

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Endothelin-1 receptor (RAT) | BDBM50065516 ((R)-4-[2-Cyano-5-(pyridin-3-ylmethoxy)-phenoxy]-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro inhibition of [125I]ET1 binding to rat A10 cell Endothelin A receptor. | J Med Chem 41: 2732-44 (1998) Article DOI: 10.1021/jm9707131 BindingDB Entry DOI: 10.7270/Q2HM594W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50065508 ((R)-4-[2-Cyano-5-(thiophen-3-ylmethoxy)-phenoxy]-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro inhibition of [125I]ET1 binding to rat A10 cell Endothelin A receptor. | J Med Chem 41: 2732-44 (1998) Article DOI: 10.1021/jm9707131 BindingDB Entry DOI: 10.7270/Q2HM594W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50065518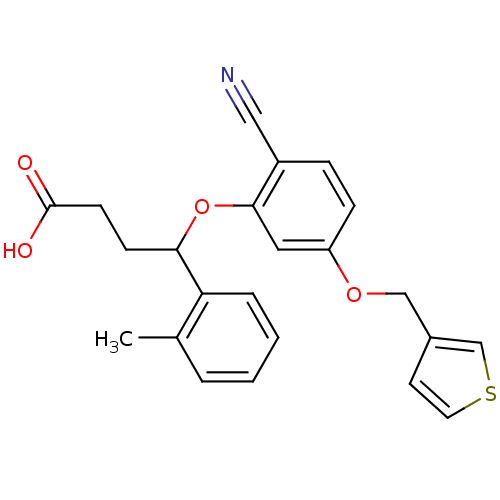 (4-[2-Cyano-5-(thiophen-3-ylmethoxy)-phenoxy]-4-o-t...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro inhibition of [125I]ET1 binding to rat A10 cell Endothelin A receptor. | J Med Chem 41: 2732-44 (1998) Article DOI: 10.1021/jm9707131 BindingDB Entry DOI: 10.7270/Q2HM594W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50065523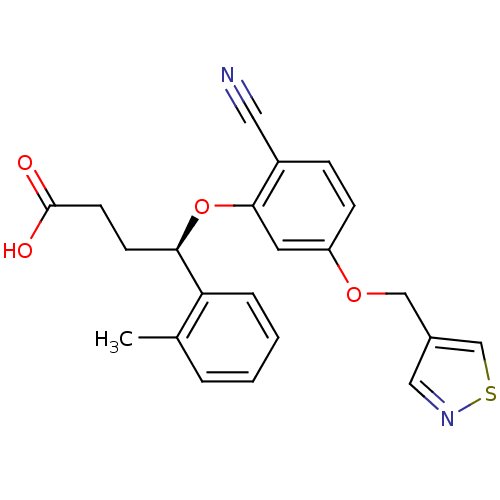 ((R)-4-[2-Cyano-5-(isothiazol-4-ylmethoxy)-phenoxy]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro inhibition of [125I]ET1 binding to rat A10 cell Endothelin A receptor. | J Med Chem 41: 2732-44 (1998) Article DOI: 10.1021/jm9707131 BindingDB Entry DOI: 10.7270/Q2HM594W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50065524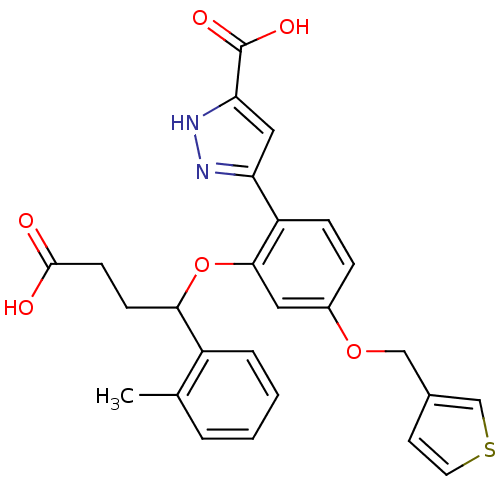 (5-[2-(3-Carboxy-1-o-tolyl-propoxy)-4-(thiophen-3-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro inhibition of [125I]ET1 binding to rat A10 cell Endothelin A receptor. | J Med Chem 41: 2732-44 (1998) Article DOI: 10.1021/jm9707131 BindingDB Entry DOI: 10.7270/Q2HM594W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50329850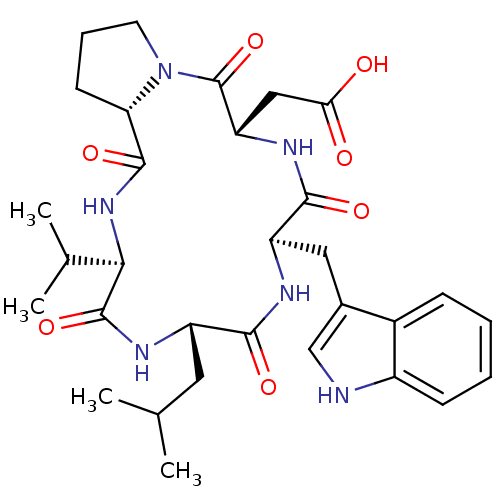 ((R)-3-Amino-4-[(S)-2-((R)-1-{(S)-1-[(R)-1-(1H-indo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro inhibition of [125I]ET1 binding to rat A10 cell Endothelin A receptor. | J Med Chem 41: 2732-44 (1998) Article DOI: 10.1021/jm9707131 BindingDB Entry DOI: 10.7270/Q2HM594W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50065525 (4-[2-(1H-Pyrazol-3-yl)-5-(thiophen-3-ylmethoxy)-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro inhibition of [125I]ET1 binding to rat A10 cell Endothelin A receptor. | J Med Chem 41: 2732-44 (1998) Article DOI: 10.1021/jm9707131 BindingDB Entry DOI: 10.7270/Q2HM594W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50065515 ((R)-4-[2-Cyano-5-(pyridin-4-ylmethoxy)-phenoxy]-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro inhibition of [125I]ET1 binding to rat A10 cell Endothelin A receptor. | J Med Chem 41: 2732-44 (1998) Article DOI: 10.1021/jm9707131 BindingDB Entry DOI: 10.7270/Q2HM594W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50065513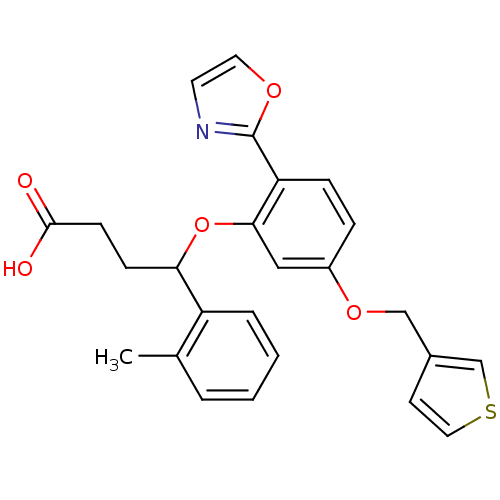 (4-[2-Oxazol-2-yl-5-(thiophen-3-ylmethoxy)-phenoxy]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro inhibition of [125I]ET1 binding to rat A10 cell Endothelin A receptor. | J Med Chem 41: 2732-44 (1998) Article DOI: 10.1021/jm9707131 BindingDB Entry DOI: 10.7270/Q2HM594W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50051901 (1-[5-(4,5-Diphenyl-1H-imidazol-2-ylsulfanyl)-penty...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against Acyl coenzyme A:cholesterol acyltransferase in microsomes from rat liver | Bioorg Med Chem Lett 6: 47-50 (1996) Article DOI: 10.1016/0960-894X(95)00555-8 BindingDB Entry DOI: 10.7270/Q2N29WXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50065521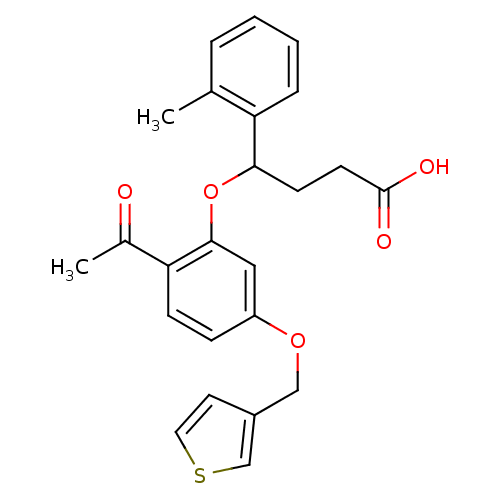 (4-[2-Acetyl-5-(thiophen-3-ylmethoxy)-phenoxy]-4-o-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro inhibition of [125I]ET1 binding to rat A10 cell Endothelin A receptor. | J Med Chem 41: 2732-44 (1998) Article DOI: 10.1021/jm9707131 BindingDB Entry DOI: 10.7270/Q2HM594W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50065512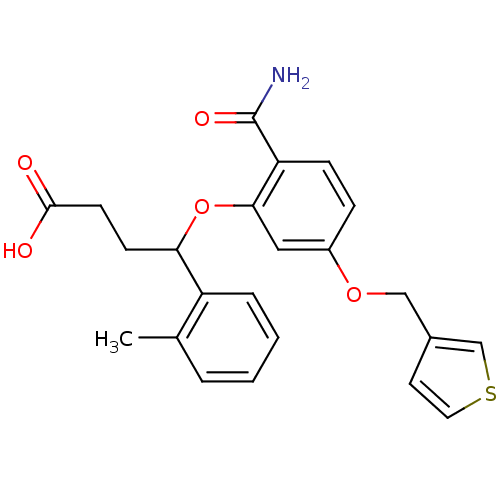 (4-[2-Carbamoyl-5-(thiophen-3-ylmethoxy)-phenoxy]-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro inhibition of [125I]ET1 binding to rat A10 cell Endothelin A receptor. | J Med Chem 41: 2732-44 (1998) Article DOI: 10.1021/jm9707131 BindingDB Entry DOI: 10.7270/Q2HM594W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50051901 (1-[5-(4,5-Diphenyl-1H-imidazol-2-ylsulfanyl)-penty...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against Acyl coenzyme A:cholesterol acyltransferase in microsomes from rabbit artery. | Bioorg Med Chem Lett 6: 47-50 (1996) Article DOI: 10.1016/0960-894X(95)00555-8 BindingDB Entry DOI: 10.7270/Q2N29WXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50065520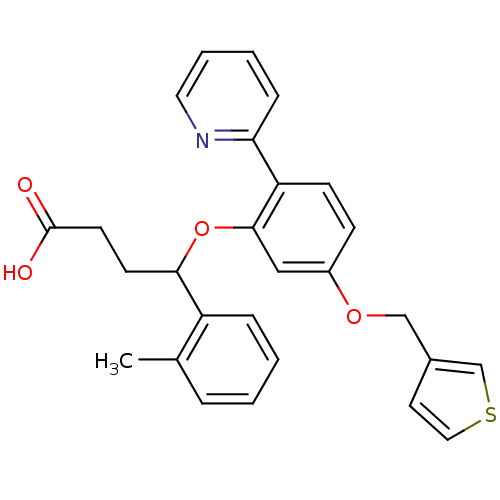 (4-[2-Pyridin-2-yl-5-(thiophen-3-ylmethoxy)-phenoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro inhibition of [125I]ET1 binding to rat A10 cell Endothelin A receptor. | J Med Chem 41: 2732-44 (1998) Article DOI: 10.1021/jm9707131 BindingDB Entry DOI: 10.7270/Q2HM594W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50051901 (1-[5-(4,5-Diphenyl-1H-imidazol-2-ylsulfanyl)-penty...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against Acyl coenzyme A:cholesterol acyltransferase in microsomes from rabbit liver. | Bioorg Med Chem Lett 6: 47-50 (1996) Article DOI: 10.1016/0960-894X(95)00555-8 BindingDB Entry DOI: 10.7270/Q2N29WXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50065522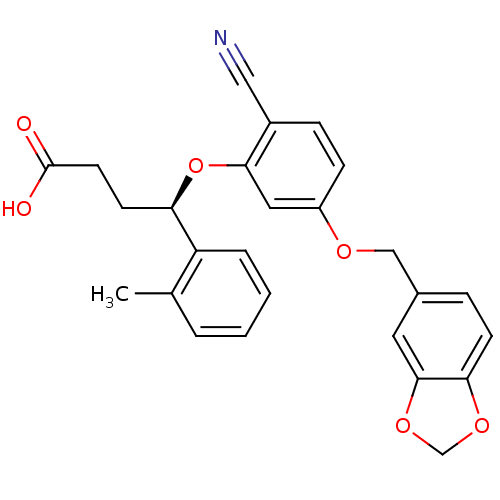 ((R)-4-[5-(Benzo[1,3]dioxol-5-ylmethoxy)-2-cyano-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro inhibition of [125I]ET1 binding to rat A10 cell Endothelin A receptor. | J Med Chem 41: 2732-44 (1998) Article DOI: 10.1021/jm9707131 BindingDB Entry DOI: 10.7270/Q2HM594W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50051901 (1-[5-(4,5-Diphenyl-1H-imidazol-2-ylsulfanyl)-penty...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against Acyl coenzyme A:cholesterol acyltransferase in microsomes from rabbit adrenal. | Bioorg Med Chem Lett 6: 47-50 (1996) Article DOI: 10.1016/0960-894X(95)00555-8 BindingDB Entry DOI: 10.7270/Q2N29WXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50051884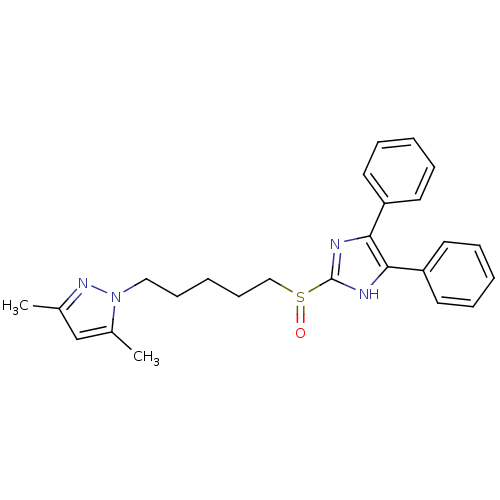 (1-[5-(4,5-Diphenyl-1H-imidazole-2-sulfinyl)-pentyl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against Acyl coenzyme A:cholesterol acyltransferase in microsomes from rat liver | Bioorg Med Chem Lett 6: 47-50 (1996) Article DOI: 10.1016/0960-894X(95)00555-8 BindingDB Entry DOI: 10.7270/Q2N29WXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50051884 (1-[5-(4,5-Diphenyl-1H-imidazole-2-sulfinyl)-pentyl...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 108 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against Acyl coenzyme A:cholesterol acyltransferase in microsomes from HEP G2 cells. | Bioorg Med Chem Lett 6: 47-50 (1996) Article DOI: 10.1016/0960-894X(95)00555-8 BindingDB Entry DOI: 10.7270/Q2N29WXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50051901 (1-[5-(4,5-Diphenyl-1H-imidazol-2-ylsulfanyl)-penty...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 108 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against Acyl coenzyme A:cholesterol acyltransferase in microsomes from rabbit liver. | Bioorg Med Chem Lett 6: 47-50 (1996) Article DOI: 10.1016/0960-894X(95)00555-8 BindingDB Entry DOI: 10.7270/Q2N29WXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50065514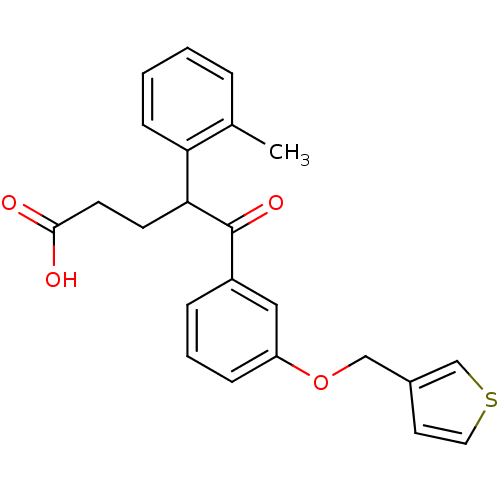 (5-Oxo-5-[3-(thiophen-3-ylmethoxy)-phenyl]-4-o-toly...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro inhibition of [125I]ET1 binding to rat A10 cell Endothelin A receptor. | J Med Chem 41: 2732-44 (1998) Article DOI: 10.1021/jm9707131 BindingDB Entry DOI: 10.7270/Q2HM594W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50065511 ((S)-4-[2-Cyano-5-(thiophen-3-ylmethoxy)-phenoxy]-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 113 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro inhibition of [125I]ET1 binding to rat A10 cell Endothelin A receptor. | J Med Chem 41: 2732-44 (1998) Article DOI: 10.1021/jm9707131 BindingDB Entry DOI: 10.7270/Q2HM594W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50051884 (1-[5-(4,5-Diphenyl-1H-imidazole-2-sulfinyl)-pentyl...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 122 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against Acyl coenzyme A:cholesterol acyltransferase in microsomes from rabbit intestine. | Bioorg Med Chem Lett 6: 47-50 (1996) Article DOI: 10.1016/0960-894X(95)00555-8 BindingDB Entry DOI: 10.7270/Q2N29WXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50051884 (1-[5-(4,5-Diphenyl-1H-imidazole-2-sulfinyl)-pentyl...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 164 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against Acyl coenzyme A:cholesterol acyltransferase in microsomes from THP-1 cells. | Bioorg Med Chem Lett 6: 47-50 (1996) Article DOI: 10.1016/0960-894X(95)00555-8 BindingDB Entry DOI: 10.7270/Q2N29WXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50065510 (2-(3-Carboxy-1-o-tolyl-propoxy)-4-(thiophen-3-ylme...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 207 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro inhibition of [125I]ET1 binding to rat A10 cell Endothelin A receptor. | J Med Chem 41: 2732-44 (1998) Article DOI: 10.1021/jm9707131 BindingDB Entry DOI: 10.7270/Q2HM594W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50051884 (1-[5-(4,5-Diphenyl-1H-imidazole-2-sulfinyl)-pentyl...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 208 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against Acyl coenzyme A:cholesterol acyltransferase in microsomes from rabbit intestine. | Bioorg Med Chem Lett 6: 47-50 (1996) Article DOI: 10.1016/0960-894X(95)00555-8 BindingDB Entry DOI: 10.7270/Q2N29WXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50051884 (1-[5-(4,5-Diphenyl-1H-imidazole-2-sulfinyl)-pentyl...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 245 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against Acyl coenzyme A:cholesterol acyltransferase in microsomes from rabbit artery. | Bioorg Med Chem Lett 6: 47-50 (1996) Article DOI: 10.1016/0960-894X(95)00555-8 BindingDB Entry DOI: 10.7270/Q2N29WXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50051884 (1-[5-(4,5-Diphenyl-1H-imidazole-2-sulfinyl)-pentyl...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against Acyl coenzyme A:cholesterol acyltransferase in microsomes from rabbit adrenal. | Bioorg Med Chem Lett 6: 47-50 (1996) Article DOI: 10.1016/0960-894X(95)00555-8 BindingDB Entry DOI: 10.7270/Q2N29WXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50065507 (4-[3-(Thiophen-3-ylmethoxy)-phenoxy]-4-o-tolyl-but...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 871 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro inhibition of [125I]ET1 binding to rat A10 cell Endothelin A receptor. | J Med Chem 41: 2732-44 (1998) Article DOI: 10.1021/jm9707131 BindingDB Entry DOI: 10.7270/Q2HM594W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50065517 (5-(2,4-Bis-benzyloxy-phenyl)-1H-pyrazole-3-carboxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro inhibition of [125I]ET1 binding to rat A10 cell Endothelin A receptor. | J Med Chem 41: 2732-44 (1998) Article DOI: 10.1021/jm9707131 BindingDB Entry DOI: 10.7270/Q2HM594W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50065524 (5-[2-(3-Carboxy-1-o-tolyl-propoxy)-4-(thiophen-3-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro inhibition of [125I]ET1 binding to rat cerebellum Endothelin B receptor. | J Med Chem 41: 2732-44 (1998) Article DOI: 10.1021/jm9707131 BindingDB Entry DOI: 10.7270/Q2HM594W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50065518 (4-[2-Cyano-5-(thiophen-3-ylmethoxy)-phenoxy]-4-o-t...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro inhibition of [125I]ET1 binding to rat cerebellum Endothelin B receptor. | J Med Chem 41: 2732-44 (1998) Article DOI: 10.1021/jm9707131 BindingDB Entry DOI: 10.7270/Q2HM594W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50065509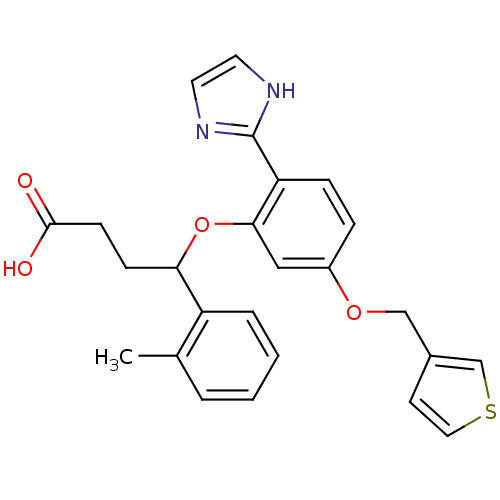 (4-[2-(1H-Imidazol-2-yl)-5-(thiophen-3-ylmethoxy)-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro inhibition of [125I]ET1 binding to rat cerebellum Endothelin B receptor. | J Med Chem 41: 2732-44 (1998) Article DOI: 10.1021/jm9707131 BindingDB Entry DOI: 10.7270/Q2HM594W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50065521 (4-[2-Acetyl-5-(thiophen-3-ylmethoxy)-phenoxy]-4-o-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro inhibition of [125I]ET1 binding to rat cerebellum Endothelin B receptor. | J Med Chem 41: 2732-44 (1998) Article DOI: 10.1021/jm9707131 BindingDB Entry DOI: 10.7270/Q2HM594W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50065512 (4-[2-Carbamoyl-5-(thiophen-3-ylmethoxy)-phenoxy]-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro inhibition of [125I]ET1 binding to rat cerebellum Endothelin B receptor. | J Med Chem 41: 2732-44 (1998) Article DOI: 10.1021/jm9707131 BindingDB Entry DOI: 10.7270/Q2HM594W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50065515 ((R)-4-[2-Cyano-5-(pyridin-4-ylmethoxy)-phenoxy]-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro inhibition of [125I]ET1 binding to rat cerebellum Endothelin B receptor. | J Med Chem 41: 2732-44 (1998) Article DOI: 10.1021/jm9707131 BindingDB Entry DOI: 10.7270/Q2HM594W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50065508 ((R)-4-[2-Cyano-5-(thiophen-3-ylmethoxy)-phenoxy]-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro inhibition of [125I]ET1 binding to rat cerebellum Endothelin B receptor. | J Med Chem 41: 2732-44 (1998) Article DOI: 10.1021/jm9707131 BindingDB Entry DOI: 10.7270/Q2HM594W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50065510 (2-(3-Carboxy-1-o-tolyl-propoxy)-4-(thiophen-3-ylme...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro inhibition of [125I]ET1 binding to rat cerebellum Endothelin B receptor. | J Med Chem 41: 2732-44 (1998) Article DOI: 10.1021/jm9707131 BindingDB Entry DOI: 10.7270/Q2HM594W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50065513 (4-[2-Oxazol-2-yl-5-(thiophen-3-ylmethoxy)-phenoxy]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro inhibition of [125I]ET1 binding to rat cerebellum Endothelin B receptor. | J Med Chem 41: 2732-44 (1998) Article DOI: 10.1021/jm9707131 BindingDB Entry DOI: 10.7270/Q2HM594W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50065516 ((R)-4-[2-Cyano-5-(pyridin-3-ylmethoxy)-phenoxy]-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro inhibition of [125I]ET1 binding to rat cerebellum Endothelin B receptor. | J Med Chem 41: 2732-44 (1998) Article DOI: 10.1021/jm9707131 BindingDB Entry DOI: 10.7270/Q2HM594W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50065525 (4-[2-(1H-Pyrazol-3-yl)-5-(thiophen-3-ylmethoxy)-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro inhibition of [125I]ET1 binding to rat cerebellum Endothelin B receptor. | J Med Chem 41: 2732-44 (1998) Article DOI: 10.1021/jm9707131 BindingDB Entry DOI: 10.7270/Q2HM594W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50065520 (4-[2-Pyridin-2-yl-5-(thiophen-3-ylmethoxy)-phenoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro inhibition of [125I]ET1 binding to rat cerebellum Endothelin B receptor. | J Med Chem 41: 2732-44 (1998) Article DOI: 10.1021/jm9707131 BindingDB Entry DOI: 10.7270/Q2HM594W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50065523 ((R)-4-[2-Cyano-5-(isothiazol-4-ylmethoxy)-phenoxy]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro inhibition of [125I]ET1 binding to rat cerebellum Endothelin B receptor. | J Med Chem 41: 2732-44 (1998) Article DOI: 10.1021/jm9707131 BindingDB Entry DOI: 10.7270/Q2HM594W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50065522 ((R)-4-[5-(Benzo[1,3]dioxol-5-ylmethoxy)-2-cyano-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro inhibition of [125I]ET1 binding to rat cerebellum Endothelin B receptor. | J Med Chem 41: 2732-44 (1998) Article DOI: 10.1021/jm9707131 BindingDB Entry DOI: 10.7270/Q2HM594W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50065511 ((S)-4-[2-Cyano-5-(thiophen-3-ylmethoxy)-phenoxy]-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro inhibition of [125I]ET1 binding to rat cerebellum Endothelin B receptor. | J Med Chem 41: 2732-44 (1998) Article DOI: 10.1021/jm9707131 BindingDB Entry DOI: 10.7270/Q2HM594W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50065514 (5-Oxo-5-[3-(thiophen-3-ylmethoxy)-phenyl]-4-o-toly...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro inhibition of [125I]ET1 binding to rat cerebellum Endothelin B receptor. | J Med Chem 41: 2732-44 (1998) Article DOI: 10.1021/jm9707131 BindingDB Entry DOI: 10.7270/Q2HM594W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50065507 (4-[3-(Thiophen-3-ylmethoxy)-phenoxy]-4-o-tolyl-but...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro inhibition of [125I]ET1 binding to rat cerebellum Endothelin B receptor. | J Med Chem 41: 2732-44 (1998) Article DOI: 10.1021/jm9707131 BindingDB Entry DOI: 10.7270/Q2HM594W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50065517 (5-(2,4-Bis-benzyloxy-phenyl)-1H-pyrazole-3-carboxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro inhibition of [125I]ET1 binding to rat cerebellum Endothelin B receptor. | J Med Chem 41: 2732-44 (1998) Article DOI: 10.1021/jm9707131 BindingDB Entry DOI: 10.7270/Q2HM594W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50329850 ((R)-3-Amino-4-[(S)-2-((R)-1-{(S)-1-[(R)-1-(1H-indo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro inhibition of [125I]ET1 binding to rat cerebellum Endothelin B receptor. | J Med Chem 41: 2732-44 (1998) Article DOI: 10.1021/jm9707131 BindingDB Entry DOI: 10.7270/Q2HM594W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50065509 (4-[2-(1H-Imidazol-2-yl)-5-(thiophen-3-ylmethoxy)-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.63E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro inhibition of [125I]ET1 binding to rat A10 cell Endothelin A receptor. | J Med Chem 41: 2732-44 (1998) Article DOI: 10.1021/jm9707131 BindingDB Entry DOI: 10.7270/Q2HM594W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||