 Found 456 hits with Last Name = 'fernandes' and Initial = 'p'
Found 456 hits with Last Name = 'fernandes' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
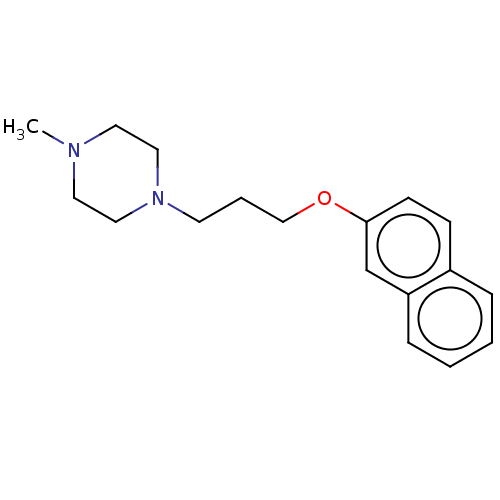
D(3) dopamine receptor
(Homo sapiens) | BDBM50610174
(CHEMBL4293814) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 2
(Homo sapiens (Human)) | BDBM82279
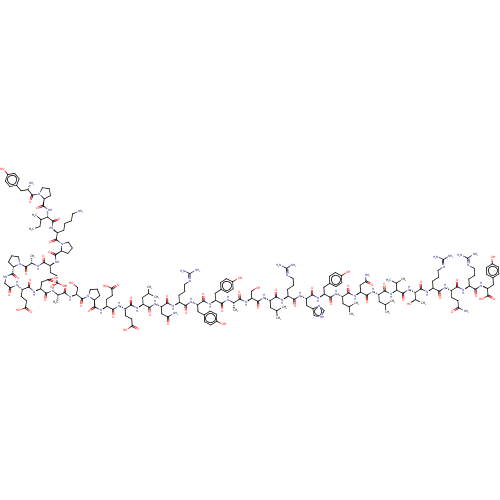
(CAS_118997-30-1 | PYY, human)Show SMILES CCC(C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)NCC(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C193H294N54O59/c1-17-99(12)154(242-181(295)141-35-25-73-245(141)187(301)115(195)80-104-37-47-110(251)48-38-104)184(298)226-125(28-18-19-67-194)188(302)246-74-26-34-140(246)180(294)223-122(59-64-149(262)263)158(272)216-102(15)186(300)244-72-24-33-139(244)179(293)212-90-146(259)217-120(58-63-148(260)261)163(277)237-136(88-152(268)269)168(282)215-101(14)157(271)240-147(92-249)306(305)247-75-27-36-142(247)182(296)224-124(61-66-151(266)267)165(279)222-123(60-65-150(264)265)166(280)227-127(77-95(4)5)170(284)235-134(86-144(197)257)175(289)220-116(29-20-68-208-190(199)200)160(274)231-131(82-106-41-51-112(253)52-42-106)173(287)232-130(81-105-39-49-111(252)50-40-105)167(281)214-100(13)156(270)239-138(91-248)178(292)230-126(76-94(2)3)169(283)219-117(30-21-69-209-191(201)202)161(275)234-133(85-109-89-207-93-213-109)174(288)233-132(83-107-43-53-113(254)54-44-107)172(286)228-128(78-96(6)7)171(285)236-135(87-145(198)258)176(290)229-129(79-97(8)9)177(291)241-153(98(10)11)183(297)243-155(103(16)250)185(299)225-119(32-23-71-211-193(205)206)159(273)221-121(57-62-143(196)256)164(278)218-118(31-22-70-210-192(203)204)162(276)238-137(189(303)304)84-108-45-55-114(255)56-46-108/h37-56,89,93-103,115-142,147,153-155,248-255H,17-36,57-88,90-92,194-195H2,1-16H3,(H2,196,256)(H2,197,257)(H2,198,258)(H,207,213)(H,212,293)(H,214,281)(H,215,282)(H,216,272)(H,217,259)(H,218,278)(H,219,283)(H,220,289)(H,221,273)(H,222,279)(H,223,294)(H,224,296)(H,225,299)(H,226,298)(H,227,280)(H,228,286)(H,229,290)(H,230,292)(H,231,274)(H,232,287)(H,233,288)(H,234,275)(H,235,284)(H,236,285)(H,237,277)(H,238,276)(H,239,270)(H,240,271)(H,241,291)(H,242,295)(H,243,297)(H,260,261)(H,262,263)(H,264,265)(H,266,267)(H,268,269)(H,303,304)(H4,199,200,208)(H4,201,202,209)(H4,203,204,210)(H4,205,206,211)/t99?,100-,101-,102-,103+,115-,116-,117-,118-,119-,120-,121-,122-,123-,124-,125-,126-,127-,128-,129-,130-,131-,132-,133-,134-,135-,136-,137-,138-,139-,140-,141-,142-,147+,153-,154-,155-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb
Curated by PDSP Ki Database
| |
J Biol Chem 270: 22661-4 (1995)
Article DOI: 10.1074/jbc.270.39.22661
BindingDB Entry DOI: 10.7270/Q2TT4PGD |
More data for this
Ligand-Target Pair | |
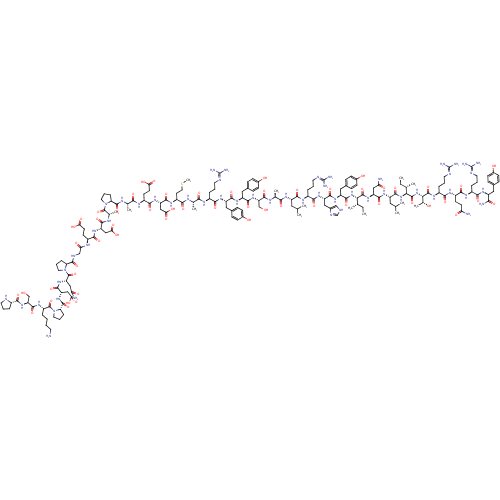
D(3) dopamine receptor
(Homo sapiens) | BDBM50610173
(CHEMBL5280702) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
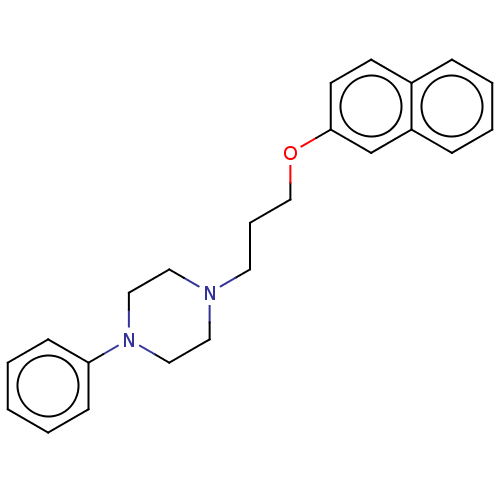
Neuropeptide Y receptor type 2
(Homo sapiens (Human)) | BDBM82277
(NPY2-36, human | NPY2-36, rat, human)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](C)NC(=O)[C@H](CCSC)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C180H276N54O55S/c1-15-88(7)140(171(284)225-121(76-132(183)243)161(274)217-116(70-87(5)6)164(277)230-141(89(8)16-2)172(285)231-142(94(13)237)173(286)213-108(32-23-64-199-180(192)193)149(262)211-110(52-55-131(182)242)154(267)209-106(30-21-62-197-178(188)189)150(263)215-114(143(185)256)71-95-36-44-100(238)45-37-95)229-165(278)119(74-98-42-50-103(241)51-43-98)220-160(273)120(75-99-81-194-85-201-99)221-152(265)107(31-22-63-198-179(190)191)210-157(270)115(69-86(3)4)216-146(259)91(10)203-166(279)126(83-235)228-159(272)118(73-97-40-48-102(240)49-41-97)219-158(271)117(72-96-38-46-101(239)47-39-96)218-151(264)105(29-20-61-196-177(186)187)207-144(257)90(9)202-148(261)112(58-68-290-14)212-162(275)123(79-138(252)253)223-155(268)111(54-57-136(248)249)208-145(258)92(11)204-169(282)129-34-25-65-232(129)174(287)93(12)205-156(269)122(78-137(250)251)222-153(266)109(53-56-135(246)247)206-134(245)82-200-168(281)128-33-24-66-233(128)176(289)125(77-133(184)244)226-163(276)124(80-139(254)255)224-170(283)130-35-26-67-234(130)175(288)113(27-17-18-59-181)214-167(280)127(84-236)227-147(260)104-28-19-60-195-104/h36-51,81,85-94,104-130,140-142,195,235-241H,15-35,52-80,82-84,181H2,1-14H3,(H2,182,242)(H2,183,243)(H2,184,244)(H2,185,256)(H,194,201)(H,200,281)(H,202,261)(H,203,279)(H,204,282)(H,205,269)(H,206,245)(H,207,257)(H,208,258)(H,209,267)(H,210,270)(H,211,262)(H,212,275)(H,213,286)(H,214,280)(H,215,263)(H,216,259)(H,217,274)(H,218,264)(H,219,271)(H,220,273)(H,221,265)(H,222,266)(H,223,268)(H,224,283)(H,225,284)(H,226,276)(H,227,260)(H,228,272)(H,229,278)(H,230,277)(H,231,285)(H,246,247)(H,248,249)(H,250,251)(H,252,253)(H,254,255)(H4,186,187,196)(H4,188,189,197)(H4,190,191,198)(H4,192,193,199)/t88-,89-,90-,91-,92-,93-,94+,104-,105-,106-,107-,108-,109-,110-,111-,112-,113-,114-,115-,116-,117-,118-,119-,120-,121-,122-,123-,124-,125-,126-,127-,128-,129-,130-,140-,141-,142-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb
Curated by PDSP Ki Database
| |
J Biol Chem 270: 22661-4 (1995)
Article DOI: 10.1074/jbc.270.39.22661
BindingDB Entry DOI: 10.7270/Q2TT4PGD |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50610180
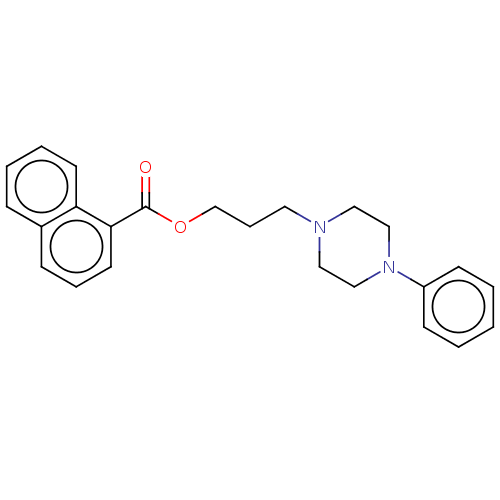
(CHEMBL5276588) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50610178

(CHEMBL5289468) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50610177

(CHEMBL5268329) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50121205
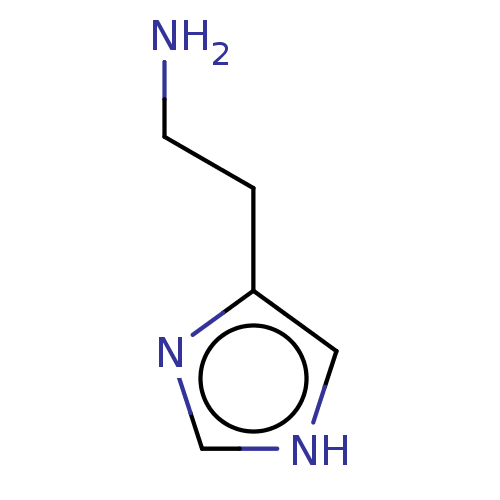
(CHEBI:18295 | Histamine)Show InChI InChI=1S/C5H9N3/c6-2-1-5-3-7-4-8-5/h3-4H,1-2,6H2,(H,7,8) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-Histamine from human histamine 4 receptor transfected in HEK293T cells incubated for 16 hrs by liquid scintillation counter anal... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115924
BindingDB Entry DOI: 10.7270/Q2833WNJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuropeptide Y receptor type 2
(Homo sapiens (Human)) | BDBM82279

(CAS_118997-30-1 | PYY, human)Show SMILES CCC(C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)NCC(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C193H294N54O59/c1-17-99(12)154(242-181(295)141-35-25-73-245(141)187(301)115(195)80-104-37-47-110(251)48-38-104)184(298)226-125(28-18-19-67-194)188(302)246-74-26-34-140(246)180(294)223-122(59-64-149(262)263)158(272)216-102(15)186(300)244-72-24-33-139(244)179(293)212-90-146(259)217-120(58-63-148(260)261)163(277)237-136(88-152(268)269)168(282)215-101(14)157(271)240-147(92-249)306(305)247-75-27-36-142(247)182(296)224-124(61-66-151(266)267)165(279)222-123(60-65-150(264)265)166(280)227-127(77-95(4)5)170(284)235-134(86-144(197)257)175(289)220-116(29-20-68-208-190(199)200)160(274)231-131(82-106-41-51-112(253)52-42-106)173(287)232-130(81-105-39-49-111(252)50-40-105)167(281)214-100(13)156(270)239-138(91-248)178(292)230-126(76-94(2)3)169(283)219-117(30-21-69-209-191(201)202)161(275)234-133(85-109-89-207-93-213-109)174(288)233-132(83-107-43-53-113(254)54-44-107)172(286)228-128(78-96(6)7)171(285)236-135(87-145(198)258)176(290)229-129(79-97(8)9)177(291)241-153(98(10)11)183(297)243-155(103(16)250)185(299)225-119(32-23-71-211-193(205)206)159(273)221-121(57-62-143(196)256)164(278)218-118(31-22-70-210-192(203)204)162(276)238-137(189(303)304)84-108-45-55-114(255)56-46-108/h37-56,89,93-103,115-142,147,153-155,248-255H,17-36,57-88,90-92,194-195H2,1-16H3,(H2,196,256)(H2,197,257)(H2,198,258)(H,207,213)(H,212,293)(H,214,281)(H,215,282)(H,216,272)(H,217,259)(H,218,278)(H,219,283)(H,220,289)(H,221,273)(H,222,279)(H,223,294)(H,224,296)(H,225,299)(H,226,298)(H,227,280)(H,228,286)(H,229,290)(H,230,292)(H,231,274)(H,232,287)(H,233,288)(H,234,275)(H,235,284)(H,236,285)(H,237,277)(H,238,276)(H,239,270)(H,240,271)(H,241,291)(H,242,295)(H,243,297)(H,260,261)(H,262,263)(H,264,265)(H,266,267)(H,268,269)(H,303,304)(H4,199,200,208)(H4,201,202,209)(H4,203,204,210)(H4,205,206,211)/t99?,100-,101-,102-,103+,115-,116-,117-,118-,119-,120-,121-,122-,123-,124-,125-,126-,127-,128-,129-,130-,131-,132-,133-,134-,135-,136-,137-,138-,139-,140-,141-,142-,147+,153-,154-,155-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb
Curated by PDSP Ki Database
| |
J Biol Chem 270: 22661-4 (1995)
Article DOI: 10.1074/jbc.270.39.22661
BindingDB Entry DOI: 10.7270/Q2TT4PGD |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50610182
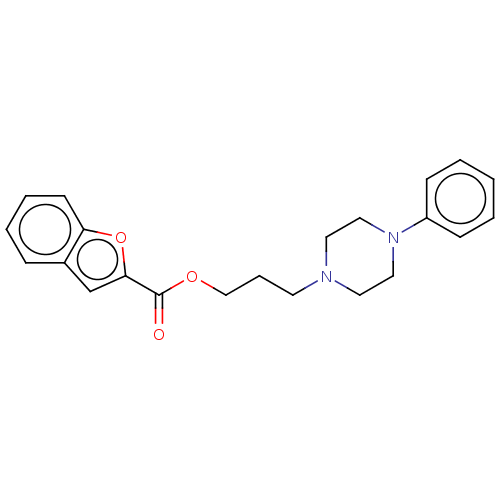
(CHEMBL5287235) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 2
(Homo sapiens (Human)) | BDBM50015490
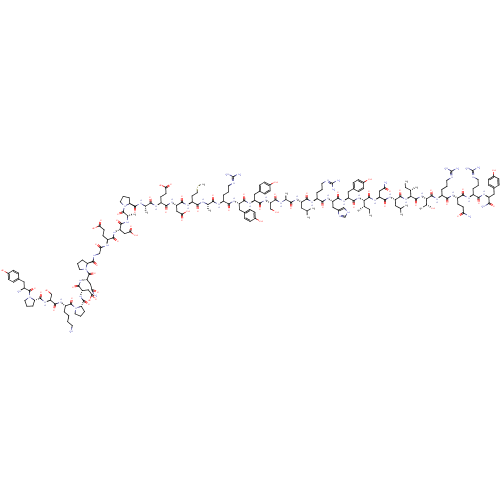
(CHEMBL438945 | H-YPSKPDNPGEDAPAEDMARYYSALRHYINLITR...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](C)NC(=O)[C@H](CCSC)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C189H285N55O57S/c1-15-93(7)148(179(295)234-128(81-140(193)254)168(284)226-123(74-92(5)6)171(287)239-149(94(8)16-2)180(296)240-150(99(13)247)181(297)222-115(31-22-67-208-189(202)203)156(272)220-117(56-59-139(192)253)161(277)218-113(29-20-65-206-187(198)199)157(273)224-121(151(195)267)76-101-38-48-107(249)49-39-101)238-172(288)126(79-104-44-54-110(252)55-45-104)229-167(283)127(80-105-86-204-90-210-105)230-159(275)114(30-21-66-207-188(200)201)219-164(280)122(73-91(3)4)225-154(270)96(10)212-173(289)133(88-245)236-166(282)125(78-103-42-52-109(251)53-43-103)228-165(281)124(77-102-40-50-108(250)51-41-102)227-158(274)112(28-19-64-205-186(196)197)216-152(268)95(9)211-155(271)119(62-72-302-14)221-169(285)130(84-146(263)264)232-162(278)118(58-61-144(259)260)217-153(269)97(11)213-176(292)136-33-24-68-241(136)182(298)98(12)214-163(279)129(83-145(261)262)231-160(276)116(57-60-143(257)258)215-142(256)87-209-175(291)135-32-23-70-243(135)185(301)132(82-141(194)255)235-170(286)131(85-147(265)266)233-177(293)138-35-26-71-244(138)184(300)120(27-17-18-63-190)223-174(290)134(89-246)237-178(294)137-34-25-69-242(137)183(299)111(191)75-100-36-46-106(248)47-37-100/h36-55,86,90-99,111-138,148-150,245-252H,15-35,56-85,87-89,190-191H2,1-14H3,(H2,192,253)(H2,193,254)(H2,194,255)(H2,195,267)(H,204,210)(H,209,291)(H,211,271)(H,212,289)(H,213,292)(H,214,279)(H,215,256)(H,216,268)(H,217,269)(H,218,277)(H,219,280)(H,220,272)(H,221,285)(H,222,297)(H,223,290)(H,224,273)(H,225,270)(H,226,284)(H,227,274)(H,228,281)(H,229,283)(H,230,275)(H,231,276)(H,232,278)(H,233,293)(H,234,295)(H,235,286)(H,236,282)(H,237,294)(H,238,288)(H,239,287)(H,240,296)(H,257,258)(H,259,260)(H,261,262)(H,263,264)(H,265,266)(H4,196,197,205)(H4,198,199,206)(H4,200,201,207)(H4,202,203,208)/t93-,94-,95-,96-,97-,98-,99+,111-,112-,113-,114-,115-,116-,117-,118-,119-,120-,121-,122-,123-,124-,125-,126-,127-,128-,129-,130-,131-,132-,133-,134-,135-,136-,137-,138-,148-,149-,150-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb
Curated by PDSP Ki Database
| |
J Biol Chem 270: 22661-4 (1995)
Article DOI: 10.1074/jbc.270.39.22661
BindingDB Entry DOI: 10.7270/Q2TT4PGD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50121205

(CHEBI:18295 | Histamine)Show InChI InChI=1S/C5H9N3/c6-2-1-5-3-7-4-8-5/h3-4H,1-2,6H2,(H,7,8) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-Histamine from human histamine 3 receptor transfected in HEK293T cells incubated for 16 hrs by liquid scintillation counter anal... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115924
BindingDB Entry DOI: 10.7270/Q2833WNJ |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 2
(Homo sapiens (Human)) | BDBM82277

(NPY2-36, human | NPY2-36, rat, human)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](C)NC(=O)[C@H](CCSC)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C180H276N54O55S/c1-15-88(7)140(171(284)225-121(76-132(183)243)161(274)217-116(70-87(5)6)164(277)230-141(89(8)16-2)172(285)231-142(94(13)237)173(286)213-108(32-23-64-199-180(192)193)149(262)211-110(52-55-131(182)242)154(267)209-106(30-21-62-197-178(188)189)150(263)215-114(143(185)256)71-95-36-44-100(238)45-37-95)229-165(278)119(74-98-42-50-103(241)51-43-98)220-160(273)120(75-99-81-194-85-201-99)221-152(265)107(31-22-63-198-179(190)191)210-157(270)115(69-86(3)4)216-146(259)91(10)203-166(279)126(83-235)228-159(272)118(73-97-40-48-102(240)49-41-97)219-158(271)117(72-96-38-46-101(239)47-39-96)218-151(264)105(29-20-61-196-177(186)187)207-144(257)90(9)202-148(261)112(58-68-290-14)212-162(275)123(79-138(252)253)223-155(268)111(54-57-136(248)249)208-145(258)92(11)204-169(282)129-34-25-65-232(129)174(287)93(12)205-156(269)122(78-137(250)251)222-153(266)109(53-56-135(246)247)206-134(245)82-200-168(281)128-33-24-66-233(128)176(289)125(77-133(184)244)226-163(276)124(80-139(254)255)224-170(283)130-35-26-67-234(130)175(288)113(27-17-18-59-181)214-167(280)127(84-236)227-147(260)104-28-19-60-195-104/h36-51,81,85-94,104-130,140-142,195,235-241H,15-35,52-80,82-84,181H2,1-14H3,(H2,182,242)(H2,183,243)(H2,184,244)(H2,185,256)(H,194,201)(H,200,281)(H,202,261)(H,203,279)(H,204,282)(H,205,269)(H,206,245)(H,207,257)(H,208,258)(H,209,267)(H,210,270)(H,211,262)(H,212,275)(H,213,286)(H,214,280)(H,215,263)(H,216,259)(H,217,274)(H,218,264)(H,219,271)(H,220,273)(H,221,265)(H,222,266)(H,223,268)(H,224,283)(H,225,284)(H,226,276)(H,227,260)(H,228,272)(H,229,278)(H,230,277)(H,231,285)(H,246,247)(H,248,249)(H,250,251)(H,252,253)(H,254,255)(H4,186,187,196)(H4,188,189,197)(H4,190,191,198)(H4,192,193,199)/t88-,89-,90-,91-,92-,93-,94+,104-,105-,106-,107-,108-,109-,110-,111-,112-,113-,114-,115-,116-,117-,118-,119-,120-,121-,122-,123-,124-,125-,126-,127-,128-,129-,130-,140-,141-,142-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb
Curated by PDSP Ki Database
| |
J Biol Chem 270: 22661-4 (1995)
Article DOI: 10.1074/jbc.270.39.22661
BindingDB Entry DOI: 10.7270/Q2TT4PGD |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50610169
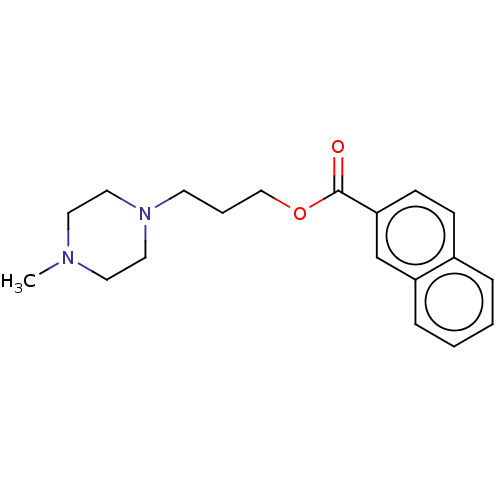
(CHEMBL5270151) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 2
(Homo sapiens (Human)) | BDBM50015490

(CHEMBL438945 | H-YPSKPDNPGEDAPAEDMARYYSALRHYINLITR...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](C)NC(=O)[C@H](CCSC)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C189H285N55O57S/c1-15-93(7)148(179(295)234-128(81-140(193)254)168(284)226-123(74-92(5)6)171(287)239-149(94(8)16-2)180(296)240-150(99(13)247)181(297)222-115(31-22-67-208-189(202)203)156(272)220-117(56-59-139(192)253)161(277)218-113(29-20-65-206-187(198)199)157(273)224-121(151(195)267)76-101-38-48-107(249)49-39-101)238-172(288)126(79-104-44-54-110(252)55-45-104)229-167(283)127(80-105-86-204-90-210-105)230-159(275)114(30-21-66-207-188(200)201)219-164(280)122(73-91(3)4)225-154(270)96(10)212-173(289)133(88-245)236-166(282)125(78-103-42-52-109(251)53-43-103)228-165(281)124(77-102-40-50-108(250)51-41-102)227-158(274)112(28-19-64-205-186(196)197)216-152(268)95(9)211-155(271)119(62-72-302-14)221-169(285)130(84-146(263)264)232-162(278)118(58-61-144(259)260)217-153(269)97(11)213-176(292)136-33-24-68-241(136)182(298)98(12)214-163(279)129(83-145(261)262)231-160(276)116(57-60-143(257)258)215-142(256)87-209-175(291)135-32-23-70-243(135)185(301)132(82-141(194)255)235-170(286)131(85-147(265)266)233-177(293)138-35-26-71-244(138)184(300)120(27-17-18-63-190)223-174(290)134(89-246)237-178(294)137-34-25-69-242(137)183(299)111(191)75-100-36-46-106(248)47-37-100/h36-55,86,90-99,111-138,148-150,245-252H,15-35,56-85,87-89,190-191H2,1-14H3,(H2,192,253)(H2,193,254)(H2,194,255)(H2,195,267)(H,204,210)(H,209,291)(H,211,271)(H,212,289)(H,213,292)(H,214,279)(H,215,256)(H,216,268)(H,217,269)(H,218,277)(H,219,280)(H,220,272)(H,221,285)(H,222,297)(H,223,290)(H,224,273)(H,225,270)(H,226,284)(H,227,274)(H,228,281)(H,229,283)(H,230,275)(H,231,276)(H,232,278)(H,233,293)(H,234,295)(H,235,286)(H,236,282)(H,237,294)(H,238,288)(H,239,287)(H,240,296)(H,257,258)(H,259,260)(H,261,262)(H,263,264)(H,265,266)(H4,196,197,205)(H4,198,199,206)(H4,200,201,207)(H4,202,203,208)/t93-,94-,95-,96-,97-,98-,99+,111-,112-,113-,114-,115-,116-,117-,118-,119-,120-,121-,122-,123-,124-,125-,126-,127-,128-,129-,130-,131-,132-,133-,134-,135-,136-,137-,138-,148-,149-,150-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb
Curated by PDSP Ki Database
| |
J Biol Chem 270: 22661-4 (1995)
Article DOI: 10.1074/jbc.270.39.22661
BindingDB Entry DOI: 10.7270/Q2TT4PGD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50552436

(CHEMBL4749654) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-Histamine from human histamine 3 receptor transfected in HEK293T cells incubated for 16 hrs by liquid scintillation counter anal... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115924
BindingDB Entry DOI: 10.7270/Q2833WNJ |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 2
(Homo sapiens (Human)) | BDBM82278
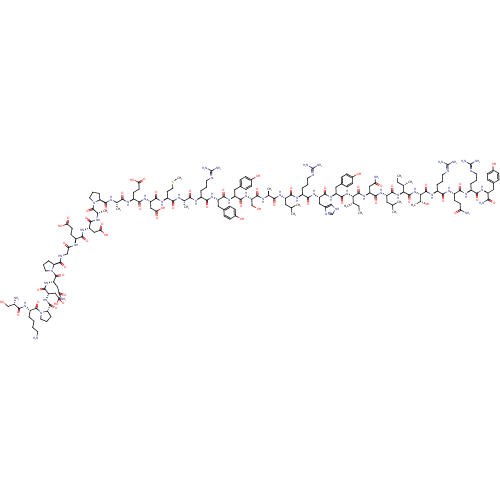
(NPY3-36)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](C)NC(=O)[C@H](CCSC)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@@H](N)CO)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C175H269N53O54S/c1-15-85(7)136(166(277)220-118(73-128(179)237)157(268)212-113(67-84(5)6)160(271)224-137(86(8)16-2)167(278)225-138(91(13)231)168(279)208-105(30-22-61-194-175(188)189)145(256)206-107(50-53-127(178)236)150(261)204-103(28-20-59-192-173(184)185)146(257)210-111(139(181)250)68-92-34-42-97(232)43-35-92)223-161(272)116(71-95-40-48-100(235)49-41-95)215-156(267)117(72-96-78-190-82-196-96)216-148(259)104(29-21-60-193-174(186)187)205-153(264)112(66-83(3)4)211-142(253)88(10)198-162(273)123(81-230)222-155(266)115(70-94-38-46-99(234)47-39-94)214-154(265)114(69-93-36-44-98(233)45-37-93)213-147(258)102(27-19-58-191-172(182)183)202-140(251)87(9)197-144(255)109(56-65-283-14)207-158(269)120(76-134(246)247)218-151(262)108(52-55-132(242)243)203-141(252)89(11)199-164(275)125-32-24-62-226(125)169(280)90(12)200-152(263)119(75-133(244)245)217-149(260)106(51-54-131(240)241)201-130(239)79-195-163(274)124-31-23-63-227(124)171(282)122(74-129(180)238)221-159(270)121(77-135(248)249)219-165(276)126-33-25-64-228(126)170(281)110(26-17-18-57-176)209-143(254)101(177)80-229/h34-49,78,82-91,101-126,136-138,229-235H,15-33,50-77,79-81,176-177H2,1-14H3,(H2,178,236)(H2,179,237)(H2,180,238)(H2,181,250)(H,190,196)(H,195,274)(H,197,255)(H,198,273)(H,199,275)(H,200,263)(H,201,239)(H,202,251)(H,203,252)(H,204,261)(H,205,264)(H,206,256)(H,207,269)(H,208,279)(H,209,254)(H,210,257)(H,211,253)(H,212,268)(H,213,258)(H,214,265)(H,215,267)(H,216,259)(H,217,260)(H,218,262)(H,219,276)(H,220,277)(H,221,270)(H,222,266)(H,223,272)(H,224,271)(H,225,278)(H,240,241)(H,242,243)(H,244,245)(H,246,247)(H,248,249)(H4,182,183,191)(H4,184,185,192)(H4,186,187,193)(H4,188,189,194)/t85-,86-,87-,88-,89-,90-,91+,101-,102-,103-,104-,105-,106-,107-,108-,109-,110-,111-,112-,113-,114-,115-,116-,117-,118-,119-,120-,121-,122-,123-,124-,125-,126-,136-,137-,138-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb
Curated by PDSP Ki Database
| |
J Biol Chem 270: 22661-4 (1995)
Article DOI: 10.1074/jbc.270.39.22661
BindingDB Entry DOI: 10.7270/Q2TT4PGD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50552434
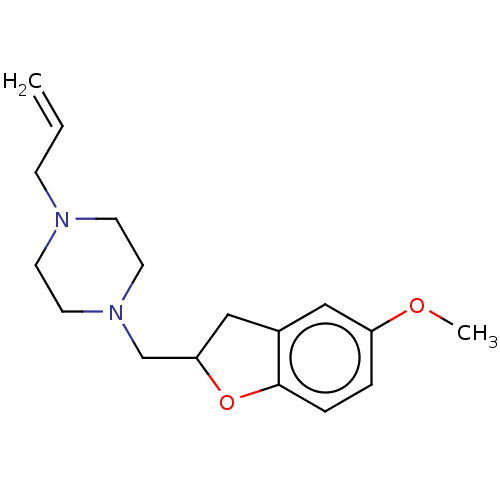
(CHEMBL4747180) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-Histamine from human histamine 3 receptor transfected in HEK293T cells incubated for 16 hrs by liquid scintillation counter anal... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115924
BindingDB Entry DOI: 10.7270/Q2833WNJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50610170

(CHEMBL5278711) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50610181
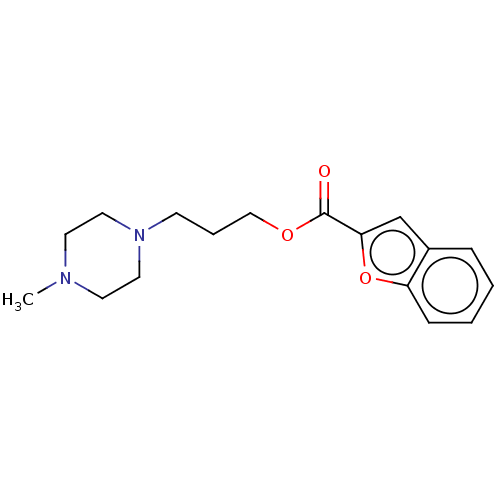
(CHEMBL5270288) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50610179

(CHEMBL5282002) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50610175
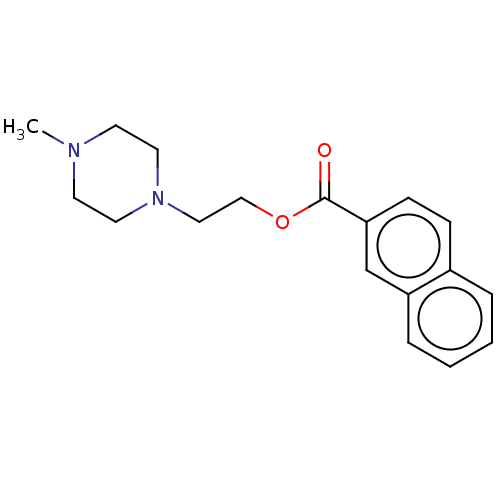
(CHEMBL5283987) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50610176
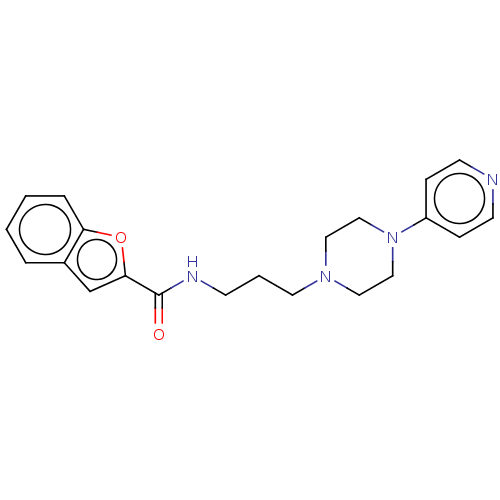
(CHEMBL5265920) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 2
(Homo sapiens (Human)) | BDBM82278

(NPY3-36)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](C)NC(=O)[C@H](CCSC)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@@H](N)CO)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C175H269N53O54S/c1-15-85(7)136(166(277)220-118(73-128(179)237)157(268)212-113(67-84(5)6)160(271)224-137(86(8)16-2)167(278)225-138(91(13)231)168(279)208-105(30-22-61-194-175(188)189)145(256)206-107(50-53-127(178)236)150(261)204-103(28-20-59-192-173(184)185)146(257)210-111(139(181)250)68-92-34-42-97(232)43-35-92)223-161(272)116(71-95-40-48-100(235)49-41-95)215-156(267)117(72-96-78-190-82-196-96)216-148(259)104(29-21-60-193-174(186)187)205-153(264)112(66-83(3)4)211-142(253)88(10)198-162(273)123(81-230)222-155(266)115(70-94-38-46-99(234)47-39-94)214-154(265)114(69-93-36-44-98(233)45-37-93)213-147(258)102(27-19-58-191-172(182)183)202-140(251)87(9)197-144(255)109(56-65-283-14)207-158(269)120(76-134(246)247)218-151(262)108(52-55-132(242)243)203-141(252)89(11)199-164(275)125-32-24-62-226(125)169(280)90(12)200-152(263)119(75-133(244)245)217-149(260)106(51-54-131(240)241)201-130(239)79-195-163(274)124-31-23-63-227(124)171(282)122(74-129(180)238)221-159(270)121(77-135(248)249)219-165(276)126-33-25-64-228(126)170(281)110(26-17-18-57-176)209-143(254)101(177)80-229/h34-49,78,82-91,101-126,136-138,229-235H,15-33,50-77,79-81,176-177H2,1-14H3,(H2,178,236)(H2,179,237)(H2,180,238)(H2,181,250)(H,190,196)(H,195,274)(H,197,255)(H,198,273)(H,199,275)(H,200,263)(H,201,239)(H,202,251)(H,203,252)(H,204,261)(H,205,264)(H,206,256)(H,207,269)(H,208,279)(H,209,254)(H,210,257)(H,211,253)(H,212,268)(H,213,258)(H,214,265)(H,215,267)(H,216,259)(H,217,260)(H,218,262)(H,219,276)(H,220,277)(H,221,270)(H,222,266)(H,223,272)(H,224,271)(H,225,278)(H,240,241)(H,242,243)(H,244,245)(H,246,247)(H,248,249)(H4,182,183,191)(H4,184,185,192)(H4,186,187,193)(H4,188,189,194)/t85-,86-,87-,88-,89-,90-,91+,101-,102-,103-,104-,105-,106-,107-,108-,109-,110-,111-,112-,113-,114-,115-,116-,117-,118-,119-,120-,121-,122-,123-,124-,125-,126-,136-,137-,138-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb
Curated by PDSP Ki Database
| |
J Biol Chem 270: 22661-4 (1995)
Article DOI: 10.1074/jbc.270.39.22661
BindingDB Entry DOI: 10.7270/Q2TT4PGD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50552435
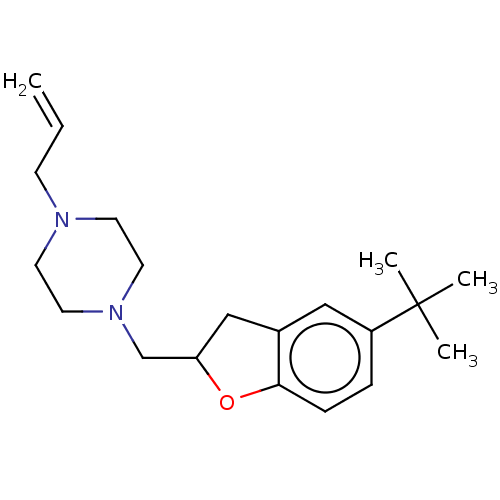
(CHEMBL4756432) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-Histamine from human histamine 3 receptor transfected in HEK293T cells incubated for 16 hrs by liquid scintillation counter anal... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115924
BindingDB Entry DOI: 10.7270/Q2833WNJ |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50405713
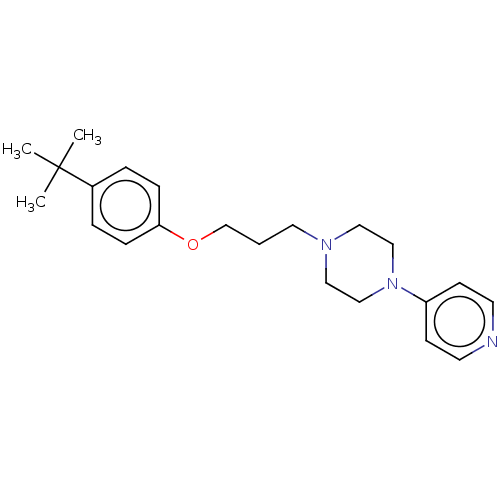
(CHEMBL4173854)Show InChI InChI=1S/C15H18ClNO3S2/c1-9(8-21)14(18)17-7-12(6-13(17)15(19)20)22-11-4-2-10(16)3-5-11/h2-5,9,12-13,21H,6-8H2,1H3,(H,19,20)/t9-,12+,13+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Protein O-GlcNAcase
(Homo sapiens (Human)) | BDBM50577843
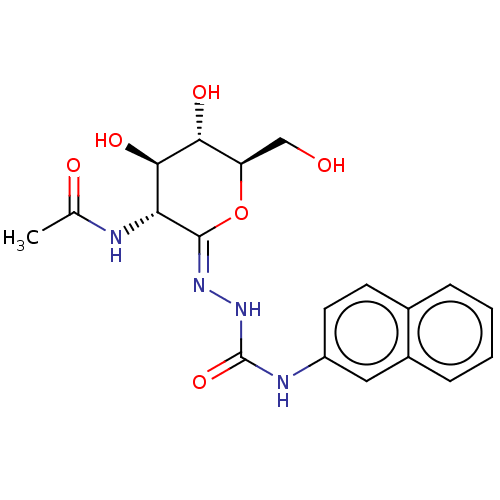
(CHEMBL4853460)Show SMILES CC(=O)N[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O\C1=N/NC(=O)Nc1ccc2ccccc2c1 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Competitive inhibition of N-terminal His-tagged human OGA using 4-Mu-GlcNAc as substrate assessed as inhibition constant by measuring liberation of n... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113649
BindingDB Entry DOI: 10.7270/Q2PV6Q63 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50552430

(CHEMBL4798369) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-Histamine from human histamine 3 receptor transfected in HEK293T cells incubated for 16 hrs by liquid scintillation counter anal... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115924
BindingDB Entry DOI: 10.7270/Q2833WNJ |
More data for this
Ligand-Target Pair | |
Beta-hexosaminidase
(Bos taurus) | BDBM50577843

(CHEMBL4853460)Show SMILES CC(=O)N[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O\C1=N/NC(=O)Nc1ccc2ccccc2c1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Competitive inhibition of cow HexA/HexB (unknown origin) using pNP-GlcNAc as substrate assessed as inhibition constant by measuring liberation of nit... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113649
BindingDB Entry DOI: 10.7270/Q2PV6Q63 |
More data for this
Ligand-Target Pair | |
Protein O-GlcNAcase
(Homo sapiens (Human)) | BDBM50531967
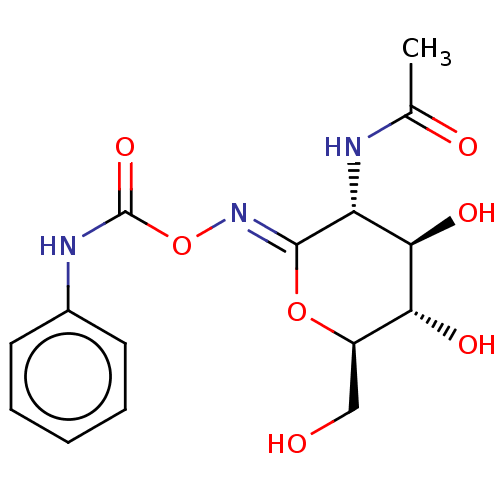
(Pugnac)Show SMILES CC(=O)N[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O\C1=N/OC(=O)Nc1ccccc1 |r| Show InChI InChI=1S/C15H19N3O7/c1-8(20)16-11-13(22)12(21)10(7-19)24-14(11)18-25-15(23)17-9-5-3-2-4-6-9/h2-6,10-13,19,21-22H,7H2,1H3,(H,16,20)(H,17,23)/b18-14-/t10-,11-,12-,13-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of OGA (unknown origin) using pNP-O-GlcNAc as substrate assessed as inhibition constant incubated for 30 mins |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113649
BindingDB Entry DOI: 10.7270/Q2PV6Q63 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(3) dopamine receptor
(Homo sapiens) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50552439

(CHEMBL4568949) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-Histamine from human histamine 4 receptor transfected in HEK293T cells incubated for 16 hrs by liquid scintillation counter anal... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115924
BindingDB Entry DOI: 10.7270/Q2833WNJ |
More data for this
Ligand-Target Pair | |
Protein O-GlcNAcase
(Homo sapiens (Human)) | BDBM50577842

(CHEMBL4866781)Show SMILES CC(=O)N[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O\C1=N/NC(=O)Nc1ccc(Cl)cc1 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Competitive inhibition of N-terminal His-tagged human OGA using 4-Mu-GlcNAc as substrate assessed as inhibition constant by measuring liberation of n... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113649
BindingDB Entry DOI: 10.7270/Q2PV6Q63 |
More data for this
Ligand-Target Pair | |
Beta-hexosaminidase
(Bos taurus) | BDBM50577838
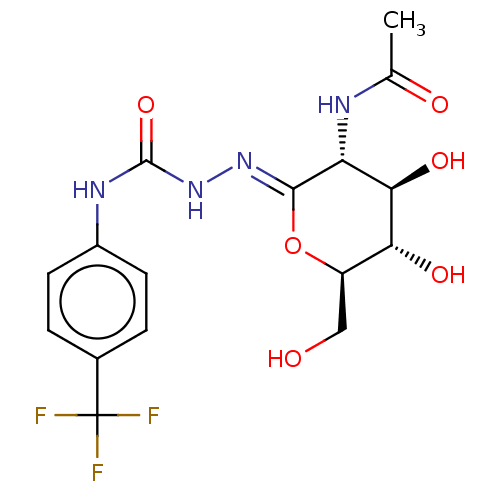
(CHEMBL4874886)Show SMILES CC(=O)N[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O\C1=N/NC(=O)Nc1ccc(cc1)C(F)(F)F |r| | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 125 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Competitive inhibition of cow HexA/HexB (unknown origin) using pNP-GlcNAc as substrate assessed as inhibition constant by measuring liberation of nit... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113649
BindingDB Entry DOI: 10.7270/Q2PV6Q63 |
More data for this
Ligand-Target Pair | |
Beta-hexosaminidase
(Bos taurus) | BDBM50577836
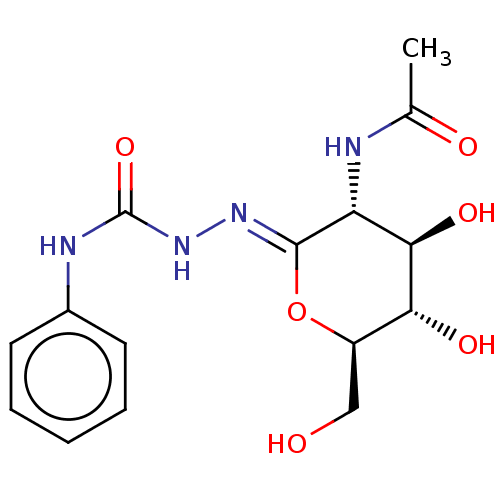
(CHEMBL4861823)Show SMILES CC(=O)N[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O\C1=N/NC(=O)Nc1ccccc1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HexA/HexB (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113649
BindingDB Entry DOI: 10.7270/Q2PV6Q63 |
More data for this
Ligand-Target Pair | |
Beta-hexosaminidase
(Bos taurus) | BDBM50577840

(CHEMBL4864246)Show SMILES CC(=O)N[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O\C1=N/NC(=O)Nc1cccc2ccccc12 |r| | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 154 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Competitive inhibition of cow HexA/HexB (unknown origin) using pNP-GlcNAc as substrate assessed as inhibition constant by measuring liberation of nit... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113649
BindingDB Entry DOI: 10.7270/Q2PV6Q63 |
More data for this
Ligand-Target Pair | |
Protein O-GlcNAcase
(Homo sapiens (Human)) | BDBM50577837
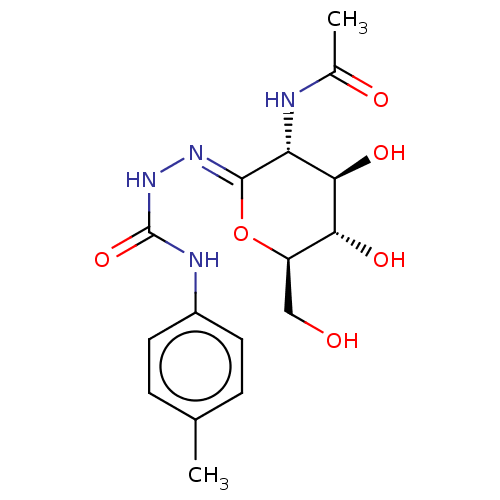
(CHEMBL4875285)Show SMILES CC(=O)N[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O\C1=N/NC(=O)Nc1ccc(C)cc1 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Competitive inhibition of N-terminal His-tagged human OGA using pNP-GlcNAc as chromogenic substrate assessed as inhibition constant by measuring libe... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113649
BindingDB Entry DOI: 10.7270/Q2PV6Q63 |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum mannosyl-oligosaccharide 1,2-alpha-mannosidase
(Homo sapiens (Human)) | BDBM50402966
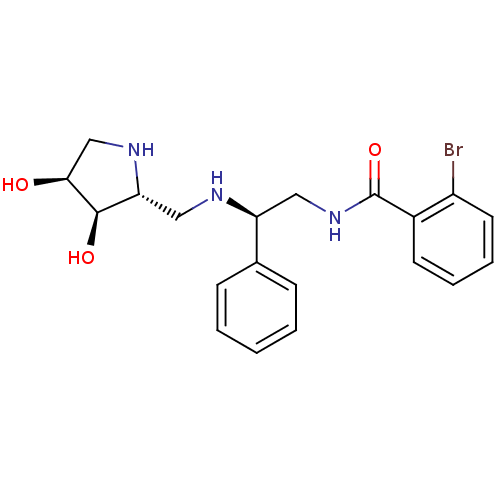
(CHEMBL2206824)Show SMILES O[C@H]1CN[C@H](CN[C@@H](CNC(=O)c2ccccc2Br)c2ccccc2)[C@H]1O |r| Show InChI InChI=1S/C20H24BrN3O3/c21-15-9-5-4-8-14(15)20(27)24-10-16(13-6-2-1-3-7-13)22-11-17-19(26)18(25)12-23-17/h1-9,16-19,22-23,25-26H,10-12H2,(H,24,27)/t16-,17+,18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Porto 687
Curated by ChEMBL
| Assay Description
Inhibition of human ER alpha mannosidase 1 |
Bioorg Med Chem 20: 6945-59 (2012)
Article DOI: 10.1016/j.bmc.2012.10.011
BindingDB Entry DOI: 10.7270/Q26T0NTC |
More data for this
Ligand-Target Pair | |
Protein O-GlcNAcase
(Homo sapiens (Human)) | BDBM50577838

(CHEMBL4874886)Show SMILES CC(=O)N[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O\C1=N/NC(=O)Nc1ccc(cc1)C(F)(F)F |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 167 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Competitive inhibition of N-terminal His-tagged human OGA using pNP-GlcNAc as chromogenic substrate assessed as inhibition constant by measuring libe... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113649
BindingDB Entry DOI: 10.7270/Q2PV6Q63 |
More data for this
Ligand-Target Pair | |
Beta-hexosaminidase
(Bos taurus) | BDBM50577842

(CHEMBL4866781)Show SMILES CC(=O)N[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O\C1=N/NC(=O)Nc1ccc(Cl)cc1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Competitive inhibition of cow HexA/HexB (unknown origin) using pNP-GlcNAc as substrate assessed as inhibition constant by measuring liberation of nit... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113649
BindingDB Entry DOI: 10.7270/Q2PV6Q63 |
More data for this
Ligand-Target Pair | |
Protein O-GlcNAcase
(Homo sapiens (Human)) | BDBM50577836

(CHEMBL4861823)Show SMILES CC(=O)N[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O\C1=N/NC(=O)Nc1ccccc1 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Competitive inhibition of N-terminal His-tagged human OGA using pNP-GlcNAc as chromogenic substrate assessed as inhibition constant by measuring libe... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113649
BindingDB Entry DOI: 10.7270/Q2PV6Q63 |
More data for this
Ligand-Target Pair | |
Beta-hexosaminidase subunit alpha
(Bos taurus) | BDBM50577836

(CHEMBL4861823)Show SMILES CC(=O)N[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O\C1=N/NC(=O)Nc1ccccc1 |r| | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 205 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Competitive inhibition of cow HexA/HexB (unknown origin) using pNP-GlcNAc as substrate assessed as inhibition constant by measuring liberation of nit... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113649
BindingDB Entry DOI: 10.7270/Q2PV6Q63 |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum mannosyl-oligosaccharide 1,2-alpha-mannosidase
(Homo sapiens (Human)) | BDBM50402972
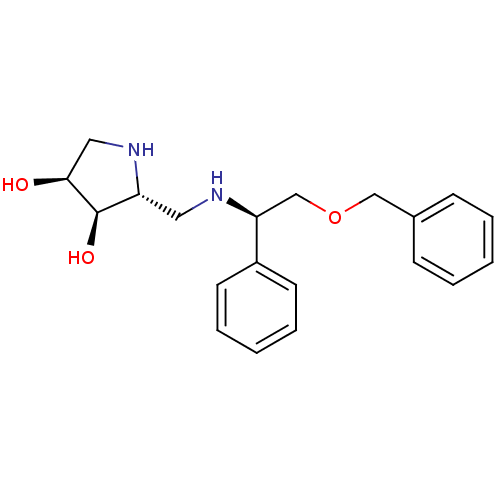
(CHEMBL2206818)Show SMILES O[C@H]1CN[C@H](CN[C@@H](COCc2ccccc2)c2ccccc2)[C@H]1O |r| Show InChI InChI=1S/C20H26N2O3/c23-19-12-22-17(20(19)24)11-21-18(16-9-5-2-6-10-16)14-25-13-15-7-3-1-4-8-15/h1-10,17-24H,11-14H2/t17-,18+,19+,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Porto 687
Curated by ChEMBL
| Assay Description
Inhibition of human ER alpha mannosidase 1 |
Bioorg Med Chem 20: 6945-59 (2012)
Article DOI: 10.1016/j.bmc.2012.10.011
BindingDB Entry DOI: 10.7270/Q26T0NTC |
More data for this
Ligand-Target Pair | |
Protein O-GlcNAcase
(Homo sapiens (Human)) | BDBM50577839

(CHEMBL4851727)Show SMILES COc1ccc(NC(=O)N\N=C2/O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2NC(C)=O)cc1 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Competitive inhibition of N-terminal His-tagged human OGA using pNP-GlcNAc as chromogenic substrate assessed as inhibition constant by measuring libe... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113649
BindingDB Entry DOI: 10.7270/Q2PV6Q63 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50610170

(CHEMBL5278711) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum mannosyl-oligosaccharide 1,2-alpha-mannosidase
(Homo sapiens (Human)) | BDBM50402967
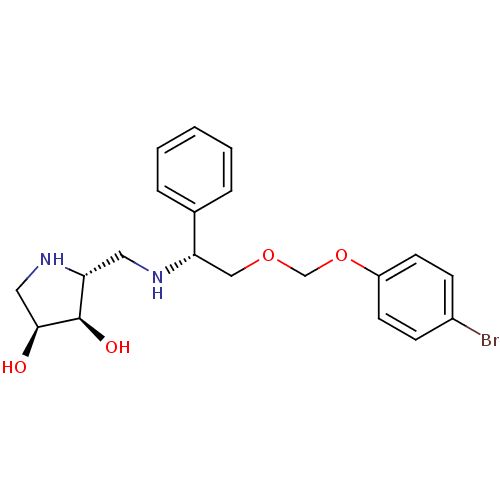
(CHEMBL2206823)Show SMILES O[C@H]1CN[C@H](CN[C@@H](COCOc2ccc(Br)cc2)c2ccccc2)[C@H]1O |r| Show InChI InChI=1S/C20H25BrN2O4/c21-15-6-8-16(9-7-15)27-13-26-12-18(14-4-2-1-3-5-14)22-10-17-20(25)19(24)11-23-17/h1-9,17-20,22-25H,10-13H2/t17-,18+,19+,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Porto 687
Curated by ChEMBL
| Assay Description
Inhibition of human ER alpha mannosidase 1 |
Bioorg Med Chem 20: 6945-59 (2012)
Article DOI: 10.1016/j.bmc.2012.10.011
BindingDB Entry DOI: 10.7270/Q26T0NTC |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum mannosyl-oligosaccharide 1,2-alpha-mannosidase
(Homo sapiens (Human)) | BDBM50402968
(CHEMBL2206822)Show SMILES O[C@H]1CN[C@H](CN[C@@H](COCOc2cccc(Br)c2)c2ccccc2)[C@H]1O |r| Show InChI InChI=1S/C20H25BrN2O4/c21-15-7-4-8-16(9-15)27-13-26-12-18(14-5-2-1-3-6-14)22-10-17-20(25)19(24)11-23-17/h1-9,17-20,22-25H,10-13H2/t17-,18+,19+,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Porto 687
Curated by ChEMBL
| Assay Description
Inhibition of human ER alpha mannosidase 1 |
Bioorg Med Chem 20: 6945-59 (2012)
Article DOI: 10.1016/j.bmc.2012.10.011
BindingDB Entry DOI: 10.7270/Q26T0NTC |
More data for this
Ligand-Target Pair | |
Beta-hexosaminidase
(Bos taurus) | BDBM50577837

(CHEMBL4875285)Show SMILES CC(=O)N[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O\C1=N/NC(=O)Nc1ccc(C)cc1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 332 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Competitive inhibition of cow HexA/HexB (unknown origin) using pNP-GlcNAc as substrate assessed as inhibition constant by measuring liberation of nit... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113649
BindingDB Entry DOI: 10.7270/Q2PV6Q63 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50552441

(CHEMBL4747747) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-Histamine from human histamine 3 receptor transfected in HEK293T cells incubated for 16 hrs by liquid scintillation counter anal... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115924
BindingDB Entry DOI: 10.7270/Q2833WNJ |
More data for this
Ligand-Target Pair | |
Beta-hexosaminidase
(Bos taurus) | BDBM50577839

(CHEMBL4851727)Show SMILES COc1ccc(NC(=O)N\N=C2/O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2NC(C)=O)cc1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 413 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Competitive inhibition of cow HexA/HexB (unknown origin) using pNP-GlcNAc as substrate assessed as inhibition constant by measuring liberation of nit... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113649
BindingDB Entry DOI: 10.7270/Q2PV6Q63 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data