

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50500526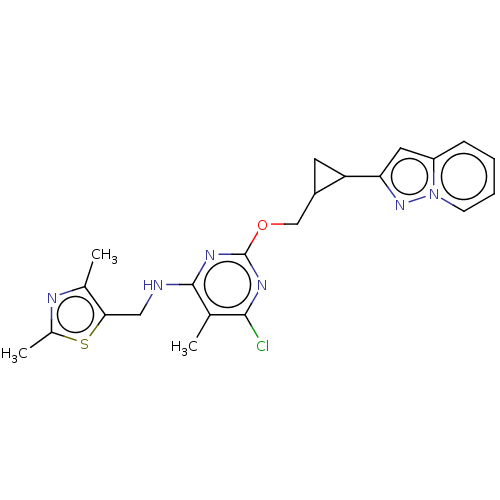 (CHEMBL3747517) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of PDE10A (unknown origin) by IMAP assay | Bioorg Med Chem Lett 26: 126-32 (2016) Article DOI: 10.1016/j.bmcl.2015.11.013 BindingDB Entry DOI: 10.7270/Q2NP27FW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50500520 (CHEMBL3746993) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of PDE10A (unknown origin) by IMAP assay | Bioorg Med Chem Lett 26: 126-32 (2016) Article DOI: 10.1016/j.bmcl.2015.11.013 BindingDB Entry DOI: 10.7270/Q2NP27FW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50500538 (CHEMBL3745790) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of PDE10A (unknown origin) by IMAP assay | Bioorg Med Chem Lett 26: 126-32 (2016) Article DOI: 10.1016/j.bmcl.2015.11.013 BindingDB Entry DOI: 10.7270/Q2NP27FW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50500521 (CHEMBL3746277) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of PDE10A (unknown origin) by IMAP assay | Bioorg Med Chem Lett 26: 126-32 (2016) Article DOI: 10.1016/j.bmcl.2015.11.013 BindingDB Entry DOI: 10.7270/Q2NP27FW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50500535 (CHEMBL3747450) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of PDE10A (unknown origin) by IMAP assay | Bioorg Med Chem Lett 26: 126-32 (2016) Article DOI: 10.1016/j.bmcl.2015.11.013 BindingDB Entry DOI: 10.7270/Q2NP27FW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50500519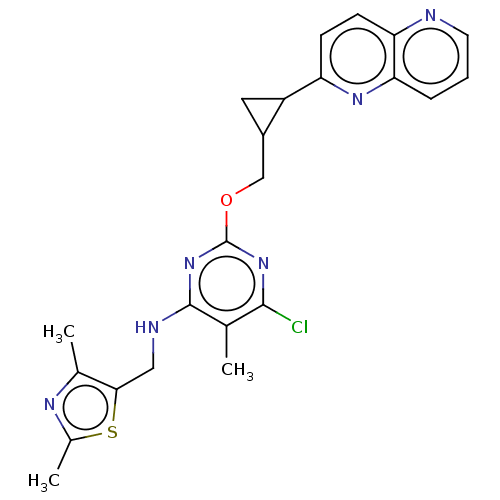 (CHEMBL3746917) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of PDE10A (unknown origin) by IMAP assay | Bioorg Med Chem Lett 26: 126-32 (2016) Article DOI: 10.1016/j.bmcl.2015.11.013 BindingDB Entry DOI: 10.7270/Q2NP27FW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1/beta-1 (Homo sapiens (Human)) | BDBM280795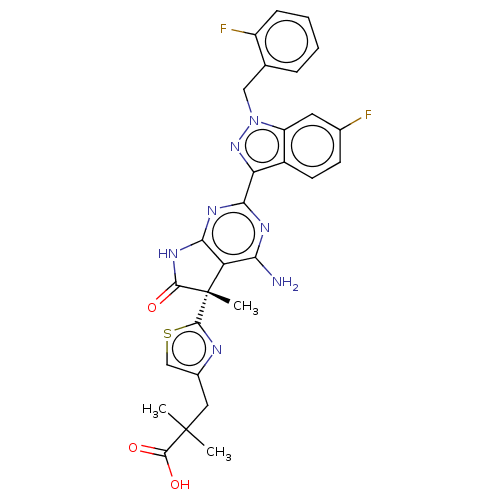 ((S)-3-(2-{4-amino-2-[6- fluoro-1-(2-fluorobenzyl)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The binding buffer was composed of 50 mM triethanolamine, pH 7.4, 3 mM MgCl2, 0.025% BSA, 2 mM dithiothreitol (DTT), 300 μM DETA/NO and 400 _... | US Patent US10428076 (2019) BindingDB Entry DOI: 10.7270/Q22N54P9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1 (Homo sapiens (Human)) | BDBM280795 ((S)-3-(2-{4-amino-2-[6- fluoro-1-(2-fluorobenzyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The binding potencies of sGC compounds to the human recombinant sGC enzyme were determined in a Size Exclusion Chromatography (SEC) competition bindi... | US Patent US10030027 (2018) BindingDB Entry DOI: 10.7270/Q22N549C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1/beta-1 (Homo sapiens (Human)) | BDBM280795 ((S)-3-(2-{4-amino-2-[6- fluoro-1-(2-fluorobenzyl)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The binding buffer was composed of 50 mM triethanolamine, pH 7.4, 3 mM MgCl2, 0.025% BSA, 2 mM dithiothreitol (DTT), 300 μM DETA/NO and 400 _... | US Patent US10428076 (2019) BindingDB Entry DOI: 10.7270/Q22N54P9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1 (Homo sapiens (Human)) | BDBM280795 ((S)-3-(2-{4-amino-2-[6- fluoro-1-(2-fluorobenzyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The binding potencies of sGC compounds to the human recombinant sGC enzyme were determined in a Size Exclusion Chromatography (SEC) competition bindi... | US Patent US10030027 (2018) BindingDB Entry DOI: 10.7270/Q22N549C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1/beta-1 (Homo sapiens (Human)) | BDBM280822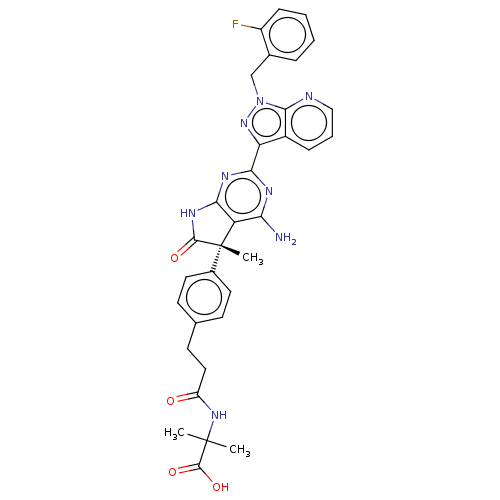 ((S)-2-(3-(4-{4-amino-2- [1-(2-fluorobenzyl)-1H- py...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The binding buffer was composed of 50 mM triethanolamine, pH 7.4, 3 mM MgCl2, 0.025% BSA, 2 mM dithiothreitol (DTT), 300 μM DETA/NO and 400 _... | US Patent US10428076 (2019) BindingDB Entry DOI: 10.7270/Q22N54P9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1 (Homo sapiens (Human)) | BDBM280822 ((S)-2-(3-(4-{4-amino-2- [1-(2-fluorobenzyl)-1H- py...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The binding potencies of sGC compounds to the human recombinant sGC enzyme were determined in a Size Exclusion Chromatography (SEC) competition bindi... | US Patent US10030027 (2018) BindingDB Entry DOI: 10.7270/Q22N549C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1 (Homo sapiens (Human)) | BDBM280822 ((S)-2-(3-(4-{4-amino-2- [1-(2-fluorobenzyl)-1H- py...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The binding potencies of sGC compounds to the human recombinant sGC enzyme were determined in a Size Exclusion Chromatography (SEC) competition bindi... | US Patent US10030027 (2018) BindingDB Entry DOI: 10.7270/Q22N549C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1/beta-1 (Homo sapiens (Human)) | BDBM280822 ((S)-2-(3-(4-{4-amino-2- [1-(2-fluorobenzyl)-1H- py...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The binding buffer was composed of 50 mM triethanolamine, pH 7.4, 3 mM MgCl2, 0.025% BSA, 2 mM dithiothreitol (DTT), 300 μM DETA/NO and 400 _... | US Patent US10428076 (2019) BindingDB Entry DOI: 10.7270/Q22N54P9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1 (Homo sapiens (Human)) | BDBM280911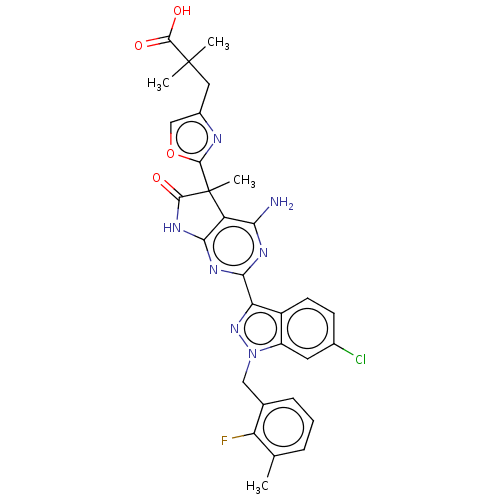 (3-(2-{4-amino-2- [6-chloro-1-(2- fluoro-3-methyl- ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The binding potencies of sGC compounds to the human recombinant sGC enzyme were determined in a Size Exclusion Chromatography (SEC) competition bindi... | US Patent US10030027 (2018) BindingDB Entry DOI: 10.7270/Q22N549C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1 (Homo sapiens (Human)) | BDBM280911 (3-(2-{4-amino-2- [6-chloro-1-(2- fluoro-3-methyl- ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The binding potencies of sGC compounds to the human recombinant sGC enzyme were determined in a Size Exclusion Chromatography (SEC) competition bindi... | US Patent US10030027 (2018) BindingDB Entry DOI: 10.7270/Q22N549C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1/beta-1 (Homo sapiens (Human)) | BDBM280911 (3-(2-{4-amino-2- [6-chloro-1-(2- fluoro-3-methyl- ...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The binding buffer was composed of 50 mM triethanolamine, pH 7.4, 3 mM MgCl2, 0.025% BSA, 2 mM dithiothreitol (DTT), 300 μM DETA/NO and 400 _... | US Patent US10428076 (2019) BindingDB Entry DOI: 10.7270/Q22N54P9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1/beta-1 (Homo sapiens (Human)) | BDBM280911 (3-(2-{4-amino-2- [6-chloro-1-(2- fluoro-3-methyl- ...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The binding buffer was composed of 50 mM triethanolamine, pH 7.4, 3 mM MgCl2, 0.025% BSA, 2 mM dithiothreitol (DTT), 300 μM DETA/NO and 400 _... | US Patent US10428076 (2019) BindingDB Entry DOI: 10.7270/Q22N54P9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM472 (5-tert-butyl-4-{[(6S)-4-hydroxy-6-[2-(4-hydroxyphe...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Tested for binding affinity against HIV protease | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50409174 (CHEMBL169119) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Tested for binding affinity against HIV protease | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM126823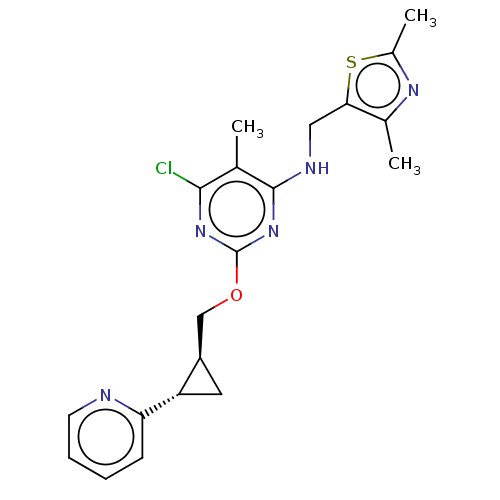 (US8785467, 1-20) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of PDE10A (unknown origin) by IMAP assay | Bioorg Med Chem Lett 26: 126-32 (2016) Article DOI: 10.1016/j.bmcl.2015.11.013 BindingDB Entry DOI: 10.7270/Q2NP27FW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM126823 (US8785467, 1-20) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of PDE10A (unknown origin) by IMAP assay | Bioorg Med Chem Lett 26: 126-32 (2016) Article DOI: 10.1016/j.bmcl.2015.11.013 BindingDB Entry DOI: 10.7270/Q2NP27FW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM472 (5-tert-butyl-4-{[(6S)-4-hydroxy-6-[2-(4-hydroxyphe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM430 (3-[(2-tert-butyl-4-hydroxy-5-methylphenyl)sulfanyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1/beta-1 (Homo sapiens (Human)) | BDBM280876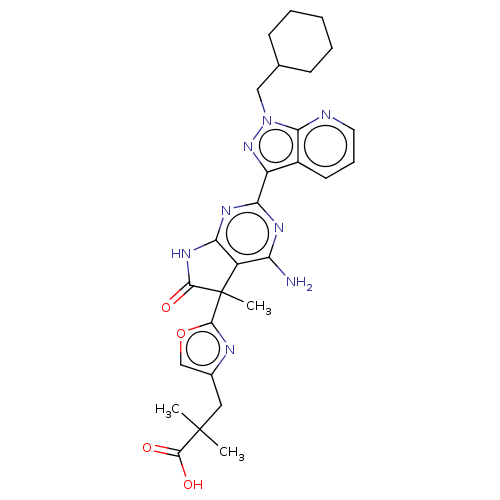 (3-(2-{4-amino- 2-[1-(cyclohexyl- methyl)-1H- pyraz...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The binding buffer was composed of 50 mM triethanolamine, pH 7.4, 3 mM MgCl2, 0.025% BSA, 2 mM dithiothreitol (DTT), 300 μM DETA/NO and 400 _... | US Patent US10428076 (2019) BindingDB Entry DOI: 10.7270/Q22N54P9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1/beta-1 (Homo sapiens (Human)) | BDBM280876 (3-(2-{4-amino- 2-[1-(cyclohexyl- methyl)-1H- pyraz...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The binding buffer was composed of 50 mM triethanolamine, pH 7.4, 3 mM MgCl2, 0.025% BSA, 2 mM dithiothreitol (DTT), 300 μM DETA/NO and 400 _... | US Patent US10428076 (2019) BindingDB Entry DOI: 10.7270/Q22N54P9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1 (Homo sapiens (Human)) | BDBM280914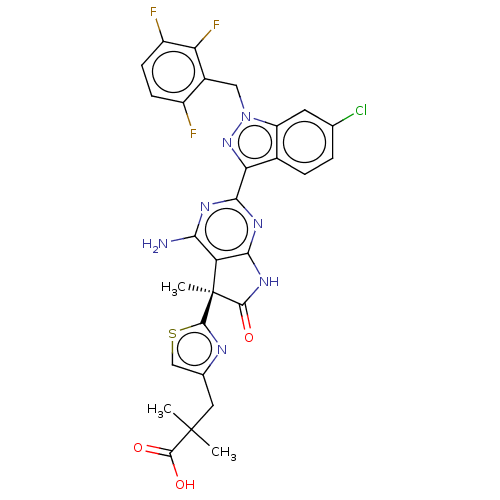 ((S)-3-(2-{4-amino- 2-[6-chloro-1- (2,3,6-trifluoro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The binding potencies of sGC compounds to the human recombinant sGC enzyme were determined in a Size Exclusion Chromatography (SEC) competition bindi... | US Patent US10030027 (2018) BindingDB Entry DOI: 10.7270/Q22N549C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1 (Homo sapiens (Human)) | BDBM280914 ((S)-3-(2-{4-amino- 2-[6-chloro-1- (2,3,6-trifluoro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The binding potencies of sGC compounds to the human recombinant sGC enzyme were determined in a Size Exclusion Chromatography (SEC) competition bindi... | US Patent US10030027 (2018) BindingDB Entry DOI: 10.7270/Q22N549C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1 (Homo sapiens (Human)) | BDBM280904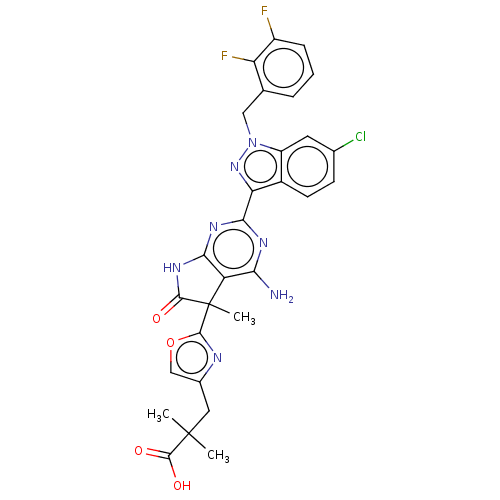 (3-(2-{4-amino-2- [6-chloro-1-(2,3- difluorobenzyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The binding potencies of sGC compounds to the human recombinant sGC enzyme were determined in a Size Exclusion Chromatography (SEC) competition bindi... | US Patent US10030027 (2018) BindingDB Entry DOI: 10.7270/Q22N549C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1 (Homo sapiens (Human)) | BDBM280904 (3-(2-{4-amino-2- [6-chloro-1-(2,3- difluorobenzyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The binding potencies of sGC compounds to the human recombinant sGC enzyme were determined in a Size Exclusion Chromatography (SEC) competition bindi... | US Patent US10030027 (2018) BindingDB Entry DOI: 10.7270/Q22N549C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1 (Homo sapiens (Human)) | BDBM280876 (3-(2-{4-amino- 2-[1-(cyclohexyl- methyl)-1H- pyraz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The binding potencies of sGC compounds to the human recombinant sGC enzyme were determined in a Size Exclusion Chromatography (SEC) competition bindi... | US Patent US10030027 (2018) BindingDB Entry DOI: 10.7270/Q22N549C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1 (Homo sapiens (Human)) | BDBM280876 (3-(2-{4-amino- 2-[1-(cyclohexyl- methyl)-1H- pyraz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The binding potencies of sGC compounds to the human recombinant sGC enzyme were determined in a Size Exclusion Chromatography (SEC) competition bindi... | US Patent US10030027 (2018) BindingDB Entry DOI: 10.7270/Q22N549C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1/beta-1 (Homo sapiens (Human)) | BDBM280914 ((S)-3-(2-{4-amino- 2-[6-chloro-1- (2,3,6-trifluoro...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The binding buffer was composed of 50 mM triethanolamine, pH 7.4, 3 mM MgCl2, 0.025% BSA, 2 mM dithiothreitol (DTT), 300 μM DETA/NO and 400 _... | US Patent US10428076 (2019) BindingDB Entry DOI: 10.7270/Q22N54P9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1/beta-1 (Homo sapiens (Human)) | BDBM280914 ((S)-3-(2-{4-amino- 2-[6-chloro-1- (2,3,6-trifluoro...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The binding buffer was composed of 50 mM triethanolamine, pH 7.4, 3 mM MgCl2, 0.025% BSA, 2 mM dithiothreitol (DTT), 300 μM DETA/NO and 400 _... | US Patent US10428076 (2019) BindingDB Entry DOI: 10.7270/Q22N54P9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1/beta-1 (Homo sapiens (Human)) | BDBM280904 (3-(2-{4-amino-2- [6-chloro-1-(2,3- difluorobenzyl)...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The binding buffer was composed of 50 mM triethanolamine, pH 7.4, 3 mM MgCl2, 0.025% BSA, 2 mM dithiothreitol (DTT), 300 μM DETA/NO and 400 _... | US Patent US10428076 (2019) BindingDB Entry DOI: 10.7270/Q22N54P9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1/beta-1 (Homo sapiens (Human)) | BDBM280904 (3-(2-{4-amino-2- [6-chloro-1-(2,3- difluorobenzyl)...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The binding buffer was composed of 50 mM triethanolamine, pH 7.4, 3 mM MgCl2, 0.025% BSA, 2 mM dithiothreitol (DTT), 300 μM DETA/NO and 400 _... | US Patent US10428076 (2019) BindingDB Entry DOI: 10.7270/Q22N54P9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1 (Homo sapiens (Human)) | BDBM280821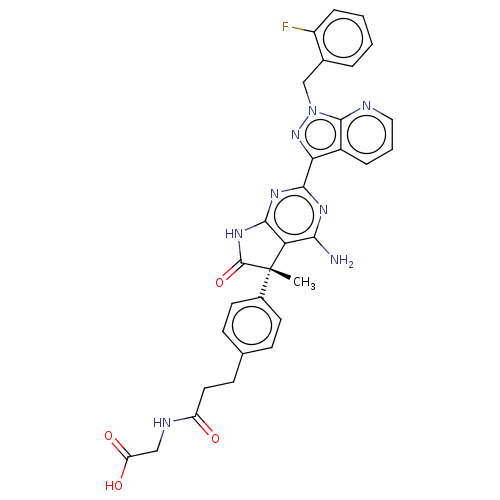 ((S)-(3-(4-{4-Amino-2-[1-(2-fluorobenzyl)-1H-pyrazo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The binding potencies of sGC compounds to the human recombinant sGC enzyme were determined in a Size Exclusion Chromatography (SEC) competition bindi... | US Patent US10030027 (2018) BindingDB Entry DOI: 10.7270/Q22N549C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1 (Homo sapiens (Human)) | BDBM280821 ((S)-(3-(4-{4-Amino-2-[1-(2-fluorobenzyl)-1H-pyrazo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The binding potencies of sGC compounds to the human recombinant sGC enzyme were determined in a Size Exclusion Chromatography (SEC) competition bindi... | US Patent US10030027 (2018) BindingDB Entry DOI: 10.7270/Q22N549C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1/beta-1 (Homo sapiens (Human)) | BDBM280821 ((S)-(3-(4-{4-Amino-2-[1-(2-fluorobenzyl)-1H-pyrazo...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The binding buffer was composed of 50 mM triethanolamine, pH 7.4, 3 mM MgCl2, 0.025% BSA, 2 mM dithiothreitol (DTT), 300 μM DETA/NO and 400 _... | US Patent US10428076 (2019) BindingDB Entry DOI: 10.7270/Q22N54P9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1/beta-1 (Homo sapiens (Human)) | BDBM280821 ((S)-(3-(4-{4-Amino-2-[1-(2-fluorobenzyl)-1H-pyrazo...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The binding buffer was composed of 50 mM triethanolamine, pH 7.4, 3 mM MgCl2, 0.025% BSA, 2 mM dithiothreitol (DTT), 300 μM DETA/NO and 400 _... | US Patent US10428076 (2019) BindingDB Entry DOI: 10.7270/Q22N54P9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50245852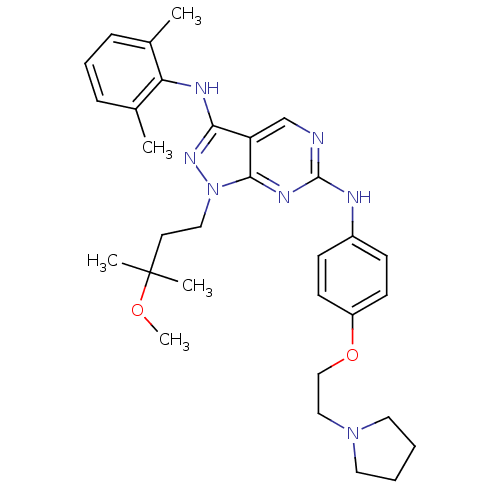 (CHEMBL458333 | N3-(2,6-dimethylphenyl)-1-(3-methox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of LCK (unknown origin) | Bioorg Med Chem Lett 18: 6352-6 (2008) Article DOI: 10.1016/j.bmcl.2008.10.092 BindingDB Entry DOI: 10.7270/Q2B56JKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1/beta-1 (Homo sapiens (Human)) | BDBM280918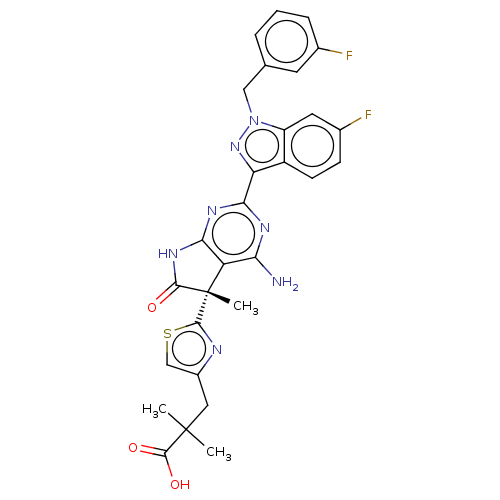 ((S)-3-(2-{4-amino- 2-[6-fluoro-1-(3- fluorobenzyl)...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The binding buffer was composed of 50 mM triethanolamine, pH 7.4, 3 mM MgCl2, 0.025% BSA, 2 mM dithiothreitol (DTT), 300 μM DETA/NO and 400 _... | US Patent US10428076 (2019) BindingDB Entry DOI: 10.7270/Q22N54P9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1/beta-1 (Homo sapiens (Human)) | BDBM280918 ((S)-3-(2-{4-amino- 2-[6-fluoro-1-(3- fluorobenzyl)...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The binding buffer was composed of 50 mM triethanolamine, pH 7.4, 3 mM MgCl2, 0.025% BSA, 2 mM dithiothreitol (DTT), 300 μM DETA/NO and 400 _... | US Patent US10428076 (2019) BindingDB Entry DOI: 10.7270/Q22N54P9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1 (Homo sapiens (Human)) | BDBM280918 ((S)-3-(2-{4-amino- 2-[6-fluoro-1-(3- fluorobenzyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The binding potencies of sGC compounds to the human recombinant sGC enzyme were determined in a Size Exclusion Chromatography (SEC) competition bindi... | US Patent US10030027 (2018) BindingDB Entry DOI: 10.7270/Q22N549C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1 (Homo sapiens (Human)) | BDBM280918 ((S)-3-(2-{4-amino- 2-[6-fluoro-1-(3- fluorobenzyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The binding potencies of sGC compounds to the human recombinant sGC enzyme were determined in a Size Exclusion Chromatography (SEC) competition bindi... | US Patent US10030027 (2018) BindingDB Entry DOI: 10.7270/Q22N549C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50514737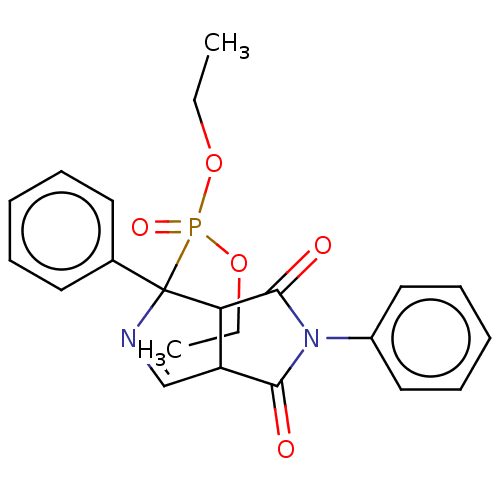 (CHEMBL4482861) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0417 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Displacement of [3H]RX821002 from alpha2-AR in human brain frontal cortex incubated for 30 mins by liquid scintillation spectrometry | J Med Chem 63: 3610-3633 (2020) Article DOI: 10.1021/acs.jmedchem.9b02080 BindingDB Entry DOI: 10.7270/Q2FB569H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1/beta-1 (Homo sapiens (Human)) | BDBM280824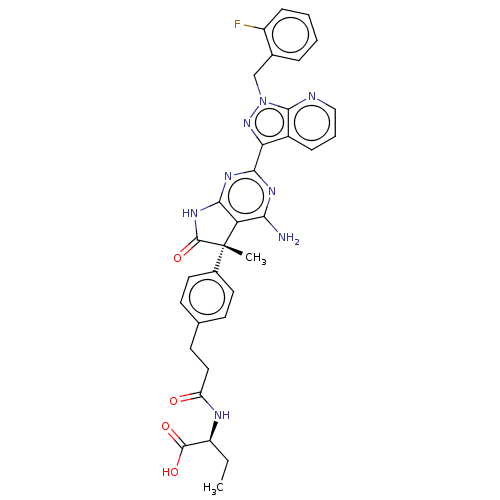 ((5S,2S)-2-(3-(4-{4- amino-2-[1-(2- fluorobenzyl)-1...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The binding buffer was composed of 50 mM triethanolamine, pH 7.4, 3 mM MgCl2, 0.025% BSA, 2 mM dithiothreitol (DTT), 300 μM DETA/NO and 400 _... | US Patent US10428076 (2019) BindingDB Entry DOI: 10.7270/Q22N54P9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1 (Homo sapiens (Human)) | BDBM280824 ((5S,2S)-2-(3-(4-{4- amino-2-[1-(2- fluorobenzyl)-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The binding potencies of sGC compounds to the human recombinant sGC enzyme were determined in a Size Exclusion Chromatography (SEC) competition bindi... | US Patent US10030027 (2018) BindingDB Entry DOI: 10.7270/Q22N549C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1/beta-1 (Homo sapiens (Human)) | BDBM280824 ((5S,2S)-2-(3-(4-{4- amino-2-[1-(2- fluorobenzyl)-1...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The binding buffer was composed of 50 mM triethanolamine, pH 7.4, 3 mM MgCl2, 0.025% BSA, 2 mM dithiothreitol (DTT), 300 μM DETA/NO and 400 _... | US Patent US10428076 (2019) BindingDB Entry DOI: 10.7270/Q22N54P9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1 (Homo sapiens (Human)) | BDBM280824 ((5S,2S)-2-(3-(4-{4- amino-2-[1-(2- fluorobenzyl)-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The binding potencies of sGC compounds to the human recombinant sGC enzyme were determined in a Size Exclusion Chromatography (SEC) competition bindi... | US Patent US10030027 (2018) BindingDB Entry DOI: 10.7270/Q22N549C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 18616 total ) | Next | Last >> |