDBM85806

(6-FLUOR-2-FENYL-9-METHYL-4-(PYRROLIDINE-1-CARBONYL...)Show SMILES Cn1c2ccc(F)cc2c2c(cn(-c3ccccc3)c(=O)c12)C(=O)N1CCCC1 Show InChI InChI=1S/C23H20FN3O2/c1-25-19-10-9-15(24)13-17(19)20-18(22(28)26-11-5-6-12-26)14-27(23(29)21(20)25)16-7-3-2-4-8-16/h2-4,7-10,13-14H,5-6,11-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| 117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Synthelabo
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 753-68 (2001)
BindingDB Entry DOI: 10.7270/Q2KS6Q34 |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5
(RAT) | BDBM85806

(6-FLUOR-2-FENYL-9-METHYL-4-(PYRROLIDINE-1-CARBONYL...)Show SMILES Cn1c2ccc(F)cc2c2c(cn(-c3ccccc3)c(=O)c12)C(=O)N1CCCC1 Show InChI InChI=1S/C23H20FN3O2/c1-25-19-10-9-15(24)13-17(19)20-18(22(28)26-11-5-6-12-26)14-27(23(29)21(20)25)16-7-3-2-4-8-16/h2-4,7-10,13-14H,5-6,11-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| 215 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Synthelabo
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 753-68 (2001)
BindingDB Entry DOI: 10.7270/Q2KS6Q34 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50517832
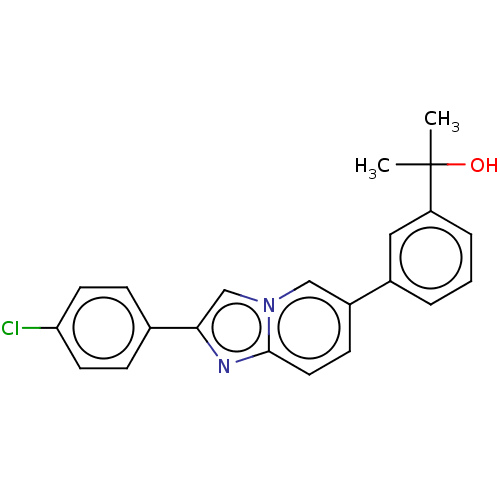
(CHEMBL4564985)Show SMILES CC(C)(O)c1cccc(c1)-c1ccc2nc(cn2c1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C22H19ClN2O/c1-22(2,26)18-5-3-4-16(12-18)17-8-11-21-24-20(14-25(21)13-17)15-6-9-19(23)10-7-15/h3-14,26H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 29: 929-932 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.024
BindingDB Entry DOI: 10.7270/Q2BG2SB9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50517811

(CHEMBL4576867)Show InChI InChI=1S/C20H15ClN2O/c21-18-7-4-15(5-8-18)19-12-23-11-17(6-9-20(23)22-19)16-3-1-2-14(10-16)13-24/h1-12,24H,13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem Lett 29: 929-932 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.024
BindingDB Entry DOI: 10.7270/Q2BG2SB9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50517833
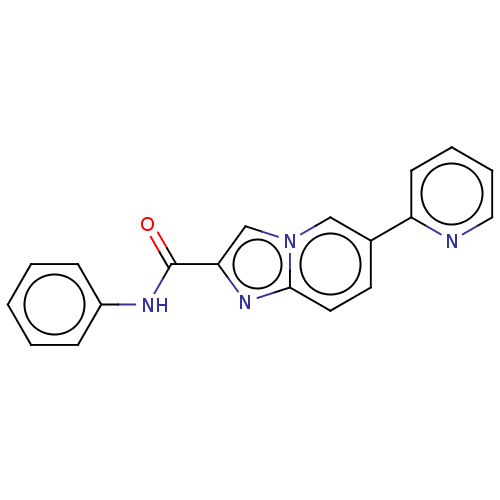
(CHEMBL4472621)Show InChI InChI=1S/C19H14N4O/c24-19(21-15-6-2-1-3-7-15)17-13-23-12-14(9-10-18(23)22-17)16-8-4-5-11-20-16/h1-13H,(H,21,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem Lett 29: 929-932 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.024
BindingDB Entry DOI: 10.7270/Q2BG2SB9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50517833

(CHEMBL4472621)Show InChI InChI=1S/C19H14N4O/c24-19(21-15-6-2-1-3-7-15)17-13-23-12-14(9-10-18(23)22-17)16-8-4-5-11-20-16/h1-13H,(H,21,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 29: 929-932 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.024
BindingDB Entry DOI: 10.7270/Q2BG2SB9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50517811

(CHEMBL4576867)Show InChI InChI=1S/C20H15ClN2O/c21-18-7-4-15(5-8-18)19-12-23-11-17(6-9-20(23)22-19)16-3-1-2-14(10-16)13-24/h1-12,24H,13H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 29: 929-932 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.024
BindingDB Entry DOI: 10.7270/Q2BG2SB9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50517833

(CHEMBL4472621)Show InChI InChI=1S/C19H14N4O/c24-19(21-15-6-2-1-3-7-15)17-13-23-12-14(9-10-18(23)22-17)16-8-4-5-11-20-16/h1-13H,(H,21,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 29: 929-932 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.024
BindingDB Entry DOI: 10.7270/Q2BG2SB9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50517811

(CHEMBL4576867)Show InChI InChI=1S/C20H15ClN2O/c21-18-7-4-15(5-8-18)19-12-23-11-17(6-9-20(23)22-19)16-3-1-2-14(10-16)13-24/h1-12,24H,13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 29: 929-932 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.024
BindingDB Entry DOI: 10.7270/Q2BG2SB9 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Homo sapiens (Human)) | BDBM50517808
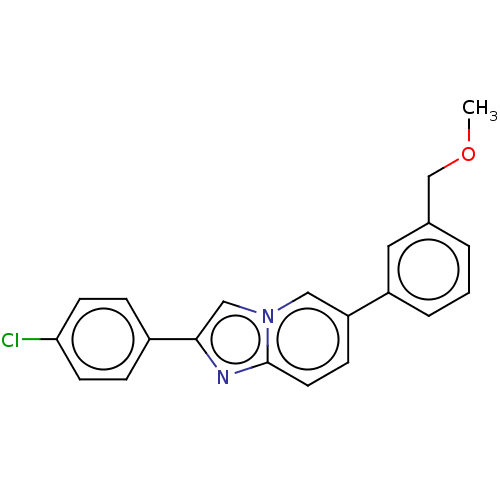
(CHEMBL4465741)Show InChI InChI=1S/C21H17ClN2O/c1-25-14-15-3-2-4-17(11-15)18-7-10-21-23-20(13-24(21)12-18)16-5-8-19(22)9-6-16/h2-13H,14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Agonist activity at full-length Gal4-fused NOT (unknown origin) expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 29: 929-932 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.024
BindingDB Entry DOI: 10.7270/Q2BG2SB9 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Homo sapiens (Human)) | BDBM50517809
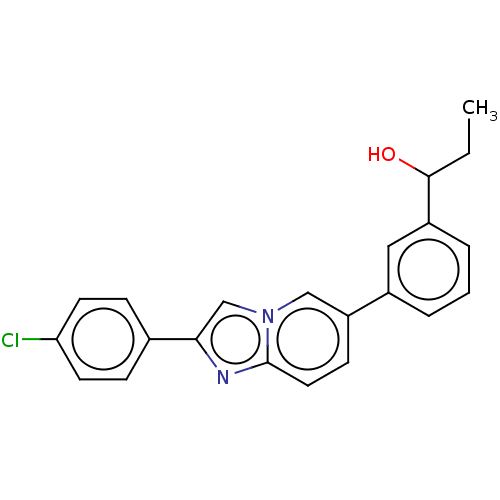
(CHEMBL4587411)Show SMILES CCC(O)c1cccc(c1)-c1ccc2nc(cn2c1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C22H19ClN2O/c1-2-21(26)17-5-3-4-16(12-17)18-8-11-22-24-20(14-25(22)13-18)15-6-9-19(23)10-7-15/h3-14,21,26H,2H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Agonist activity at full-length Gal4-fused NOT (unknown origin) expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 29: 929-932 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.024
BindingDB Entry DOI: 10.7270/Q2BG2SB9 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Mus musculus) | BDBM50517810
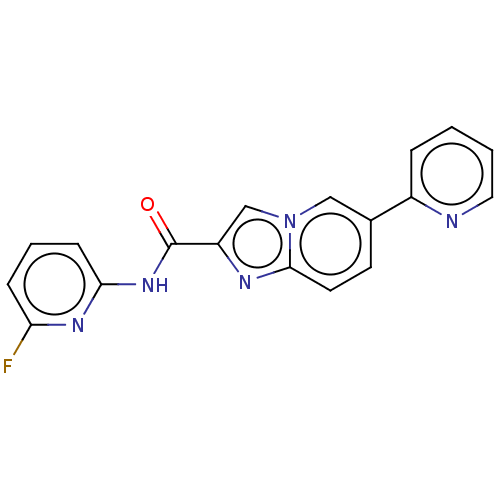
(CHEMBL4579076)Show InChI InChI=1S/C18H12FN5O/c19-15-5-3-6-16(22-15)23-18(25)14-11-24-10-12(7-8-17(24)21-14)13-4-1-2-9-20-13/h1-11H,(H,22,23,25) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Agonist activity at Nurr1 in mouse N2A cells harboring NBRE by luciferase reporter gene assay |
Bioorg Med Chem Lett 29: 929-932 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.024
BindingDB Entry DOI: 10.7270/Q2BG2SB9 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Mus musculus) | BDBM50517811

(CHEMBL4576867)Show InChI InChI=1S/C20H15ClN2O/c21-18-7-4-15(5-8-18)19-12-23-11-17(6-9-20(23)22-19)16-3-1-2-14(10-16)13-24/h1-12,24H,13H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Agonist activity at Nurr1 in mouse N2A cells harboring NBRE by luciferase reporter gene assay |
Bioorg Med Chem Lett 29: 929-932 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.024
BindingDB Entry DOI: 10.7270/Q2BG2SB9 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Homo sapiens (Human)) | BDBM50517812
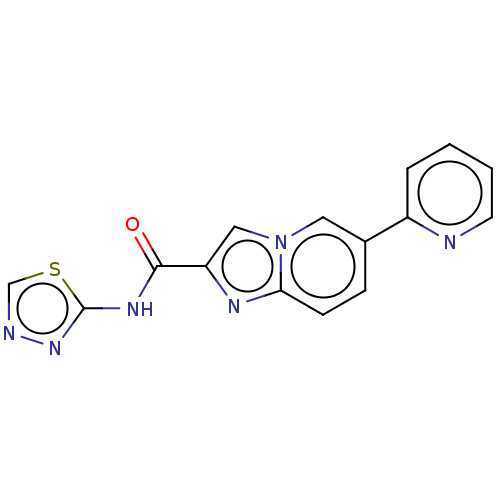
(CHEMBL4454933)Show InChI InChI=1S/C15H10N6OS/c22-14(19-15-20-17-9-23-15)12-8-21-7-10(4-5-13(21)18-12)11-3-1-2-6-16-11/h1-9H,(H,19,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Agonist activity at full-length Gal4-fused NOT (unknown origin) expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 29: 929-932 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.024
BindingDB Entry DOI: 10.7270/Q2BG2SB9 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Homo sapiens (Human)) | BDBM50517813
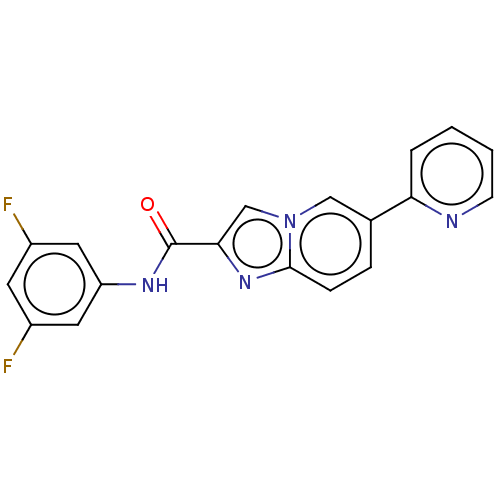
(CHEMBL4464547)Show SMILES Fc1cc(F)cc(NC(=O)c2cn3cc(ccc3n2)-c2ccccn2)c1 Show InChI InChI=1S/C19H12F2N4O/c20-13-7-14(21)9-15(8-13)23-19(26)17-11-25-10-12(4-5-18(25)24-17)16-3-1-2-6-22-16/h1-11H,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Agonist activity at full-length Gal4-fused NOT (unknown origin) expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 29: 929-932 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.024
BindingDB Entry DOI: 10.7270/Q2BG2SB9 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Homo sapiens (Human)) | BDBM50517814
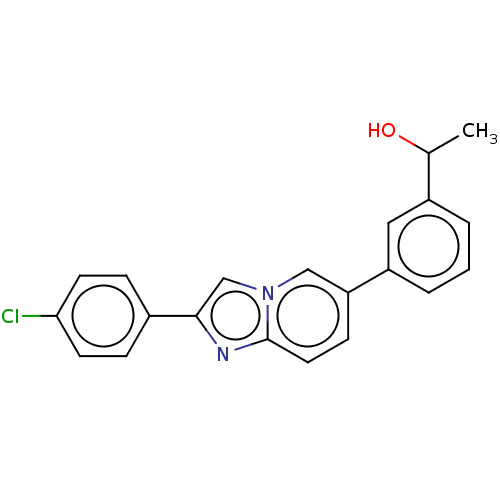
(CHEMBL4461137)Show SMILES CC(O)c1cccc(c1)-c1ccc2nc(cn2c1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C21H17ClN2O/c1-14(25)16-3-2-4-17(11-16)18-7-10-21-23-20(13-24(21)12-18)15-5-8-19(22)9-6-15/h2-14,25H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 219 | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Agonist activity at full-length Gal4-fused NOT (unknown origin) expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 29: 929-932 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.024
BindingDB Entry DOI: 10.7270/Q2BG2SB9 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Homo sapiens (Human)) | BDBM50517815

(CHEMBL4559712)Show SMILES CC(O)(CO)c1cccc(c1)-c1ccc2nc(cn2c1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C22H19ClN2O2/c1-22(27,14-26)18-4-2-3-16(11-18)17-7-10-21-24-20(13-25(21)12-17)15-5-8-19(23)9-6-15/h2-13,26-27H,14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Agonist activity at full-length Gal4-fused NOT (unknown origin) expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 29: 929-932 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.024
BindingDB Entry DOI: 10.7270/Q2BG2SB9 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Homo sapiens (Human)) | BDBM50517816
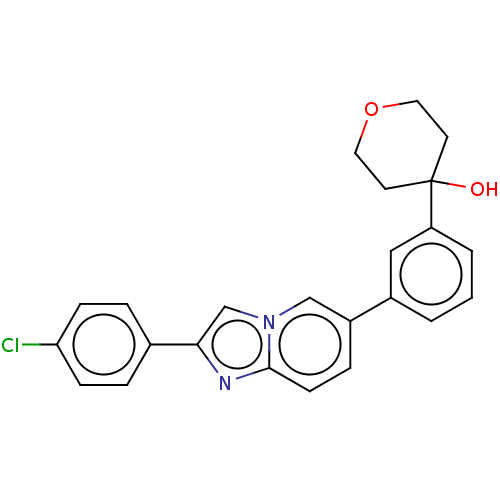
(CHEMBL4440984)Show SMILES OC1(CCOCC1)c1cccc(c1)-c1ccc2nc(cn2c1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C24H21ClN2O2/c25-21-7-4-17(5-8-21)22-16-27-15-19(6-9-23(27)26-22)18-2-1-3-20(14-18)24(28)10-12-29-13-11-24/h1-9,14-16,28H,10-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Agonist activity at full-length Gal4-fused NOT (unknown origin) expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 29: 929-932 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.024
BindingDB Entry DOI: 10.7270/Q2BG2SB9 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Homo sapiens (Human)) | BDBM50517817

(CHEMBL4446736)Show SMILES OC1(COC1)c1cccc(c1)-c1ccc2nc(cn2c1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C22H17ClN2O2/c23-19-7-4-15(5-8-19)20-12-25-11-17(6-9-21(25)24-20)16-2-1-3-18(10-16)22(26)13-27-14-22/h1-12,26H,13-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 323 | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Agonist activity at full-length Gal4-fused NOT (unknown origin) expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 29: 929-932 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.024
BindingDB Entry DOI: 10.7270/Q2BG2SB9 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Homo sapiens (Human)) | BDBM50517814

(CHEMBL4461137)Show SMILES CC(O)c1cccc(c1)-c1ccc2nc(cn2c1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C21H17ClN2O/c1-14(25)16-3-2-4-17(11-16)18-7-10-21-23-20(13-24(21)12-18)15-5-8-19(22)9-6-15/h2-14,25H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 75 | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Agonist activity at full-length Gal4-fused NOT (unknown origin) expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 29: 929-932 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.024
BindingDB Entry DOI: 10.7270/Q2BG2SB9 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Homo sapiens (Human)) | BDBM50517818

(CHEMBL4453694)Show InChI InChI=1S/C21H17ClN2O/c1-14-2-3-16(10-18(14)13-25)17-6-9-21-23-20(12-24(21)11-17)15-4-7-19(22)8-5-15/h2-12,25H,13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Agonist activity at full-length Gal4-fused NOT (unknown origin) expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 29: 929-932 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.024
BindingDB Entry DOI: 10.7270/Q2BG2SB9 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Homo sapiens (Human)) | BDBM50517819

(CHEMBL4475176)Show SMILES OCc1c(F)ccc(c1F)-c1ccc2nc(cn2c1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C20H13ClF2N2O/c21-14-4-1-12(2-5-14)18-10-25-9-13(3-8-19(25)24-18)15-6-7-17(22)16(11-26)20(15)23/h1-10,26H,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 38 | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Agonist activity at full-length Gal4-fused NOT (unknown origin) expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 29: 929-932 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.024
BindingDB Entry DOI: 10.7270/Q2BG2SB9 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Homo sapiens (Human)) | BDBM50517820
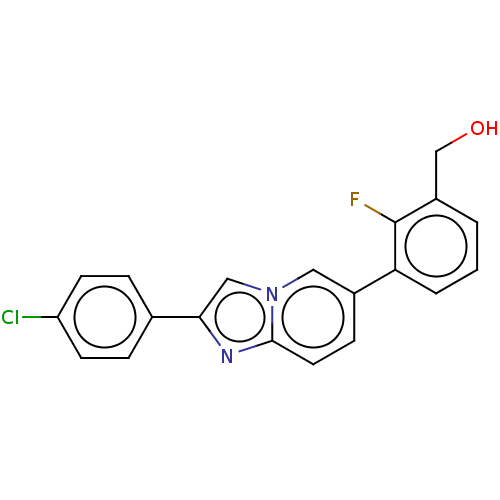
(CHEMBL4473232)Show InChI InChI=1S/C20H14ClFN2O/c21-16-7-4-13(5-8-16)18-11-24-10-14(6-9-19(24)23-18)17-3-1-2-15(12-25)20(17)22/h1-11,25H,12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Agonist activity at full-length Gal4-fused NOT (unknown origin) expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 29: 929-932 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.024
BindingDB Entry DOI: 10.7270/Q2BG2SB9 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Homo sapiens (Human)) | BDBM50517821

(CHEMBL4521080)Show InChI InChI=1S/C16H11N5O2/c22-16(19-12-7-18-23-10-12)14-9-21-8-11(4-5-15(21)20-14)13-3-1-2-6-17-13/h1-10H,(H,19,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Agonist activity at full-length Gal4-fused NOT (unknown origin) expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 29: 929-932 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.024
BindingDB Entry DOI: 10.7270/Q2BG2SB9 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Homo sapiens (Human)) | BDBM50517822
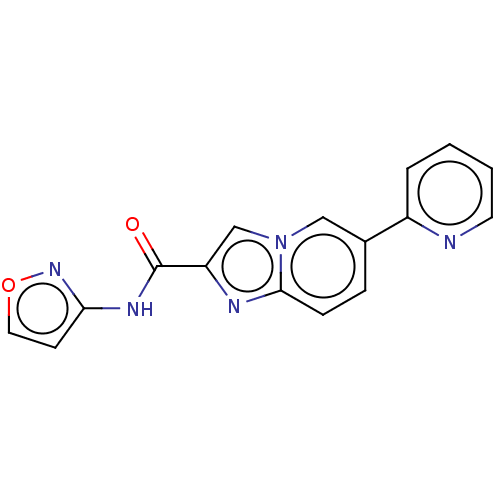
(CHEMBL4450849)Show InChI InChI=1S/C16H11N5O2/c22-16(19-14-6-8-23-20-14)13-10-21-9-11(4-5-15(21)18-13)12-3-1-2-7-17-12/h1-10H,(H,19,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Agonist activity at full-length Gal4-fused NOT (unknown origin) expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 29: 929-932 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.024
BindingDB Entry DOI: 10.7270/Q2BG2SB9 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Homo sapiens (Human)) | BDBM50517823

(CHEMBL4435806)Show InChI InChI=1S/C16H11N5OS/c22-15(20-16-18-7-8-23-16)13-10-21-9-11(4-5-14(21)19-13)12-3-1-2-6-17-12/h1-10H,(H,18,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Agonist activity at full-length Gal4-fused NOT (unknown origin) expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 29: 929-932 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.024
BindingDB Entry DOI: 10.7270/Q2BG2SB9 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Homo sapiens (Human)) | BDBM50517824

(CHEMBL4455780)Show InChI InChI=1S/C18H13N5O/c24-18(22-16-6-2-4-10-20-16)15-12-23-11-13(7-8-17(23)21-15)14-5-1-3-9-19-14/h1-12H,(H,20,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Agonist activity at full-length Gal4-fused NOT (unknown origin) expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 29: 929-932 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.024
BindingDB Entry DOI: 10.7270/Q2BG2SB9 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Homo sapiens (Human)) | BDBM50517825

(CHEMBL4473547)Show InChI InChI=1S/C20H13N5O/c21-11-14-5-1-2-7-17(14)24-20(26)18-13-25-12-15(8-9-19(25)23-18)16-6-3-4-10-22-16/h1-10,12-13H,(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Agonist activity at full-length Gal4-fused NOT (unknown origin) expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 29: 929-932 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.024
BindingDB Entry DOI: 10.7270/Q2BG2SB9 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Homo sapiens (Human)) | BDBM50517826
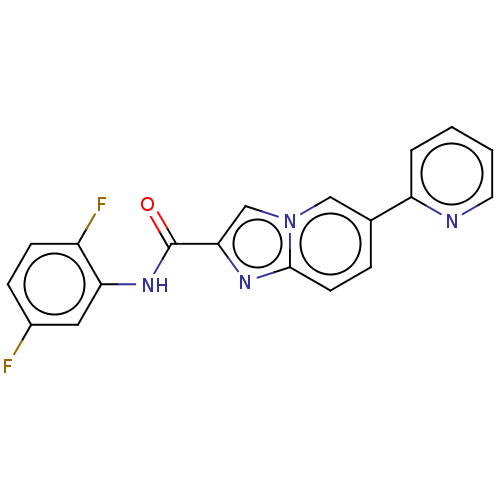
(CHEMBL4453191)Show SMILES Fc1ccc(F)c(NC(=O)c2cn3cc(ccc3n2)-c2ccccn2)c1 Show InChI InChI=1S/C19H12F2N4O/c20-13-5-6-14(21)16(9-13)24-19(26)17-11-25-10-12(4-7-18(25)23-17)15-3-1-2-8-22-15/h1-11H,(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Agonist activity at full-length Gal4-fused NOT (unknown origin) expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 29: 929-932 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.024
BindingDB Entry DOI: 10.7270/Q2BG2SB9 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Homo sapiens (Human)) | BDBM50517827

(CHEMBL4528015)Show InChI InChI=1S/C19H13ClN4O/c20-14-5-1-2-7-16(14)23-19(25)17-12-24-11-13(8-9-18(24)22-17)15-6-3-4-10-21-15/h1-12H,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Agonist activity at full-length Gal4-fused NOT (unknown origin) expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 29: 929-932 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.024
BindingDB Entry DOI: 10.7270/Q2BG2SB9 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Homo sapiens (Human)) | BDBM50517811

(CHEMBL4576867)Show InChI InChI=1S/C20H15ClN2O/c21-18-7-4-15(5-8-18)19-12-23-11-17(6-9-20(23)22-19)16-3-1-2-14(10-16)13-24/h1-12,24H,13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 45 | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Agonist activity at full-length Gal4-fused NOT (unknown origin) expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 29: 929-932 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.024
BindingDB Entry DOI: 10.7270/Q2BG2SB9 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Mus musculus) | BDBM50517821

(CHEMBL4521080)Show InChI InChI=1S/C16H11N5O2/c22-16(19-12-7-18-23-10-12)14-9-21-8-11(4-5-15(21)20-14)13-3-1-2-6-17-13/h1-10H,(H,19,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 55 | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Agonist activity at Nurr1 in mouse N2A cells harboring NBRE by luciferase reporter gene assay |
Bioorg Med Chem Lett 29: 929-932 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.024
BindingDB Entry DOI: 10.7270/Q2BG2SB9 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Mus musculus) | BDBM50517828

(CHEMBL4465777)Show InChI InChI=1S/C16H12N6O/c23-16(20-14-6-8-18-21-14)13-10-22-9-11(4-5-15(22)19-13)12-3-1-2-7-17-12/h1-10H,(H2,18,20,21,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 21 | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Agonist activity at Nurr1 in mouse N2A cells harboring NBRE by luciferase reporter gene assay |
Bioorg Med Chem Lett 29: 929-932 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.024
BindingDB Entry DOI: 10.7270/Q2BG2SB9 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Mus musculus) | BDBM50517822

(CHEMBL4450849)Show InChI InChI=1S/C16H11N5O2/c22-16(19-14-6-8-23-20-14)13-10-21-9-11(4-5-15(21)18-13)12-3-1-2-7-17-12/h1-10H,(H,19,20,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Agonist activity at Nurr1 in mouse N2A cells harboring NBRE by luciferase reporter gene assay |
Bioorg Med Chem Lett 29: 929-932 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.024
BindingDB Entry DOI: 10.7270/Q2BG2SB9 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Mus musculus) | BDBM50517829
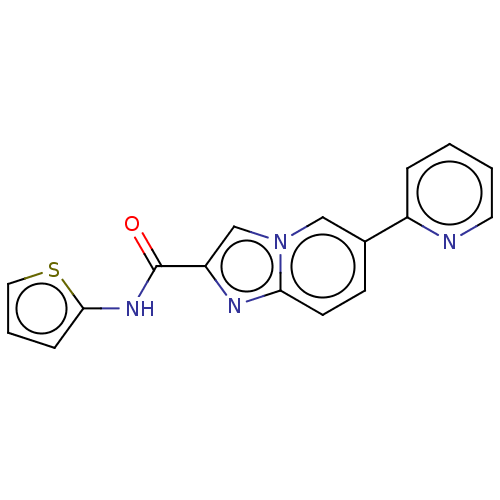
(CHEMBL4542579)Show InChI InChI=1S/C17H12N4OS/c22-17(20-16-5-3-9-23-16)14-11-21-10-12(6-7-15(21)19-14)13-4-1-2-8-18-13/h1-11H,(H,20,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Agonist activity at Nurr1 in mouse N2A cells harboring NBRE by luciferase reporter gene assay |
Bioorg Med Chem Lett 29: 929-932 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.024
BindingDB Entry DOI: 10.7270/Q2BG2SB9 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Mus musculus) | BDBM50517830
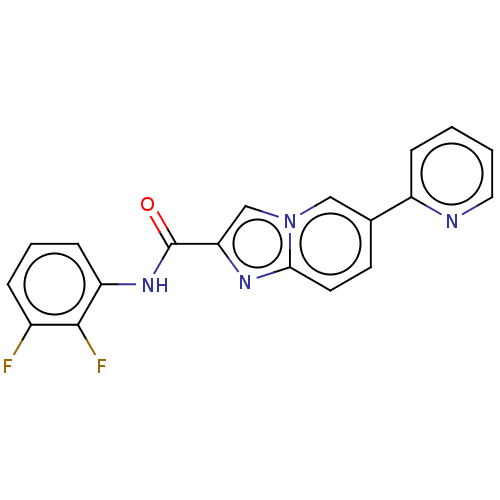
(CHEMBL4475835)Show InChI InChI=1S/C19H12F2N4O/c20-13-4-3-6-15(18(13)21)24-19(26)16-11-25-10-12(7-8-17(25)23-16)14-5-1-2-9-22-14/h1-11H,(H,24,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Agonist activity at Nurr1 in mouse N2A cells harboring NBRE by luciferase reporter gene assay |
Bioorg Med Chem Lett 29: 929-932 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.024
BindingDB Entry DOI: 10.7270/Q2BG2SB9 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Mus musculus) | BDBM50517826

(CHEMBL4453191)Show SMILES Fc1ccc(F)c(NC(=O)c2cn3cc(ccc3n2)-c2ccccn2)c1 Show InChI InChI=1S/C19H12F2N4O/c20-13-5-6-14(21)16(9-13)24-19(26)17-11-25-10-12(4-7-18(25)23-17)15-3-1-2-8-22-15/h1-11H,(H,24,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Agonist activity at Nurr1 in mouse N2A cells harboring NBRE by luciferase reporter gene assay |
Bioorg Med Chem Lett 29: 929-932 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.024
BindingDB Entry DOI: 10.7270/Q2BG2SB9 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Mus musculus) | BDBM50517831

(CHEMBL4516628)Show InChI InChI=1S/C19H13FN4O/c20-14-5-1-2-7-16(14)23-19(25)17-12-24-11-13(8-9-18(24)22-17)15-6-3-4-10-21-15/h1-12H,(H,23,25) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Agonist activity at Nurr1 in mouse N2A cells harboring NBRE by luciferase reporter gene assay |
Bioorg Med Chem Lett 29: 929-932 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.024
BindingDB Entry DOI: 10.7270/Q2BG2SB9 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Mus musculus) | BDBM50517827

(CHEMBL4528015)Show InChI InChI=1S/C19H13ClN4O/c20-14-5-1-2-7-16(14)23-19(25)17-12-24-11-13(8-9-18(24)22-17)15-6-3-4-10-21-15/h1-12H,(H,23,25) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 27 | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Agonist activity at Nurr1 in mouse N2A cells harboring NBRE by luciferase reporter gene assay |
Bioorg Med Chem Lett 29: 929-932 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.024
BindingDB Entry DOI: 10.7270/Q2BG2SB9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data