

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50499349 (CHEMBL4299370) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC6 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50499361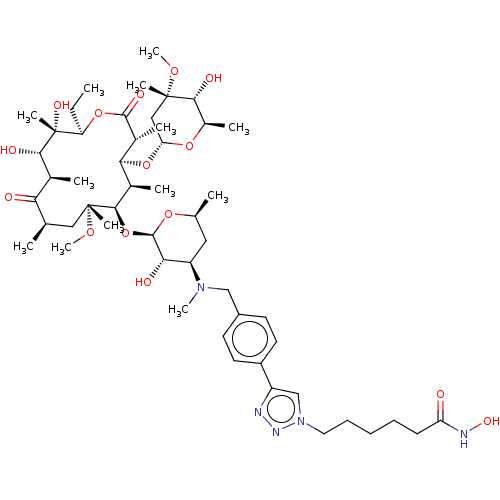 (CHEMBL4299435) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC6 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50499346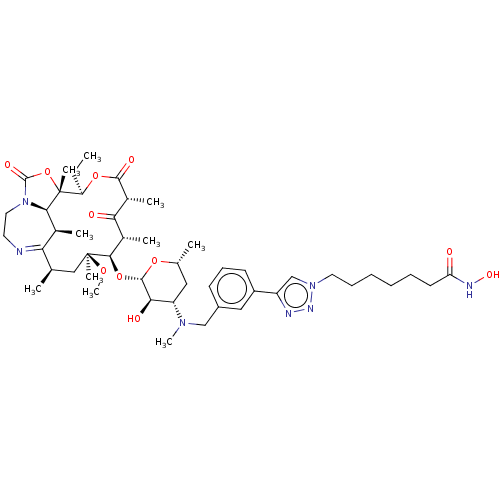 (CHEMBL3736314) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC6 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50499327 (CHEMBL4299447) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC6 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50209796 (CHEMBL3885011) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC6 (unknown origin) after 20 hrs by SAMDI mass spectroscopic analysis | Bioorg Med Chem 25: 1202-1218 (2017) Article DOI: 10.1016/j.bmc.2016.12.032 BindingDB Entry DOI: 10.7270/Q2F76FJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50499350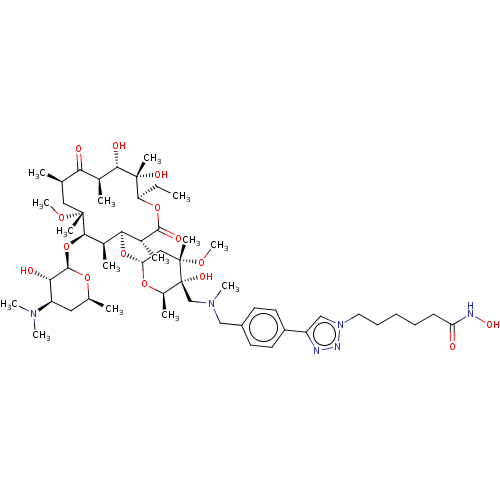 (CHEMBL4299426) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC6 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50499330 (CHEMBL4299491) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC6 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50499357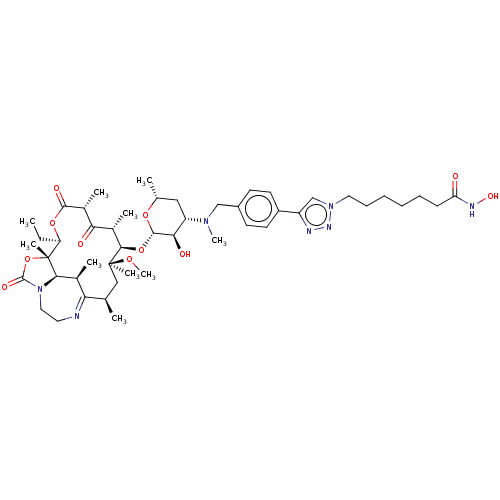 (CHEMBL3736342) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC6 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50499331 (CHEMBL4299417) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC6 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50499320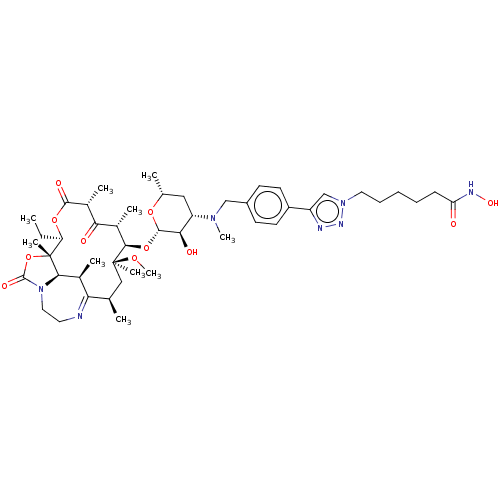 (CHEMBL3735736) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC6 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50499345 (CHEMBL4299449) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC6 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50209789 (CHEMBL3885174) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC6 (unknown origin) after 20 hrs by SAMDI mass spectroscopic analysis | Bioorg Med Chem 25: 1202-1218 (2017) Article DOI: 10.1016/j.bmc.2016.12.032 BindingDB Entry DOI: 10.7270/Q2F76FJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50499362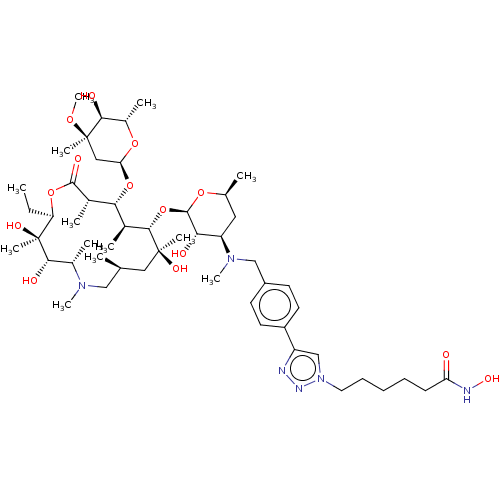 (CHEMBL4299470) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC6 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50499343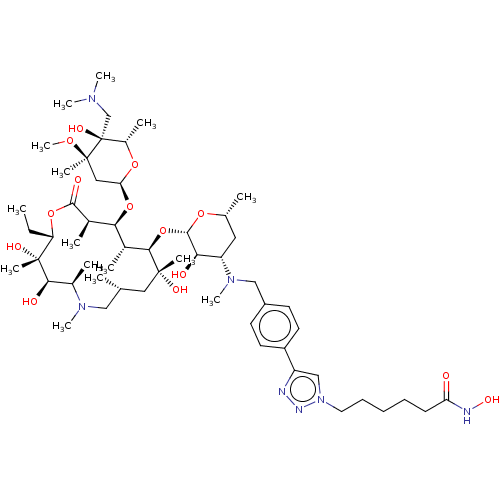 (CHEMBL4299468) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC6 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50499327 (CHEMBL4299447) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC1 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50545002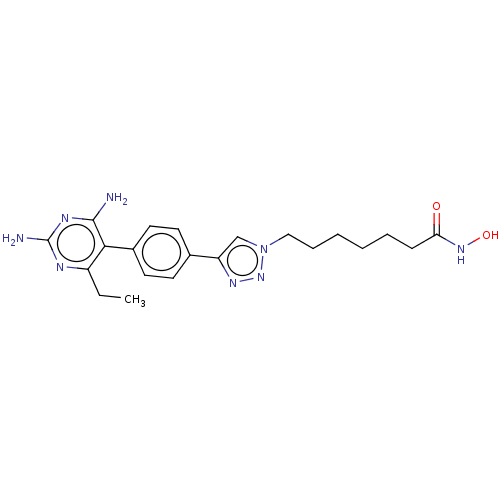 (CHEMBL4635479) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC6 using fluorogenic HDAC substrate 3 preincubated for 30 mins followed by substrate addition and measured after 1... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115345 BindingDB Entry DOI: 10.7270/Q2PR80K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50499319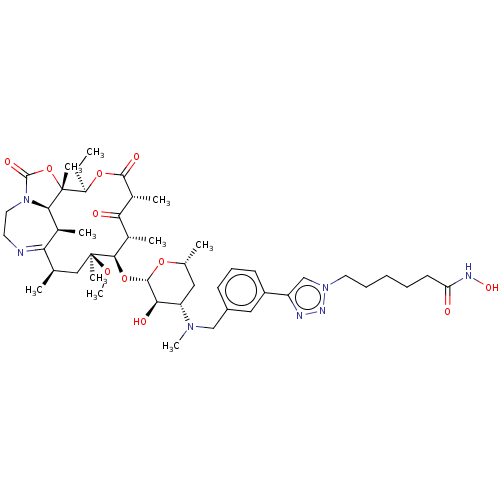 (CHEMBL3735212) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC6 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50499358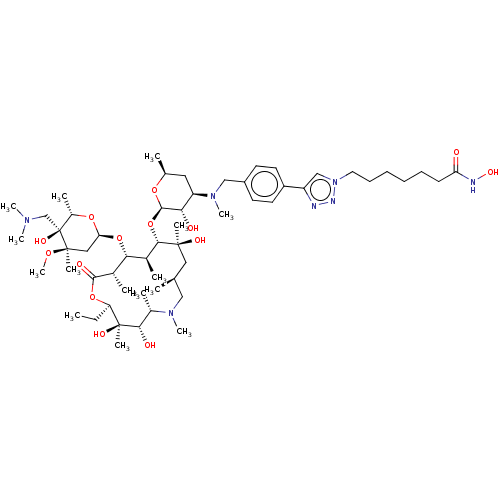 (CHEMBL4299467) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC6 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50545003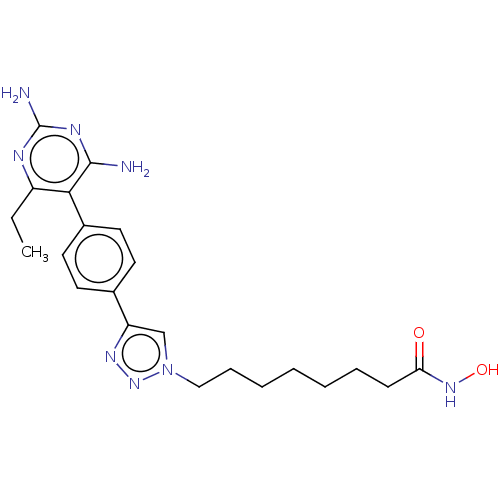 (CHEMBL4636452) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC6 using fluorogenic HDAC substrate 3 preincubated for 30 mins followed by substrate addition and measured after 1... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115345 BindingDB Entry DOI: 10.7270/Q2PR80K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50499357 (CHEMBL3736342) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC1 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50209788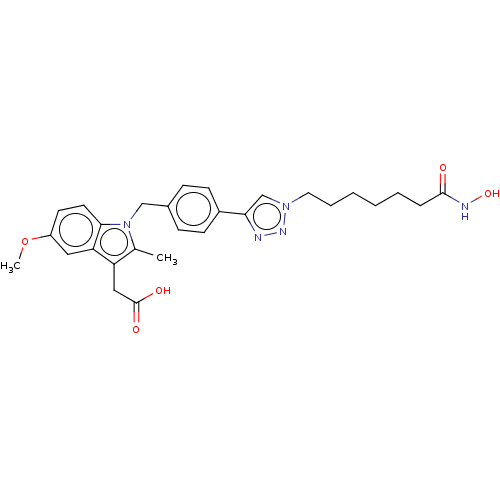 (CHEMBL3884506) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC6 (unknown origin) after 20 hrs by SAMDI mass spectroscopic analysis | Bioorg Med Chem 25: 1202-1218 (2017) Article DOI: 10.1016/j.bmc.2016.12.032 BindingDB Entry DOI: 10.7270/Q2F76FJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50499349 (CHEMBL4299370) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC1 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50499355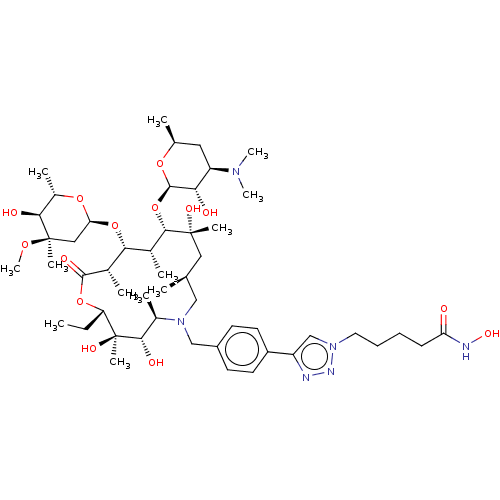 (CHEMBL4299469) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC6 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50499325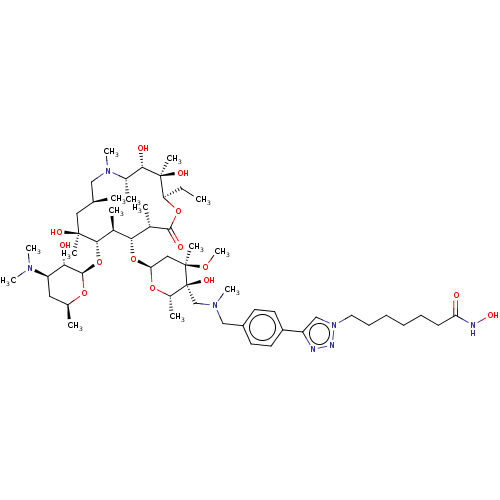 (CHEMBL4299488) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC6 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50499365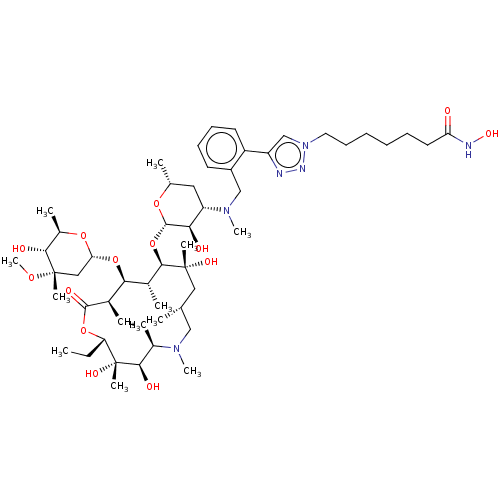 (CHEMBL4299472) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC6 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50499348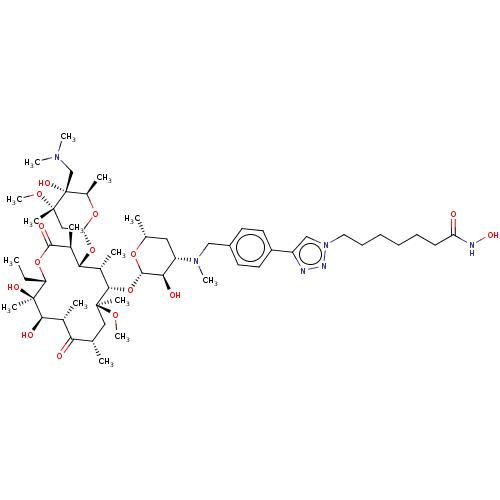 (CHEMBL4299407) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC6 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50499323 (CHEMBL4299428) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC6 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50209791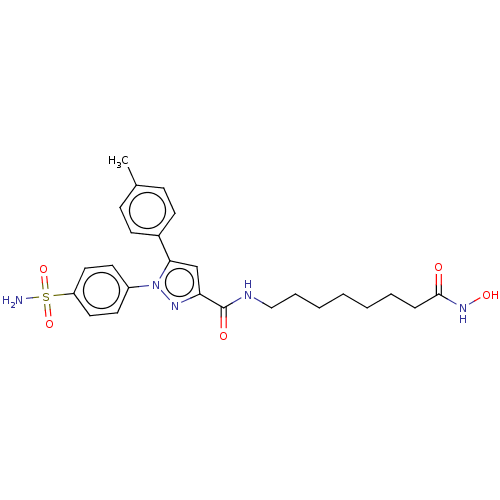 (CHEMBL3883560) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC2 (unknown origin) after 24 hrs by SAMDI mass spectroscopic analysis | Bioorg Med Chem 25: 1202-1218 (2017) Article DOI: 10.1016/j.bmc.2016.12.032 BindingDB Entry DOI: 10.7270/Q2F76FJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50499348 (CHEMBL4299407) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC1 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50499336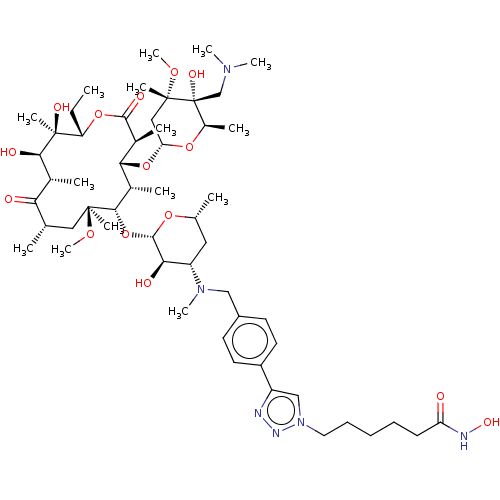 (CHEMBL4299384) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC6 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50209791 (CHEMBL3883560) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC3 (unknown origin) after 5 hrs by SAMDI mass spectroscopic analysis | Bioorg Med Chem 25: 1202-1218 (2017) Article DOI: 10.1016/j.bmc.2016.12.032 BindingDB Entry DOI: 10.7270/Q2F76FJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC6 using fluorogenic HDAC substrate 3 preincubated for 30 mins followed by substrate addition and measured after 1... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115345 BindingDB Entry DOI: 10.7270/Q2PR80K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50209789 (CHEMBL3885174) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC3 (unknown origin) after 5 hrs by SAMDI mass spectroscopic analysis | Bioorg Med Chem 25: 1202-1218 (2017) Article DOI: 10.1016/j.bmc.2016.12.032 BindingDB Entry DOI: 10.7270/Q2F76FJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50499321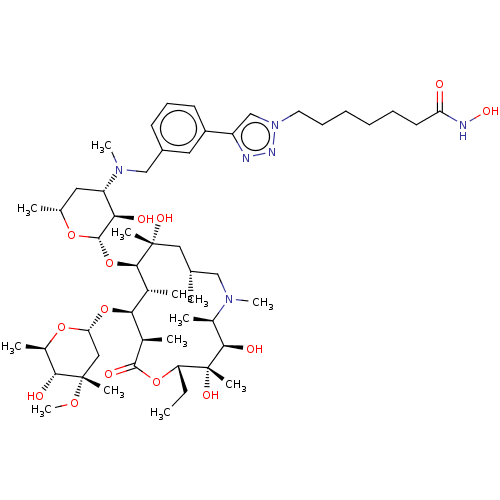 (CHEMBL4299419) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC6 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using fluorogenic HDAC substrate preincubated for 30 mins followed by substrate addition and measured after 15 ... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115345 BindingDB Entry DOI: 10.7270/Q2PR80K2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50499338 (CHEMBL4299411) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC6 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50545002 (CHEMBL4635479) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using fluorogenic HDAC substrate preincubated for 30 mins followed by substrate addition and measured after 15 ... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115345 BindingDB Entry DOI: 10.7270/Q2PR80K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50545001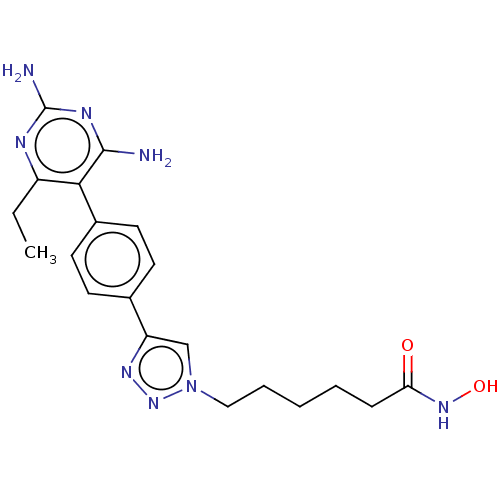 (CHEMBL4649487) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC6 using fluorogenic HDAC substrate 3 preincubated for 30 mins followed by substrate addition and measured after 1... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115345 BindingDB Entry DOI: 10.7270/Q2PR80K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50209790 (CHEMBL3885367) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC6 (unknown origin) after 20 hrs by SAMDI mass spectroscopic analysis | Bioorg Med Chem 25: 1202-1218 (2017) Article DOI: 10.1016/j.bmc.2016.12.032 BindingDB Entry DOI: 10.7270/Q2F76FJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50209794 (CHEMBL3884118) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC6 (unknown origin) after 20 hrs by SAMDI mass spectroscopic analysis | Bioorg Med Chem 25: 1202-1218 (2017) Article DOI: 10.1016/j.bmc.2016.12.032 BindingDB Entry DOI: 10.7270/Q2F76FJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50499325 (CHEMBL4299488) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC1 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50209834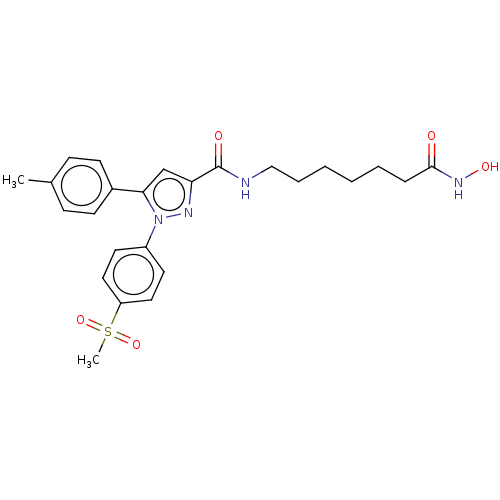 (CHEMBL3884141) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC6 (unknown origin) after 20 hrs by SAMDI mass spectroscopic analysis | Bioorg Med Chem 25: 1202-1218 (2017) Article DOI: 10.1016/j.bmc.2016.12.032 BindingDB Entry DOI: 10.7270/Q2F76FJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50209797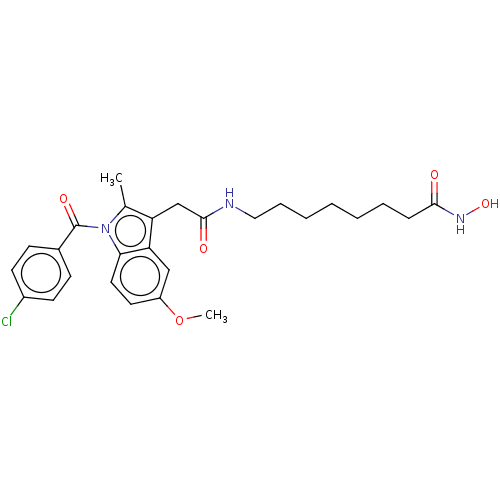 (CHEMBL3885245) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC6 (unknown origin) after 20 hrs by SAMDI mass spectroscopic analysis | Bioorg Med Chem 25: 1202-1218 (2017) Article DOI: 10.1016/j.bmc.2016.12.032 BindingDB Entry DOI: 10.7270/Q2F76FJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50499346 (CHEMBL3736314) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC1 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50209788 (CHEMBL3884506) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC3 (unknown origin) after 5 hrs by SAMDI mass spectroscopic analysis | Bioorg Med Chem 25: 1202-1218 (2017) Article DOI: 10.1016/j.bmc.2016.12.032 BindingDB Entry DOI: 10.7270/Q2F76FJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50209791 (CHEMBL3883560) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC1 (unknown origin) after 24 hrs by SAMDI mass spectroscopic analysis | Bioorg Med Chem 25: 1202-1218 (2017) Article DOI: 10.1016/j.bmc.2016.12.032 BindingDB Entry DOI: 10.7270/Q2F76FJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50209829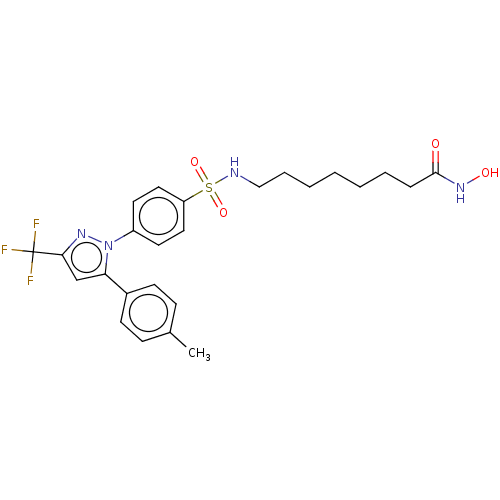 (CHEMBL3883830) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC6 (unknown origin) after 20 hrs by SAMDI mass spectroscopic analysis | Bioorg Med Chem 25: 1202-1218 (2017) Article DOI: 10.1016/j.bmc.2016.12.032 BindingDB Entry DOI: 10.7270/Q2F76FJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50209835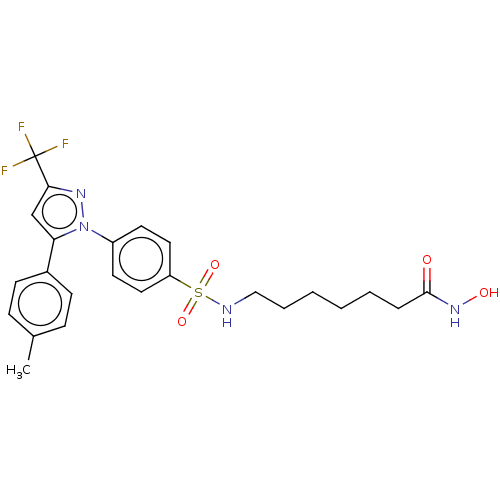 (CHEMBL3885496) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HDAC6 (unknown origin) after 20 hrs by SAMDI mass spectroscopic analysis | Bioorg Med Chem 25: 1202-1218 (2017) Article DOI: 10.1016/j.bmc.2016.12.032 BindingDB Entry DOI: 10.7270/Q2F76FJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50499331 (CHEMBL4299417) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC1 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50499351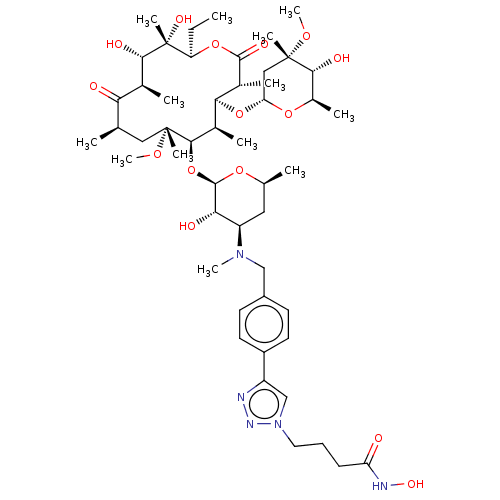 (CHEMBL4299406) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC6 (unknown origin) expressed in Escherichia coli BL21(DE3) after 60 mins using trichostatin A by label-free mass spectr... | Bioorg Med Chem 23: 7543-64 (2015) Article DOI: 10.1016/j.bmc.2015.10.045 BindingDB Entry DOI: 10.7270/Q2RX9G2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 236 total ) | Next | Last >> |