 Found 435 hits with Last Name = 'fei' and Initial = 'f'
Found 435 hits with Last Name = 'fei' and Initial = 'f' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50495338
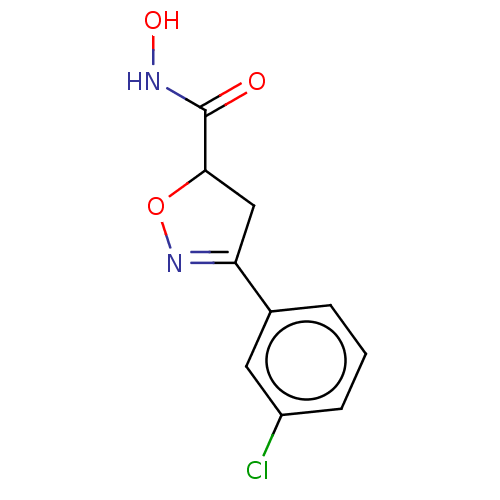
(CHEMBL3110147)Show InChI InChI=1S/C10H9ClN2O3/c11-7-3-1-2-6(4-7)8-5-9(16-13-8)10(14)12-15/h1-4,9,15H,5H2,(H,12,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 133 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Militar de Engenharia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase-1-mediated CO2 hydration by stopped-flow assay |
J Med Chem 57: 298-308 (2014)
Article DOI: 10.1021/jm400902y
BindingDB Entry DOI: 10.7270/Q28K7D2G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50495344
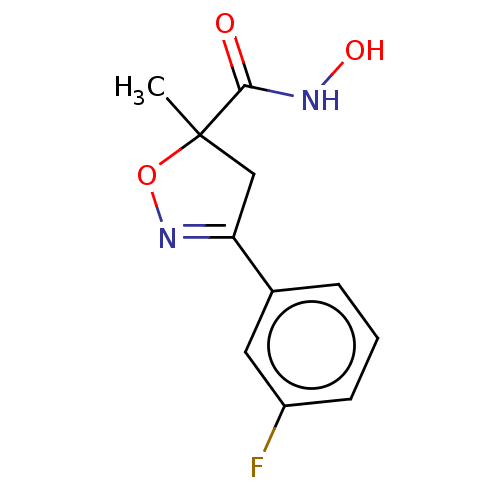
(CHEMBL3110154)Show InChI InChI=1S/C11H11FN2O3/c1-11(10(15)13-16)6-9(14-17-11)7-3-2-4-8(12)5-7/h2-5,16H,6H2,1H3,(H,13,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 257 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Militar de Engenharia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase-2-mediated CO2 hydration by stopped-flow assay |
J Med Chem 57: 298-308 (2014)
Article DOI: 10.1021/jm400902y
BindingDB Entry DOI: 10.7270/Q28K7D2G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50495345
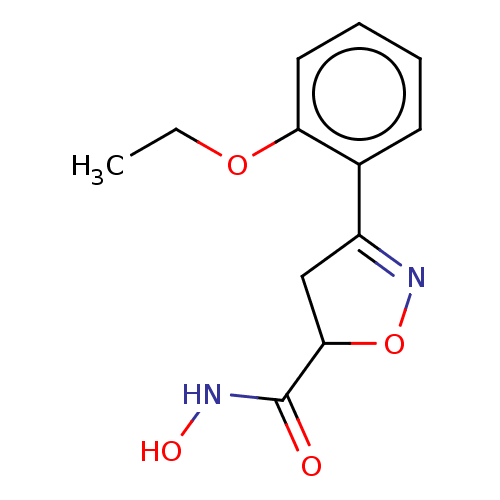
(CHEMBL3110149)Show InChI InChI=1S/C12H14N2O4/c1-2-17-10-6-4-3-5-8(10)9-7-11(18-14-9)12(15)13-16/h3-6,11,16H,2,7H2,1H3,(H,13,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 297 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Militar de Engenharia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase-2-mediated CO2 hydration by stopped-flow assay |
J Med Chem 57: 298-308 (2014)
Article DOI: 10.1021/jm400902y
BindingDB Entry DOI: 10.7270/Q28K7D2G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50495339
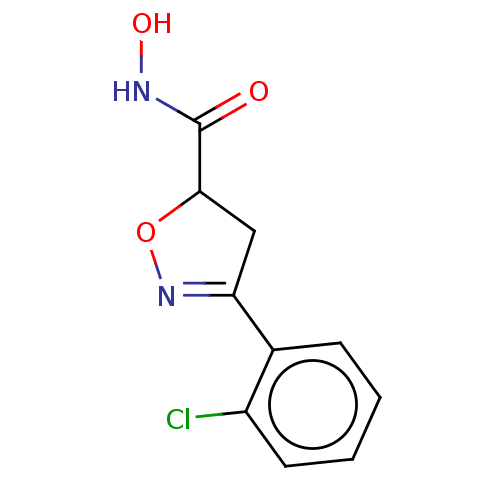
(CHEMBL3110146)Show InChI InChI=1S/C10H9ClN2O3/c11-7-4-2-1-3-6(7)8-5-9(16-13-8)10(14)12-15/h1-4,9,15H,5H2,(H,12,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 516 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Militar de Engenharia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase-1-mediated CO2 hydration by stopped-flow assay |
J Med Chem 57: 298-308 (2014)
Article DOI: 10.1021/jm400902y
BindingDB Entry DOI: 10.7270/Q28K7D2G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50495349
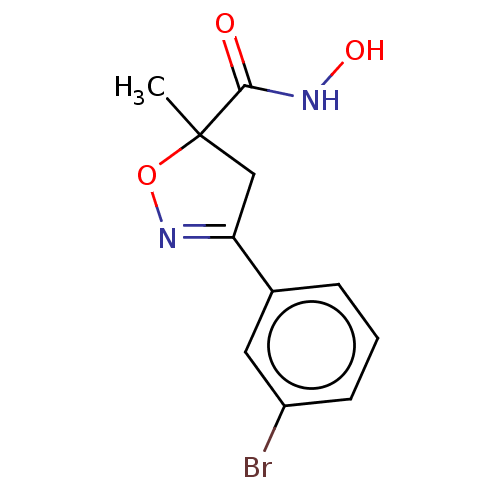
(CHEMBL3110153)Show InChI InChI=1S/C11H11BrN2O3/c1-11(10(15)13-16)6-9(14-17-11)7-3-2-4-8(12)5-7/h2-5,16H,6H2,1H3,(H,13,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 598 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Militar de Engenharia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase-1-mediated CO2 hydration by stopped-flow assay |
J Med Chem 57: 298-308 (2014)
Article DOI: 10.1021/jm400902y
BindingDB Entry DOI: 10.7270/Q28K7D2G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50495343
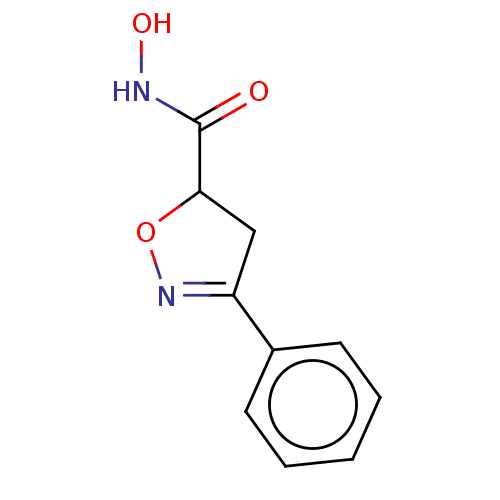
(CHEMBL3110145)Show InChI InChI=1S/C10H10N2O3/c13-10(11-14)9-6-8(12-15-9)7-4-2-1-3-5-7/h1-5,9,14H,6H2,(H,11,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 612 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Militar de Engenharia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase-1-mediated CO2 hydration by stopped-flow assay |
J Med Chem 57: 298-308 (2014)
Article DOI: 10.1021/jm400902y
BindingDB Entry DOI: 10.7270/Q28K7D2G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50495342
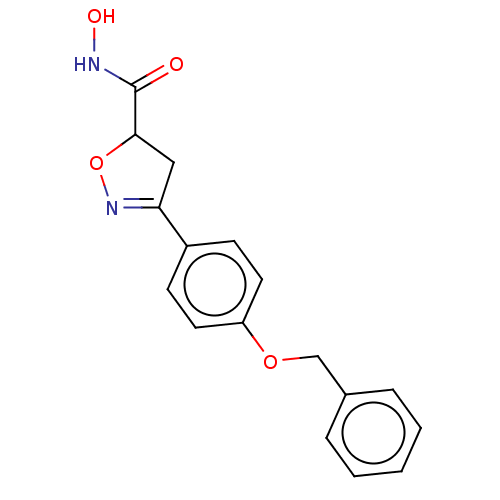
(CHEMBL3110151)Show SMILES ONC(=O)C1CC(=NO1)c1ccc(OCc2ccccc2)cc1 |c:6| Show InChI InChI=1S/C17H16N2O4/c20-17(18-21)16-10-15(19-23-16)13-6-8-14(9-7-13)22-11-12-4-2-1-3-5-12/h1-9,16,21H,10-11H2,(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 641 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Militar de Engenharia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase-1-mediated CO2 hydration by stopped-flow assay |
J Med Chem 57: 298-308 (2014)
Article DOI: 10.1021/jm400902y
BindingDB Entry DOI: 10.7270/Q28K7D2G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50495349

(CHEMBL3110153)Show InChI InChI=1S/C11H11BrN2O3/c1-11(10(15)13-16)6-9(14-17-11)7-3-2-4-8(12)5-7/h2-5,16H,6H2,1H3,(H,13,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 733 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Militar de Engenharia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase-2-mediated CO2 hydration by stopped-flow assay |
J Med Chem 57: 298-308 (2014)
Article DOI: 10.1021/jm400902y
BindingDB Entry DOI: 10.7270/Q28K7D2G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50495343

(CHEMBL3110145)Show InChI InChI=1S/C10H10N2O3/c13-10(11-14)9-6-8(12-15-9)7-4-2-1-3-5-7/h1-5,9,14H,6H2,(H,11,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 802 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Militar de Engenharia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase-2-mediated CO2 hydration by stopped-flow assay |
J Med Chem 57: 298-308 (2014)
Article DOI: 10.1021/jm400902y
BindingDB Entry DOI: 10.7270/Q28K7D2G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50495341
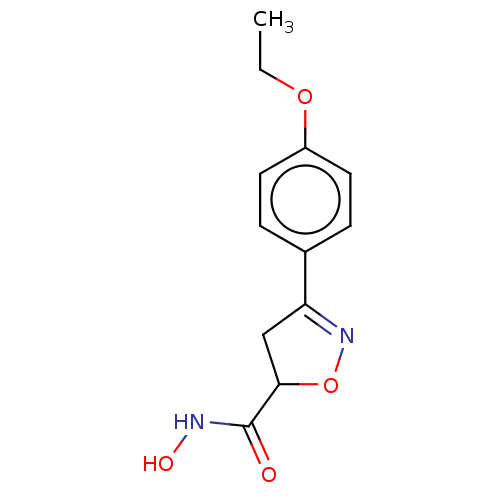
(CHEMBL3110150)Show InChI InChI=1S/C12H14N2O4/c1-2-17-9-5-3-8(4-6-9)10-7-11(18-14-10)12(15)13-16/h3-6,11,16H,2,7H2,1H3,(H,13,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 808 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Militar de Engenharia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase-2-mediated CO2 hydration by stopped-flow assay |
J Med Chem 57: 298-308 (2014)
Article DOI: 10.1021/jm400902y
BindingDB Entry DOI: 10.7270/Q28K7D2G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50495342

(CHEMBL3110151)Show SMILES ONC(=O)C1CC(=NO1)c1ccc(OCc2ccccc2)cc1 |c:6| Show InChI InChI=1S/C17H16N2O4/c20-17(18-21)16-10-15(19-23-16)13-6-8-14(9-7-13)22-11-12-4-2-1-3-5-12/h1-9,16,21H,10-11H2,(H,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 815 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Militar de Engenharia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase-2-mediated CO2 hydration by stopped-flow assay |
J Med Chem 57: 298-308 (2014)
Article DOI: 10.1021/jm400902y
BindingDB Entry DOI: 10.7270/Q28K7D2G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50495347
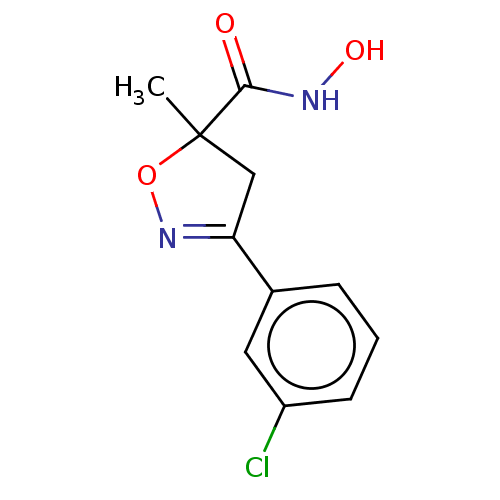
(CHEMBL3110152)Show InChI InChI=1S/C11H11ClN2O3/c1-11(10(15)13-16)6-9(14-17-11)7-3-2-4-8(12)5-7/h2-5,16H,6H2,1H3,(H,13,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 847 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Militar de Engenharia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase-2-mediated CO2 hydration by stopped-flow assay |
J Med Chem 57: 298-308 (2014)
Article DOI: 10.1021/jm400902y
BindingDB Entry DOI: 10.7270/Q28K7D2G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50495346
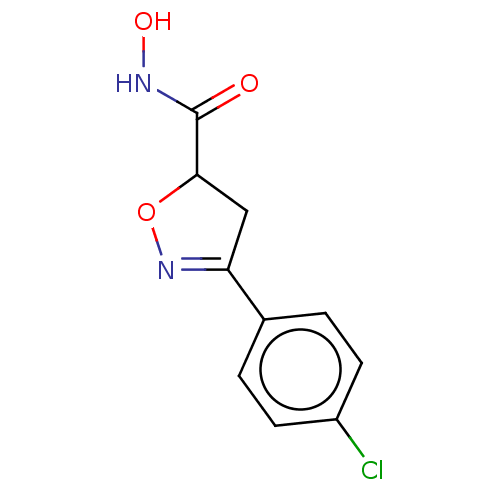
(CHEMBL3110148)Show InChI InChI=1S/C10H9ClN2O3/c11-7-3-1-6(2-4-7)8-5-9(16-13-8)10(14)12-15/h1-4,9,15H,5H2,(H,12,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Militar de Engenharia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase-1-mediated CO2 hydration by stopped-flow assay |
J Med Chem 57: 298-308 (2014)
Article DOI: 10.1021/jm400902y
BindingDB Entry DOI: 10.7270/Q28K7D2G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50495340
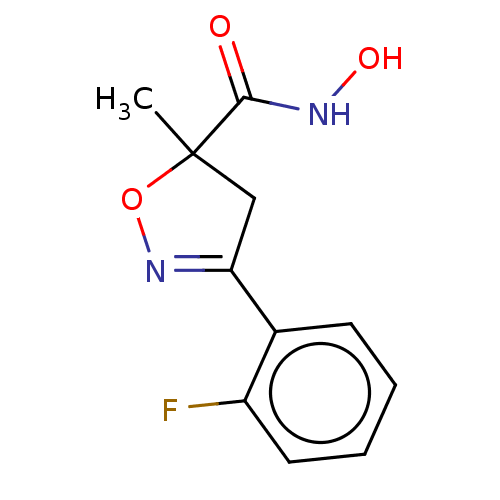
(CHEMBL3110155)Show InChI InChI=1S/C11H11FN2O3/c1-11(10(15)13-16)6-9(14-17-11)7-4-2-3-5-8(7)12/h2-5,16H,6H2,1H3,(H,13,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Militar de Engenharia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase-2-mediated CO2 hydration by stopped-flow assay |
J Med Chem 57: 298-308 (2014)
Article DOI: 10.1021/jm400902y
BindingDB Entry DOI: 10.7270/Q28K7D2G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50495339

(CHEMBL3110146)Show InChI InChI=1S/C10H9ClN2O3/c11-7-4-2-1-3-6(7)8-5-9(16-13-8)10(14)12-15/h1-4,9,15H,5H2,(H,12,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Militar de Engenharia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase-2-mediated CO2 hydration by stopped-flow assay |
J Med Chem 57: 298-308 (2014)
Article DOI: 10.1021/jm400902y
BindingDB Entry DOI: 10.7270/Q28K7D2G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50495347

(CHEMBL3110152)Show InChI InChI=1S/C11H11ClN2O3/c1-11(10(15)13-16)6-9(14-17-11)7-3-2-4-8(12)5-7/h2-5,16H,6H2,1H3,(H,13,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Militar de Engenharia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase-1-mediated CO2 hydration by stopped-flow assay |
J Med Chem 57: 298-308 (2014)
Article DOI: 10.1021/jm400902y
BindingDB Entry DOI: 10.7270/Q28K7D2G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50495341

(CHEMBL3110150)Show InChI InChI=1S/C12H14N2O4/c1-2-17-9-5-3-8(4-6-9)10-7-11(18-14-10)12(15)13-16/h3-6,11,16H,2,7H2,1H3,(H,13,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Militar de Engenharia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase-1-mediated CO2 hydration by stopped-flow assay |
J Med Chem 57: 298-308 (2014)
Article DOI: 10.1021/jm400902y
BindingDB Entry DOI: 10.7270/Q28K7D2G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50495338

(CHEMBL3110147)Show InChI InChI=1S/C10H9ClN2O3/c11-7-3-1-2-6(4-7)8-5-9(16-13-8)10(14)12-15/h1-4,9,15H,5H2,(H,12,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Militar de Engenharia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase-2-mediated CO2 hydration by stopped-flow assay |
J Med Chem 57: 298-308 (2014)
Article DOI: 10.1021/jm400902y
BindingDB Entry DOI: 10.7270/Q28K7D2G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50495344

(CHEMBL3110154)Show InChI InChI=1S/C11H11FN2O3/c1-11(10(15)13-16)6-9(14-17-11)7-3-2-4-8(12)5-7/h2-5,16H,6H2,1H3,(H,13,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Militar de Engenharia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase-1-mediated CO2 hydration by stopped-flow assay |
J Med Chem 57: 298-308 (2014)
Article DOI: 10.1021/jm400902y
BindingDB Entry DOI: 10.7270/Q28K7D2G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50495340

(CHEMBL3110155)Show InChI InChI=1S/C11H11FN2O3/c1-11(10(15)13-16)6-9(14-17-11)7-4-2-3-5-8(7)12/h2-5,16H,6H2,1H3,(H,13,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Militar de Engenharia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase-1-mediated CO2 hydration by stopped-flow assay |
J Med Chem 57: 298-308 (2014)
Article DOI: 10.1021/jm400902y
BindingDB Entry DOI: 10.7270/Q28K7D2G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50495345

(CHEMBL3110149)Show InChI InChI=1S/C12H14N2O4/c1-2-17-10-6-4-3-5-8(10)9-7-11(18-14-9)12(15)13-16/h3-6,11,16H,2,7H2,1H3,(H,13,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Militar de Engenharia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase-1-mediated CO2 hydration by stopped-flow assay |
J Med Chem 57: 298-308 (2014)
Article DOI: 10.1021/jm400902y
BindingDB Entry DOI: 10.7270/Q28K7D2G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50495346

(CHEMBL3110148)Show InChI InChI=1S/C10H9ClN2O3/c11-7-3-1-6(2-4-7)8-5-9(16-13-8)10(14)12-15/h1-4,9,15H,5H2,(H,12,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Militar de Engenharia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase-2-mediated CO2 hydration by stopped-flow assay |
J Med Chem 57: 298-308 (2014)
Article DOI: 10.1021/jm400902y
BindingDB Entry DOI: 10.7270/Q28K7D2G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50495348
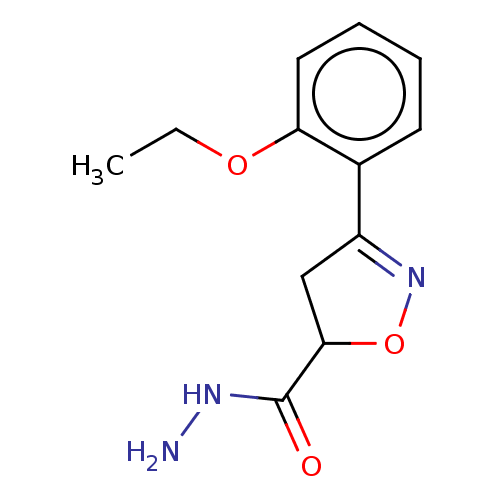
(CHEMBL3110156)Show InChI InChI=1S/C12H15N3O3/c1-2-17-10-6-4-3-5-8(10)9-7-11(18-15-9)12(16)14-13/h3-6,11H,2,7,13H2,1H3,(H,14,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Militar de Engenharia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase-1-mediated CO2 hydration by stopped-flow assay |
J Med Chem 57: 298-308 (2014)
Article DOI: 10.1021/jm400902y
BindingDB Entry DOI: 10.7270/Q28K7D2G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50495337
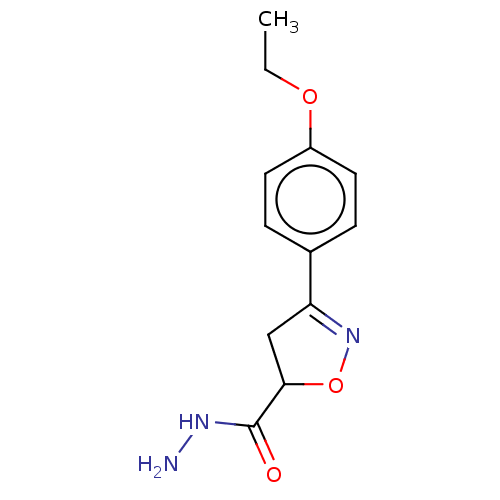
(CHEMBL3110157)Show InChI InChI=1S/C12H15N3O3/c1-2-17-9-5-3-8(4-6-9)10-7-11(18-15-10)12(16)14-13/h3-6,11H,2,7,13H2,1H3,(H,14,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Militar de Engenharia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase-1-mediated CO2 hydration by stopped-flow assay |
J Med Chem 57: 298-308 (2014)
Article DOI: 10.1021/jm400902y
BindingDB Entry DOI: 10.7270/Q28K7D2G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50495337

(CHEMBL3110157)Show InChI InChI=1S/C12H15N3O3/c1-2-17-9-5-3-8(4-6-9)10-7-11(18-15-10)12(16)14-13/h3-6,11H,2,7,13H2,1H3,(H,14,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Militar de Engenharia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase-2-mediated CO2 hydration by stopped-flow assay |
J Med Chem 57: 298-308 (2014)
Article DOI: 10.1021/jm400902y
BindingDB Entry DOI: 10.7270/Q28K7D2G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50495348

(CHEMBL3110156)Show InChI InChI=1S/C12H15N3O3/c1-2-17-10-6-4-3-5-8(10)9-7-11(18-15-9)12(16)14-13/h3-6,11H,2,7,13H2,1H3,(H,14,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Militar de Engenharia
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase-2-mediated CO2 hydration by stopped-flow assay |
J Med Chem 57: 298-308 (2014)
Article DOI: 10.1021/jm400902y
BindingDB Entry DOI: 10.7270/Q28K7D2G |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50493576

(CHEMBL2435828)Show SMILES CCCc1nc2c(C)cc(NC(=O)CCc3ccccc3)cc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O Show InChI InChI=1S/C34H33N3O3/c1-3-9-31-36-33-23(2)20-27(35-32(38)19-16-24-10-5-4-6-11-24)21-30(33)37(31)22-25-14-17-26(18-15-25)28-12-7-8-13-29(28)34(39)40/h4-8,10-15,17-18,20-21H,3,9,16,19,22H2,1-2H3,(H,35,38)(H,39,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Sar1Ile8-angiotensin 2 from angiotensin 2 AT1 receptor (unknown origin) after 180 mins by gamma counting analysis |
Eur J Med Chem 69: 44-54 (2013)
Article DOI: 10.1016/j.ejmech.2013.08.014
BindingDB Entry DOI: 10.7270/Q2PK0K32 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50493578
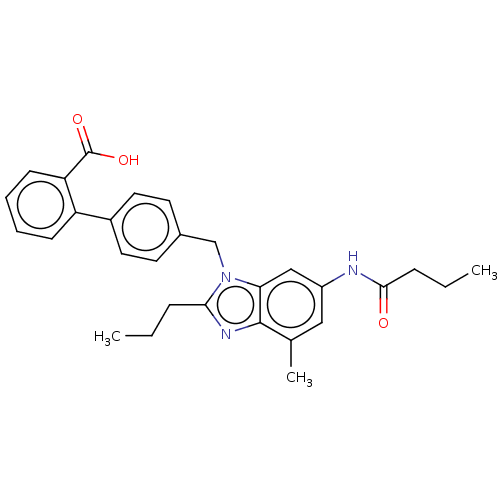
(CHEMBL2435824)Show SMILES CCCC(=O)Nc1cc(C)c2nc(CCC)n(Cc3ccc(cc3)-c3ccccc3C(O)=O)c2c1 Show InChI InChI=1S/C29H31N3O3/c1-4-8-26-31-28-19(3)16-22(30-27(33)9-5-2)17-25(28)32(26)18-20-12-14-21(15-13-20)23-10-6-7-11-24(23)29(34)35/h6-7,10-17H,4-5,8-9,18H2,1-3H3,(H,30,33)(H,34,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Sar1Ile8-angiotensin 2 from angiotensin 2 AT1 receptor (unknown origin) after 180 mins by gamma counting analysis |
Eur J Med Chem 69: 44-54 (2013)
Article DOI: 10.1016/j.ejmech.2013.08.014
BindingDB Entry DOI: 10.7270/Q2PK0K32 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM82258

(CAS_114798-26-4 | Losartan | NSC_3961)Show SMILES CCCCc1nc(Cl)c(CO)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C22H23ClN6O/c1-2-3-8-20-24-21(23)19(14-30)29(20)13-15-9-11-16(12-10-15)17-6-4-5-7-18(17)22-25-27-28-26-22/h4-7,9-12,30H,2-3,8,13-14H2,1H3,(H,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemical Engineering& the Environment
Curated by ChEMBL
| Assay Description
Inhibition of angiotensin AT1 receptor |
Bioorg Med Chem 20: 4208-16 (2012)
Article DOI: 10.1016/j.bmc.2012.05.056
BindingDB Entry DOI: 10.7270/Q2GT5P7J |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50043280

(4'-((1,4'-dimethyl-2'-propyl(2,6'-bi-1H-benzimidaz...)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O)-c1nc2ccccc2n1C Show InChI InChI=1S/C33H30N4O2/c1-4-9-30-35-31-21(2)18-24(32-34-27-12-7-8-13-28(27)36(32)3)19-29(31)37(30)20-22-14-16-23(17-15-22)25-10-5-6-11-26(25)33(38)39/h5-8,10-19H,4,9,20H2,1-3H3,(H,38,39) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [125I]Sar1 Ile8-Ang 2 from angiotensin 2 AT2 receptor after 180 mins by gamma counting |
Eur J Med Chem 49: 183-90 (2012)
Article DOI: 10.1016/j.ejmech.2012.01.009
BindingDB Entry DOI: 10.7270/Q29K4BPW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50591330

(CHEMBL5197431)Show SMILES CC(C)S(=O)(=O)N1CCC(CC1)NC(=O)NC12CC3CC(C)(CC(C1)c1ccccc31)C2 |TLB:30:18:23.24.22:31,25:23:18.19.17:31,26:25:24:20.22.31,21:20:24:30.25.18.17,THB:19:18:24:20.22.31,19:20:24:30.25.18.17| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00515
BindingDB Entry DOI: 10.7270/Q21R6VHV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50591331
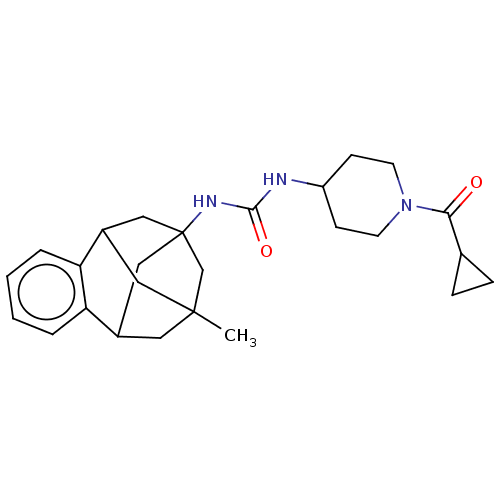
(CHEMBL5197282)Show SMILES CC12CC3CC(CC(C1)c1ccccc31)(C2)NC(=O)NC1CCN(CC1)C(=O)C1CC1 |TLB:14:3:7.6.8:15,9:7:3.2.4:15,10:9:6:1.8.15,0:1:6:14.9.3.4,THB:2:3:6:1.8.15,2:1:6:14.9.3.4| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00515
BindingDB Entry DOI: 10.7270/Q21R6VHV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50591334
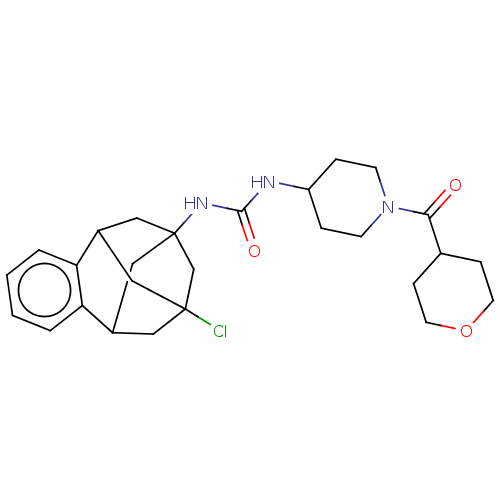
(CHEMBL5191146)Show SMILES ClC12CC3CC(CC(C1)c1ccccc31)(C2)NC(=O)NC1CCN(CC1)C(=O)C1CCOCC1 |TLB:14:3:7.6.8:15,9:7:3.2.4:15,10:9:6:1.8.15,0:1:6:14.9.3.4,THB:2:3:6:1.8.15,2:1:6:14.9.3.4| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00515
BindingDB Entry DOI: 10.7270/Q21R6VHV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50591327
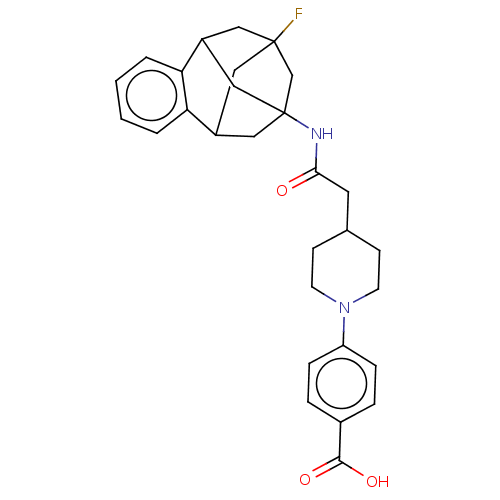
(CHEMBL5177544)Show SMILES OC(=O)c1ccc(cc1)N1CCC(CC(=O)NC23CC4CC(F)(CC(C2)c2ccccc42)C3)CC1 |TLB:31:19:24.25.23:32,26:24:18.19.20:32,27:26:25:21.23.32,22:21:25:31.26.18.19,THB:20:19:25:21.23.32,20:21:25:31.26.18.19| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00515
BindingDB Entry DOI: 10.7270/Q21R6VHV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM158481

(US9029401, 1728 (t-TUCB))Show SMILES OC(=O)c1ccc(O[C@H]2CC[C@@H](CC2)NC(=O)Nc2ccc(OC(F)(F)F)cc2)cc1 |r,wU:8.7,wD:11.14,(10,,;8.67,-.77,;8.67,-2.31,;7.34,,;6,-.77,;4.67,,;4.67,1.54,;3.33,2.31,;2,1.54,;2,,;.67,-.77,;-.67,,;-.67,1.54,;.67,2.31,;-2,-.77,;-3.33,,;-3.33,1.54,;-4.67,-.77,;-6,,;-6,1.54,;-7.34,2.31,;-8.67,1.54,;-10,2.31,;-10,3.85,;-10,5.39,;-8.67,4.62,;-11.34,4.62,;-8.67,,;-7.34,-.77,;6,2.31,;7.34,1.54,)| Show InChI InChI=1S/C21H21F3N2O5/c22-21(23,24)31-18-11-5-15(6-12-18)26-20(29)25-14-3-9-17(10-4-14)30-16-7-1-13(2-8-16)19(27)28/h1-2,5-8,11-12,14,17H,3-4,9-10H2,(H,27,28)(H2,25,26,29)/t14-,17- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00515
BindingDB Entry DOI: 10.7270/Q21R6VHV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM409005
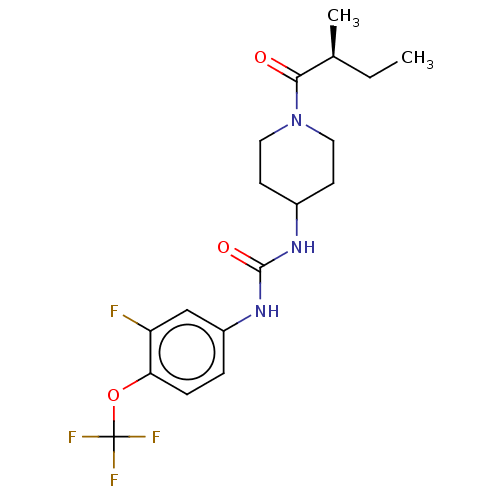
(US10377744, Compound No. 26 | US11123311, Compound...)Show SMILES CC[C@H](C)C(=O)N1CCC(CC1)NC(=O)Nc1ccc(OC(F)(F)F)c(F)c1 |r| Show InChI InChI=1S/C18H23F4N3O3/c1-3-11(2)16(26)25-8-6-12(7-9-25)23-17(27)24-13-4-5-15(14(19)10-13)28-18(20,21)22/h4-5,10-12H,3,6-9H2,1-2H3,(H2,23,24,27)/t11-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00515
BindingDB Entry DOI: 10.7270/Q21R6VHV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM50581728
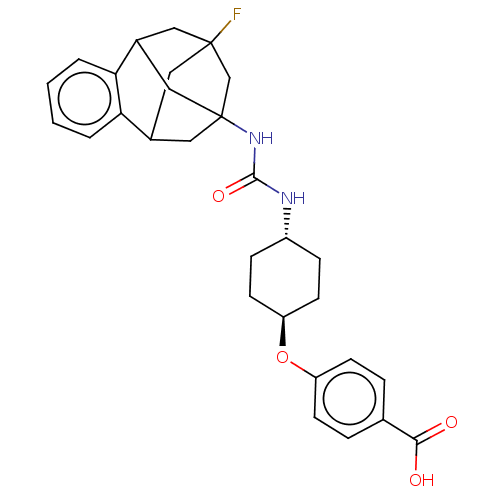
(CHEMBL5093683)Show SMILES OC(=O)c1ccc(O[C@H]2CC[C@@H](CC2)NC(=O)NC23CC4CC(F)(CC(C2)c2ccccc42)C3)cc1 |r,wU:8.7,wD:11.14,TLB:32:20:25.26.24:33,28:27:19:21.22.33,31:32:19:21.22.33,THB:24:25:19:21.22.33,24:22:19:32.25.26.27,27:25:20.19.21:33,23:22:19:32.25.26.27,(74.96,-46.38,;74.97,-44.84,;76.3,-44.08,;73.64,-44.07,;72.3,-44.83,;70.97,-44.06,;70.98,-42.52,;69.65,-41.74,;68.32,-42.51,;68.31,-44.05,;66.98,-44.81,;65.65,-44.03,;65.64,-42.5,;66.98,-41.73,;64.31,-44.8,;62.99,-44.04,;62.99,-42.52,;61.68,-44.8,;60.36,-44.04,;59.36,-45.3,;57.97,-44.75,;57.96,-43.18,;58.99,-41.95,;58.59,-40.47,;57.65,-42.43,;57.66,-43.9,;58.98,-44.38,;55.38,-44.08,;53.9,-44.49,;53.51,-45.99,;54.63,-47.08,;56.11,-46.65,;56.47,-45.16,;60.37,-42.53,;72.31,-41.75,;73.64,-42.52,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rat recombinant sEH using CMNPC as substrate incubated for 5 mins by fluorescent based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01601
BindingDB Entry DOI: 10.7270/Q2H70KPP |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM50581727
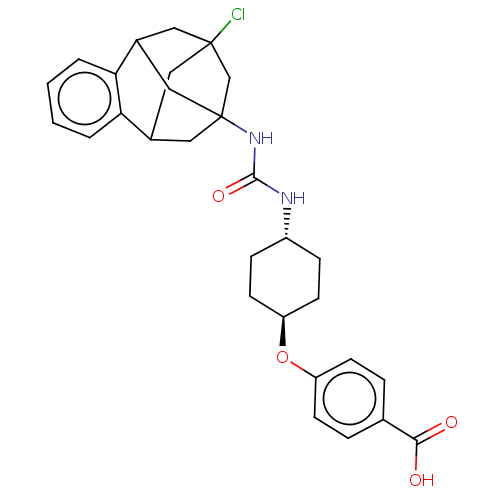
(CHEMBL5084744)Show SMILES OC(=O)c1ccc(O[C@H]2CC[C@@H](CC2)NC(=O)NC23CC4CC(Cl)(CC(C2)c2ccccc42)C3)cc1 |r,wU:8.7,wD:11.14,TLB:32:20:25.26.24:33,28:27:19:21.22.33,31:32:19:21.22.33,THB:24:25:19:21.22.33,24:22:19:32.25.26.27,27:25:20.19.21:33,23:22:19:32.25.26.27,(49.77,-46.61,;49.78,-45.07,;51.11,-44.3,;48.45,-44.3,;47.11,-45.06,;45.78,-44.28,;45.79,-42.75,;44.46,-41.97,;43.13,-42.73,;43.12,-44.28,;41.79,-45.04,;40.46,-44.26,;40.45,-42.72,;41.79,-41.96,;39.12,-45.03,;37.8,-44.27,;37.8,-42.74,;36.49,-45.03,;35.17,-44.27,;34.17,-45.53,;32.78,-44.97,;32.77,-43.4,;33.8,-42.18,;33.4,-40.69,;32.46,-42.65,;32.47,-44.12,;33.79,-44.61,;30.19,-44.31,;28.71,-44.72,;28.32,-46.22,;29.44,-47.3,;30.92,-46.88,;31.28,-45.39,;35.18,-42.76,;47.12,-41.98,;48.45,-42.75,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rat recombinant sEH using CMNPC as substrate incubated for 5 mins by fluorescent based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01601
BindingDB Entry DOI: 10.7270/Q2H70KPP |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM50581725
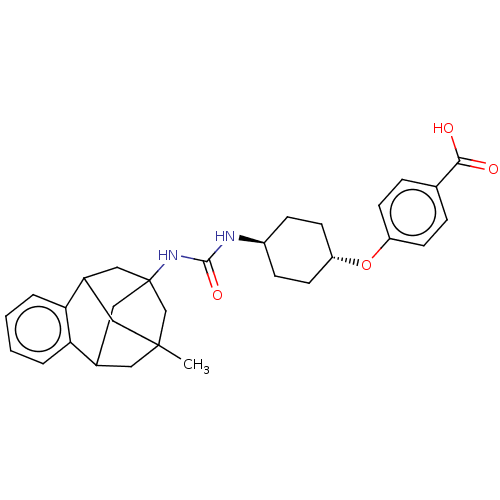
(CHEMBL5081862)Show SMILES CC12CC3CC(CC(C1)c1ccccc31)(C2)NC(=O)N[C@H]1CC[C@@H](CC1)Oc1ccc(cc1)C(O)=O |r,wU:23.30,wD:20.23,TLB:14:3:7.6.8:15,10:9:4:2.1.15,13:14:4:2.1.15,THB:8:7:4:2.1.15,8:1:4:14.7.6.9,9:7:3.4.2:15,0:1:4:14.7.6.9,(54.65,-28.03,;55.05,-29.51,;54.03,-30.74,;54.03,-32.3,;55.42,-32.86,;56.42,-31.6,;55.04,-31.94,;53.73,-31.46,;53.72,-29.99,;51.45,-31.64,;49.96,-32.05,;49.58,-33.55,;50.69,-34.63,;52.17,-34.21,;52.54,-32.72,;56.43,-30.09,;57.74,-32.36,;59.06,-31.6,;59.06,-30.07,;60.38,-32.36,;61.71,-31.59,;63.04,-32.37,;64.37,-31.61,;64.38,-30.07,;63.05,-29.29,;61.71,-30.06,;65.72,-29.3,;67.05,-30.08,;67.03,-31.61,;68.36,-32.39,;69.7,-31.63,;69.7,-30.08,;68.37,-29.31,;71.03,-32.4,;71.03,-33.94,;72.37,-31.64,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rat recombinant sEH using CMNPC as substrate incubated for 5 mins by fluorescent based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01601
BindingDB Entry DOI: 10.7270/Q2H70KPP |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Mus musculus (Mouse)) | BDBM50581727

(CHEMBL5084744)Show SMILES OC(=O)c1ccc(O[C@H]2CC[C@@H](CC2)NC(=O)NC23CC4CC(Cl)(CC(C2)c2ccccc42)C3)cc1 |r,wU:8.7,wD:11.14,TLB:32:20:25.26.24:33,28:27:19:21.22.33,31:32:19:21.22.33,THB:24:25:19:21.22.33,24:22:19:32.25.26.27,27:25:20.19.21:33,23:22:19:32.25.26.27,(49.77,-46.61,;49.78,-45.07,;51.11,-44.3,;48.45,-44.3,;47.11,-45.06,;45.78,-44.28,;45.79,-42.75,;44.46,-41.97,;43.13,-42.73,;43.12,-44.28,;41.79,-45.04,;40.46,-44.26,;40.45,-42.72,;41.79,-41.96,;39.12,-45.03,;37.8,-44.27,;37.8,-42.74,;36.49,-45.03,;35.17,-44.27,;34.17,-45.53,;32.78,-44.97,;32.77,-43.4,;33.8,-42.18,;33.4,-40.69,;32.46,-42.65,;32.47,-44.12,;33.79,-44.61,;30.19,-44.31,;28.71,-44.72,;28.32,-46.22,;29.44,-47.3,;30.92,-46.88,;31.28,-45.39,;35.18,-42.76,;47.12,-41.98,;48.45,-42.75,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mouse recombinant sEH using CMNPC as substrate incubated for 5 mins by fluorescent based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01601
BindingDB Entry DOI: 10.7270/Q2H70KPP |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM409005

(US10377744, Compound No. 26 | US11123311, Compound...)Show SMILES CC[C@H](C)C(=O)N1CCC(CC1)NC(=O)Nc1ccc(OC(F)(F)F)c(F)c1 |r| Show InChI InChI=1S/C18H23F4N3O3/c1-3-11(2)16(26)25-8-6-12(7-9-25)23-17(27)24-13-4-5-15(14(19)10-13)28-18(20,21)22/h4-5,10-12H,3,6-9H2,1-2H3,(H2,23,24,27)/t11-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant sEH using CMNPC as substrate incubated for 5 mins by fluorescent based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01601
BindingDB Entry DOI: 10.7270/Q2H70KPP |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50581727

(CHEMBL5084744)Show SMILES OC(=O)c1ccc(O[C@H]2CC[C@@H](CC2)NC(=O)NC23CC4CC(Cl)(CC(C2)c2ccccc42)C3)cc1 |r,wU:8.7,wD:11.14,TLB:32:20:25.26.24:33,28:27:19:21.22.33,31:32:19:21.22.33,THB:24:25:19:21.22.33,24:22:19:32.25.26.27,27:25:20.19.21:33,23:22:19:32.25.26.27,(49.77,-46.61,;49.78,-45.07,;51.11,-44.3,;48.45,-44.3,;47.11,-45.06,;45.78,-44.28,;45.79,-42.75,;44.46,-41.97,;43.13,-42.73,;43.12,-44.28,;41.79,-45.04,;40.46,-44.26,;40.45,-42.72,;41.79,-41.96,;39.12,-45.03,;37.8,-44.27,;37.8,-42.74,;36.49,-45.03,;35.17,-44.27,;34.17,-45.53,;32.78,-44.97,;32.77,-43.4,;33.8,-42.18,;33.4,-40.69,;32.46,-42.65,;32.47,-44.12,;33.79,-44.61,;30.19,-44.31,;28.71,-44.72,;28.32,-46.22,;29.44,-47.3,;30.92,-46.88,;31.28,-45.39,;35.18,-42.76,;47.12,-41.98,;48.45,-42.75,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant sEH using CMNPC as substrate incubated for 5 mins by fluorescent based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01601
BindingDB Entry DOI: 10.7270/Q2H70KPP |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50493577
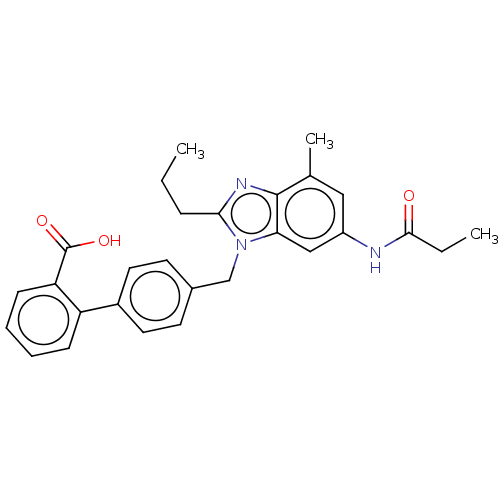
(CHEMBL2435823)Show SMILES CCCc1nc2c(C)cc(NC(=O)CC)cc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O Show InChI InChI=1S/C28H29N3O3/c1-4-8-25-30-27-18(3)15-21(29-26(32)5-2)16-24(27)31(25)17-19-11-13-20(14-12-19)22-9-6-7-10-23(22)28(33)34/h6-7,9-16H,4-5,8,17H2,1-3H3,(H,29,32)(H,33,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Sar1Ile8-angiotensin 2 from angiotensin 2 AT1 receptor (unknown origin) after 180 mins by gamma counting analysis |
Eur J Med Chem 69: 44-54 (2013)
Article DOI: 10.1016/j.ejmech.2013.08.014
BindingDB Entry DOI: 10.7270/Q2PK0K32 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50604191
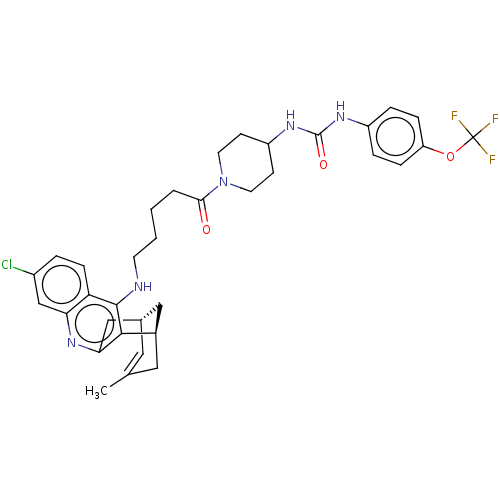
(CHEMBL5207628)Show SMILES [H][C@]12Cc3nc4cc(Cl)ccc4c(NCCCCC(=O)N4CCC(CC4)NC(=O)Nc4ccc(OC(F)(F)F)cc4)c3[C@]([H])(CC(C)=C1)C2 |r,c:50| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02150
BindingDB Entry DOI: 10.7270/Q2F76HMM |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50604188
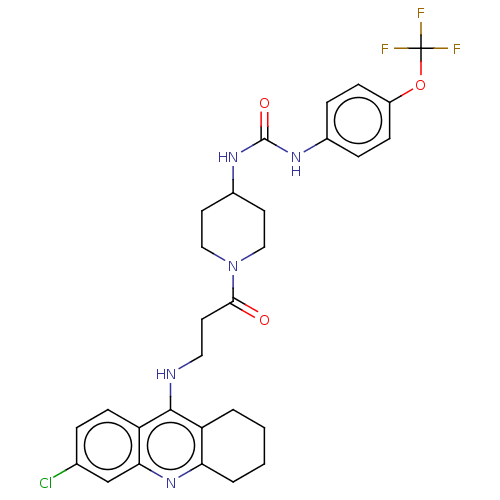
(CHEMBL5204900)Show SMILES FC(F)(F)Oc1ccc(NC(=O)NC2CCN(CC2)C(=O)CCNc2c3CCCCc3nc3cc(Cl)ccc23)cc1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02150
BindingDB Entry DOI: 10.7270/Q2F76HMM |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Mus musculus (Mouse)) | BDBM50591345

(CHEMBL5196519)Show SMILES ClC12CC3CC(CC(C1)c1ccccc31)(C2)NC(=O)NC1CCN(CC1)C(=O)CCC1(CCC#C)N=N1 |c:40,TLB:14:3:7.6.8:15,9:7:3.2.4:15,10:9:6:1.8.15,0:1:6:14.9.3.4,THB:2:3:6:1.8.15,2:1:6:14.9.3.4| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00515
BindingDB Entry DOI: 10.7270/Q21R6VHV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Mus musculus (Mouse)) | BDBM50591344
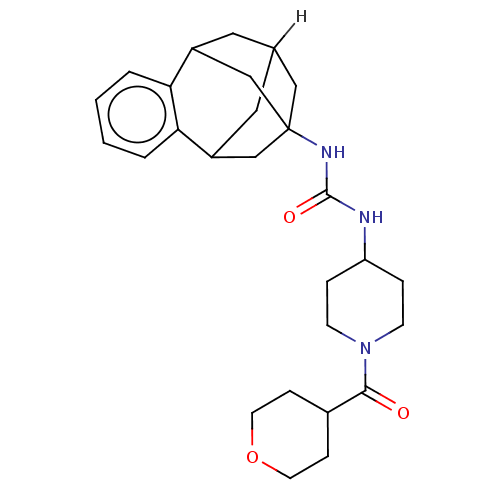
(CHEMBL5208857)Show SMILES [2H]C12CC3CC(CC(C1)c1ccccc31)(C2)NC(=O)NC1CCN(CC1)C(=O)C1CCOCC1 |TLB:14:3:7.6.8:15,9:7:3.2.4:15,10:9:6:1.8.15,0:1:6:14.9.3.4,THB:2:3:6:1.8.15,2:1:6:14.9.3.4| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00515
BindingDB Entry DOI: 10.7270/Q21R6VHV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Mus musculus (Mouse)) | BDBM50591341
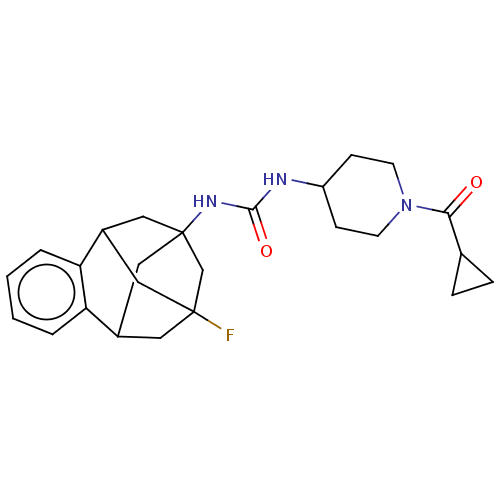
(CHEMBL5177372)Show SMILES FC12CC3CC(CC(C1)c1ccccc31)(C2)NC(=O)NC1CCN(CC1)C(=O)C1CC1 |TLB:14:3:7.6.8:15,9:7:3.2.4:15,10:9:6:1.8.15,0:1:6:14.9.3.4,THB:2:3:6:1.8.15,2:1:6:14.9.3.4| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00515
BindingDB Entry DOI: 10.7270/Q21R6VHV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Mus musculus (Mouse)) | BDBM50591337
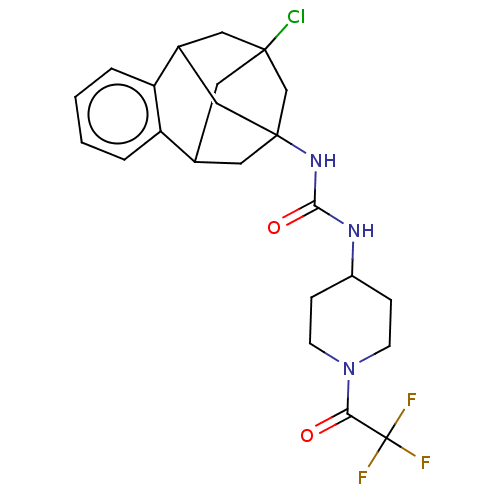
(CHEMBL5192445)Show SMILES FC(F)(F)C(=O)N1CCC(CC1)NC(=O)NC12CC3CC(Cl)(CC(C1)c1ccccc31)C2 |TLB:30:18:23.24.22:31,25:23:18.19.17:31,26:25:24:20.22.31,21:20:24:30.25.18.17,THB:19:18:24:20.22.31,19:20:24:30.25.18.17| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00515
BindingDB Entry DOI: 10.7270/Q21R6VHV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Mus musculus (Mouse)) | BDBM50591336

(CHEMBL5179027)Show SMILES ClC12CC3CC(CC(C1)c1ccccc31)(C2)NC(=O)NC1CCN(CC1)C(=O)C1CC1 |TLB:14:3:7.6.8:15,9:7:3.2.4:15,10:9:6:1.8.15,0:1:6:14.9.3.4,THB:2:3:6:1.8.15,2:1:6:14.9.3.4| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00515
BindingDB Entry DOI: 10.7270/Q21R6VHV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data