 Found 370 hits with Last Name = 'ferguson' and Initial = 'h'
Found 370 hits with Last Name = 'ferguson' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604026

(CHEMBL5192384)Show SMILES CC(C)(O)c1cc(ncc1-c1ccc(cc1)C1(COC1)C(=O)Nc1ccc(F)cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604023
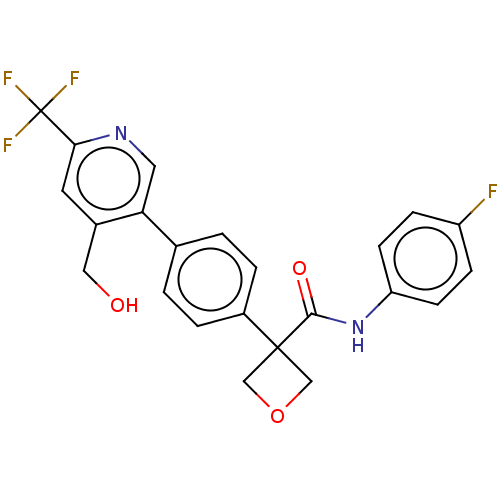
(CHEMBL5192977)Show SMILES OCc1cc(ncc1-c1ccc(cc1)C1(COC1)C(=O)Nc1ccc(F)cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604021
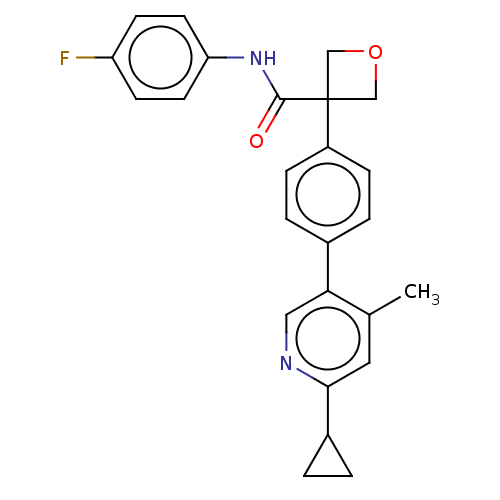
(CHEMBL5207194)Show SMILES Cc1cc(ncc1-c1ccc(cc1)C1(COC1)C(=O)Nc1ccc(F)cc1)C1CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50538503
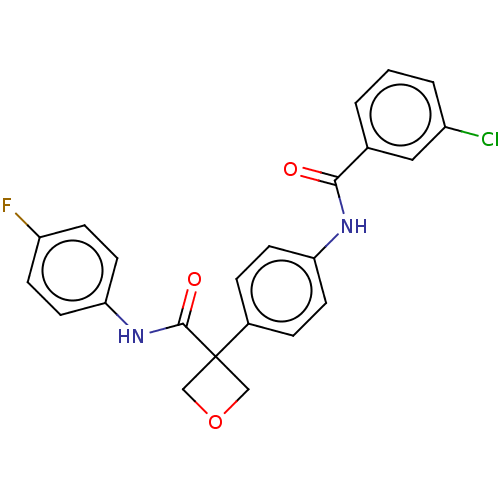
(CHEMBL4645108)Show SMILES Fc1ccc(NC(=O)C2(COC2)c2ccc(NC(=O)c3cccc(Cl)c3)cc2)cc1 Show InChI InChI=1S/C23H18ClFN2O3/c24-17-3-1-2-15(12-17)21(28)26-19-8-4-16(5-9-19)23(13-30-14-23)22(29)27-20-10-6-18(25)7-11-20/h1-12H,13-14H2,(H,26,28)(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50567132
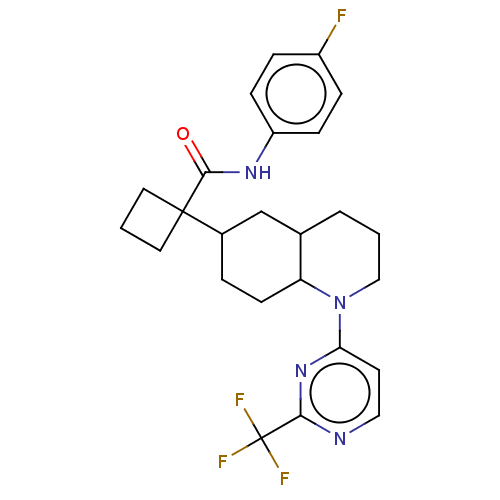
(CHEMBL4863767)Show SMILES Fc1ccc(NC(=O)C2(CCC2)C2CCC3C(CCCN3c3ccnc(n3)C(F)(F)F)C2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human whole blood assessed as unbound concentration |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128314
BindingDB Entry DOI: 10.7270/Q2TT4VP5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50567128
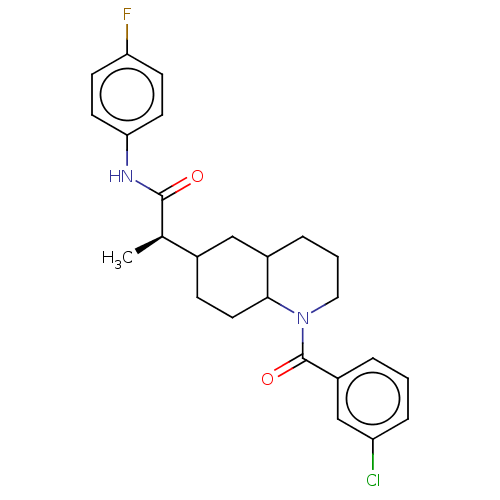
(CHEMBL4855797)Show SMILES C[C@H](C1CCC2C(CCCN2C(=O)c2cccc(Cl)c2)C1)C(=O)Nc1ccc(F)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human whole blood assessed as unbound concentration |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128314
BindingDB Entry DOI: 10.7270/Q2TT4VP5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604026

(CHEMBL5192384)Show SMILES CC(C)(O)c1cc(ncc1-c1ccc(cc1)C1(COC1)C(=O)Nc1ccc(F)cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50567131

(CHEMBL4848630)Show SMILES Cc1nccc(n1)N1CCCC2CC(CCC12)C1(CCC1)C(=O)Nc1ccc(F)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human whole blood assessed as unbound concentration |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128314
BindingDB Entry DOI: 10.7270/Q2TT4VP5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50568395

(CHEMBL4848098)Show SMILES Clc1ccc(NC(=O)C2(CCC2)c2ccc3N(CCc3n2)c2ccccn2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 assessed as unbound compound concentration by human whole blood assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128214
BindingDB Entry DOI: 10.7270/Q29K4G0K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50568383

(CHEMBL4872023)Show SMILES Fc1ccc(NC(=O)C2(CCC2)c2ccc3N(CCc3c2)C(=O)OC2CC2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 assessed as unbound compound concentration by human whole blood assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128214
BindingDB Entry DOI: 10.7270/Q29K4G0K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50543295
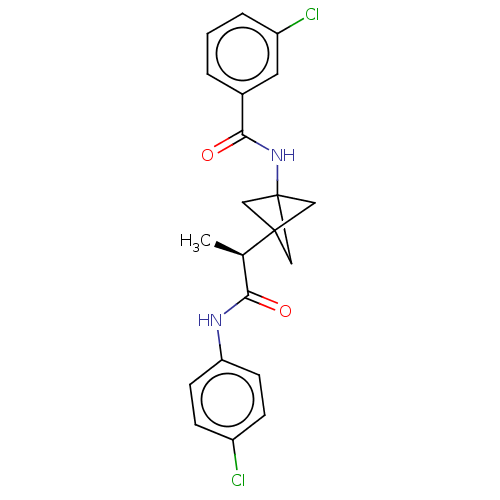
(CHEMBL4642946)Show SMILES C[C@H](C(=O)Nc1ccc(Cl)cc1)C12CC(C1)(C2)NC(=O)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C21H20Cl2N2O2/c1-13(18(26)24-17-7-5-15(22)6-8-17)20-10-21(11-20,12-20)25-19(27)14-3-2-4-16(23)9-14/h2-9,13H,10-12H2,1H3,(H,24,26)(H,25,27)/t13-,20?,21?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFN-gamma stimulated human HeLa cells using L-tryptophan as substrate incubated for 48 hrs by fluorescence assay |
ACS Med Chem Lett 11: 1548-1554 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00195
BindingDB Entry DOI: 10.7270/Q2RB786J |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50568397
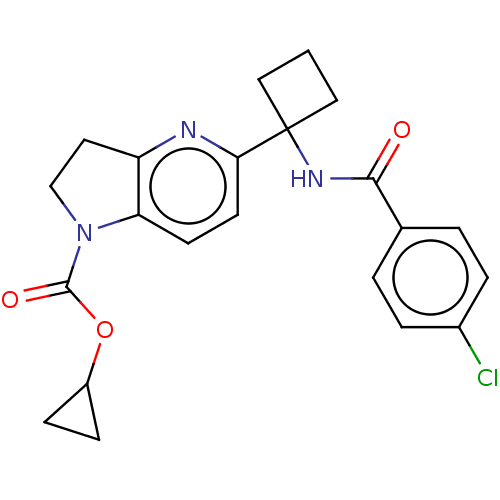
(CHEMBL4869600)Show SMILES Clc1ccc(cc1)C(=O)NC1(CCC1)c1ccc2N(CCc2n1)C(=O)OC1CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 assessed as unbound compound concentration by human whole blood assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128214
BindingDB Entry DOI: 10.7270/Q29K4G0K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50567134

(CHEMBL4848366)Show SMILES CC(N1CCC2C(CCCN2C(=O)OC2CC2)C1)C(=O)Nc1ccc(Cl)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human whole blood assessed as unbound concentration |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128314
BindingDB Entry DOI: 10.7270/Q2TT4VP5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604025
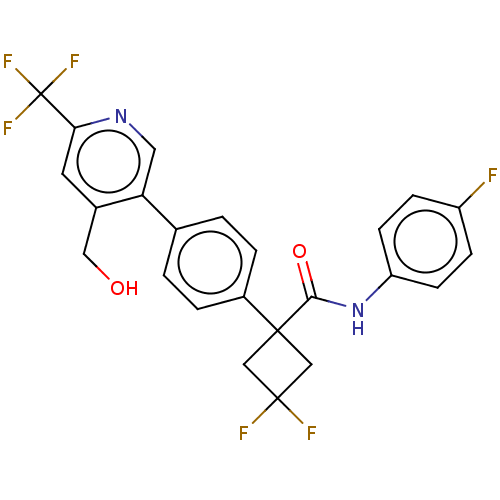
(CHEMBL5173861)Show SMILES OCc1cc(ncc1-c1ccc(cc1)C1(CC(F)(F)C1)C(=O)Nc1ccc(F)cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552941

(CHEMBL4764710)Show SMILES C[C@@H](NC(=O)c1ccc(F)cc1)c1ccc2N(CCCc2n1)C(=O)OC1CCC1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50568391
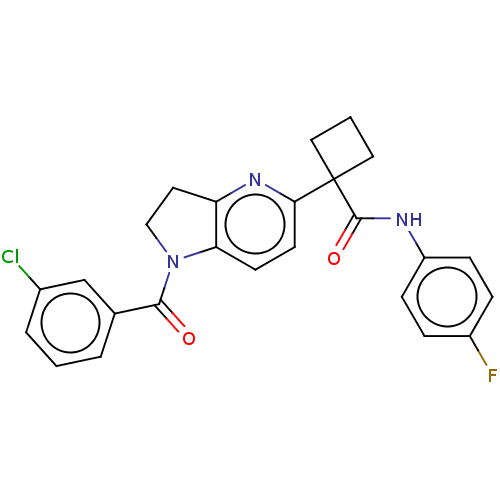
(CHEMBL4855740)Show SMILES Fc1ccc(NC(=O)C2(CCC2)c2ccc3N(CCc3n2)C(=O)c2cccc(Cl)c2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 assessed as unbound compound concentration by human whole blood assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128214
BindingDB Entry DOI: 10.7270/Q29K4G0K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50567130

(CHEMBL4858599)Show SMILES Fc1ccc(NC(=O)C2(CCC2)C2CCC3C(CCCN3C(=O)C3CC3)C2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human whole blood assessed as unbound concentration |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128314
BindingDB Entry DOI: 10.7270/Q2TT4VP5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50567129
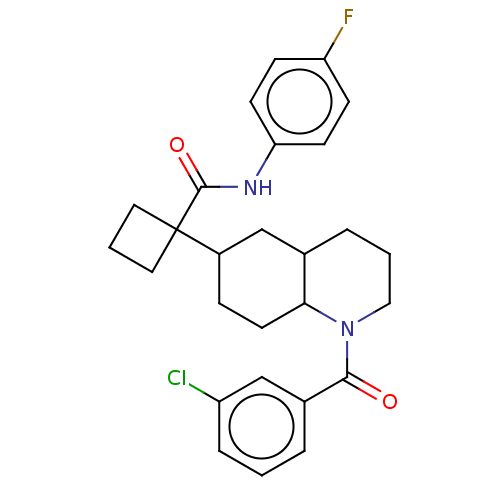
(CHEMBL4849622)Show SMILES Fc1ccc(NC(=O)C2(CCC2)C2CCC3C(CCCN3C(=O)c3cccc(Cl)c3)C2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human whole blood assessed as unbound concentration |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128314
BindingDB Entry DOI: 10.7270/Q2TT4VP5 |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM329724
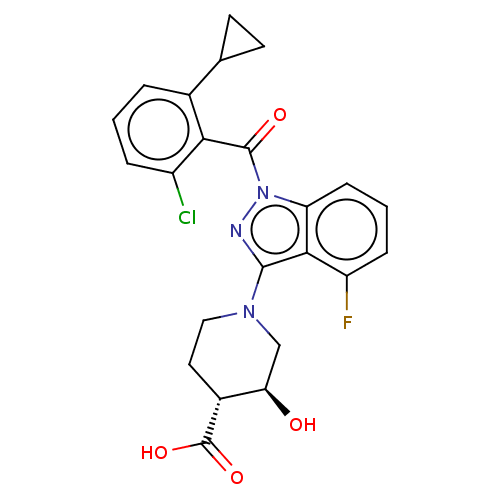
((3S,4R)-1-(1-(2-chloro-6-cyclopropylbenzoyl)-4-flu...)Show SMILES O[C@@H]1CN(CC[C@H]1C(O)=O)c1nn(C(=O)c2c(Cl)cccc2C2CC2)c2cccc(F)c12 Show InChI InChI=1S/C23H21ClFN3O4/c24-15-4-1-3-13(12-7-8-12)19(15)22(30)28-17-6-2-5-16(25)20(17)21(26-28)27-10-9-14(23(31)32)18(29)11-27/h1-6,12,14,18,29H,7-11H2,(H,31,32)/t14-,18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Allosteric inhibition of recombinant His6-tagged RORgammat LBD (unknown origin) expressed in Escherichia coli BL21(DE3) assessed as inhibition of bio... |
ACS Med Chem Lett 11: 114-119 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00431
BindingDB Entry DOI: 10.7270/Q24Q7Z9C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50567128

(CHEMBL4855797)Show SMILES C[C@H](C1CCC2C(CCCN2C(=O)c2cccc(Cl)c2)C1)C(=O)Nc1ccc(F)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human HeLa cells assessed as production of N-formylkynurenine using tryptophan as substrate in presence of inactivated fetal bo... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128314
BindingDB Entry DOI: 10.7270/Q2TT4VP5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50567128

(CHEMBL4855797)Show SMILES C[C@H](C1CCC2C(CCCN2C(=O)c2cccc(Cl)c2)C1)C(=O)Nc1ccc(F)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human HeLa cells assessed as production of N-formylkynurenine using tryptophan as substrate in presence of inactivated fetal bo... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128314
BindingDB Entry DOI: 10.7270/Q2TT4VP5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604024

(CHEMBL5193283)Show SMILES OCc1cc(ncc1-c1ccc(cc1)C1(CCC1)C(=O)Nc1ccc(F)cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50568383

(CHEMBL4872023)Show SMILES Fc1ccc(NC(=O)C2(CCC2)c2ccc3N(CCc3c2)C(=O)OC2CC2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 in human Hela cells |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128214
BindingDB Entry DOI: 10.7270/Q29K4G0K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552934

(CHEMBL4744727)Show SMILES C[C@@H](NC(=O)c1ccc(F)cc1)c1ccc2N(CCCc2n1)C(=O)c1cccc(Cl)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604025

(CHEMBL5173861)Show SMILES OCc1cc(ncc1-c1ccc(cc1)C1(CC(F)(F)C1)C(=O)Nc1ccc(F)cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50567130

(CHEMBL4858599)Show SMILES Fc1ccc(NC(=O)C2(CCC2)C2CCC3C(CCCN3C(=O)C3CC3)C2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human HeLa cells assessed as production of N-formylkynurenine using tryptophan as substrate in presence of inactivated fetal bo... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128314
BindingDB Entry DOI: 10.7270/Q2TT4VP5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50567131

(CHEMBL4848630)Show SMILES Cc1nccc(n1)N1CCCC2CC(CCC12)C1(CCC1)C(=O)Nc1ccc(F)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human HeLa cells assessed as production of N-formylkynurenine using tryptophan as substrate in presence of inactivated fetal bo... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128314
BindingDB Entry DOI: 10.7270/Q2TT4VP5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552931

(CHEMBL4755227)Show SMILES C[C@@H](NC(=O)c1ccc(Cl)cc1)c1ccc2N(CCCc2n1)C(=O)c1cccc(Cl)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50568387
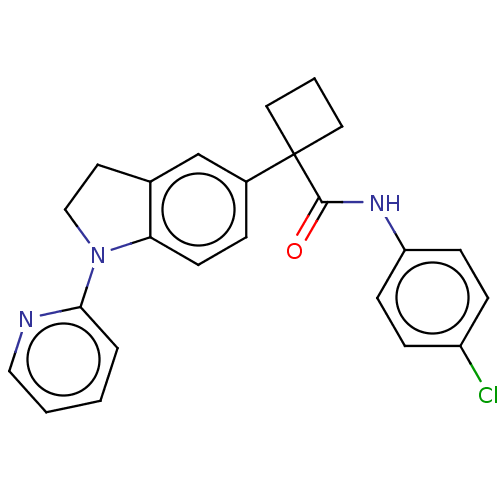
(CHEMBL4875994)Show SMILES Clc1ccc(NC(=O)C2(CCC2)c2ccc3N(CCc3c2)c2ccccn2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 in human Hela cells |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128214
BindingDB Entry DOI: 10.7270/Q29K4G0K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50567131

(CHEMBL4848630)Show SMILES Cc1nccc(n1)N1CCCC2CC(CCC12)C1(CCC1)C(=O)Nc1ccc(F)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human HeLa cells assessed as production of N-formylkynurenine using tryptophan as substrate in presence of inactivated fetal bo... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128314
BindingDB Entry DOI: 10.7270/Q2TT4VP5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50538499
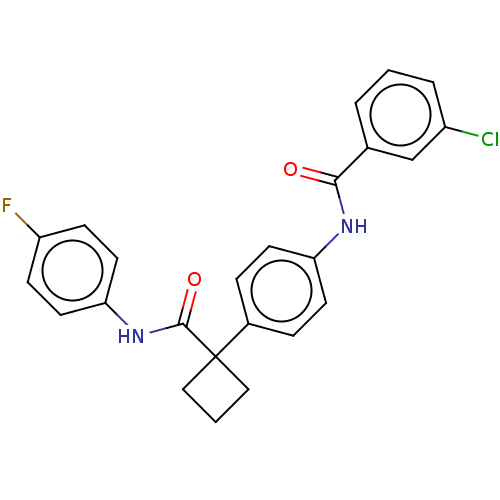
(CHEMBL4638223)Show SMILES Fc1ccc(NC(=O)C2(CCC2)c2ccc(NC(=O)c3cccc(Cl)c3)cc2)cc1 Show InChI InChI=1S/C24H20ClFN2O2/c25-18-4-1-3-16(15-18)22(29)27-20-9-5-17(6-10-20)24(13-2-14-24)23(30)28-21-11-7-19(26)8-12-21/h1,3-12,15H,2,13-14H2,(H,27,29)(H,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFN-gamma stimulated human HeLa cells using L-tryptophan as substrate incubated for 48 hrs by fluorescence assay |
ACS Med Chem Lett 11: 1548-1554 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00195
BindingDB Entry DOI: 10.7270/Q2RB786J |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552948

(CHEMBL4779920)Show SMILES C[C@@H](NC(=O)c1ccc(F)cc1)c1ccc2N(CCCc2n1)c1ccncn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552950

(CHEMBL4783395)Show SMILES C[C@@H](NC(=O)c1ccc(F)cc1)c1ccc2N(CCCc2n1)c1ccnc(n1)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50567132

(CHEMBL4863767)Show SMILES Fc1ccc(NC(=O)C2(CCC2)C2CCC3C(CCCN3c3ccnc(n3)C(F)(F)F)C2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human HeLa cells assessed as production of N-formylkynurenine using tryptophan as substrate in presence of inactivated fetal bo... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128314
BindingDB Entry DOI: 10.7270/Q2TT4VP5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50567134

(CHEMBL4848366)Show SMILES CC(N1CCC2C(CCCN2C(=O)OC2CC2)C1)C(=O)Nc1ccc(Cl)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human HeLa cells assessed as production of N-formylkynurenine using tryptophan as substrate in presence of inactivated fetal bo... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128314
BindingDB Entry DOI: 10.7270/Q2TT4VP5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50567128

(CHEMBL4855797)Show SMILES C[C@H](C1CCC2C(CCCN2C(=O)c2cccc(Cl)c2)C1)C(=O)Nc1ccc(F)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human HeLa cells assessed as production of N-formylkynurenine using tryptophan as substrate in presence of inactivated fetal bo... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128314
BindingDB Entry DOI: 10.7270/Q2TT4VP5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50543291
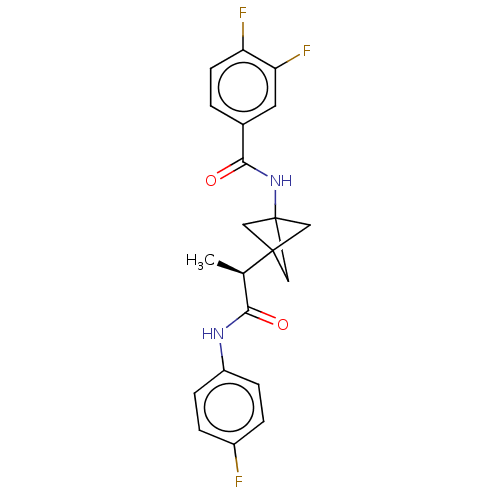
(CHEMBL4644751)Show SMILES C[C@H](C(=O)Nc1ccc(F)cc1)C12CC(C1)(C2)NC(=O)c1ccc(F)c(F)c1 |r| Show InChI InChI=1S/C21H19F3N2O2/c1-12(18(27)25-15-5-3-14(22)4-6-15)20-9-21(10-20,11-20)26-19(28)13-2-7-16(23)17(24)8-13/h2-8,12H,9-11H2,1H3,(H,25,27)(H,26,28)/t12-,20?,21?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFN-gamma stimulated human HeLa cells using L-tryptophan as substrate incubated for 48 hrs by fluorescence assay |
ACS Med Chem Lett 11: 1548-1554 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00195
BindingDB Entry DOI: 10.7270/Q2RB786J |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50567128

(CHEMBL4855797)Show SMILES C[C@H](C1CCC2C(CCCN2C(=O)c2cccc(Cl)c2)C1)C(=O)Nc1ccc(F)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human HeLa cells assessed as production of N-formylkynurenine using tryptophan as substrate in presence of inactivated fetal bo... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128314
BindingDB Entry DOI: 10.7270/Q2TT4VP5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50567132

(CHEMBL4863767)Show SMILES Fc1ccc(NC(=O)C2(CCC2)C2CCC3C(CCCN3c3ccnc(n3)C(F)(F)F)C2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human HeLa cells assessed as production of N-formylkynurenine using tryptophan as substrate in presence of inactivated fetal bo... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128314
BindingDB Entry DOI: 10.7270/Q2TT4VP5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50567132

(CHEMBL4863767)Show SMILES Fc1ccc(NC(=O)C2(CCC2)C2CCC3C(CCCN3c3ccnc(n3)C(F)(F)F)C2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human HeLa cells assessed as production of N-formylkynurenine using tryptophan as substrate in presence of inactivated fetal bo... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128314
BindingDB Entry DOI: 10.7270/Q2TT4VP5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604023

(CHEMBL5192977)Show SMILES OCc1cc(ncc1-c1ccc(cc1)C1(COC1)C(=O)Nc1ccc(F)cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50106301
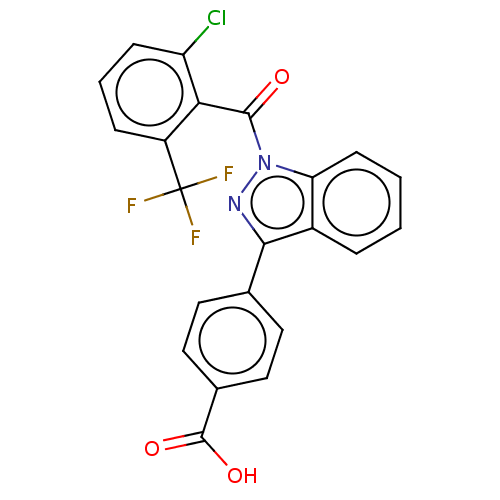
(CHEMBL3598140)Show SMILES OC(=O)c1ccc(cc1)-c1nn(C(=O)c2c(Cl)cccc2C(F)(F)F)c2ccccc12 Show InChI InChI=1S/C22H12ClF3N2O3/c23-16-6-3-5-15(22(24,25)26)18(16)20(29)28-17-7-2-1-4-14(17)19(27-28)12-8-10-13(11-9-12)21(30)31/h1-11H,(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Allosteric inhibition of recombinant His6-tagged RORgammat LBD (unknown origin) expressed in Escherichia coli BL21(DE3) assessed as inhibition of bio... |
ACS Med Chem Lett 11: 114-119 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00431
BindingDB Entry DOI: 10.7270/Q24Q7Z9C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM329726
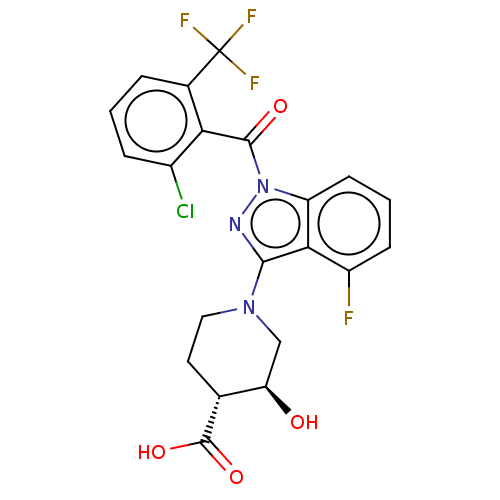
((3S,4R)-1-(1-(2- chloro-6- (trifluoromethyl)benzoy...)Show SMILES O[C@@H]1CN(CC[C@H]1C(O)=O)c1nn(C(=O)c2c(Cl)cccc2C(F)(F)F)c2cccc(F)c12 Show InChI InChI=1S/C21H16ClF4N3O4/c22-12-4-1-3-11(21(24,25)26)16(12)19(31)29-14-6-2-5-13(23)17(14)18(27-29)28-8-7-10(20(32)33)15(30)9-28/h1-6,10,15,30H,7-9H2,(H,32,33)/t10-,15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Allosteric inhibition of recombinant His6-tagged RORgammat LBD (unknown origin) expressed in Escherichia coli BL21(DE3) assessed as inhibition of bio... |
ACS Med Chem Lett 11: 114-119 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00431
BindingDB Entry DOI: 10.7270/Q24Q7Z9C |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50568389
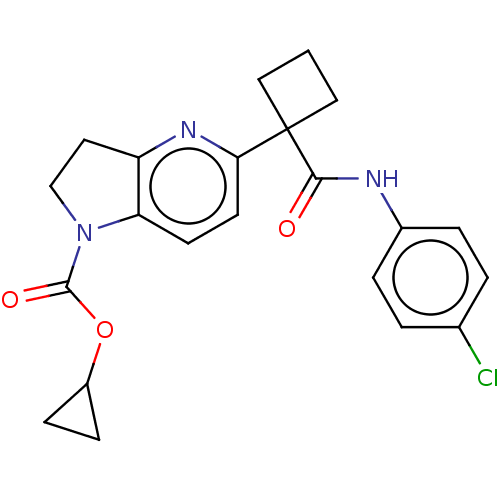
(CHEMBL4865753)Show SMILES Clc1ccc(NC(=O)C2(CCC2)c2ccc3N(CCc3n2)C(=O)OC2CC2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 in human Hela cells |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128214
BindingDB Entry DOI: 10.7270/Q29K4G0K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50543298

(CHEMBL4647172)Show SMILES C[C@H](C(=O)Nc1ccc(F)cc1)C12CC(C1)(C2)NC(=O)c1cc(F)cc(Cl)c1 |r| Show InChI InChI=1S/C21H19ClF2N2O2/c1-12(18(27)25-17-4-2-15(23)3-5-17)20-9-21(10-20,11-20)26-19(28)13-6-14(22)8-16(24)7-13/h2-8,12H,9-11H2,1H3,(H,25,27)(H,26,28)/t12-,20?,21?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFN-gamma stimulated human HeLa cells using L-tryptophan as substrate incubated for 48 hrs by fluorescence assay |
ACS Med Chem Lett 11: 1548-1554 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00195
BindingDB Entry DOI: 10.7270/Q2RB786J |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50538503

(CHEMBL4645108)Show SMILES Fc1ccc(NC(=O)C2(COC2)c2ccc(NC(=O)c3cccc(Cl)c3)cc2)cc1 Show InChI InChI=1S/C23H18ClFN2O3/c24-17-3-1-2-15(12-17)21(28)26-19-8-4-16(5-9-19)23(13-30-14-23)22(29)27-20-10-6-18(25)7-11-20/h1-12H,13-14H2,(H,26,28)(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50543297

(CHEMBL4648765)Show SMILES C[C@H](C(=O)Nc1ccc(F)cc1)C12CC(C1)(C2)NC(=O)c1ccc(F)c(Cl)c1 |r| Show InChI InChI=1S/C21H19ClF2N2O2/c1-12(18(27)25-15-5-3-14(23)4-6-15)20-9-21(10-20,11-20)26-19(28)13-2-7-17(24)16(22)8-13/h2-8,12H,9-11H2,1H3,(H,25,27)(H,26,28)/t12-,20?,21?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFN-gamma stimulated human HeLa cells using L-tryptophan as substrate incubated for 48 hrs by fluorescence assay |
ACS Med Chem Lett 11: 1548-1554 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00195
BindingDB Entry DOI: 10.7270/Q2RB786J |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604022
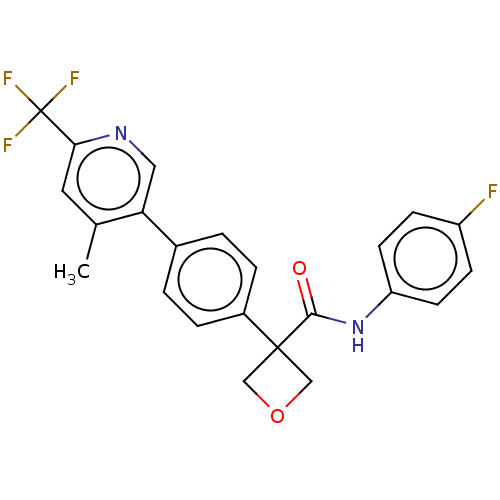
(CHEMBL5180607)Show SMILES Cc1cc(ncc1-c1ccc(cc1)C1(COC1)C(=O)Nc1ccc(F)cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50568395

(CHEMBL4848098)Show SMILES Clc1ccc(NC(=O)C2(CCC2)c2ccc3N(CCc3n2)c2ccccn2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 in human Hela cells |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128214
BindingDB Entry DOI: 10.7270/Q29K4G0K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604021

(CHEMBL5207194)Show SMILES Cc1cc(ncc1-c1ccc(cc1)C1(COC1)C(=O)Nc1ccc(F)cc1)C1CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data